Symbiodiniaceae and Ruegeria sp. Co-Cultivation to Enhance Nutrient Exchanges in Coral Holobiont
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Procedures
2.2. Isolation of Bacterial Strains and Symbiodiniaceae from Corals
2.3. Functional Screening for Probiotic Bacteria Using 16S/ITS2 rDNA Gene Sequencing
2.4. Symbiodiniaceae-Bacteria Co-Culture
2.5. Labeling Bacteria with Stable Isotope 15N-NH4Cl
2.6. SIP-Bacteria and Symbiodiniaceae Co-Culture
2.7. Determination of Photosynthetic Pigments
2.8. Single-Cell Raman Spectrum Analysis
2.9. Raman Spectra Analysis
3. Results
3.1. Screening Results of Coral Symbiotic Microorganisms
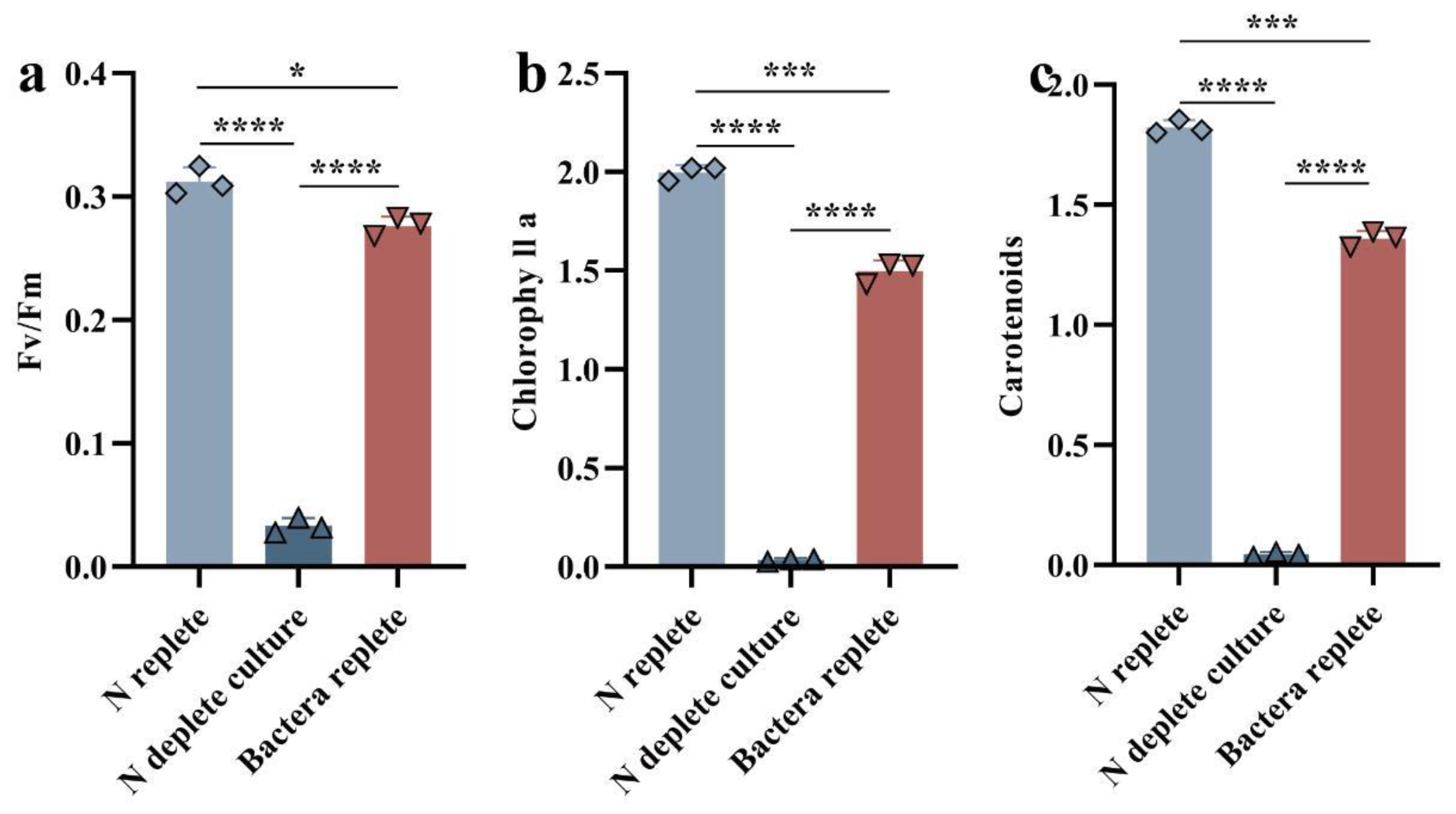
3.2. Physiological and Photosynthetic Response to Co-Cultivation of Symbiodiniaceae and Bacteria
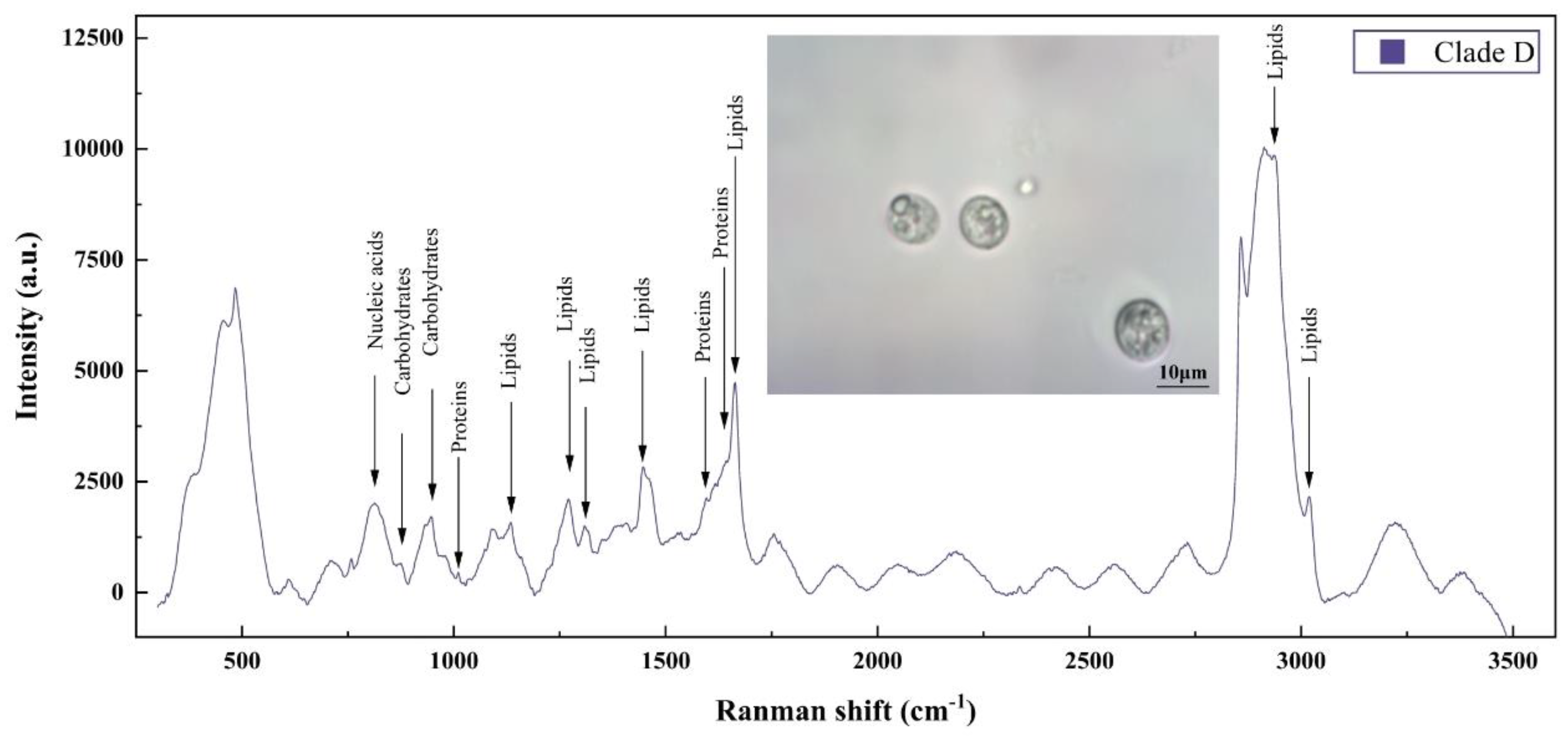
3.3. Raman Spectra of Single Microalgal Cell
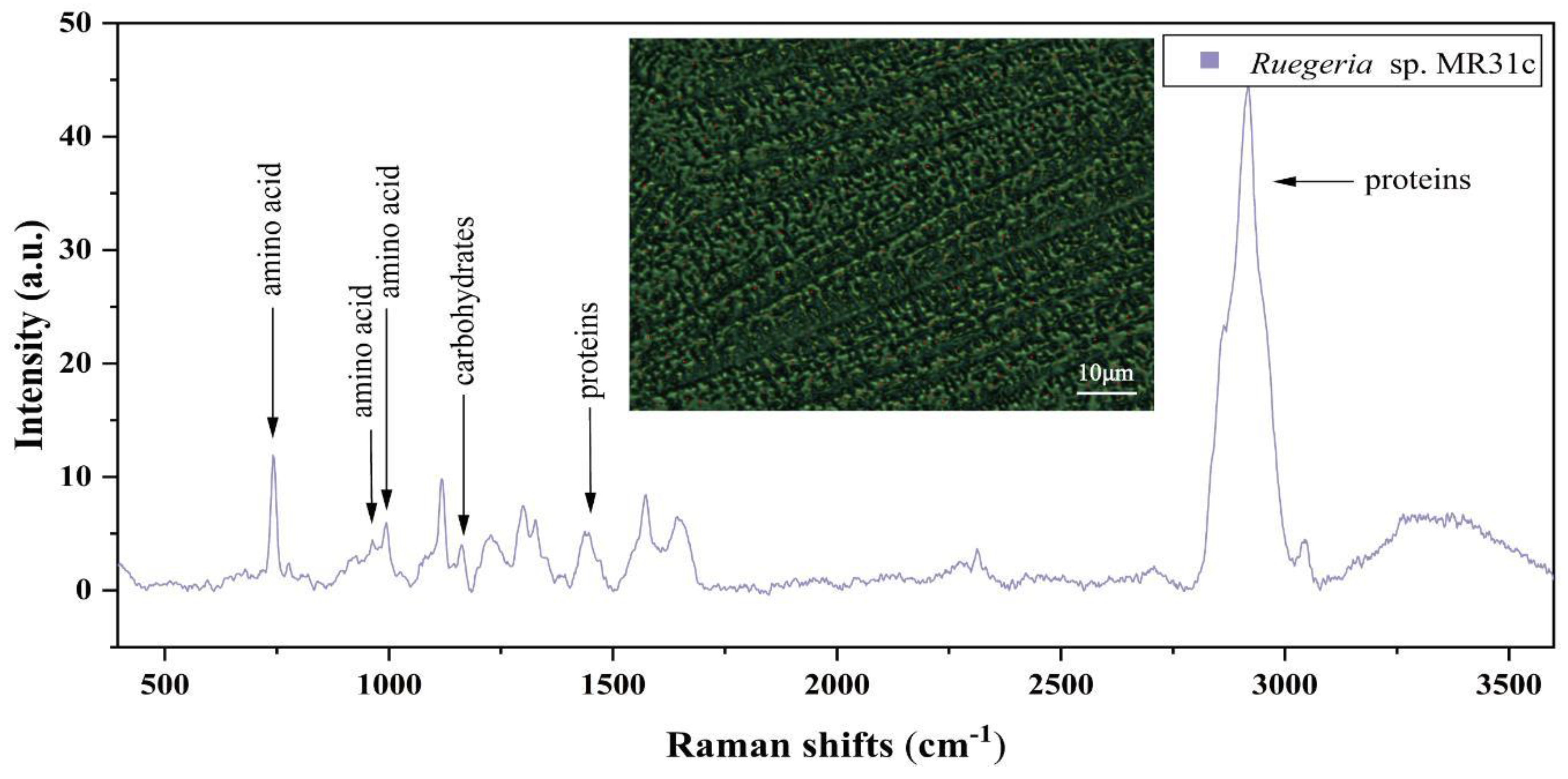
3.4. Raman Spectra of Single Bacterial Cell
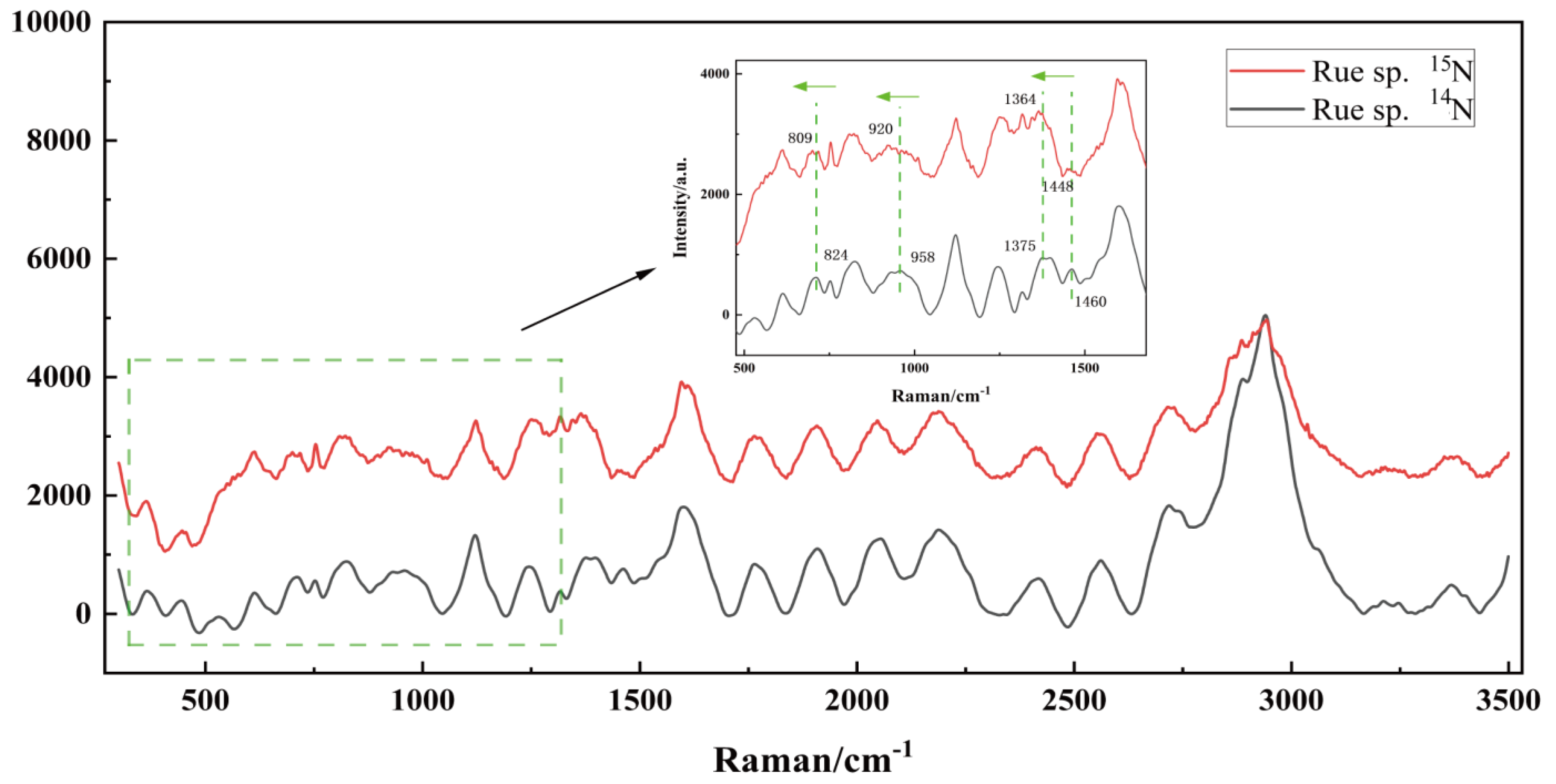
3.5. SCRS Dynamics of Forward 15N-Labelling in the Culture of Ruegeria sp. MR31c
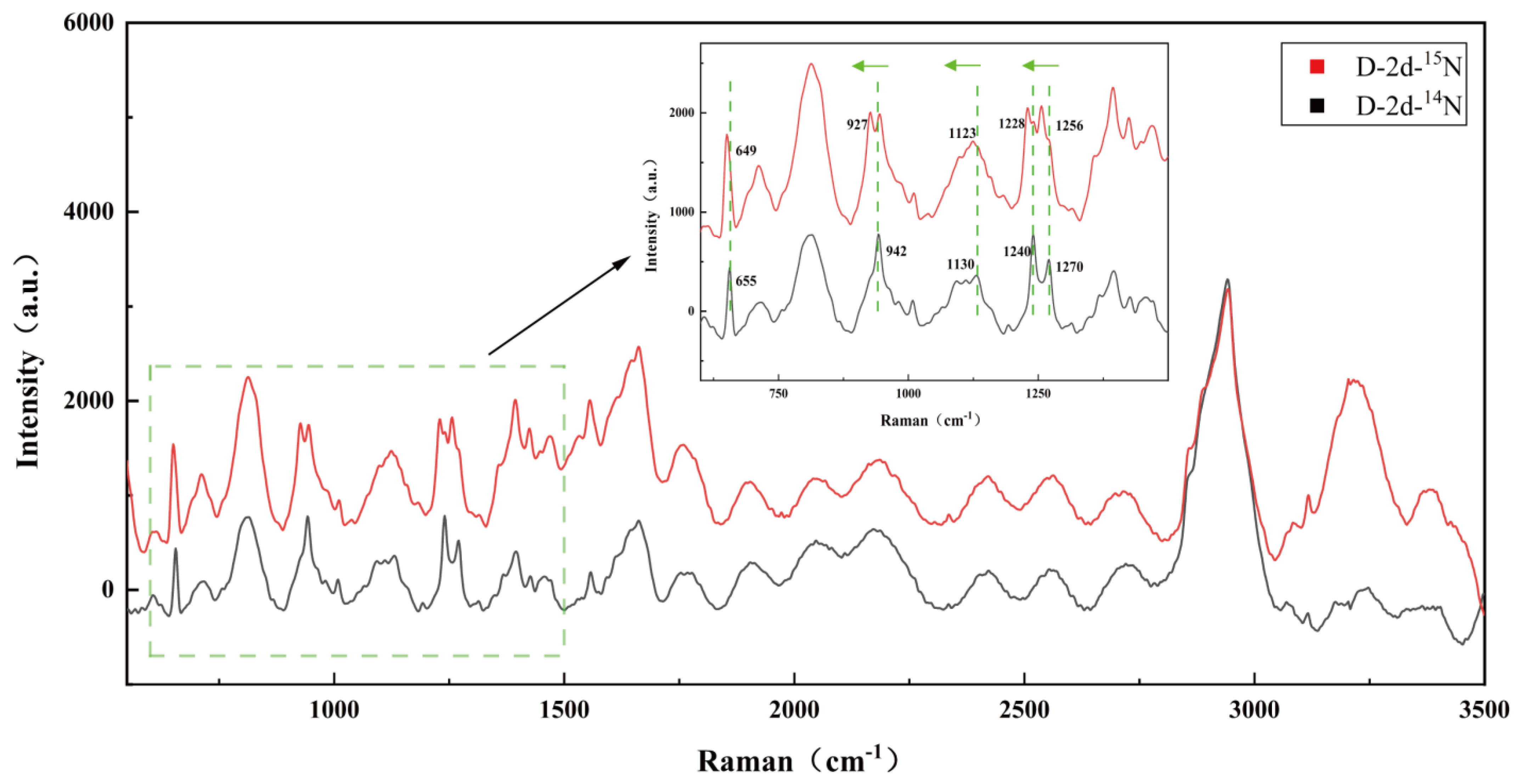
3.6. Dynamics of SCRS Characteristic Bands in Algal and Bacteria Co-Culture
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary production of the biosphere: Integrating terrestrial and oceanic components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef]
- Karl, D.M.; Hebel, D.V.; Björkman, K.; Letelier, R.M. The role of dissolved organic matter release in the productivity of the oligotrophic North Pacific Ocean. Limnol. Oceanogr. 2003, 43, 1270–1286. [Google Scholar] [CrossRef]
- Mague, T.H.; Friberg, E.; Hughes, D.J.; Morris, I. Extracellular Release of Carbon by Marine Phytoplankton: A Physiological Approach. Limnol. Oceanogr. 1980, 25, 262–279. [Google Scholar] [CrossRef]
