Validation of a Pseudovirus Neutralization Assay for Severe Acute Respiratory Syndrome Coronavirus 2: A High-Throughput Method for the Evaluation of Vaccine Immunogenicity
Abstract
1. Introduction
2. Materials and Methods
2.1. Assay Procedure
2.2. Samples
2.3. Validation Assays
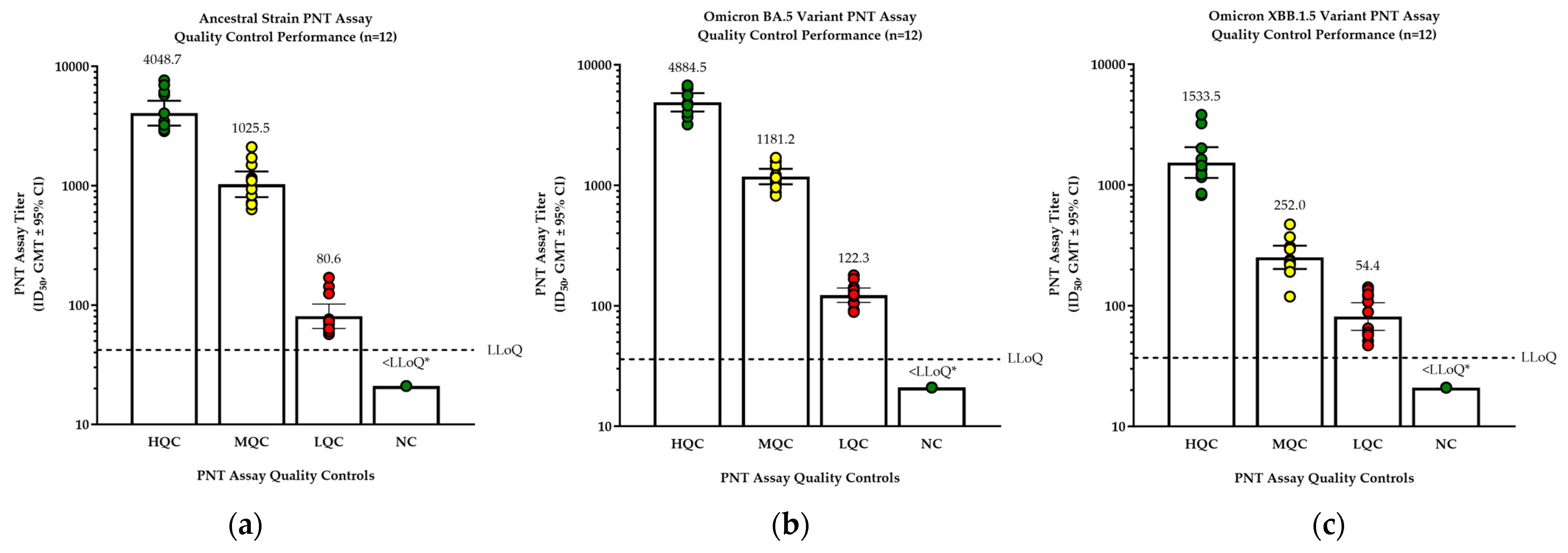
2.3.1. Precision
2.3.2. Specificity
2.3.3. Linearity
2.3.4. Sensitivity and Analytical Range
2.3.5. Conversion to WHO International Standard Units (IU/mL)
2.4. Variant Assays
2.5. Correlation Analyses
3. Results
3.1. Ancestral Strain Validation Parameters
3.2. Assay Validations for Variants
3.3. Correlation between the PNT Assay and the Whole-Virus-Based MN Assay
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. David J. Spencer CDC Museum—COVID-19 Timeline. Available online: https://www.cdc.gov/museum/timeline/covid19.html (accessed on 16 June 2023).
