Sustainable Remediation of Soil and Water Utilizing Arbuscular Mycorrhizal Fungi: A Review
Abstract
:1. Introduction
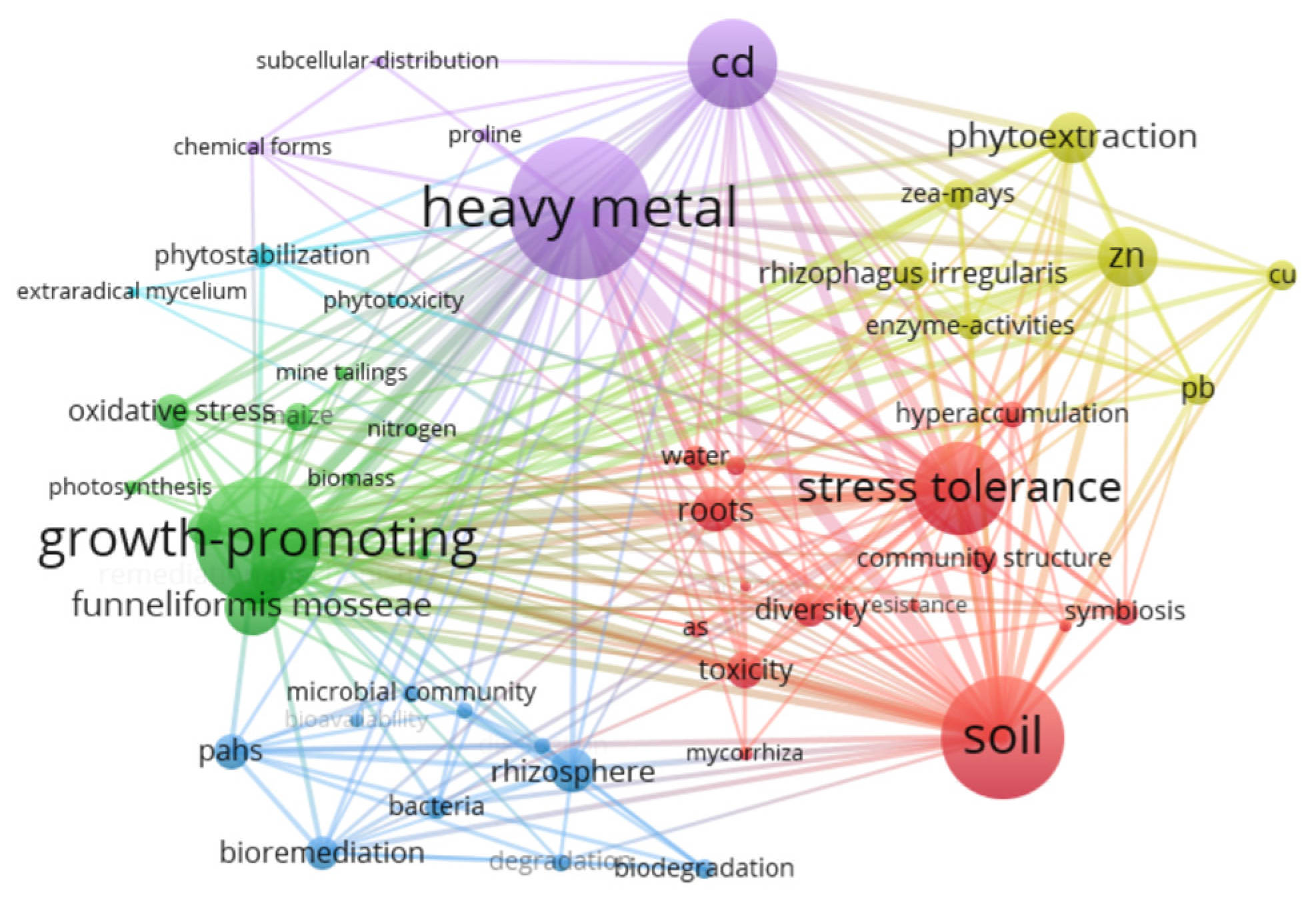
2. Bibliometric Analyses
3. AMF-Assisted Phytoremediation of Contaminated Soil
3.1. Soil Contaminated with Heavy Metal
3.2. Soil Contaminated with Organic Pollutants
3.2.1. Petroleum Hydrocarbons (PHCs) and Polycyclic Aromatic Hydrocarbons (PAHs)
3.2.2. Polychlorinated Biphenyls (PCBs)
3.2.3. Antibiotics
4. AMF-Assisted Phytoremediation of Contaminated Water
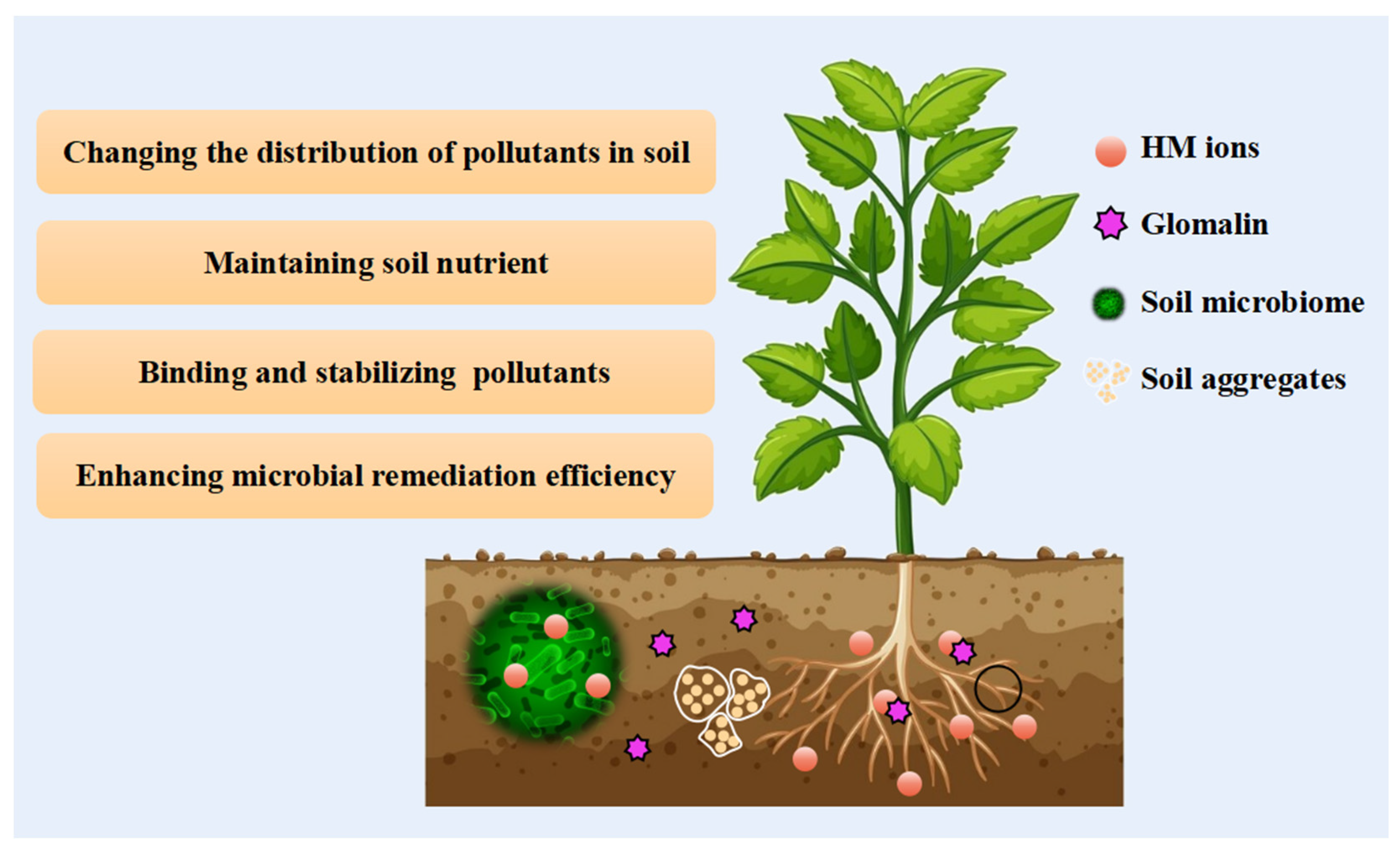
5. Direct Influence of AMF on Pollutant Removal
5.1. Binding and Stabilization of Pollutants by Glomalin
5.2. Pollutant Retention inside Fungal Structure
5.3. Trophic Interaction between AMF and Host Plant
6. The Role of AMF in Host Plant’s Tolerance Strategy
6.1. The Role of AMF in Host Plant’s Tolerance Strategy
6.2. AMF Response to Host Plant’s Antioxidant Defense
6.3. AMF Regulate Gene Expression Resistance to Pollutant Stress
7. Conclusions and Perspectives
- The lack of oxygen in water and sediment is one of the factors that limit the effectiveness of AMF. To overcome this, it is important to study and identify AMF strains that can adapt to anaerobic environments and have a wide range of applications. Additionally, it is essential to optimize the combination of AMF strains with plant species to maximize the efficiency of remediation.
- Pollution can affect the community structure of AMF applied to contaminated sites. Only a few AMF species with resistance to pollutant stress can survive in highly polluted areas, which can diminish the expected remediation effect. Future studies focusing on screening and isolating bacterial species that closely interact with AMF in highly polluted environments and understanding the interactions between them should be conducted. Developing strategies that utilize AMF and microbial communities, which interact with AMF, rather than inoculation with AMF alone, is essential for the remediation of contaminated soil and water.
- Existing research has focused on the remediation of a single type of pollution via AMF–plant symbiosis, while the interactions between different types of pollutants have been neglected. Remediation of composite polluted media with HMs and organic compounds should be emphasized in the future. To address the above challenges, medium-scale remediation trials of actual pollution and site construction should be conducted to accelerate the transition of AMF–plant symbiosis system remediation technology from simulation trials to practical applications.
Author Contributions
Funding
Conflicts of Interest
References
- Chakraborty, S.; Qamruzzaman, M.; Zaman, M.; Alam, M.; Hossain, D.; Pramanik, B.; Nguyen, L.; Nghiem, L.; Zhou, J.; Mondal, I.; et al. Metals in e-waste: Occurrence, fate, impacts and remediation technologies. Process. Saf. Environ. Prot. 2022, 162, 230–252. [Google Scholar] [CrossRef]
- Verma, S.; Bhatt, P.; Verma, A.; Mudila, H.; Prasher, P.; Rene, E.R. Microbial technologies for heavy metal remediation: Effect of process conditions and current practices. Clean Technol. Environ. Policy 2021, 25, 1485–1507. [Google Scholar] [CrossRef]
- Maurin, N.; Sayen, S.; Guillon, E. Gas chromatography–mass spectrometry analysis of organic pollutants in French soils irrigated with agro-industrial wastewater. Front. Environ. Sci. 2023, 11, 1125487. [Google Scholar] [CrossRef]
- Khorasani, H.; Xu, J.; Nguyen, T.; Kralles, Z.; Westerhoff, P.; Dai, N.; Zhu, Z. Contribution of wastewater- versus non-wastewater-derived sources to haloacetonitriles formation potential in a wastewater-impacted river. Sci. Total Environ. 2021, 792, 148355. [Google Scholar] [CrossRef]
- He, X.; Song, S.; Huang, Y.; Huang, X.; Huang, H.; Zhang, T.; Sun, H. Contamination of neonicotinoid insecticides in source water and their fate during drinking water treatment in the Dongguan section of the Pearl River. Sci. Total Environ. 2023, 901, 165935. [Google Scholar] [CrossRef]
- He, J.; Strezov, V.; Kumar, R.; Weldekidan, H.; Jahan, S.; Dastjerdi, B.H.; Zhou, X.; Kan, T. Pyrolysis of heavy metal contaminated Avicennia marina biomass from phytoremediation: Characterisation of biomass and pyrolysis products. J. Clean. Prod. 2019, 234, 1235–1245. [Google Scholar] [CrossRef]
- Tarla, D.N.; Erickson, L.E.; Hettiarachchi, G.M.; Amadi, S.I.; Galkaduwa, M.; Davis, L.C.; Nurzhanova, A.; Pidlisnyuk, V. Phytoremediation and Bioremediation of Pesticide-Contaminated Soil. Appl. Sci. 2020, 10, 1217. [Google Scholar] [CrossRef]