- Hellebust, J.A. Excretion of some organic compounds by marine phytoplankton. Limnol. Oceanogr. 1965, 10, 192–206. [Google Scholar] [CrossRef]
- Gascuel, D.; Bozec, Y.M.; Chassot, E.; Colomb, A.; Laurans, M. The trophic spectrum: Theory and application as an ecosystem indicator. ICES J. Mar. Sci. 2005, 62, 443–452. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Mumby, P.J.; Hooten, A.J.; Steneck, R.S.; Greenfield, P.; Gomez, E.; Harvell, C.D.; Sale, P.F.; Edwards, A.J.; Caldeira, K.; et al. Coral Reefs Under Rapid Climate Change and Ocean Acidification. Science 2007, 318, 1737–1742. [Google Scholar] [CrossRef]
- Hughes, T.; Baird, A.H.; Bellwood, D.R.; Card, M.; Connolly, S.R.; Folke, C.; Grosberg, R.; Hoegh-Guldberg, O.; Jackson, J.B.C.; Kleypas, J.; et al. Climate change, human impact, and the resilience of coral reefs. Science 2003, 301, 929–933. [Google Scholar] [CrossRef]
- Haine, E.R. Symbiont-mediated protection. Proc. R. Soc. B Biol. Sci. 2008, 275, 353–361. [Google Scholar] [CrossRef]
- Kushmaro, A.; Loya, Y.; Fine, M.; Rosenberg, E. Bacterial infection and coral bleaching. Nature 1996, 380, 396. [Google Scholar] [CrossRef]
- Barott, K.L.; Rodriguez-Brito, B.; Janouškovec, J.; Marhaver, K.L.; Smith, J.E.; Keeling, P.; Rohwer, F.L. Microbial diversity associated with four functional groups of benthic reef algae and the reef-building coral Montastraea annularis. Environ Microbiol. 2011, 13, 1192–1204. [Google Scholar] [CrossRef]
- Reshef, L.; Koren, O.; Loya, Y.; Zilber-Rosenberg, I.; Rosenberg, E. The coral probiotic hypothesis. Environ. Microbiol. 2006, 8, 2068–2073. [Google Scholar] [CrossRef]