- Fendler, A.; de Vries, E.G.E.; GeurtsvanKessel, C.H.; Haanen, J.B.; Wormann, B.; Turajlic, S.; von Lilienfeld-Toal, M. COVID-19 vaccines in patients with cancer: Immunogenicity, efficacy and safety. Nat. Rev. Clin. Oncol. 2022, 19, 385–401. [Google Scholar] [CrossRef] [PubMed]
- Fiolet, T.; Kherabi, Y.; MacDonald, C.J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2022, 28, 202–221. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Rocklov, J. The effective reproductive number of the Omicron variant of SARS-CoV-2 is several times relative to Delta. J. Travel. Med. 2022, 29, taac037. [Google Scholar] [CrossRef] [PubMed]
- Van Tilbeurgh, M.; Lemdani, K.; Beignon, A.S.; Chapon, C.; Tchitchek, N.; Cheraitia, L.; Marcos Lopez, E.; Pascal, Q.; Le Grand, R.; Maisonnasse, P.; et al. Predictive Markers of Immunogenicity and Efficacy for Human Vaccines. Vaccines 2021, 9, 579. [Google Scholar] [CrossRef] [PubMed]
- Pang, N.Y.; Pang, A.S.; Chow, V.T.; Wang, D.Y. Understanding neutralising antibodies against SARS-CoV-2 and their implications in clinical practice. Mil. Med. Res. 2021, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.; Theel, E.S. Immunity to SARS-CoV-2: What Do We Know and Should We Be Testing for It? J. Clin. Microbiol. 2022, 60, e0048221. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, X.E. Construction and applications of SARS-CoV-2 pseudoviruses: A mini review. Int. J. Biol. Sci. 2021, 17, 1574–1580. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Kalkeri, R.; Zhu, M.; Cloney-Clark, S.; Haner, B.; Wang, M.; Osman, B.; Dent, D.; Feng, S.-L.; Longacre, Z.; et al. A pseudovirus-based neutralization assay for SARS-COV-2 variants: A rapid, cost-effective, BSL-2–based high-throughput assay useful for vaccine immunogenicity evaluation. Microrganisms 2024, 12, 501. [Google Scholar] [CrossRef] [PubMed]
- Keech, C.; Albert, G.; Cho, I.; Robertson, A.; Reed, P.; Neal, S.; Plested, J.S.; Zhu, M.; Cloney-Clark, S.; Zhou, H.; et al. Phase 1-2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. N. Engl. J. Med. 2020, 383, 2320–2332. [Google Scholar] [CrossRef] [PubMed]
- Sholukh, A.M.; Fiore-Gartland, A.; Ford, E.S.; Miner, M.D.; Hou, Y.J.; Tse, L.V.; Kaiser, H.; Zhu, H.; Lu, J.; Madarampalli, B.; et al. Evaluation of Cell-Based and Surrogate SARS-CoV-2 Neutralization Assays. J. Clin. Microbiol. 2021, 59, e0052721. [Google Scholar] [CrossRef] [PubMed]
- Bhiman, J.N.; Richardson, S.I.; Lambson, B.E.; Kgagudi, P.; Mzindle, N.; Kaldine, H.; Crowther, C.; Gray, G.; Bekker, L.G.; Novavax Trial Clinical Lead Author Group; et al. Novavax NVX-COV2373 triggers neutralization of Omicron sub-lineages. Sci. Rep. 2023, 13, 1222. [Google Scholar] [CrossRef] [PubMed]
- Fong, Y.; Huang, Y.; Benkeser, D.; Carpp, L.N.; Anez, G.; Woo, W.; McGarry, A.; Dunkle, L.M.; Cho, I.; Houchens, C.R.; et al. Immune correlates analysis of the PREVENT-19 COVID-19 vaccine efficacy clinical trial. Nat. Commun. 2023, 14, 331. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Mohlenberg, M.; Wang, P.; Zhou, H. Immune evasion of neutralizing antibodies by SARS-CoV-2 Omicron. Cytokine Growth Factor. Rev. 2023, 70, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liang, J.; Hu, H.; Li, X.; Wang, L.; Wang, Z. Research progress in methods for detecting neutralizing antibodies against SARS-CoV-2. Anal. Biochem. 2023, 673, 115199. [Google Scholar] [CrossRef] [PubMed]
- Riepler, L.; Rossler, A.; Falch, A.; Volland, A.; Borena, W.; von Laer, D.; Kimpel, J. Comparison of Four SARS-CoV-2 Neutralization Assays. Vaccines 2020, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Updated COVID-19 Vaccines for Use in the United States for 2023–2024. Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines-2023-2024#:~:text=The%20FDA%20has%20approved%20and,19%20caused%20by%20circulating%20variants (accessed on 11 June 2024).
- World Health Organization. Statement on the Antigen Composition of COVID-19 Vaccines. Available online: https://www.who.int/news/item/18-05-2023-statement-on-the-antigen-composition-of-covid-19-vaccines (accessed on 28 June 2023).
