Abstract
Previous reports have mainly investigated the long-term effects (>30 d), such as gut microbiota dysbiosis and systemic low-grade inflammation, in mice fed fried oil. However, short-term intake of deep-fried oil is more likely to occur in daily life, and such studies are lacking. This study aimed to investigate the short-term effects of fried oil intake on systemic low-grade inflammation. Male Kunming mice were fed non-fried soybean oil or low (25%), medium (50%), or high (100%)—fried oil at 4.4 g/kg for 6 d. Serum and fecal samples were collected on day 7. In all groups fed fried oil, the serum levels of tumor necrosis factor (TNF-α) were significantly elevated 2-4-fold. Among the gut microbiota, the abundance of Alloprevotella significantly decreased by up to 76%, while Lactobacilli significantly increased by up to 385%. The fecal valeric acid content was significantly increased and positively correlated with TNF-α levels. Both valeric acid and TNF-α levels were positively correlated with the abundance of Lactobacilli and negatively correlated with that of Alloprevotella. In summary, a short-term ingestion of even low doses of fried oil alters the gut microbiota Alloprevotella and Lactobacilli and increases fecal valeric acid content, which correlates with increased serum TNF-α levels.
1. Introduction
Many consumers favor fried food because of its crisp taste, quick preparation, and convenience. To reduce costs, reusing fried oil has become a common practice in many fast-food restaurants. Cooking oil is easily oxidized by frequent heating to high temperatures, which not only reduces its nutritional value but also can form toxic chemicals, including benzopyrene and heterocyclic amines, that can leach into food. Eating oil-fried food can lead to obesity, metabolic disorders, systemic low-grade inflammation, and other diseases [1,2].
The consumption of fried food commonly induces systemic low-grade inflammation. This state is non-specific, persistent, and mild, as evidenced by a 2-4-fold increase in plasma inflammatory factors such as TNF-α [3], a cytokine considered to be a key factor in age-related diseases, autoimmune disorders, cancer, cardiovascular diseases, and diabetes [4]. Therefore, it is important to investigate the low-grade inflammation mechanism caused by the repeated use of oil for frying to understand the mechanistic targets and their regulation.
Some studies suggest that the damage caused by fried oil to the body systems is a result of oxidative stress [5]. However, there is now strong evidence that the gut microbiota and its metabolic disturbances are also responsible for the induction of systemic low-grade inflammation, especially by changes in the composition and levels of short-chain fatty acids (SCFAs), which are mostly the metabolic end products of the gut microbiota [6]. They regulate body inflammatory responses by inducing or inhibiting cytokine production [7]. However, the changes in the microbiota and associated metabolite composition caused by different inducers and biomarkers of systemic low-grade inflammation are different. Therefore, clarifying the effects of fried oil on gut microbiota structure and SCFA levels is important for identifying the mechanisms by which fried oil induces systemic low-grade inflammation. Based on this, we hypothesized that a short-term consumption of repeatedly fried soybean oil would induce systemic low-grade inflammation by altering the structure of the gut microbiota community and the production of SCFAs, thus providing a basis for exploring targeted interventions to mitigate the health risks associated with a frequent consumption of fried foods.
The current literature on the harm caused by fried oil is mostly based on a long-term (≥30 days) and excessive consumption of fried oil [8,9], and there is a lack of research on the effects of short-term (one week) intake of fried oil. The short-term consumption of fried oil aligns with the frequency of the individual consumption of fried foods in daily life. Investigating the mechanism of induction of systemic low-grade inflammation following short-term exposure may offer more precise health recommendations.
This study explored the mechanism of low-grade inflammation induced by the short-term dietary intake of fried oil to provide theoretical support for exploring specific control targets.
2. Materials and Methods
2.1. Preparation of Fried Oil
Chicken fillets, French fries, and dough were fried in fresh soybean oil for 10 min each hour for 8 h (from 9 a.m. to 5 p.m.) for 4 consecutive days, for a total of 32 times. Four sets of soybean-fried oils were prepared based on the percentage of heated soybean oil in the mix. We used unheated soybean oil (C), 25% fried soybean oil +75% unheated soybean oil (low dose: LD), 50% fried soybean oil +50% unheated soybean oil (medium dose: MD), and 100% fried soybean oil (high dose: HD).
2.2. Animal Study
Six-week-old male Kunming mice (30 ± 2 g) were purchased from Changsha Tianqin Biotechnology Co., Ltd., Changsha, China (SPF, SCXK [Xiang] 2019-0014). The experimental animals were maintained at room temperature (RT: 25 ± 2 °C), a humidity of 40–70%, and a 12 h light/dark cycle. The experimental animals were approved by the Animal Ethics Committee of Guangdong Ocean University (GDOU-LAE-2020-018) and raised in strict accordance with the operating standards of the Experimental Animal Center of Guangdong Ocean University (SYXK2014-0053). The standard polypropylene cages used in the experiments were 53 cm in length, 39 cm in width, and 20 cm in height, which provided ample living space for the mice and complied with animal welfare and ethical standards. Water bottles, cages, and bedding materials were sterilized using high-pressure steam, and the water bottles and bedding materials were changed three times a week. After one week of adaptive feeding, 20 mice were randomly divided into 4 groups with 5 animals each: C, LD, MD, and HD. All mice received an intragastric administration of either unheated soybean oil (C) or fried oil (LD, MD, HD) at 4.4 g/kg, respectively, once daily for 6 d. The body weights of the mice were measured daily during the experimental period.
2.3. Serum TNF-α
After 6 days of gavage, blood was collected from the eyeballs of mice on day 7, and centrifuged at 4 °C, 8300 r/min, for 5min in a cryo-centrifuge (ST16R, Thermo Fisher Technology [China] Co., Ltd., Shanghai, China). The supernatant was aspirated and placed in a −80 °C ultra-low-temperature refrigerator (JW-86-158-LA). Serum TNF-α concentration was measured using a TNF-α ELISA kit (Shenzhen Xinbosheng Biotechnology Co., Ltd., Shenzhen, China) procedure.
2.4. Gut Microbiota
On day 7, the fecal samples of mice were collected into 1.5 mL sterile EP tubes and stored in an ultra-low-temperature refrigerator at −80 °C. Fecal samples were sent to Hangzhou GH Information Technology Co., Ltd., Hangzhou, China. for 16S rRNA gene sequencing and detection using an Illumina HiSeq4000 pair-end 2 × 150 bp sequencing platform. Gut microbiota strain categories were identified by database comparisons.
2.5. Fecal SCFAs
Fecal SCFA levels were detected using GC-MS (GCMS-TQ8050 NX, Shimadzu Co., Ltd., Kyoto City, Japan). Fecal samples (50 mg) were added to 100 μL of 15% phosphoric acid, followed by 100 μL of a 125 μg/mL isocaproic acid solution and 900 μL diethyl ether, and then homogenized and centrifuged for 10 min. The mixed solution was filtered using a 0.22 μm organic microporous membrane. The GC conditions were as follows: a VF-17MS capillary column; inlet temperature of 250 °C; ion source temperature of 230 °C; transmission line temperature of 250 °C; and four-bar temperature of 150 °C. MS conditions were the following: an electron bombardment ionization source, single-ion scanning mode, and electron energy of 70 eV.
2.6. Statistical Analysis
The experimental data were statistically analyzed using SPSS27.0 software. Pearson’s method was used to analyze data correlation. Data are expressed as the “mean ± SD”, and a one-way analysis of variance was used to compare the means of each group, with p < 0.05 indicating a statistically significant difference.