- Barott, K.L.; Rodriguez-Mueller, B.; Youle, M.; Marhaver, K.L.; Vermeij, M.J.; Smith, J.E.; Rohwer, F.L. Microbial to reef scale interactions between the reef-building coral Montastraea annularis and benthic algae. Proc. R. Soc. B Biol. Sci. 2011, 279, 1655–1664. [Google Scholar] [CrossRef]
- Kuhl, M.; Cohen, Y.; Dalsgaard, T.; Jørgensen, B.B.; Revsbech, N.P. Microenvironment and photosynthesis of zooxanthellae in scleractinian corals studied with microsensors for O2, pH and light. Mar. Ecol. Prog. Ser. 1995, 117, 159–172. [Google Scholar] [CrossRef]
- Muscatine, L.; McCloskey, L.R.; Marian, R.E. Marian, and Oceanography, Estimating the daily contribution of carbon from zooxanthellae to coral animal respiration 1. Limnol. Oceanogr. 1981, 26, 601–611. [Google Scholar] [CrossRef]
- Fine, M.; Loya, Y. Endolithic algae: An alternative source of photoassimilates during coral bleaching. Proc. R. Soc. B Biol. Sci. 2002, 269, 1205–1210. [Google Scholar] [CrossRef]
- Lema, K.A.; Willis, B.L.; Bourne, D.G. Corals Form Characteristic Associations with Symbiotic Nitrogen-Fixing Bacteria. Appl. Environ. Microbiol. 2012, 78, 3136–3144. [Google Scholar] [CrossRef]
- Mouchka, M.E.; Hewson, I.; Harvell, C.D. Coral-Associated Bacterial Assemblages: Current Knowledge and the Potential for Climate-Driven Impacts. Integr. Comp. Biol. 2010, 50, 662. [Google Scholar] [CrossRef]
- Amin, S.A.; Hmelo, L.R.; van Tol, H.M.; Durham, B.P.; Carlson, L.T.; Heal, K.R.; Morales, R.L.; Berthiaume, C.T.; Parker, M.S.; Djunaedi, B.; et al. Interaction and signalling between a cosmopolitan phytoplankton and associated bacteria. Nature 2015, 522, 98–101. [Google Scholar] [CrossRef]