- Jamil, F.; Mukhtar, H.; Fouillaud, M.; Dufossé, L. Rhizosphere Signaling: Insights into Plant–Rhizomicrobiome Interactions for Sustainable Agronomy. Microorganisms 2022, 10, 899. [Google Scholar] [CrossRef]
- Srivastava, S.; Shukla, A.; Rajput, V.D.; Kumar, K.; Minkina, T.; Mandzhieva, S.; Shmaraeva, A.; Suprasanna, P. Arsenic Remediation through Sustainable Phytoremediation Approaches. Minerals 2021, 11, 936. [Google Scholar] [CrossRef]
- Jajoo, A.; Mathur, S. Role of arbuscular mycorrhizal fungi as an underground saviuor for protecting plants from abiotic stresses. Physiol. Mol. Biol. Plants 2021, 27, 2589–2603. [Google Scholar] [CrossRef]
- Lanfranco, L.; Bonfante, P.; Genre, A. The Mutualistic Interaction between Plants and Arbuscular Mycorrhizal Fungi. Microbiol. Spectr. 2016, 4, 727–747. [Google Scholar] [CrossRef]
- Roth, R.; Paszkowski, U. Plant carbon nourishment of arbuscular mycorrhizal fungi. Curr. Opin. Plant Biol. 2017, 39, 50–56. [Google Scholar] [CrossRef]
- Liu, J.; Chen, J.; Xie, K.; Tian, Y.; Yan, A.; Liu, J.; Huang, Y.; Wang, S.; Zhu, Y.; Chen, A.; et al. A mycorrhiza-specific H+-ATPase is essential for arbuscule development and symbiotic phosphate and nitrogen uptake. Plant Cell Environ. 2020, 43, 1069–1083. [Google Scholar] [CrossRef]
- Adeyemi, N.O.; Atayese, M.O.; Sakariyawo, O.S.; Azeez, J.O.; Olubode, A.; Ridwan, M.; Adebayo, R.; Adeoye, S. A Growth and Phosphorus Uptake of Soybean (Glycine max L.) in Response to Arbuscular Mycorrhizal Fungus Rhizophagus Intraradices Inoculation in Heavy Metal-contaminated Soils. Soil Sediment Contam. Int. J. 2021, 30, 1–16. [Google Scholar] [CrossRef]
- Diagne, N.; Ngom, M.; Djighaly, P.I.; Fall, D.; Hocher, V.; Svistoonoff, S. Roles of Arbuscular Mycorrhizal Fungi on Plant Growth and Performance: Importance in Biotic and Abiotic Stressed Regulation. Diversity 2020, 12, 370. [Google Scholar] [CrossRef]
- Tiwari, J.; Ma, Y.; Bauddh, K. Arbuscular mycorrhizal fungi: An ecological accelerator of phytoremediation of metal contaminated soils. Arch. Agron. Soil Sci. 2020, 68, 283–296. [Google Scholar] [CrossRef]
- Faggioli, V.; Menoyo, E.; Geml, J.; Kemppainen, M.; Pardo, A.; Salazar, M.J.; Becerra, A.G. Soil lead pollution modifies the structure of arbuscular mycorrhizal fungal communities. Mycorrhiza 2019, 29, 363–373. [Google Scholar] [CrossRef]
- Silva-Castro, G.A.; Cano, C.; Moreno-Morillas, S.; Bago, A.; García-Romera, I. Inoculation of Indigenous Arbuscular Mycorrhizal Fungi as a Strategy for the Recovery of Long-Term Heavy Metal-Contaminated Soils in a Mine-Spill Area. J. Fungi 2022, 9, 56. [Google Scholar] [CrossRef]
- Cabral, L.; Soares, C.R.F.S.; Giachini, A.J.; Siqueira, J.O. Arbuscular mycorrhizal fungi in phytoremediation of contaminated areas by trace elements: Mechanisms and major benefits of their applications. World J. Microbiol. Biotechnol. 2015, 31, 1655–1664. [Google Scholar] [CrossRef]
- Gou, X.; Ni, H.; Sadowsky, M.J.; Chang, X.; Liu, W.; Wei, X. Arbuscular mycorrhizal fungi alleviate erosion-induced soil nutrient losses in experimental agro-ecosystems. CATENA 2023, 220, 106687. [Google Scholar] [CrossRef]
- Yin, L.; Dijkstra, F.A.; Phillips, R.P.; Zhu, B.; Wang, P.; Cheng, W. Arbuscular mycorrhizal trees cause a higher carbon to nitrogen ratio of soil organic matter decomposition via rhizosphere priming than ectomycorrhizal trees. Soil Biol. Biochem. 2021, 157, 108246. [Google Scholar] [CrossRef]
- Ghuge, S.A.; Nikalje, G.C.; Kadam, U.S.; Suprasanna, P.; Hong, J.C. Comprehensive mechanisms of heavy metal toxicity in plants, detoxification, and remediation. J. Hazard. Mater. 2023, 450, 131039. [Google Scholar] [CrossRef]
- Mwelwa, S.; Chungu, D.; Tailoka, F.; Beesigamukama, D.; Tanga, C. Biotransfer of heavy metals along the soil-plant-edible insect-human food chain in Africa. Sci. Total Environ. 2023, 881, 163150. [Google Scholar] [CrossRef]
- Wen, J.; Zhou, J.; Zhang, R.; Ren, W.; Zhao, J.; Cai, D. Current advances of the valorization technologies for heavy metal containing hyperaccumulators. Ind. Crop. Prod. 2024, 209, 118051. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, S.; Liu, S.; Peng, J.; Zhang, H.; Zhao, Q.; Zheng, L.; Chen, Y.; Shen, Z.; Xu, X.; et al. Enhancing the Phytoremediation of Heavy Metals by Combining Hyperaccumulator and Heavy Metal-Resistant Plant Growth-Promoting Bacteria. Front. Plant Sci. 2022, 13, 912350. [Google Scholar] [CrossRef]