3. Results
3.1. Effects of Fried Oil on Body Weights
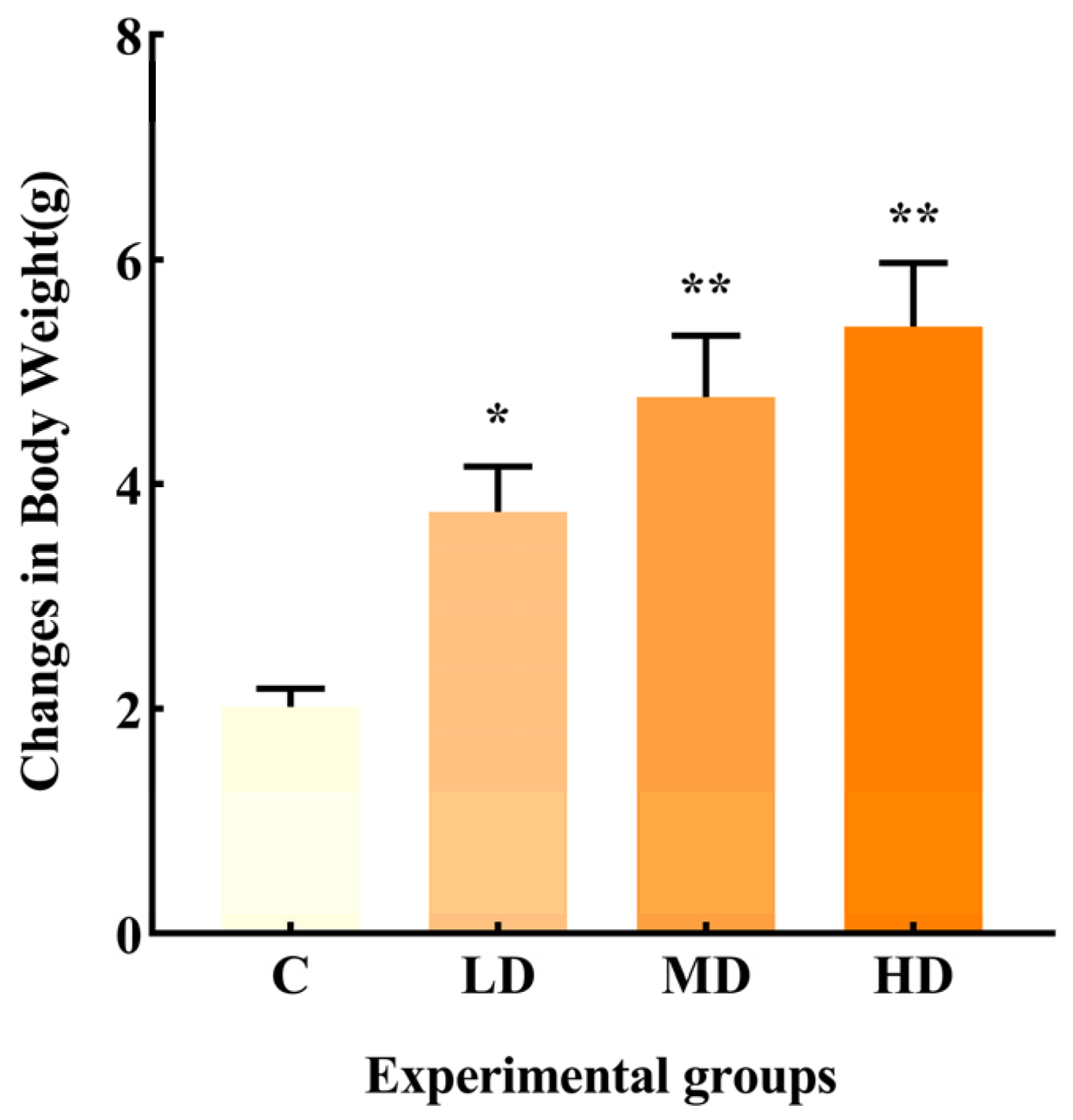
As shown in Figure 1, the change in body weight in the LD, MD, and HD groups was significantly higher than that in group C, with increases of 86.3% (p < 0.05), 137%, and 168% (p < 0.01), respectively.
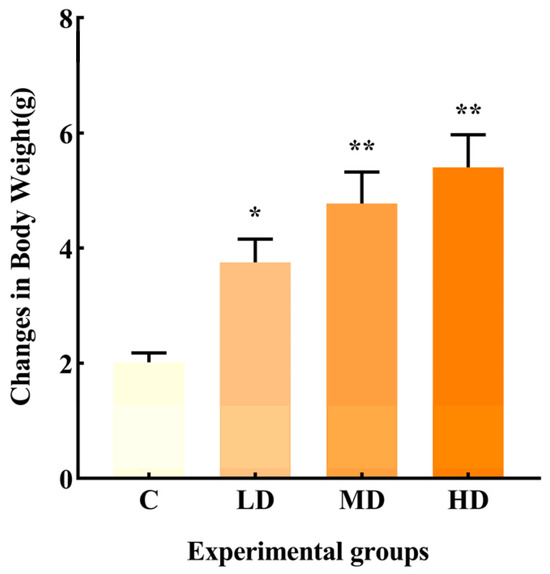
Figure 1.
Effects of different doses of fried oil on body weight of mice after six days of intragastric administration. Data are expressed as mean ± SD. * p < 0.05, ** p < 0.01 vs. C.
3.2. Effects of Fried Oil on Serum TNF-α
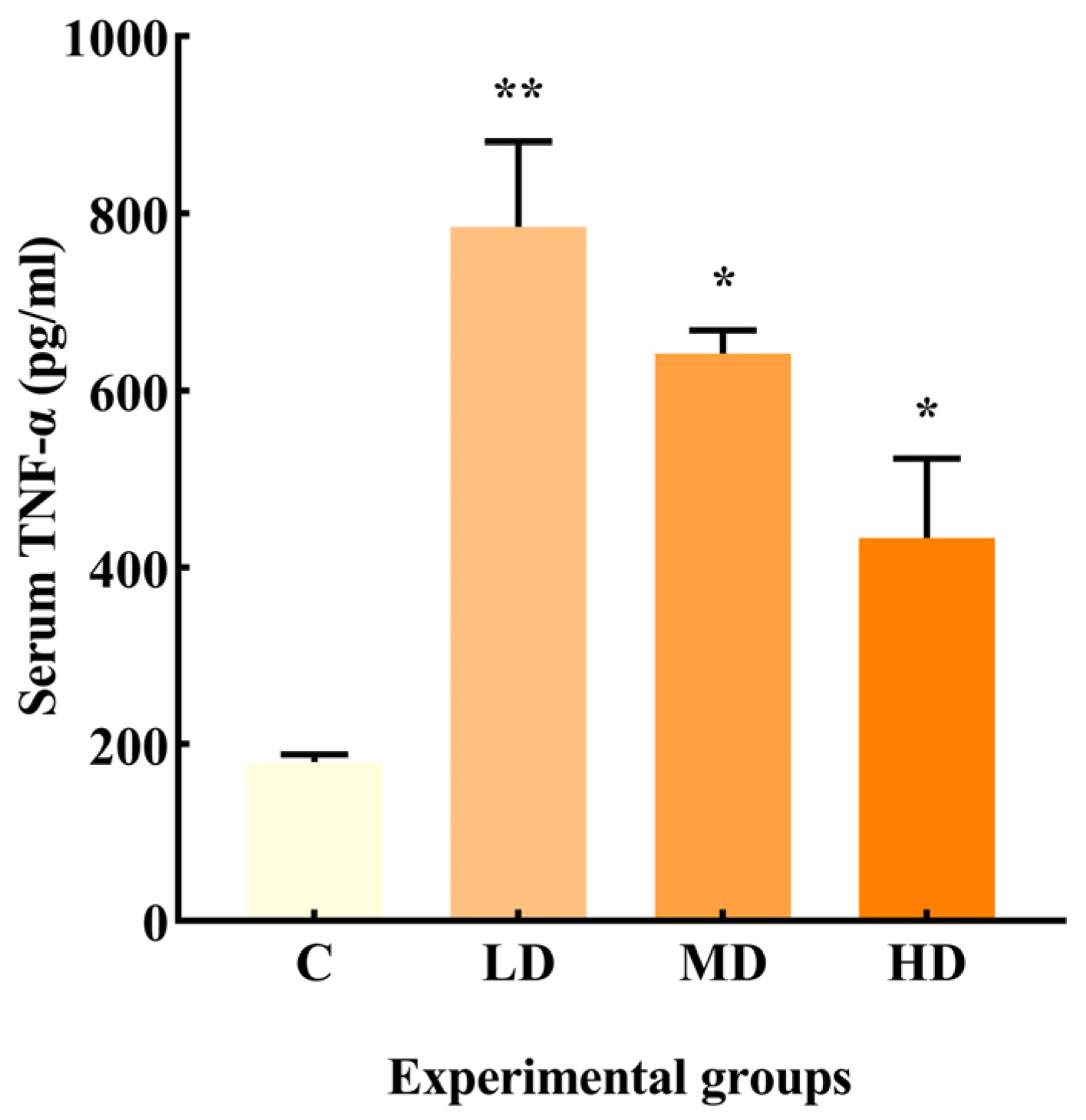
As illustrated in Figure 2, the LD, MD, and HD groups exhibited a significant increase in TNF-α production by 335% (p < 0.01) and 256% and 140% (p < 0.05), respectively, compared to the C group.