- de-Bashan, L.E.; Mayali, X.; Bebout, B.M.; Weber, P.K.; Detweiler, A.M.; Hernandez, J.-P.; Prufert-Bebout, L.; Bashan, Y. Establishment of stable synthetic mutualism without co-evolution between microalgae and bacteria demonstrated by mutual transfer of metabolites (NanoSIMS isotopic imaging) and persistent physical association (Fluorescent in situ hybridization). Algal Res. 2016, 15, 179–186. [Google Scholar] [CrossRef]
- Jarvis, R.M.; Goodacre, R. Goodacre, Discrimination of Bacterial Using Surface-Enhanced Raman Spectroscopy. Anal. Chem. 2004, 76, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, Y.; Tao, Y.; Muhamadali, H.; Goodacre, R.; Zhou, N.-Y.; Preston, G.M.; Xu, J.; Huang, W.E. Reverse and Multiple Stable Isotope Probing to Study Bacterial Metabolism and Interactions at the Single Cell Level. Anal. Chem. 2016, 88, 9443–9450. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Zhang, D.; Halas, N.J.; Hartgerink, J.D. Aromatic Amino Acids Providing Characteristic Motifs in the Raman and SERS Spectroscopy of Peptides. J. Phys. Chem. B 2008, 112, 9158–9164. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.E.; Bailey, M.J.; Thompson, I.P.; Whiteley, A.S.; Spiers, A.J. Single-Cell Raman Spectral Profiles of Pseudomonas fluorescens SBW25 Reflects in vitro and in planta Metabolic History. Microb. Ecol. 2007, 53, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Rösch, P.; Harz, M.; Schmitt, M.; Peschke, K.-D.; Ronneberger, O.; Burkhardt, H.; Motzkus, H.-W.; Lankers, M.; Hofer, S.; Thiele, H.; et al. Chemotaxonomic Identification of Single Bacteria by Micro-Raman Spectroscopy: Application to Clean-Room-Relevant Biological Contaminations. Appl. Environ. Microbiol. 2005, 71, 1626–1637. [Google Scholar] [CrossRef] [PubMed]