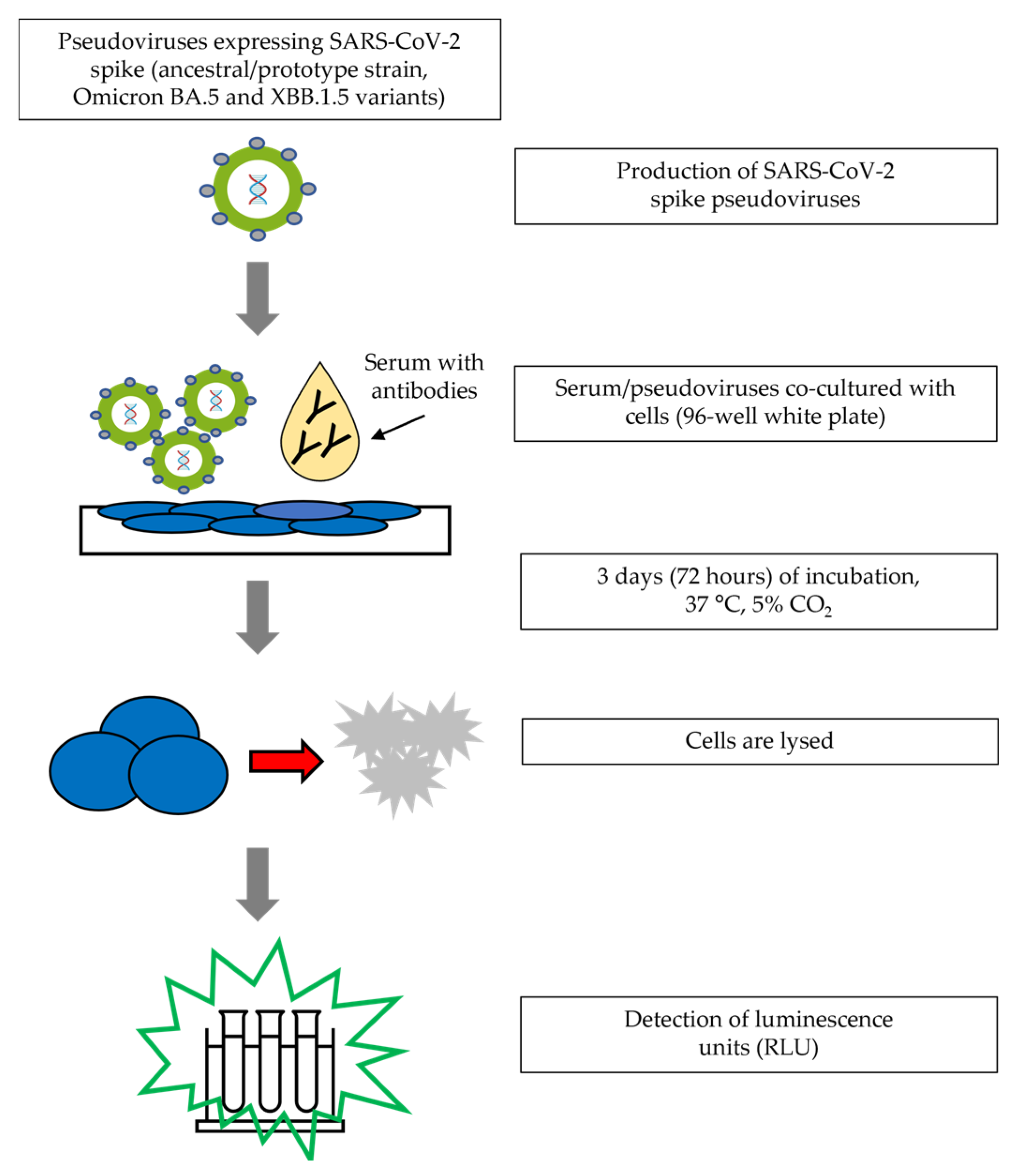


| Sample | N 1 | PNT Titer GMT 2 | Inter-Assay %GCV | Intra-Assay %GCV | Total %GCV |
|---|---|---|---|---|---|
| Overall 3 | N/A | N/A | 6.6 | 42.8 | 43.4 |
| 1 | 24 | 4048.7 | 46.6 | 34.2 | 60.0 |
| 2 | 24 | 1025.5 | 12.9 | 42.4 | 44.7 |
| 3 | 24 | 80.6 | 39.8 | 33.2 | 53.4 |
| 4 | 24 | 20.02 | 0.0 | 0.0 | 0.0 |
| 5 | 24 | 104.0 | 0.0 | 35.9 | 35.9 |
| 6 | 24 | 281.8 | 10.9 | 25.5 | 27.9 |
| 7 | 24 | 12,248.5 | 9.0 | 35.5 | 36.8 |
| 8 | 24 | 172.3 | 0.0 | 41.8 | 41.8 |
| 9 | 24 | 193.9 | 0.0 | 40.4 | 40.4 |
| 10 | 24 | 11,589.0 | 6.6 | 40.7 | 41.4 |
| 11 | 24 | 8463.1 | 12.4 | 36.2 | 38.6 |
| 12 | 24 | 2128.9 | 25.9 | 56.4 | 63.7 |
| 13 | 23 | 28.0 | 0.0 | 49.4 | 49.4 |
| 14 | 24 | 816.0 | 7.3 | 37.8 | 38.6 |
| 15 | 24 | 2424.7 | 13.8 | 34.5 | 37.5 |
| 16 | 24 | 397.0 | 0.0 | 43.9 | 43.9 |
| 17 | 24 | 2269.5 | 10.6 | 43.6 | 45.1 |
| 18 | 24 | 375.9 | 13.5 | 38.4 | 41.0 |
| 19 | 24 | 783.7 | 3.6 | 43.2 | 43.4 |
| 20 | 24 | 1978.5 | 44.8 | 49.4 | 70.3 |
| 21 | 24 | 1322.5 | 18.1 | 42.9 | 47.2 |
| 22 | 24 | 3044.9 | 50.8 | 41.0 | 68.5 |
| 23 | 24 | 5885.3 | 38.9 | 36.3 | 55.1 |
| 24 | 24 | 1330.1 | 0.0 | 52.9 | 52.9 |
| 25 | 24 | 546.9 | 37.8 | 33.0 | 51.7 |
| 26 | 22 | 230.2 | 27.5 | 25.2 | 37.9 |
| 27 | 18 | 73.0 | 60.5 | 39.6 | 76.1 |
| 28 | 21 | 27.8 | 17.8 | 36.6 | 41.2 |
| 29 | 22 | 20.4 | 3.0 | 4.7 | 5.6 |
| 30 | 24 | 32.8 | 24.6 | 50.6 | 57.6 |
| 31 | 24 | 34.4 | 45.8 | 49.3 | 71.0 |
| 32 | 24 | 14,863.5 | 0.0 | 59.8 | 59.8 |
| 33 | 24 | 5386.6 | 47.2 | 42.6 | 66.7 |
| 34 | 24 | 2136.1 | 7.6 | 43.5 | 44.3 |
| 35 | 24 | 468.2 | 0.0 | 40.7 | 40.7 |
| 36 | 21 | 66.0 | 0.0 | 23.5 | 23.5 |
| 37 | 19 | 44.1 | 0.0 | 41.1 | 41.1 |
| 38 | 23 | 42.8 | 0.0 | 46.1 | 46.1 |
| 39 | 24 | 38.1 | 31.8 | 46.4 | 58.1 |
| WHO Reference 20/136 | 16 | 4680.9 | 0.0 | 49.0 | 49.0 |
| Pre-COVID-19 healthy human sera | |||||||
|---|---|---|---|---|---|---|---|
| Humanserum | PNT titer | ||||||