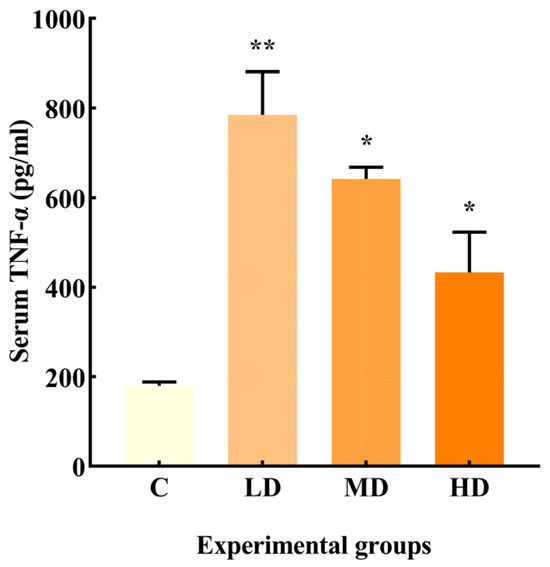
Figure 2.
Effects of different doses of fried oil on serum TNF-α in mice. Data are expressed as mean ± SD. * p < 0.05, ** p < 0.01 vs. C.
3.3. Effects of Fried Oil on the Gut Microbiota Diversity
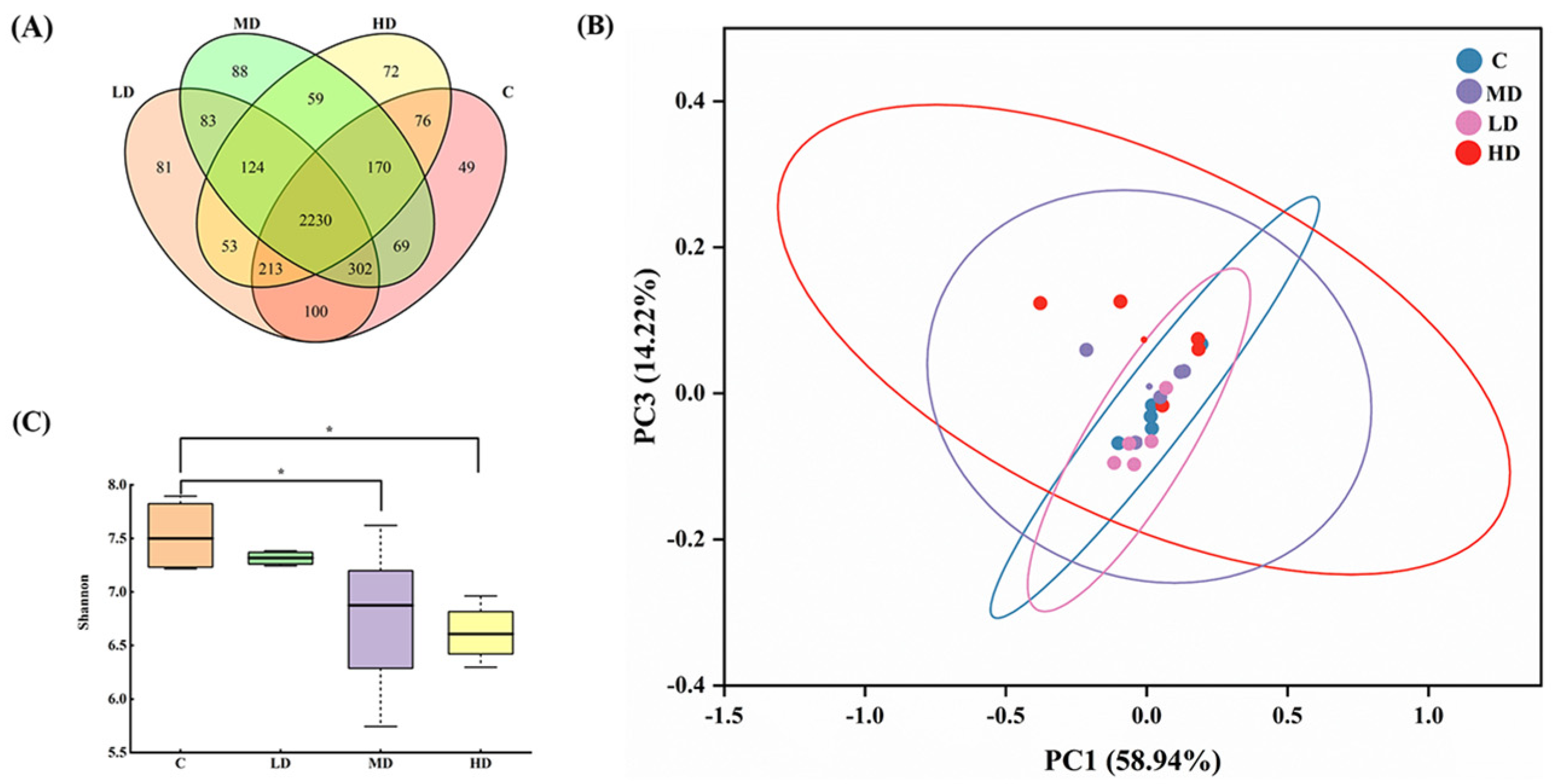
As shown in Figure 3A, 3209, 3186, 3125, and 2997 OTUs were obtained in the C, LD, MD, and HD groups, respectively. With an increase in the fried oil dose, the OTU values of the gut microbiota in mice gradually decreased. These results indicate that a certain dose of fried oil can reduce the species diversity of the gut microbiota in mice in the short term. The total OUT counts in the LD, MD, HD, and C groups were 2845, 2771, and 2689, respectively. As the dosage of fried oil increased, the population of OTUs shared in the gut microbiota of mice treated with fried oil and non-fried oil gradually declined, indicating an increasing divergence in the gut microbiota between the two groups. A principal coordinate analysis using UniFrac indicated differences in the gut microbiota between samples and revealed different clusters of microbiota composition in each group. As illustrated in Figure 3B, the differences in the gut microbiota between samples were insignificant, indicating that the structural characteristics of the gut microbiota were analogous in all groups. In Figure 3C, the Shannon index of the MD and HD groups was significantly lower than that of the C group (p < 0.05).
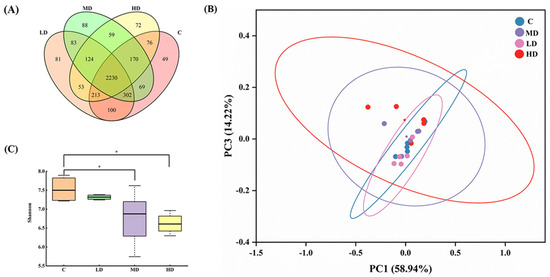
Figure 3.