- Pätzold, R.; Keuntje, M.; Theophile, K.; Müller, J.; Mielcarek, E.; Ngezahayo, A.; Anders-von Ahlften, A. In situ mapping of nitrifiers and anammox bacteria in microbial aggregates by means of confocal resonance Raman microscopy. J. Microbiol. Methods 2008, 72, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Hatzenpichler, R.; Krukenberg, V.; Spietz, R.L.; Jay, Z.J. Next-generation physiology approaches to study microbiome function at single cell level. Nat. Rev. Microbiol. 2020, 18, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Azemtsop Matanfack, G.; Pistiki, A.; Rösch, P.; Popp, J. Raman 18O-labeling of bacteria in visible and deep UV-ranges. J. Biophotonics 2021, 14, e202100013. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Gong, Y.; Xu, T.; Davison, P.A.; MacGregor-Chatwin, C.; Hunter, C.N.; Xu, L.; Meng, Y.; Ji, Y.; Ma, B.; et al. Revealing CO2-Fixing SAR11 Bacteria in the Ocean by Raman-Based Single-Cell Metabolic Profiling and Genomics. BioDesign Res. 2022, 2022, 9782712. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhu, P.; Zhang, L.; Gong, Y.; Wang, C.; Sun, L.; Wang, L.; Chen, R.; Mao, Y.; Fu, X.; et al. Single-Cell Identification, Drug Susceptibility Test, and Whole-genome Sequencing of Helicobacter pylori Directly from Gastric Biopsy by Clinical Antimicrobial Susceptibility Test Ramanometry. Clin. Chem. 2022, 68, 1064–1074. [Google Scholar] [CrossRef]
- Dana, J.D.; Wilkes, C. Structure and Classification Of Zoophytes, United States Exploring Expedition 1838–1842. Available online: https://www.biodiversitylibrary.org/bibliography/4789 (accessed on 30 May 2024).
- Veron, J.E.N. Corals of Australia and the Indo-Pacific. Environmental Science, Biology, Geology. 1993. Available online: https://www.semanticscholar.org/paper/Corals-of-Australia-and-the-Indo-Pacific-Veron/f236d971121dfa985122da86342eb5cae0421e89 (accessed on 30 May 2024).