- Heggo, A.; Angle, J.; Chaney, R. Effects of vesicular-arbuscular mycorrhizal fungi on heavy metal uptake by soybeans. Soil Biol. Biochem. 1990, 22, 865–869. [Google Scholar] [CrossRef]
- Khan, A. Relationships between chromium biomagnification ratio, accumulation factor, and mycorrhizae in plants growing on tannery effluent-polluted soil. Environ. Int. 2001, 26, 417–423. [Google Scholar] [CrossRef]
- Cabral, L.; Siqueira, J.O.; Soares, C.R.F.S.; Pinto, J.E.B.P. Retenção de metais pesados em micélio de fungos micorrízicos arbusculares. Quim. Nova 2010, 33, 25–29. [Google Scholar] [CrossRef]
- Pedroso, D.d.F.; Barbosa, M.V.; dos Santos, J.V.; Pinto, F.A.; Siqueira, J.O.; Carneiro, M.A.C. Arbuscular Mycorrhizal Fungi Favor the Initial Growth of Acacia mangium, Sorghum bicolor, and Urochloa brizantha in Soil Contaminated with Zn, Cu, Pb, and Cd. Bull. Environ. Contam. Toxicol. 2018, 101, 386–391. [Google Scholar] [CrossRef]
- Dagher, D.J.; de la Providencia, I.E.; Pitre, F.E.; St-Arnaud, M.; Hijri, M. Arbuscular Mycorrhizal Fungal Assemblages Significantly Shifted upon Bacterial Inoculation in Non-Contaminated and Petroleum-Contaminated Environments. Microorganisms 2020, 8, 602. [Google Scholar] [CrossRef]
- Wang, F.; Adams, C.A.; Yang, W.; Sun, Y.; Shi, Z. Benefits of arbuscular mycorrhizal fungi in reducing organic contaminant residues in crops: Implications for cleaner agricultural production. Crit. Rev. Environ. Sci. Technol. 2019, 50, 1580–1612. [Google Scholar] [CrossRef]
- Rajtor, M.; Piotrowska-Seget, Z. Prospects for arbuscular mycorrhizal fungi (AMF) to assist in phytoremediation of soil hydrocarbon contaminants. Chemosphere 2016, 162, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Puschenreiter, M.; Gerhard, S.; Schöftner, P.; Yousaf, S.; Wang, A.; Syed, J.H.; Reichenauer, T.G. Rhizoremediation of petroleum hydrocarbon-contaminated soils: Improvement opportunities and field applications. Environ. Exp. Bot. 2018, 147, 202–219. [Google Scholar] [CrossRef]
- Iffis, B.; St-Arnaud, M.; Hijri, M. Petroleum hydrocarbon contamination, plant identity and arbuscular mycorrhizal fungal (AMF) community determine assemblages of the AMF spore-associated microbes. Environ. Microbiol. 2016, 18, 2689–2704. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Kong, M.; St-Arnaud, M.; Hijri, M. Arbuscular Mycorrhizal Fungal Communities of Native Plant Species under High Petroleum Hydrocarbon Contamination Highlights Rhizophagus as a Key Tolerant Genus. Microorganisms 2020, 8, 872. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, A.; Davies, F.T.; Autenrieth, R.L.; Zuberer, D.A. Arbuscular Mycorrhiza and Petroleum-Degrading Microorganisms Enhance Phytoremediation of Petroleum-Contaminated Soil. Int. J. Phytoremediat. 2008, 10, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Wei, Y.; Li, J.; Han, C.; Deng, Y.; Su, G. Polycyclic aromatic hydrocarbons (PAHs) and their derivatives (oxygenated PAHs, azaarenes, and sulfur/oxygen-containing heterocyclic PAHs) in surface soils from a typical city, south China. Chemosphere 2021, 283, 131190. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Cheng, Z.; Ling, W.; Huang, J. Arbuscular mycorrhizal fungal hyphae contribute to the uptake of polycyclic aromatic hydrocarbons by plant roots. Bioresource Technol. 2010, 101, 6895–6901. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, S.; Guo, J.; Xu, Z.; Wang, S.; Sang, Y. Effects of arbuscular mycorrhizal inoculation on the phytoremediation of PAH-contaminated soil: A meta-analysis. Chemosphere 2022, 307, 136033. [Google Scholar] [CrossRef]
- Song, S.; Xue, J.; Lu, Y.; Zhang, H.; Wang, C.; Cao, X.; Li, Q. Are unintentionally produced polychlorinated biphenyls the main source of polychlorinated biphenyl occurrence in soils? Environ. Pollut. 2018, 243, 492–500. [Google Scholar] [CrossRef]
- Qin, H.; Brookes, P.C.; Xu, J. Arbuscular Mycorrhizal Fungal Hyphae Alter Soil Bacterial Community and Enhance Polychlorinated Biphenyls Dissipation. Front. Microbiol. 2016, 7, 939. [Google Scholar] [CrossRef]
- Lu, Y.-F.; Lu, M.; Peng, F.; Wan, Y.; Liao, M.-H. Remediation of polychlorinated biphenyl-contaminated soil by using a combination of ryegrass, arbuscular mycorrhizal fungi and earthworms. Chemosphere 2014, 106, 44–50. [Google Scholar] [CrossRef]
- Teng, Y.; Luo, Y.; Sun, X.; Tu, C.; Xu, L.; Liu, W.; Li, Z.; Christie, P. Influence of Arbuscular Mycorrhiza and Rhizobium on Phytoremediation by Alfalfa of an Agricultural Soil Contaminated with Weathered PCBs: A Field Study. Int. J. Phytoremediat. 2010, 12, 516–533. [Google Scholar] [CrossRef]