Effects of fried oil on gut microbiota diversity in mice. (A) Venn diagram display of mouse gut microbiota in each group. (B) Principal coordinate analysis showing mouse gut microbiota structure. (C) Shannon index of mouse gut microbiota. Data are expressed as mean ± SD. * p < 0.05 vs. C.
In conclusion, this indicates that in terms of alpha diversity, intake of medium and high doses of fried oil can reduce the richness and diversity of the gut microbiota.
3.4. Effects of Fried Oil on the Abundance of Gut Microbiota at the Phylum Level
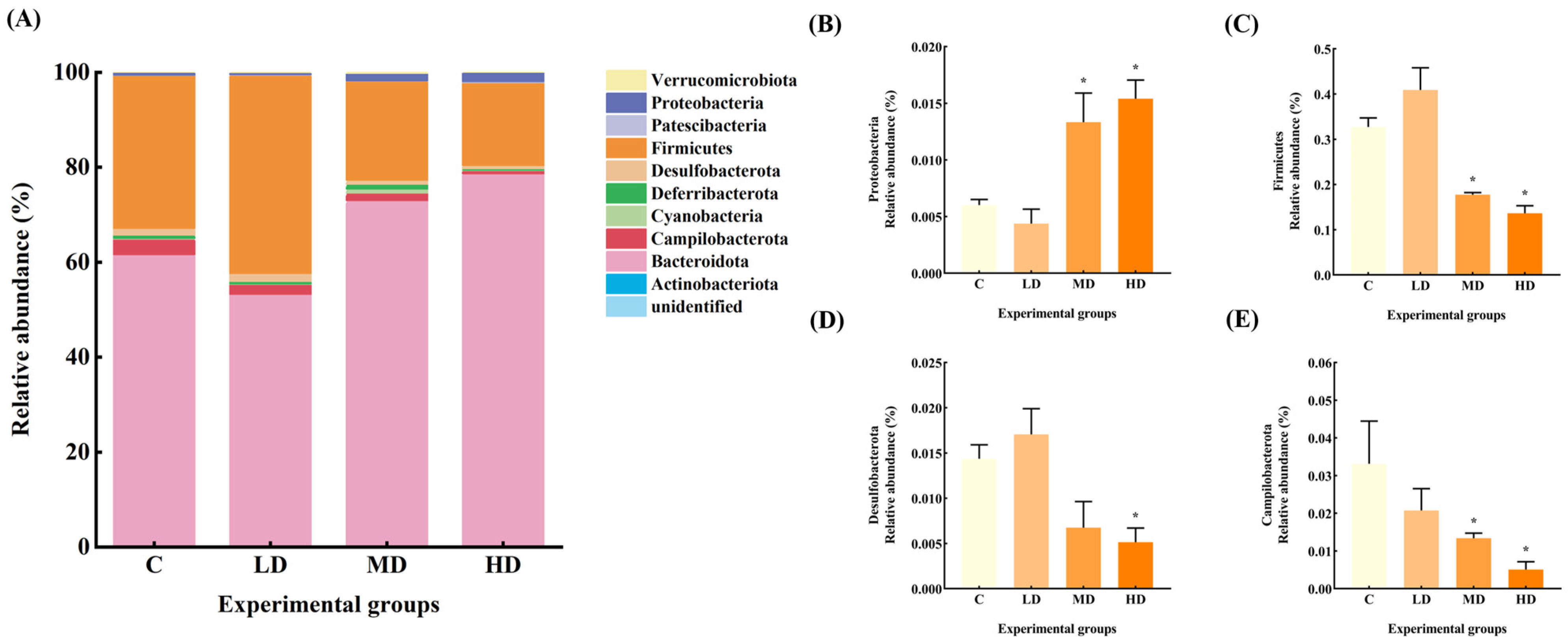
The gut microbiota constitutive abundance of the mice in each group at the phylum level is shown in Figure 4. The relative abundances of gut microbiota Firmicutes and Desulfobacterota in the LD group increased by 25% and 19%, respectively, compared to the C group. The relative abundance of Deferribacterota and Campilobacterota decreased by 31% and 37%, respectively.
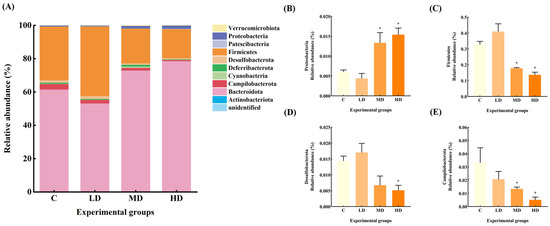
Figure 4.
(A) Abundance plots of species composition at the phylum level for each group of mice gut microbiota. (B) Proteobacteria relative abundance. (C) Firmicutes relative abundance. (D) Desulfobacterota relative abundance. (E) Campilobacterota relative abundance. Data are expressed as mean ± SD. * p < 0.05 vs. C.
The relative abundance of Proteobacteria in the gut microbiota of mice in the MD group increased by 122% (p < 0.05), and that of Deferribacterota increased by 27%. The relative abundance of Firmicutes and Campilobacterota decreased by 46% and 60%, respectively (p < 0.05), and Desulfobacterota decreased by 53%.
The relative abundance of Proteobacteria in the HD group was significantly increased by 156% (p < 0.05), whereas the relative abundance of Desulfobacterota, Firmicutes, and Campilobacterota was significantly decreased by 64%, 58%, and 85%, respectively (p < 0.05).
3.5. Effects of Fried Oil on Gut Microbiota Abundance at Genus Level
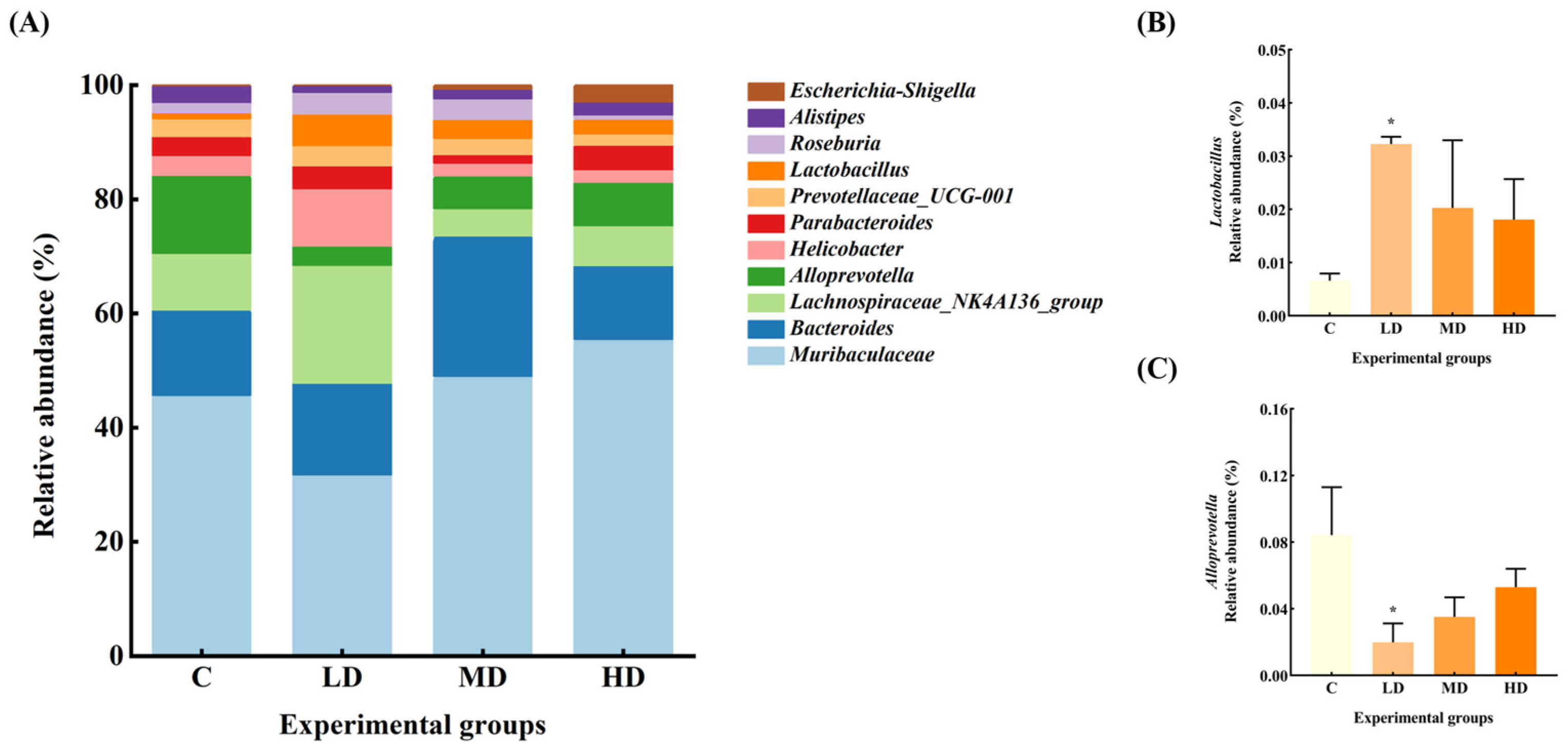
The gut microbiota of the mice in each group at the genus level are shown in Figure 5. The relative abundance of Muribaculaceae and Alistipes in the gut microbiota of mice in the LD group was reduced by 37% and 60%, respectively, compared with the C group. Alloprevotella significantly decreased by 76% (p < 0.05). The relative abundance of Lachnospiraceae_NK4A136_group, Helicobacter, and Roseburia increased by 96%, 170%, and 123%, respectively. Notably, the abundance of Lactobacillus significantly increased by 385% (p < 0.05).