- Guillard, R. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals; Smith, W.L., Chanley, M.H., Eds.; Springer: Boston, MA, USA, 1975. [Google Scholar]
- Peixoto, R.S.; Rosado, P.M.; Leite DC, D.A.; Rosado, A.S.; Bourne, D.G. Beneficial Microorganisms for Corals (BMC): Proposed Mechanisms for Coral Health and Resilience. Front. Microbiol. 2017, 8, 236713. [Google Scholar] [CrossRef]
- Wiedenmann, J.; D’Angelo, C.; Smith, E.G.; Hunt, A.N.; Legiret, F.E.; Postle, A.D.; Achterberg, E.P. Nutrient enrichment can increase the susceptibility of reef corals to bleaching. Nat. Clim. Change 2013, 3, 160–164. [Google Scholar] [CrossRef]
- Muscatine, L.; Ferrier-Pages, C.; Blackburn, A.; Gates, R.D.; Baghdasarian, G.; Allemand, D. Cell-specific density of symbiotic dinoflagellates in tropical anthozoans. Coral Reefs 1998, 17, 329–337. [Google Scholar] [CrossRef]
- He, Y.; Zhang, P.; Huang, S.; Wang, T.; Ji, Y.; Xu, J. Label-free, simultaneous quantification of starch, protein and triacylglycerol in single microalgal cells. Biotechnol. Biofuels 2017, 10, 275. [Google Scholar] [CrossRef]
- Huang, W.E.; Griffiths, R.I.; Thompson, I.P.; Bailey, M.J.; Whiteley, A.S. Raman microscopic analysis of single microbial cells. Anal. Chem. 2004, 76, 4452–4458. [Google Scholar] [CrossRef]
- Huang, W.E.; Ward, A.D.; Whiteley, A.S. Raman tweezers sorting of single microbial cells. Environ. Microbiol. Rep. 2009, 1, 44–49. [Google Scholar] [CrossRef]
- Cui, L.; Yang, K.; Li, H.-Z.; Zhang, H.; Su, J.-Q.; Paraskevaidi, M.; Martin, F.L.; Ren, B.; Zhu, Y.-G. Functional Single-Cell Approach to Probing Nitrogen-Fixing Bacteria in Soil Communities by Resonance Raman Spectroscopy with 15N2 Labeling. Anal. Chem. 2018, 90, 5082–5089. [Google Scholar] [CrossRef] [PubMed]
- Mary, Y.S.; Ushakumari, L.; Harikumar, B.; Varghese, H.T.; Panicker, C.Y. FT-IR, FT-raman and SERS spectra of L-proline. J. Iran. Chem. Soc. 2009, 6, 138–144. [Google Scholar] [CrossRef]
- Thomas, G.J.; Prescott, B.; Olins, D.E. Secondary Structure of Histones and DNA in Chromatin. Science 1977, 197, 385–388. [Google Scholar] [CrossRef]
- Cui, L.; Yang, K.; Zhou, G.; Huang, W.E.; Zhu, Y.G. Surface-Enhanced Raman Spectroscopy Combined with Stable Isotope Probing to Monitor Nitrogen Assimilation at Both Bulk and Single-Cell Level. Anal. Chem. 2017, 89, 5793–5800. [Google Scholar] [CrossRef]
- Lesser, M.P.; Mazel, C.H.; Gorbunov, M.Y.; Falkowski, P.G. Discovery of Symbiotic Nitrogen-Fixing Cyanobacteria in Corals. Science 2004, 305, 997–1000. [Google Scholar] [CrossRef]
- Lesser, M.P.; Falcón, L.I.; Rodríguez-Román, A.; Enríquez, S.; Hoegh-Guldberg, O.; Iglesias-Prieto, R. Nitrogen fixation by symbiotic cyanobacteria provides a source of nitrogen for the scleractinian coral Montastraea cavernosa. Mar. Ecol. Prog. Ser. 2007, 346, 143–152. [Google Scholar] [CrossRef]
- Paillard, C.; Le Roux, F.; Borrego, J.J. Bacterial disease in marine bivalves, a review of recent studies: Trends and evolution. Aquat. Living Resour. 2004, 17, 477–498. [Google Scholar] [CrossRef]
- Miura, N.; Motone, K.; Takagi, T.; Aburaya, S.; Watanabe, S.; Aoki, W.; Ueda, M. Ruegeria sp. Strains Isolated from the Reef-Building Coral Galaxea fascicularis Inhibit Growth of the Temperature-Dependent Pathogen Vibrio coralliilyticus. Mar. Biotechnol. 2019, 21, 1–8. [Google Scholar]
- Rees TA, V. Are Symbiotic Algae Nutrient Deficient? Proc. R. Soc. Lond. Ser. B Biol. Sci. 1991, 243, 227–233. [Google Scholar]
- Schmollinger, S.; Mühlhaus, T.; Boyle, N.R.; Blaby, I.K.; Casero, D.; Mettler, T.; Moseley, J.L.; Kropat, J.; Sommer, F.; Strenkert, D.; et al. Nitrogen-Sparing Mechanisms in Chlamydomonas Affect the Transcriptome, the Proteome, and Photosynthetic Metabolism. Plant Cell 2014, 26, 1410–1435. [Google Scholar] [CrossRef] [PubMed]
- Bottrill, D.E.; Possingham, J.V.; Kriedemann, P.E. The effect of nutrient deficiencies on phosynthesis and respiration in spinach. Plant Soil 1970, 32, 424–438. [Google Scholar] [CrossRef]
- Bongaerts, J.; Krämer, M.; Müller, U.; Raeven, L.; Wubbolts, M. Metabolic Engineering for Microbial Production of Aromatic Amino Acids and Derived Compounds. Metab. Eng. 2001, 3, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Gosset, G. Production of aromatic compounds in bacteria. Curr. Opin. Biotechnol. 2009, 20, 651–658. [Google Scholar] [CrossRef]
- Saibi, W.; Feki, K.; Yacoubi, I.; Brini, F. Bridging Between Proline Structure, Functions, Metabolism, and Involvement in Organism Physiology. Appl. Biochem. Biotechnol. 2015, 176, 2107–2119. [Google Scholar] [CrossRef]