- Qin, H.; Brookes, P.C.; Xu, J.; Feng, Y. Bacterial degradation of Aroclor 1242 in the mycorrhizosphere soils of zucchini (Cucurbita pepo L.) inoculated with arbuscular mycorrhizal fungi. Environ. Sci. Pollut. Res. 2014, 21, 12790–12799. [Google Scholar] [CrossRef]
- Zand, A.D.; Tabrizi, A.M.; Heir, A.V. The influence of association of plant growth-promoting rhizobacteria and zero-valent iron nanoparticles on removal of antimony from soil by Trifolium repens. Environ. Sci. Pollut. Res. 2020, 27, 42815–42829. [Google Scholar] [CrossRef]
- Gil-Díaz, M.; Pérez, R.A.; Alonso, J.; Miguel, E.; Diez-Pascual, S.; Lobo, M.C. Iron nanoparticles to recover a co-contaminated soil with Cr and PCBs. Sci. Rep. 2022, 12, 3541. [Google Scholar] [CrossRef]
- Lou, Y.; Cai, Y.; Tong, Y.; Hsieh, L.; Li, X.; Xu, W.; Shi, K.; Shen, C.; Xu, X.; Lou, L. Interaction between pollutants during the removal of polychlorinated biphenyl-heavy metal combined pollution by modified nanoscale zero-valent iron. Sci. Total Environ. 2019, 673, 120–127. [Google Scholar] [CrossRef]
- Sun, D.; Hu, J.; Bai, J.; Qin, H.; Wang, J.; Wang, J.; Lin, X. Arbuscular mycorrhizal fungus facilitates ryegrass (Lolium perenne L.) growth and polychlorinated biphenyls degradation in a soil applied with nanoscale zero-valent iron. Ecotoxicol. Environ. Saf. 2021, 215, 112170. [Google Scholar] [CrossRef]
- Wen, S.; Sun, D.; Qin, H.; Wang, J.; Lin, X.; Bai, J.; Hu, J. Moderate input of nanoscale zero-valent iron accelerated soil polychlorinated biphenyls dissipation with limited inhibition on arbuscular mycorrhizal fungal diversity. J. Soils Sediments 2024, 24, 1139–1147. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, K.S.; Jeon, J.H.; Lee, S.H. Antibiotic resistance in soil. Lancet Infect. Dis. 2018, 18, 1306–1307. [Google Scholar] [CrossRef]
- Wu, J.; Wang, J.; Li, Z.; Guo, S.; Li, K.; Xu, P.; Ok, Y.S.; Jones, D.L.; Zou, J. Antibiotics and antibiotic resistance genes in agricultural soils: A systematic analysis. Crit. Rev. Environ. Sci. Technol. 2022, 53, 847–864. [Google Scholar] [CrossRef]
- Thiele-Bruhn, S. Pharmaceutical antibiotic compounds in soils—A review. J. Plant Nutr. Soil Sci. 2003, 166, 145–167, Erratum in J. Plant Nutr. Soil Sci. 2003, 166, 546. [Google Scholar] [CrossRef]
- Wang, J.; Huang, R.; Liang, Y.; Long, X.; Wu, S.; Han, Z.; Liu, H.; Huangfu, X. Prediction of antibiotic sorption in soil with machine learning and analysis of global antibiotic resistance risk. J. Hazard. Mater. 2024, 466, 133563. [Google Scholar] [CrossRef]
- Cao, J.; Ji, D.; Wang, C. Interaction between earthworms and arbuscular mycorrhizal fungi on the degradation of oxytetracycline in soils. Soil Biol. Biochem. 2015, 90, 283–292. [Google Scholar] [CrossRef]
- Cao, J.; Wang, C.; Dou, Z.; Liu, M.; Ji, D. Hyphospheric impacts of earthworms and arbuscular mycorrhizal fungus on soil bacterial community to promote oxytetracycline degradation. J. Hazard. Mater. 2018, 341, 346–354. [Google Scholar] [CrossRef]
- Cao, J.; Wang, C.; Ji, D. Improvement of the soil nitrogen content and maize growth by earthworms and arbuscular mycorrhizal fungi in soils polluted by oxytetracycline. Sci. Total Environ. 2016, 571, 926–934. [Google Scholar] [CrossRef]
- Cao, Q.; Wang, H.; Chen, X.; Wang, R.; Liu, J. Composition and distribution of microbial communities in natural river wetlands and corresponding constructed wetlands. Ecol. Eng. 2017, 98, 40–48. [Google Scholar] [CrossRef]
- Jamiołkowska, A.; Księżniak, A.; Gałązka, A.; Hetman, B.; Kopacki, M.; Skwaryło-Bednarz, B. Impact of abiotic factors on development of the community of arbuscular mycorrhizal fungi in the soil: A Review. Int. Agrophys. 2018, 32, 133–140. [Google Scholar] [CrossRef]
- Fusconi, A.; Mucciarelli, M. How important is arbuscular mycorrhizal colonization in wetland and aquatic habitats? Environ. Exp. Bot. 2018, 155, 128–141. [Google Scholar] [CrossRef]
- Xu, Z.; Lv, Y.; Fang, M.; Liu, J.; Zeng, H.; Ban, Y. Diverse and abundant arbuscular mycorrhizal fungi in ecological floating beds used to treat eutrophic water. Appl. Microbiol. Biotechnol. 2021, 105, 6959–6975. [Google Scholar] [CrossRef]
- Xu, Z.; Ban, Y.; Jiang, Y.; Zhang, X.; Liu, X. Arbuscular Mycorrhizal Fungi in Wetland Habitats and Their Application in Constructed Wetland: A Review. Pedosphere 2016, 26, 592–617. [Google Scholar] [CrossRef]