| Positive Control-1 | 4048.7 | ||||||
| Positive Control-2 | 1025.5 | ||||||
| Positive Control-3 | 80.6 | ||||||
| Pre-COVID-1 | <LLoQ | ||||||
| Pre-COVID-2 | <LLoQ | ||||||
| Pre-COVID-3 | <LLoQ | ||||||
| Pre-COVID-4 | <LLoQ | ||||||
| Pre-COVID-5 | <LLoQ | ||||||
| Pre-COVID-6 | <LLoQ | ||||||
| Pre-COVID-7 | <LLoQ | ||||||
| Pre-COVID-8 | <LLoQ | ||||||
| RSV F protein-vaccinated sera | |||||||
| Participant ID | Anti-RSV F protein antibody | Wuhan D614 prototype PNT titer | |||||
| Pre-RSV F vaccination (day 0) | Post-RSV F vaccination (day 14) | Post/pre ratio | Pre-RSV F vaccination (day 0) | Post-RSV F vaccination (day 14) | Post/pre ratio | ||
| 41 | 509 | 43,767 | 86 | <LLoQ | <LLoQ | 1 | |
| 42 | 474 | 20,188 | 42.6 | <LLoQ | <LLoQ | 1 | |
| 43 | 483 | 9721 | 20.1 | <LLoQ | <LLoQ | 1 | |
| 44 | 632 | 10,958 | 17.3 | <LLoQ | <LLoQ | 1 | |
| 45 | 772 | 15,869 | 20.6 | <LLoQ | <LLoQ | 1 | |
| Influenza-vaccinated sera | |||||||
| Participant ID | Influenza HAI titer (A/Kansas/14/2017) (H3N2) | Wuhan D614 prototype PNT titer | |||||
| Pre-influenza vaccination (day 0) | Post-influenza vaccination (day 28) | Post/pre-vaccination ratio | Pre-influenza vaccination (day 0) | Post-influenza vaccination (day 28) | Post/pre-vaccination ratio | ||
| 46 | 10 | 640 | 64 | <LLoQ | <LLoQ | 1 | |
| 47 | 20 | 1280 | 64 | <LLoQ | <LLoQ | 1 | |
| 48 | 10 | 1280 | 128 | <LLoQ | <LLoQ | 1 | |
| 49 | 20 | 2560 | 128 | <LLoQ | <LLoQ | 1 | |
| 50 | 40 | 2560 | 64 | <LLoQ | <LLoQ | 1 | |
| Parameter | Estimate | 95% LCL | 95% UCL |
|---|---|---|---|
| Slope | 1.000 | 0.847 | 1.153 |
| Intercept | −0.046 | −0.492 | 0.400 |
| R2 | 0.983 | N/A | |
| Dilution | N | Observed PNT GMT | Expected PNT GMT | %Relative Bias | Inter-Assay %GCV | Intra-Assay %GCV | Total %GCV |
|---|---|---|---|---|---|---|---|
| 1 | 12 | 14,863.2 | 14,863.2 | 0.0 | 0.0 | 59.8 | 59.8 |
| 2 | 12 | 5386.6 | 7431.6 | −27.5 | 47.2 1 | 42.6 | 66.7 |