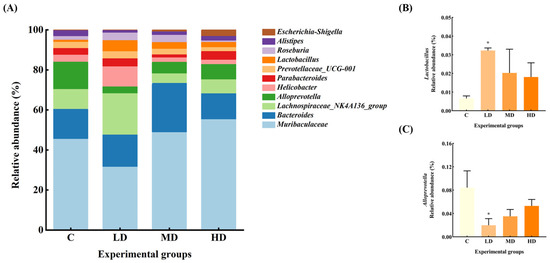
Figure 5.
(A) Abundance plots of species composition at the genus level for each group of mouse gut microbiota. (B) Lactobacillus relative abundance. (C) Alloprevotella relative abundance. Data are expressed as mean ± SD. * p < 0.05 vs. C.
The relative abundance of Escherichia–Shigella in the MD group increased 8-fold, and Bacteroides, Roseburia, Lactobacillus, and Muribaculaceae by 62%, 95%, 207%, and 6%, respectively, whereas the relative abundance of Lachnospiraceae_NK4A136_group, Alloprevotella, Parabacteroides, and Alistipes decreased by 52%, 58%, 56%, and 42%, respectively.
The relative abundance of Escherichia–Shigella increased by 39-fold in the HD group. Parabacteroides, Muribaculaceae, and Lactobacillus increased by 45%, 37%, and 174%, respectively, while the abundance of Bacteroides, Helicobacter, Alloprevotella, and Alistipes decreased by 3%, 29%, 37%, and 13%, respectively.
3.6. Effects of Fried Oil on Fecal SCFAs
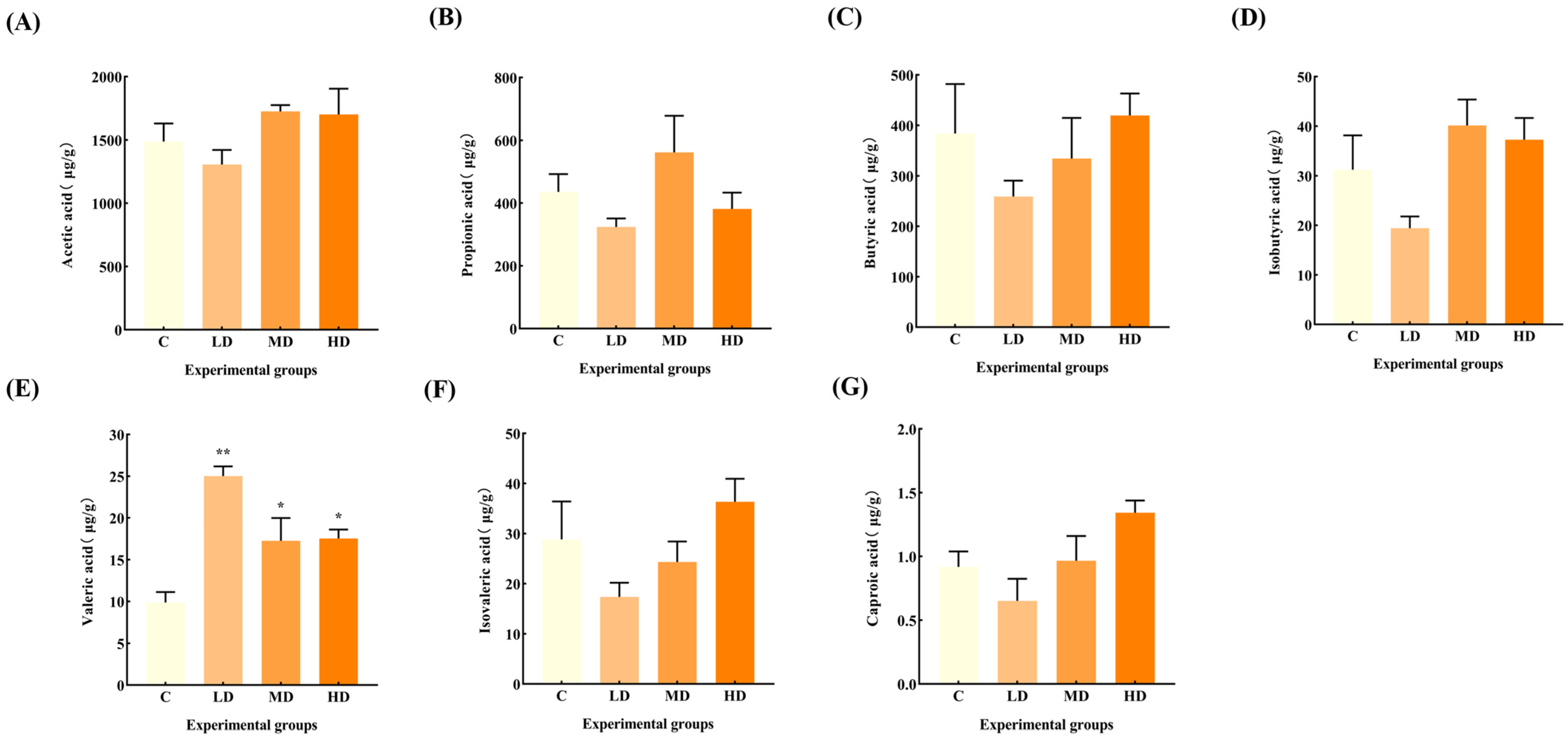
Figure 6 reveals that there was no significant change in the levels of acetic acid, propionic acid, butyric acid, isobutyric acid, isovaleric acid, and caproic acid in the feces of mice in the fried oil group compared with the C group, but the levels of valeric acid were significantly increased by 153% (p < 0.01), 89%, and 78%, respectively (p < 0.05).