- Ban, Y.; Xiao, Z.; Wu, C.; Lv, Y.; Meng, F.; Wang, J.; Xu, Z. The positive effects of inoculation using arbuscular mycorrhizal fungi and/or dark septate endophytes on the purification efficiency of CuO-nanoparticles-polluted wastewater in constructed wetland. J. Hazard. Mater. 2021, 416, 126095. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, Y.; Xu, Z.; Chen, X.; Zhang, X.; Chen, Z.; Ban, Y. Arbuscular mycorrhizal fungi enhanced the drinking water treatment residue-based vertical flow constructed wetlands on the purification of arsenic-containing wastewater. J. Hazard. Mater. 2024, 465, 133241. [Google Scholar] [CrossRef]
- Hu, B.; Hu, S.; Vymazal, J.; Chen, Z. Application of arbuscular mycorrhizal fungi for pharmaceuticals and personal care productions removal in constructed wetlands with different substrate. J. Clean. Prod. 2022, 339, 130760. [Google Scholar] [CrossRef]
- Zhouying, X.; Kaiguo, L.; Wenxuan, L.; Chen, W.; Xi, C.; Jun, H.; Xiangling, Z.; Yihui, B. The positive effects of arbuscular mycorrhizal fungi inoculation and/or additional aeration on the purification efficiency of combined heavy metals in vertical flow constructed wetlands. Environ. Sci. Pollut. Res. 2022, 29, 68950–68964. [Google Scholar]
- Xu, Z.; Huang, J.; Chu, Z.; Meng, F.; Liu, J.; Li, K.; Chen, X.; Jiang, Y.; Ban, Y. Plant and microbial communities responded to copper and/or tetracyclines in mycorrhizal enhanced vertical flow constructed wetlands microcosms with Canna indica L. J. Hazard. Mater. 2023, 451, 131114. [Google Scholar] [CrossRef]
- Hu, B.; Hu, S.; Chen, Z.; Vymazal, J. Employ of arbuscular mycorrhizal fungi for pharmaceuticals ibuprofen and diclofenac removal in mesocosm-scale constructed wetlands. J. Hazard. Mater. 2020, 409, 124524. [Google Scholar] [CrossRef]
- Ban, Y.; Tan, J.; Xiong, Y.; Mo, X.; Jiang, Y.; Xu, Z. Transcriptome analysis reveals the molecular mechanisms of Phragmites australis tolerance to CuO-nanoparticles and/or flood stress induced by arbuscular mycorrhizal fungi. J. Hazard. Mater. 2023, 442, 130118. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, Y.; Liu, Y.; Ban, Y.; Li, K.; Li, X.; Zhang, X.; Xu, Z. Removal of sulfamethoxazole and Cu, Cd compound pollution by arbuscular mycorrhizal fungi enhanced vertical flow constructed wetlands. Environ. Res. 2024, 245, 117982. [Google Scholar] [CrossRef]
- Lv, Y.; Liu, J.; Fan, Z.; Fang, M.; Xu, Z.; Ban, Y. The function and community structure of arbuscular mycorrhizal fungi in ecological floating beds used for remediation of Pb contaminated wastewater. Sci. Total Environ. 2023, 872, 162233. [Google Scholar] [CrossRef]
- Gao, P.; Wang, X.; Sang, Y.; Wang, S.; Dai, D. AM fungi enhance the function of ecological floating bed in the treatment of saline industrial wastewater. Environ. Sci. Pollut. Res. 2020, 27, 16656–16667. [Google Scholar] [CrossRef]
- Palacios, Y.M.; Gleadow, R.; Davidson, C.; Gan, W.; Winfrey, B. Do mycorrhizae increase plant growth and pollutant removal in stormwater biofilters? Water Res. 2021, 202, 117381. [Google Scholar] [CrossRef]
- Calheiros, C.S.C.; Pereira, S.I.A.; Franco, A.R.; Castro, P.M.L. Diverse Arbuscular Mycorrhizal Fungi (AMF) Communities Colonize Plants Inhabiting a Constructed Wetland for Wastewater Treatment. Water 2019, 11, 1535. [Google Scholar] [CrossRef]
- Driver, J.D.; Holben, W.E.; Rillig, M.C. Characterization of glomalin as a hyphal wall component of arbuscular mycorrhizal fungi. Soil Biol. Biochem. 2005, 37, 101–106. [Google Scholar] [CrossRef]
- Singh, A.K.; Zhu, X.; Chen, C.; Wu, J.; Yang, B.; Zakari, S.; Jiang, X.J.; Singh, N.; Liu, W. The role of glomalin in mitigation of multiple soil degradation problems. Crit. Rev. Environ. Sci. Technol. 2020, 52, 1604–1638. [Google Scholar] [CrossRef]
- Cornejo, P.; Meier, S.; Borie, G.; Rillig, M.C.; Borie, F. Glomalin-related soil protein in a Mediterranean ecosystem affected by a copper smelter and its contribution to Cu and Zn sequestration. Sci. Total Environ. 2008, 406, 154–160. [Google Scholar] [CrossRef]
- Singh, P.K. In Vitro Cu-Sequestration by Glomalin from Acaulospora spinosa Walker and Trappe. Natl. Acad. Sci. Lett. 2014, 38, 183–185. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, H.; Chen, J.; Jiang, Y.; Williams, M.A.; Wu, S.; Li, J.; Liu, J.; Yang, G.; Yan, C. Interactions of soil metals with glomalin-related soil protein as soil pollution bioindicators in mangrove wetland ecosystems. Sci. Total Environ. 2020, 709, 136051. [Google Scholar] [CrossRef]