| 8 | 12 | 2136.2 | 1857.9 | 15.0 | 7.6 | 43.5 | 44.3 |
| 32 | 12 | 468.2 | 464.5 | 0.8 | 0.0 | 40.7 | 40.7 |
| 128 | 12 | 66.7 | 116.1 | −42.6 | 0.0 | 23.5 | 23.5 |
| 256 | 12 | 39.9 | 58.1 | −31.3 | 0.0 | 41.1 | 41.1 |
| 512 | 12 | 41.6 | 29.0 | 43.4 | 0.0 | 46.1 | 46.1 |
| 1024 | 12 | 38.0 | 14.5 | 161.9 | 31.8 1 | 46.4 | 58.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Z.; Kalkeri, R.; Wang, M.; Haner, B.; Dent, D.; Osman, B.; Skonieczny, P.; Ross, J.; Feng, S.-L.; Cai, R.; et al. Validation of a Pseudovirus Neutralization Assay for Severe Acute Respiratory Syndrome Coronavirus 2: A High-Throughput Method for the Evaluation of Vaccine Immunogenicity. Microorganisms 2024, 12, 1201. https://doi.org/10.3390/microorganisms12061201
Cai Z, Kalkeri R, Wang M, Haner B, Dent D, Osman B, Skonieczny P, Ross J, Feng S-L, Cai R, et al. Validation of a Pseudovirus Neutralization Assay for Severe Acute Respiratory Syndrome Coronavirus 2: A High-Throughput Method for the Evaluation of Vaccine Immunogenicity. Microorganisms. 2024; 12(6):1201. https://doi.org/10.3390/microorganisms12061201
Chicago/Turabian StyleCai, Zhaohui, Raj Kalkeri, Mi Wang, Benjamin Haner, Dominic Dent, Bahar Osman, Paul Skonieczny, Jeremy Ross, Sheau-Line Feng, Rongman Cai, and et al. 2024. "Validation of a Pseudovirus Neutralization Assay for Severe Acute Respiratory Syndrome Coronavirus 2: A High-Throughput Method for the Evaluation of Vaccine Immunogenicity" Microorganisms 12, no. 6: 1201. https://doi.org/10.3390/microorganisms12061201
APA StyleCai, Z., Kalkeri, R., Wang, M., Haner, B., Dent, D., Osman, B., Skonieczny, P., Ross, J., Feng, S.-L., Cai, R., Zhu, M., Cloney-Clark, S., & Plested, J. S. (2024). Validation of a Pseudovirus Neutralization Assay for Severe Acute Respiratory Syndrome Coronavirus 2: A High-Throughput Method for the Evaluation of Vaccine Immunogenicity. Microorganisms, 12(6), 1201. https://doi.org/10.3390/microorganisms12061201