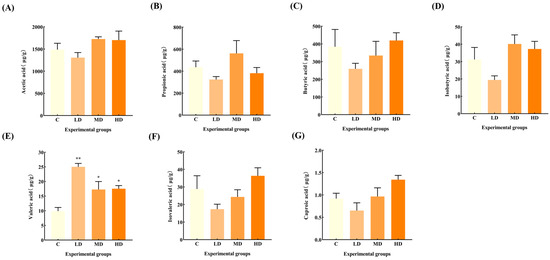
Figure 6.
Effects of fried oil on the SCFA concentration in mice feces. (A) Acetic acid. (B) Propionic acid. (C) Butyric acid. (D) Isobutyric acid. (E) Valeric acid. (F) Isovaleric acid. (G) Caproic acid. Data are expressed as mean ± SD. * p < 0.05, ** p < 0.01 vs. C.
3.7. Correlation Analysis of Gut Microbiota, SCFA Concentration, and TNF-α Levels
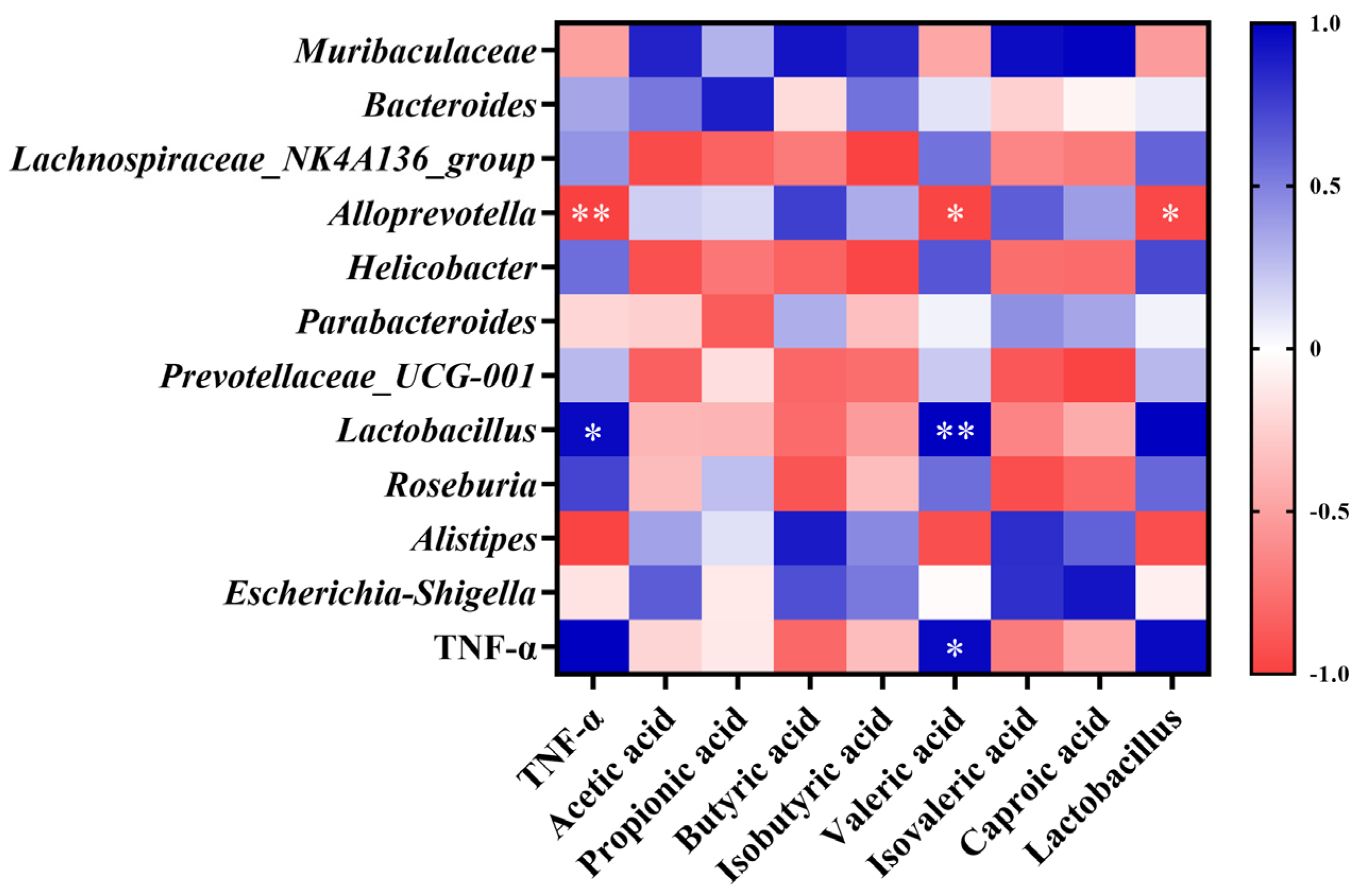
As shown in Figure 7, Alloprevotella abundance was significantly negatively correlated with TNF-α levels (p < 0.01). Lactobacillus abundance was significantly positively correlated with TNF-α levels (p < 0.05). Valeric acid concentration was significantly positively correlated with TNF-α levels (p < 0.05) and Lactobacillus abundance (p < 0.01), and significantly negatively correlated with Alloprevotella abundance (p < 0.05). Lactobacillus abundance was significantly negatively correlated with the abundance of Alloprevotella (p < 0.05).
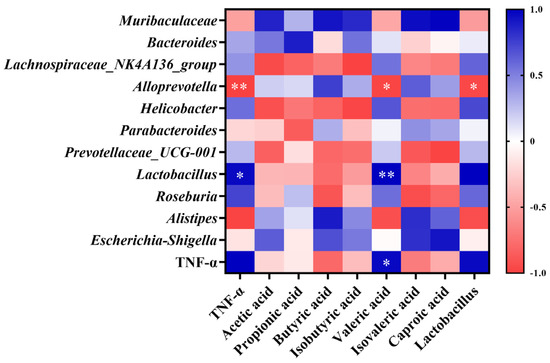
Figure 7.
Pearson correlation analysis of gut microbiota structure, SCFA concentration, and TNF-α levels. Data are expressed as mean ± SD. * p < 0.05, ** p < 0.01.
4. Discussion
Previous research indicates that obese individuals with lower bacterial richness gain more weight over time [10]. Additionally, a prolonged consumption of high-fat diets can also alter the gut microbiota composition, leading to an increased inflammatory state and obesity [11]. In the current study, a significant increase in body weight was observed in mice in the LD, MD, and HD groups compared to the C group, suggesting that even a short-term intake of a certain dose of fried oil can significantly increase body weight, while systemic low-grade inflammation can contribute to obesity.
Among the pro-inflammatory cytokines, TNF-α can mediate macrophages to release cytokines, causing low-grade inflammation and apoptosis [12]. Long-term intake of fried oil can increase plasma pro-inflammatory cytokines, but short-term intake of fried oil significantly increased by two to four times in body systems is rarely reported [13]. In this study, serum TNF-α was significantly increased by two to four times in mice administered fried oil within a week compared to that in the C group.
The Shannon index analysis showed that the richness and diversity of gut microbiota in the MD and HD groups were significantly reduced at 6d, which is consistent with the results of a previous study in mice fed fried corn oil and lard for six weeks [14].
The relative abundance of Proteobacteria is associated with toxins, which contain a variety of pathogenic bacteria (such as Escherichia, Shigella, etc.) that mediate cytokine aggregation and the formation and exacerbation of inflammation, which is a potential diagnostic indicator of ecological disorders and disease risk, and an increase in the abundance of Proteobacteria may further facilitate invasion by exogenous pathogens [15]. In intestinal inflammatory syndromes, such as irritable bowel syndrome (IBS) and metabolic syndrome, an increase in the abundance of Proteobacteria has been evident [16]. In our study, the relative abundance of Proteobacteria in gut microbiota of mice in the MD and HD groups was significantly increased by 122% and 156%, respectively, with the relative abundance of Escherichia–Shigella increased by 8 and 39 times, respectively. Furthermore, the abundance of Lactobacillus in the LD group was significantly increased, whereas that of Alloprevotella significantly decreased. All these factors point to a disturbance in the gut microbiota upon ingesting fried oil, even in the short term.