- Jia, X.; Zhao, Y.; Liu, T.; Huang, S.; Chang, Y. Elevated CO2 increases glomalin-related soil protein (GRSP) in the rhizosphere of Robinia pseudoacacia L. seedlings in Pb- and Cd-contaminated soils. Environ. Pollut. 2016, 218, 349–357. [Google Scholar] [CrossRef]
- Fang, X.; Zhong, X.; Cui, Z.; Zhang, Y.; Du, L.; Yang, Y.; Lv, J. Distribution and Remediation Techniques of Heavy Metals in Soil Aggregates Perspective: A Review. Water Air Soil Pollut. 2023, 234, 631. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.; Hu, J.; Zhang, T.; Wu, X.; Yang, Y. Arbuscular Mycorrhizal Fungi and Glomalin Play a Crucial Role in Soil Aggregate Stability in Pb-Contaminated Soil. Int. J. Environ. Res. Public Health 2022, 19, 5029. [Google Scholar] [CrossRef]
- Xiao, Z.; Duan, C.; Li, S.; Chen, J.; Peng, C.; Che, R.; Liu, C.; Huang, Y.; Mei, R.; Xu, L.; et al. The microbial mechanisms by which long-term heavy metal contamination affects soil organic carbon levels. Chemosphere 2023, 340, 139770. [Google Scholar] [CrossRef]
- Gujre, N.; Agnihotri, R.; Rangan, L.; Sharma, M.P.; Mitra, S. Deciphering the dynamics of glomalin and heavy metals in soils contaminated with hazardous municipal solid wastes. J. Hazard. Mater. 2021, 416, 125869. [Google Scholar] [CrossRef]
- Singh, G.; Bhattacharyya, R.; Das, T.; Sharma, A.; Ghosh, A.; Das, S.; Jha, P. Crop rotation and residue management effects on soil enzyme activities, glomalin and aggregate stability under zero tillage in the Indo-Gangetic Plains. Soil Tillage Res. 2018, 184, 291–300. [Google Scholar] [CrossRef]
- Wang, L.; Wang, G.; Ma, F.; You, Y. Symbiosis between hyperaccumulators and arbuscular mycorrhizal fungi and their synergistic effect on the absorption and accumulation of heavy metals: A review. Sheng Wu Gong Cheng Xue Bao 2021, 37, 3604–3621. [Google Scholar] [CrossRef]
- Carotenuto, G.; Volpe, V.; Russo, G.; Politi, M.; Sciascia, I.; de Almeida-Engler, J.; Genre, A. Local endoreduplication as a feature of intracellular fungal accommodation in arbuscular mycorrhizas. New Phytol. 2019, 223, 430–446. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, X.; Sun, Y.; Wu, Z.; Li, T.; Hu, Y.; Lv, J.; Li, G.; Zhang, Z.; Zhang, J.; et al. Chromium immobilization by extra- and intraradical fungal structures of arbuscular mycorrhizal symbioses. J. Hazard. Mater. 2016, 316, 34–42. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, X.; Chen, B.; Wu, Z.; Li, T.; Hu, Y.; Sun, Y.; Wang, Y. Chromium immobilization by extraradical mycelium of arbuscular mycorrhiza contributes to plant chromium tolerance. Environ. Exp. Bot. 2016, 122, 10–18. [Google Scholar] [CrossRef]
- Sujkowska-Rybkowska, M.; Lisek, A.; Sumorok, B.; Derkowska, E.; Szymańska, M.; Sas-Paszt, L. Arbuscular Mycorrhizal Fungi as an Important Factor Enabling the Adaptation of Anthyllis vulneraria L. to Zn-Pb-Polluted Tailings. Plants 2023, 12, 2092. [Google Scholar] [CrossRef]
- Alvarado-López, C.J.; Dasgupta-Schubert, N.; Ambriz, J.E.; Arteaga-Velazquez, J.C.; Villegas, J.A. Lead uptake by the symbiotic Daucus carota L.–Glomus intraradices system and its effect on the morphology of extra- and intraradical fungal microstructures. Environ. Sci. Pollut. Res. 2018, 26, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Fu, L.; Xia, Y.; Zheng, L.; Chen, C.; Shen, Z.; Chen, Y. Correction: Arbuscular mycorrhizal fungi enhance the copper tolerance of Tagetes patula through the sorption and barrier mechanisms of intraradical hyphae. Metallomics 2017, 9, 989. [Google Scholar] [CrossRef]
- Dhalaria, R.; Kumar, D.; Kumar, H.; Nepovimova, E.; Kuča, K.; Islam, M.T.; Verma, R. Arbuscular Mycorrhizal Fungi as Potential Agents in Ameliorating Heavy Metal Stress in Plants. Agronomy 2020, 10, 815. [Google Scholar] [CrossRef]
- Salazar, M.J.; Menoyo, E.; Faggioli, V.; Geml, J.; Cabello, M.; Rodriguez, J.H.; Marro, N.; Pardo, A.; Pignata, M.L.; Becerra, A.G. Pb accumulation in spores of arbuscular mycorrhizal fungi. Sci. Total Environ. 2018, 643, 238–246. [Google Scholar] [CrossRef]
- Chagnon, P.-L.; Bradley, R.L.; Maherali, H.; Klironomos, J.N. A trait-based framework to understand life history of mycorrhizal fungi. Trends Plant Sci. 2013, 18, 484–491. [Google Scholar] [CrossRef]
- Rich, M.K.; Nouri, E.; Courty, P.-E.; Reinhardt, D. Diet of Arbuscular Mycorrhizal Fungi: Bread and Butter? Trends Plant Sci. 2017, 22, 652–660. [Google Scholar] [CrossRef]