- Liu, W.; Hancock, C.N.; Fischer, J.W.; Harman, M.; Phang, J.M. Proline biosynthesis augments tumor cell growth and aerobic glycolysis: Involvement of pyridine nucleotides. Sci. Rep. 2015, 5, 17206. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Philips, S.; Laanbroek, H.J.; Verstraete, W. Origin, causes and effects of increased nitrite concentrations in aquatic environments. Rev. Environ. Sci. Biotechnol. 2002, 1, 115–141. [Google Scholar] [CrossRef]
- Williams, S.K.; Hodson, R.C. Transport of urea at low concentrations in Chlamydomonas reinhardi. J. Bacteriol. 1977, 130, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Wijffels, R.H.; Barbosa, M.J. An Outlook on Microalgal Biofuels. Science 2010, 329, 796–799. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Dragone, G.; Guimarães, P.M.R.; Silva, J.P.A.; Carneiro, L.M.; Roberto, I.C.; Vicente, A.; Domingues, L.; Teixeira, J.A. Technological trends, global market, and challenges of bio-ethanol production. Biotechnol. Adv. 2010, 28, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Breuer, G.; de Jaeger, L.; Artus, V.G.; Martens, D.E.; Springer, J.; Draaisma, R.B.; Eggink, G.; Wijffels, R.H. Superior triacylglycerol (TAG) accumulation in starchless mutants of Scenedesmus obliquus: (II) evaluation of TAG yield and productivity in controlled photobioreactors. Biotechnol. Biofuels 2014, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Dixon, G.K.; Syrett, P.J. The growth of dinoflagellates in laboratory cultures. New Phytol. 1988, 109, 297–302. [Google Scholar] [CrossRef]
- Apprill, A.; Marlow, H.Q.; Martindale, M.Q.; Rappé, M.S. The onset of microbial associations in the coral Pocillopora meandrina. ISME J. 2009, 3, 685–699. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M.; Alavi, M.; Miller, T.; Erlandson, K.; Schneider, R.; Belas, R. Bacterial community associated with Pfiesteria-like dinoflagellate cultures. Environ. Microbiol. 2001, 3, 380–396. [Google Scholar] [CrossRef]
- Krohn-Molt, I.; Alawi, M.; Förstner, K.U.; Wiegandt, A.; Burkhardt, L.; Indenbirken, D.; Thieß, M.; Grundhoff, A.; Kehr, J.; Tholey, A.; et al. Insights into Microalga and Bacteria Interactions of Selected Phycosphere Biofilms Using Metagenomic, Transcriptomic, and Proteomic Approaches. Front. Microbiol. 2017, 8, 1941. [Google Scholar] [CrossRef]

| Genus | No. | Name | Genbank Accession Number | Medium | Affiliated Coral Types | Identity |
|---|---|---|---|---|---|---|
| Vibrio | JW-1 | Vibrio owensii | CP045859.1 | LB; L1 | A. hyacinthus | (1412/1412) 100% |
| JW-2 | Vibrio vulnificus | MN860081.1 | LB | A. hyacinthus | (1448/1448) 100% | |
| JW-3 | Vibrio coralliilyticus | CP031472.1 | LB | A. hyacinthus | (1394/1408) 99% | |
| JW-4 | Vibrio sp. Strain JC009 | CP092106.1 | L1 | A. hyacinthus | (1452/1452) 100% | |
| JW-5 | Vibrio alginolyticus | CP054700.1 | MA2216E | G. fascicularis | (1389/1390) 99.9% | |
| JW-6 | Vibrio rotiferianus | AP019798.1 | MA2216E | G. fascicularis | (1415/1416) 99.9% | |
| Ruegeria | JW-7 | Ruegeria conchae | CP031472.1 | MA2216E | A. hyacinthus | (1358/1358) 100% |
| JW-8 | Ruegeria sp. MR31c | HQ439523.1 | MA2216E | A. hyacinthus | (1334/1348) 99% | |
| JW-9 | Ruegeria sp. atlantica | MW828512.1 | MA2216E | A. hyacinthus | (1308/1308) 100 | |
| JW-10 | Ruegeria sp. LR4 | KU560503.1 | MA2216E | A. hyacinthus | (1341/1354) 99% | |
| JW-11 | Ruegeria sp. strain MP15.1 | OQ435566.1 | MA2216E | A. hyacinthus; G. fascicularis | (1317/1330) 99% | |
| JW-12 | Ruegeria arenilitoris | MG896151.1 | MA2216E | G. fascicularis | (1309/1322) 99% | |
| JW-13 | Ruegeria lacuscaerulensis | MH283799.1 | L1 | G. fascicularis | (1432/1446) 99% | |
| Bacillus | JW-14 | Bacillus horikoshii | DQ289065.1 | MA2216E | G. fascicularis | (1434/1448) 99% |
| JW-15 | Bacillus weihaiensis | CP016020.1 | MA2216E | G. fascicularis | (1434/1434) 100% | |
| JW-16 | Bacillus coahuilensis | EF014447.1 | MA2216E | G. fascicularis | (1449/1449) 100% | |
| Thalassotalea | JW-17 | Thalassotalea euphylliae | MW828496.1 | L1 | A. hyacinthus; G. fascicularis | (1379/1392) 99% |
| JW-18 | Thalassomonas loyana | HQ439553.1 | MA2216E | G. fascicularis | (1408/1422) 99% | |
| JW-19 | Thalassomonas agarivorans | HQ439504.1 | MA2216E | G. fascicularis | (14111425) 99% | |
| Thalassospira | JW-20 | Thalassospira sp. 2ta1 | FJ952805.1 | MA2216E | A. hyacinthus | (1361/1374) 99% |
| Microbulbifer | JW-21 | Microbulbifer sp. Alg-AMLN-14-8 | MK453424.1 | MA2216E | A. hyacinthus | (1406/1406) 100% |
| Phaeobacter | JW-22 | Phaeobacter sp. strain 088 | MK801649.1 | MA2216E | A. hyacinthus | (1333/1333) 100% |
| Alteromonas | JW-23 | Alteromonas aestuariivivens | NR157790.1 | L1 | A. hyacinthus | (1457/1517) 96% |