Lactic acid bacteria can aid in balancing the gut microbiota and boosting immunity. Lactobacillus converts lactose into lactic acid, which is an important metabolic intermediate in SCFA metabolism but can also serve as a fuel source for cancer cells, promoting inflammation, angiogenesis, and metastasis [17]. The immunomodulatory effect of Lactobacillus can also be achieved through the release of cytokines induced by its metabolites, including SCFAs, tumor necrosis factor, interferon, and transforming growth factor [18]. A long-term high-fat diet can lead to a reduced number of Lactobacilli in the gut; conversely, Lactobacillus can alleviate high-fat diet-induced obesity in mice [19,20]. However, in our study, the relative abundance of Lactobacillus in the LD group significantly increased by up to 385% in the short term. According to the Pearson correlation analysis, the relative abundance of Lactobacillus was significantly positively correlated with TNF-α levels. This result contradicts the common belief that an increase in Lactobacillus abundance can reduce the levels of inflammatory factors. It seems that an increase in Lactobacilli does not necessarily mean better health, and in fact a large increase in Lactobacillus abundance may promote an increase in inflammatory factor levels [21,22].
Alloprevotella can decompose protein- and carbohydrate-containing foods. It can also function as a conditional pathogen that induces intestinal inflammation. Alloprevotella is more abundant in vegetarians compared with non-vegetarians [23], and is generally considered to be a bacterium associated with a healthy plant-based diet, acting as a “probiotic” in the human body and therefore any reduction can lead to disease [24]. Recent human studies have linked increased Alloprevotella abundance to local and systemic low-grade inflammation [25]. In our study, Alloprevotella abundance decreased significantly in the LD group and was significantly negatively correlated with Lactobacillus abundance and TNF-α levels, suggesting that frying increased soybean oil acidity, making the intestinal environment more favorable for Lactobacillus growth. The proliferation of Lactobacillus produces metabolites such as lactic acid and valeric acid, which further increase gut acidity and inhibit the growth, reproduction, and activity of Alloprevotella, culminating in a reduced abundance [26]. It has been suggested that the decrease in Alloprevotella abundance may indirectly contribute to reducing the inflammatory response and promoting gut ecological balance in the context of fried oil intake. In some studies, in rats fed fried soybean oil for six weeks, it was found that gut Lactobacillus abundance was significantly increased and Alloprevotella abundance was significantly decreased, which is consistent with our study. It is speculated that the immune system has the capacity to suppress multiple oxidative stress products formed during feeding of fried oil by promoting the proliferation of Lactobacillus, whereas the decrease in Alloprevotella abundance is related to the fermentation and utilization of carbohydrates and monosaccharides [27].
Most previous studies on the induction mechanism of systemic low-grade inflammation have primarily focused on other signaling pathways such as nuclear factor kappa-B (NF-κB) and mitogen-activated protein kinase (MAPK). However, research has linked gut microbiota metabolites and SCFAs to act as anti-inflammatory agents via G protein-coupled receptors and histone deacetylase [28,29]. Recent research has indicated that valeric acid, a branched short-chain fatty acid, is negatively correlated with good health. Excessive blood valerate concentration exacerbates inflammation and damages brain cells and dendritic branches; however, gut microbiota changes and elevated blood valerate levels can be alleviated by appropriate exercise [30]. The fecal valeric acid concentration in children with autism is higher than that of acetic acid and butyric acids [31]. A significant increase in fecal valeric acid levels occurs in patients with IBS, and fecal valeric acid levels are significantly reduced following treatment [32], suggesting that an excessive accumulation of valeric acid in the gut is detrimental to health. The Pearson correlation analysis showed a significant positive correlation between fecal valeric acid and TNF-α levels, which suggests that fried oil consumption affects health, even in the short term. Thus, an increase in fecal valeric acid levels, a metabolic product of the gut microbiota, increases TNF-α levels, and induces low-grade inflammation in the body. This result also provides an important theoretical basis for the search for control targets for systemic low-grade inflammation induced by fried oil.
This study indicated that fried oil, even at a low concentration and for a short duration, can cause gut microbiota changes. Such disturbances can result in the overgrowth of Lactobacillus and a reduction in the proliferation of Alloprevotella. Subsequently, there was an increase in the bacterial metabolite valeric acid, contributing to a significant increase in serum TNF-α levels. However, upon exposure to a high concentration of fried oil, TNF-α declined, but the gut microbiota metabolites isovaleric acid, butyric acid, and caproic acid showed an increasing trend with minimal changes in SCFAs. Notably, this phenomenon may stem from the non-linear dose–response relationships that are common in biological systems. Within a certain range, increasing exposure levels may attenuate, rather than enhance, the strength of an organism’s response, reflecting the complex regulatory mechanisms in the body, including adaptive responses and negative feedback inhibitory pathways [33]. For example, initial exposure to low doses of fried oil may stimulate a rapid TNF-α response; however, the subsequent activation of negative feedback mechanisms may have led to a decrease in its levels at the time point of our observations [34]. In addition, our study focuses on short-term exposure effects, where organisms may utilize adaptive mechanisms to respond effectively to such inflammatory stimuli, explaining the observation of a short-term decline in TNF-α. However, long-term exposure scenarios, where these adaptive mechanisms may gradually fail and cumulative damage exceeds repair capacity, may eventually manifest as a persistent or exacerbated inflammatory response, leading to increased TNF-α levels, a direction worthy of further exploration in future studies [35]. Previous reports have shown that an increase in gut butyric acid concentration induces an inhibitory effect on TNF-α levels [36]. Whether butyric acid influences TNF-α levels can be tested by extending the test period in future studies. A future goal would be to regulate gut microbiota to stimulate the growth of specific microorganisms, thereby increasing the proportion of target gut microbiota or finding a substance that can inhibit the increase in the gut metabolite valeric acid, thereby minimizing inflammation in the body. According to our study, consuming a certain dose of fried oil even for a short period can lead to an increase or decrease in selective gut microbiota, resulting in an imbalance in microbiota homeostasis and an increase in fecal valeric acid and inflammatory factor TNF-α in serum, inducing a low-degree inflammation in the body.
5. Conclusions
The consumption of fried oil, even for a short period, promoted the growth of Lactobacillus and inhibited Alloprevotella, leading to an imbalance in the gut microbiota. Gut dysbiosis was associated with a significant increase in fecal valeric acid and a significant increase in serum TNF-α levels by up to 4-fold, both of which induced systemic low-grade inflammation in mice. Future studies should consider higher levels of evidence, such as clinical observations or human interventional studies, to validate and extend the current results observed in mouse models. In summary, this study provides a theoretical basis for evaluating the effects of short-term intake of fried oil on human health and to identify a compound that can mitigate the upsurge in inflammation in the body caused by the consumption of fried oil in the diet.