- Huang, J.; Wang, C.; Qi, L.; Zhang, X.; Tang, G.; Li, L.; Guo, J.; Jia, Y.; Dou, X.; Lu, M. Phosphorus is more effective than nitrogen in restoring plant communities of heavy metals polluted soils. Environ. Pollut. 2020, 266, 115259. [Google Scholar] [CrossRef]
- Quan, L.; Zhang, J.; Wei, Q.; Wang, Y.; Qin, C.; Hu, F.; Chen, Y.; Shen, Z.; Xia, Y. Promotion of Zinc Tolerance, Acquisition and Translocation of Phosphorus in Mimosa pudica L. Mediated by Arbuscular Mycorrhizal Fungi. Bull. Environ. Contam. Toxicol. 2021, 106, 507–515. [Google Scholar] [CrossRef]
- Zhan, F.; Li, B.; Jiang, M.; Yue, X.; He, Y.; Xia, Y.; Wang, Y. Arbuscular mycorrhizal fungi enhance antioxidant defense in the leaves and the retention of heavy metals in the roots of maize. Environ. Sci. Pollut. Res. 2018, 25, 24338–24347. [Google Scholar] [CrossRef]
- Ivanov, S.; Austin, J.; Berg, R.H.; Harrison, M.J. Extensive membrane systems at the host–arbuscular mycorrhizal fungus interface. Nat. Plants 2019, 5, 194–203. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Grønlund, M.; Jakobsen, I.; Grotemeyer, M.S.; Rentsch, D.; Miyao, A.; Hirochika, H.; Kumar, C.S.; Sundaresan, V.; Salamin, N.; et al. Nonredundant Regulation of Rice Arbuscular Mycorrhizal Symbiosis by Two Members of the PHOSPHATE TRANSPORTER1 Gene Family. Plant Cell 2012, 24, 4236–4251. [Google Scholar] [CrossRef]
- You, Y.; Ju, C.; Wang, L.; Wang, X.; Ma, F.; Wang, G.; Wang, Y. The mechanism of arbuscular mycorrhizal enhancing cadmium uptake in Phragmites australis depends on the phosphorus concentration. J. Hazard. Mater. 2022, 440, 129800. [Google Scholar] [CrossRef]
- Lam, C.-M.; Lai, H.-Y. Effect of inoculation with arbuscular mycorrhizal fungi and blanching on the bioaccessibility of heavy metals in water spinach (Ipomoea aquatica Forsk.). Ecotoxicol. Environ. Saf. 2018, 162, 563–570. [Google Scholar] [CrossRef]
- Sharma, S.S.; Dietz, K.-J.; Mimura, T. Vacuolar compartmentalization as indispensable component of heavy metal detoxification in plants: Vacuolar functions in HM detoxification. Plant Cell Environ. 2016, 39, 1112–1126. [Google Scholar] [CrossRef]
- Pan, J.; Cao, S.; Xu, G.; Rehman, M.; Li, X.; Luo, D.; Wang, C.; Fang, W.; Xiao, H.; Liao, C.; et al. Comprehensive analysis reveals the underlying mechanism of arbuscular mycorrhizal fungi in kenaf cadmium stress alleviation. Chemosphere 2023, 314, 137566. [Google Scholar] [CrossRef]
- Han, Y.; Zveushe, O.K.; Dong, F.; Ling, Q.; Chen, Y.; Sajid, S.; Zhou, L.; de Dios, V.R. Unraveling the effects of arbuscular mycorrhizal fungi on cadmium uptake and detoxification mechanisms in perennial ryegrass (Lolium perenne). Sci. Total Environ. 2021, 798, 149222. [Google Scholar] [CrossRef]
- Gao, M.Y.; Chen, X.W.; Huang, W.X.; Wu, L.; Yu, Z.S.; Xiang, L.; Mo, C.H.; Li, Y.W.; Cai, Q.Y.; Wong, M.H.; et al. Cell wall modification induced by an arbuscular mycorrhizal fungus enhanced cadmium fixation in rice root. J. Hazard. Mater. 2021, 416, 125894. [Google Scholar] [CrossRef]
- Park, J.; Chung, H.; Kim, S.H.; An, J.; Nam, K. Effect of neutralizing agents on the type of As co-precipitates formed by in situ Fe oxides synthesis and its impact on the bioaccessibility of As in soil. Sci. Total Environ. 2020, 743, 140686. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Hu, Z.-H.; Yan, T.-X.; Lu, R.-R.; Peng, C.-L.; Li, S.-S.; Jing, Y.-X. Arbuscular mycorrhizal fungi alleviate Cd phytotoxicity by altering Cd subcellular distribution and chemical forms in Zea mays. Ecotoxicol. Environ. Saf. 2019, 171, 352–360. [Google Scholar] [CrossRef]
- Li, H.; Luo, N.; Zhang, L.J.; Zhao, H.M.; Li, Y.W.; Cai, Q.Y.; Wong, M.H.; Mo, C.H. Do arbuscular mycorrhizal fungi affect cadmium uptake kinetics, subcellular distribution and chemical forms in rice? Sci. Total Environ. 2016, 571, 1183–1190. [Google Scholar] [CrossRef]
- Mukherjee, A.; Agrawal, S.B.; Agrawal, M. Responses of tropical tree species to urban air pollutants: ROS/RNS formation and scavenging. Sci. Total Environ. 2019, 710, 136363. [Google Scholar] [CrossRef]
- Wu, B.; Qi, F.; Liang, Y. Fuels for ROS signaling in plant immunity. Trends Plant Sci. 2023, 28, 1124–1131. [Google Scholar] [CrossRef]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef]
- Luo, J.; Li, X.; Jin, Y.; Traore, I.; Dong, L.; Yang, G.; Wang, Y. Effects of Arbuscular Mycorrhizal Fungi Glomus mosseae on the Growth and Medicinal Components of Dysosma versipellis Under Copper Stress. Bull. Environ. Contam. Toxicol. 2020, 107, 924–930. [Google Scholar] [CrossRef]