| JW-24 | Alteromonas macleodii | OX359243.1 | L1; CDA | A. hyacinthus; G. fascicularis | (1394/1408) 99% | |
| Roseovarius | JW-25 | Roseovarius sp. | MZ262971.1 | MA2216E | A. hyacinthus | (1311/1324) 99% |
| Roseobacter-aceae | JW-26 | Shima sp. LR11 | KU560500.1 | NSW | A. hyacinthus | (1352/1352) 100% |
| JW-27 | Shimia isoporae | MH283808.1 | L1 | G. fascicularis | (1355/1355) 100% | |
| Marinobacter | JW-28 | Marinobacter sp. | MT210870.1 | MA2216E | A. hyacinthus | (1503/1503) 100% |
| Labrenzia | JW-29 | Labrenzia sp. | MK493531.1 | MA2216E | A. hyacinthus | (1389/1403) 99% |
| Psychrosphaera | JW-30 | Psychrosphaera sp. | MZ262895.1 | L1 | A. hyacinthus | (1385/1385) 100% |
| Microbacterium | JW-31 | Microbacterium esteraromaticum | MT453933.1 | MA2216E | G. fascicularis | (1393/1393) 100% |
| JW-32 | Microbacterium sp. OB57 | JN942151.1 | MA2216E | G. fascicularis | (1418/1432) 99% | |
| Rossellomorea | JW-33 | Rossellomorea aquimaris | MK256784.1 | MA2216E | G. fascicularis | (1451/1451) 100% |
| Tropicibacter | JW-34 | Tropicibacter sp. | MK801651.1 | MA2216E | G. fascicularis | (1336/1336) 100% |
| Stutzerimonas | JW-35 | Stutzerimonas stutzeri | MT356167.1 | CDA | G. fascicularis | (1461/1475) 99% |
| Acinetobacter | JW-36 | Acinetobacter seifertii | OP114754.1 | CDA | G. fascicularis | (1409/1423) 99% |
| JW-37 | Acinetobacter soli | OP854766.1 | CDA | G. fascicularis | 1403/1403 100% | |
| Enterobacter | JW-38 | Enterobacter cancerogenus | CP025225.1 | CDA | G. fascicularis | (1406/1406) 100% |
| Marinomonas | JW-39 | Marinomonas sp. | MG099520.1 | CDA | G. fascicularis | (1462/1476) 99% |
| Aerococcus | JW-40 | Aerococcus viridans | MT502756.1 | MA2216E | G. fascicularis | (1423/1437) 99% |
| Pseudoalteromonas | JW-41 | Pseudoalteromonas shioyasakiensis | KU321310.1 | MA2216E | G. fascicularis | (1407/1421) 99% |
| Component | Raman Bands (cm−1) | Assignment |
|---|---|---|
| unknown | 655.246 | v (C-S) gauche |
| unknown | 754.235 | Symmetric breathing of tryptophan |
| nucleic acids | 810.75 | C-O-P-O-C in RNA backbone |
| carbohydrates | 872.62 | C-C stretching, Hydroxyproline |
| carbohydrates | 943.17 | C-O stretching; C-O-C and C-O-H deformation; α-helix C-C backbone |
| lipids | 972.30 | V (C-C) wagging |
| proteins | 1008.68 | C-C aromatic |
| unknown | 1090.82 | C-O stretching |
| proteins | 1130.31 | C-N stretching |
| lipids | 1269.12 | Amide III random, lipids |
| lipids | 1305.86 | CH3/CH2 twisting or bending mode of lipids |
| unknown | 1364.78 | vs (CH3) Adenine, guanine, tyrosine, tryptophan |
| unknown | 1405.91 | v (COO-) |
| lipids | 1446.82 | CH2, CH3 bending modes |
| proteins | 1595.28 | C=N and C=C stretching in quinoid ring |
| proteins | 1609.89 | Cytosine (NH2) |
| proteins | 1643.04 | Amide I band (protein band) |
| lipids | 1663.69 | (C=C) cis, lipids, fatty acids |
| lipids | 2857.48 | CH2 symmetric stretch of lipids |
| lipids | 3017.76 | v=CH of lipids |
| Component | Raman Bands (cm−1) | Assignment |
|---|---|---|
| amino acid | 743.26 | C-S stretch |
| amino acid | 958.87 | C-N stretching |
| amino acid | 824.35 | aromatic ring vibration |
| amino acid | 994.42 | C-C aromatic and symmetric ring breath |
| carbohydrates | 1157.26 | C-C, C=C band stretch |
| unknown | 1231.47 | Amide III, C-N stretch, N-H coupling |
| unknown | 1329.66 | DNA, Phospholipids, purine |
| nucleobase | 1375.36 | Thymine, adenine, guanine |
| proteins | 1460.45 | CH2 bending mode, C-H vibrations |
| unknown | 1573.91 | Amide II, nucleic acid, Peptidoglycan |
| lipids | 2918.07 | C-H vibrations |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wu, H.; Shu, Y.; Hua, Y.; Fu, P. Symbiodiniaceae and Ruegeria sp. Co-Cultivation to Enhance Nutrient Exchanges in Coral Holobiont. Microorganisms 2024, 12, 1217. https://doi.org/10.3390/microorganisms12061217
Liu Y, Wu H, Shu Y, Hua Y, Fu P. Symbiodiniaceae and Ruegeria sp. Co-Cultivation to Enhance Nutrient Exchanges in Coral Holobiont. Microorganisms. 2024; 12(6):1217. https://doi.org/10.3390/microorganisms12061217
Chicago/Turabian StyleLiu, Yawen, Huan Wu, Yang Shu, Yanying Hua, and Pengcheng Fu. 2024. "Symbiodiniaceae and Ruegeria sp. Co-Cultivation to Enhance Nutrient Exchanges in Coral Holobiont" Microorganisms 12, no. 6: 1217. https://doi.org/10.3390/microorganisms12061217
APA StyleLiu, Y., Wu, H., Shu, Y., Hua, Y., & Fu, P. (2024). Symbiodiniaceae and Ruegeria sp. Co-Cultivation to Enhance Nutrient Exchanges in Coral Holobiont. Microorganisms, 12(6), 1217. https://doi.org/10.3390/microorganisms12061217