Author Contributions
Methodology, writing—original draft preparation, and investigation, L.H. (Lianhua Hu); investigation and formal analysis, L.H. (Ling Huang); funding acquisition and writing—review and editing, Z.F.; validation and data curation, C.W.; conceptualization, J.L.; conceptualization and software, Q.D.; visualization and methodology, D.X.; project administration, supervision, and resources, L.S.; writing—review and editing, R.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (No. 32172215), the GuangDong Basic and Applied Basic Research Foundation (No. 2024A1515011711), and the Special Project of Science and Technology Development of Zhanjiang (No. 2022A01014).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mozaffarian, D.; Lemaitre, R.N.; Kuller, L.H.; Burke, G.L.; Tracy, R.P.; Siscovick, D.S. Cardiac benefits of fish consumption may depend on the type of fish meal consumed: The Cardiovascular Health Study. Circulation 2003, 107, 1372–1377. [Google Scholar] [CrossRef]
- Sayon-Orea, C.; Carlos, S.; Martínez-gonzalez, M.A. Does cooking with vegetable oils increase the risk of chronic diseases?: A systematic review. Br. J. Nutr. 2015, 113 (Suppl. S2), S36–S48. [Google Scholar] [CrossRef]
- Arias, M.; Cobo, M.; Jaime-Sánchez, P.; Pastor, J.; Marijuan, P.; Pardo, J.; Rezusta, A.; Del Campo, R. Gut microbiota and systemic inflammation changes after bread consumption: The ingredients and the processing influence. J. Funct. Foods 2017, 32, 98–105. [Google Scholar] [CrossRef]
- Couzin-Frankel, J. Inflammation Bares a Dark Side. Science 2010, 330, 1621. [Google Scholar] [CrossRef]
- Wang, Z.; Liao, T.; Zhou, Z.; Wang, Y.; Diao, Y.; Strappe, P.; Prenzler, P.; Ayton, J.; Blanchard, C. Construction of local gene network for revealing different liver function of rats fed deep-fried oil with or without resistant starch. Toxicol. Lett. 2016, 258, 168–174. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- Gasaly, N.; De Vos, P.; Hermoso, M.A. Impact of Bacterial Metabolites on Gut Barrier Function and Host Immunity: A Focus on Bacterial Metabolism and Its Relevance for Intestinal Inflammation. Front. Immunol. 2021, 12, 658354. [Google Scholar] [CrossRef] [PubMed]
- Leong, X.F.; Mustafa, M.R.; Das, S.; Jaarin, K. Association of elevated blood pressure and impaired vasorelaxation in experimental Sprague-Dawley rats fed with heated vegetable oil. Lipids Health Dis. 2010, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Chuang, H.C.; Huang, C.F.; Chang, Y.C.; Lin, Y.S.; Chao, P.M. Gestational ingestion of oxidized frying oil by C57BL/6J mice differentially affects the susceptibility of the male and female offspring to diet-induced obesity in adulthood. J. Nutr. 2013, 143, 267–273. [Google Scholar] [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Effect of High-Fat Diets on Oxidative Stress, Cellular Inflammatory Response and Cognitive Function. Nutrients 2019, 11, 2579. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Zhang, H.; Baloch, Z. Pathogenetic and Therapeutic Applications of Tumor Necrosis Factor-α (TNF-α) in Major Depressive Disorder: A Systematic Review. Int. J. Mol. Sci. 2016, 17, 733. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, X.; Yang, R.; Ma, Q.; Qi, W.; Sanidad, K.Z.; Park, Y.; Kim, D.; Decker, E.A.; Zhang, G. Thermally Processed Oil Exaggerates Colonic Inflammation and Colitis-Associated Colon Tumorigenesis in Mice. Cancer Prev. Res. 2019, 12, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Kwek, E.; Yan, C.; Ding, H.; Hao, W.; He, Z.; Ma, K.Y.; Liu, J.; Zhu, H.; Chen, Z.Y. Effects of Thermally-Oxidized Frying Oils (Corn Oil and Lard) on Gut Microbiota in Hamsters. Antioxidants 2022, 11, 1732. [Google Scholar] [CrossRef] [PubMed]
- Litvak, Y.; Byndloss, M.X.; Tsolis, R.M.; Bäumler, A.J. Dysbiotic Proteobacteria expansion: A microbial signature of epithelial dysfunction. Curr. Opin. Microbiol. 2017, 39, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Gao, H.; Qi, Q.; Liu, X.; Li, J.; Gao, J.; Li, P.; Wang, Y.; Du, L.; Wang, C. High fat diet, gut microbiome and gastrointestinal cancer. Theranostics 2021, 11, 5889–5910. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, E.; Corr, S.C. Lactobacillus spp. for Gastrointestinal Health: Current and Future Perspectives. Front. Immunol. 2022, 13, 840245. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Song, C.; Li, L.; Wang, T.; Hu, J.; Zhu, L.; Yue, T. Lactobacillus alleviated obesity induced by high-fat diet in mice. J. Food Sci. 2021, 86, 5439–5451. [Google Scholar] [CrossRef]
- Xie, N.; Cui, Y.; Yin, Y.N.; Zhao, X.; Yang, J.W.; Wang, Z.G.; Fu, N.; Tang, Y.; Wang, X.H.; Liu, X.W.; et al. Effects of two Lactobacillus strains on lipid metabolism and intestinal microflora in rats fed a high-cholesterol diet. BMC Complement. Altern. Med. 2011, 11, 53. [Google Scholar] [CrossRef]
- Wells, J.M. Immunomodulatory mechanisms of lactobacilli. Microb. Cell Factories 2011, 10 (Suppl. S1), S17. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Nie, S.P.; Zhu, K.X.; Ding, Q.; Li, C.; Xiong, T.; Xie, M.Y. Lactobacillus plantarum NCU116 improves liver function, oxidative stress and lipid metabolism in rats with high fat diet induced non-alcoholic fatty liver disease. Food Funct. 2014, 5, 3216–3223. [Google Scholar] [CrossRef] [PubMed]
- Precup, G.; Vodnar, D.C. Gut Prevotella as a possible biomarker of diet and its eubiotic versus dysbiotic roles: A comprehensive literature review. Br. J. Nutr. 2019, 122, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Tett, A.; Pasolli, E.; Masetti, G.; Ercolini, D.; Segata, N. Prevotella diversity, niches and interactions with the human host. Nature Reviews. Microbiology 2021, 19, 585–599. [Google Scholar] [CrossRef]
- Larsen, J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 2017, 151, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Goburdhun, D.; Jhaumeer-Laulloo, S.B.; Musruck, R. Evaluation of soybean oil quality during conventional frying by FTIR and some chemical indexes. Int. J. Food Sci. Nutr. 2001, 52, 31–42. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, Y.; Jiang, Y.; Diao, Y.; Strappe, P.; Prenzler, P.; Ayton, J.; Blanchard, C. Deep-fried oil consumption in rats impairs glycerolipid metabolism, gut histology and microbiota structure. Lipids Health Dis. 2016, 15, 86. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, Y.; Li, X.; Sun, B. Dynamic balancing of intestinal short-chain fatty acids: The crucial role of bacterial metabolism. Trends Food Sci. Technol. 2020, 100, 118–130. [Google Scholar] [CrossRef]
- Lai, Z.; Shan, W.; Li, J.; Min, J.; Zeng, X.; Zuo, Z. Appropriate exercise level attenuates gut dysbiosis and valeric acid increase to improve neuroplasticity and cognitive function after surgery in mice. Mol. Psychiatry 2021, 26, 7167–7187. [Google Scholar] [CrossRef]
- Liu, S.; Li, E.; Sun, Z.; Fu, D.; Duan, G.; Jiang, M.; Yu, Y.; Mei, L.; Yang, P.; Tang, Y.; et al. Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder. Sci. Rep. 2019, 9, 287. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Guo, Q.; Wen, Z.; Tan, S.; Chen, J.; Lin, L.; Chen, P.; He, J.; Wen, J.; Chen, Y. The multiple effects of fecal microbiota transplantation on diarrhea-predominant irritable bowel syndrome (IBS-D) patients with anxiety and depression behaviors. Microb. Cell Factories 2021, 20, 233. [Google Scholar] [CrossRef] [PubMed]
- Billman, G.E. Homeostasis: The Underappreciated and Far Too Often Ignored Central Organizing Principle of Physiology. Front. Physiol. 2020, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Cano-Martínez, A.; Rubio-Ruiz, M.E.; Guarner-Lans, V. Homeostasis and evolution in relation to regeneration and repair. J. Physiol. 2024, 602, 2627–2648. [Google Scholar] [CrossRef]
- Prescott, J.A.; Mitchell, J.P.; Cook, S.J. Inhibitory feedback control of NF-κB signalling in health and disease. Biochem. J. 2021, 478, 2619–2664. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).