- Hu, S.; Hu, B.; Chen, Z.; Vosátka, M.; Vymazal, J. Antioxidant response in arbuscular mycorrhizal fungi inoculated wetland plant under Cr stress. Environ. Res. 2020, 191, 110203. [Google Scholar] [CrossRef]
- Lenoir, I.; Fontaine, J.; Tisserant, B.; Laruelle, F.; Sahraoui, A.L.-H. Beneficial contribution of the arbuscular mycorrhizal fungus, Rhizophagus irregularis, in the protection of Medicago truncatula roots against benzo[a]pyrene toxicity. Mycorrhiza 2017, 27, 465–476. [Google Scholar] [CrossRef]
- Tan, Z.; Xuan, Z.; Wu, C.; Cheng, Y.; Xu, C.; Ma, X.; Wang, D. Effects of Selenium on the AsA-GSH System and Photosynthesis of Pakchoi (Brassica chinensis L.) Under Lead Stress. J. Soil Sci. Plant Nutr. 2022, 22, 5111–5122. [Google Scholar] [CrossRef]
- Sharma, S.; Anand, G.; Singh, N.; Kapoor, R. Arbuscular Mycorrhiza Augments Arsenic Tolerance in Wheat (Triticum aestivum L.) by Strengthening Antioxidant Defense System and Thiol Metabolism. Front. Plant Sci. 2017, 8, 906. [Google Scholar] [CrossRef]
- Li, H.; Wang, H.; Zhao, J.; Zhang, L.; Li, Y.; Wang, H.; Teng, H.; Yuan, Z.; Yuan, Z. Physio-Biochemical and Transcriptomic Features of Arbuscular Mycorrhizal Fungi Relieving Cadmium Stress in Wheat. Antioxidants 2022, 11, 2390. [Google Scholar] [CrossRef]
- Aalipour, H.; Nikbakht, A.; Etemadi, N. Co-inoculation of Arizona cypress with arbuscular mycorrhiza fungi and Pseudomonas fluorescens under fuel pollution. Mycorrhiza 2019, 29, 277–289. [Google Scholar] [CrossRef]
- Pandey, A.; Wu, L.-B.; Murugaiyan, V.; Schaaf, G.; Ali, J.; Frei, M. Differential effects of arsenite and arsenate on rice (Oryza sativa) plants differing in glutathione S-transferase gene expression. Environ. Sci. Pollut. Res. 2023, 30, 92268–92281. [Google Scholar] [CrossRef]
- Wang, H.-R.; Zhao, X.-Y.; Zhang, J.-M.; Lu, C.; Feng, F.-J. Arbuscular mycorrhizal fungus regulates cadmium accumulation, migration, transport, and tolerance in Medicago sativa. J. Hazard. Mater. 2022, 435, 129077. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yang, F.; Liu, J.-L.; Wu, H.-T.; Yang, H.; Shi, Y.; Liu, J.; Zhang, Y.-F.; Luo, Y.-R.; Chen, K.-M. Heavy metal transporters: Functional mechanisms, regulation, and application in phytoremediation. Sci. Total Environ. 2021, 809, 151099. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Yuan, X.; Li, L.; Zeng, M.; Yang, J.; Tang, H.; Duan, C. Genome-Wide Analysis of the ATP-Binding Cassette (ABC) Transporter Family in Zea mays L. and Its Response to Heavy Metal Stresses. Int. J. Mol. Sci. 2022, 23, 2109. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Fan, X.; Feng, Y.; Wang, X.; Gao, H.; Song, F. Arbuscular mycorrhizal fungi influence the uptake of cadmium in industrial hemp (Cannabis sativa L.). Chemosphere 2023, 330, 138728. [Google Scholar] [CrossRef] [PubMed]
- Bucher, M. Functional biology of plant phosphate uptake at root and mycorrhiza interfaces. New Phytol. 2006, 173, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Sawers, R.J.H.; Svane, S.F.; Quan, C.; Grønlund, M.; Wozniak, B.; Gebreselassie, M.; González-Muñoz, E.; Montes, R.A.C.; Baxter, I.; Goudet, J.; et al. Phosphorus acquisition efficiency in arbuscular mycorrhizal maize is correlated with the abundance of root-external hyphae and the accumulation of transcripts encoding PHT1 phosphate transporters. New Phytol. 2017, 214, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Watts-Williams, S.J.; Tyerman, S.D.; Cavagnaro, T.R. The dual benefit of arbuscular mycorrhizal fungi under soil zinc deficiency and toxicity: Linking plant physiology and gene expression. Plant Soil 2017, 420, 375–388. [Google Scholar] [CrossRef]
- Li, J.; Sun, Y.; Jiang, X.; Chen, B.; Zhang, X. Arbuscular mycorrhizal fungi alleviate arsenic toxicity to Medicago sativa by influencing arsenic speciation and partitioning. Ecotoxicol. Environ. Saf. 2018, 157, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Tufail, A.; Li, H.; Naeem, A.; Li, T.X. Leaf cell membrane stability-based mechanisms of zinc nutrition in mitigating salinity stress in rice. Plant Biol. 2018, 20, 338–345. [Google Scholar] [CrossRef]
- Botella, C.; Jouhet, J.; A Block, M. Importance of phosphatidylcholine on the chloroplast surface. Prog. Lipid Res. 2016, 65, 12–23. [Google Scholar] [CrossRef]
- Cui, X.; Jia, B.; Diao, F.; Li, X.; Xu, J.; Zhang, Z.; Li, F.Y.; Guo, W. Transcriptomic analysis reveals the molecular mechanisms of arbuscular mycorrhizal fungi and nitrilotriacetic acid on Suaeda salsa tolerance to combined stress of cadmium and salt. Process. Saf. Environ. Prot. 2022, 160, 210–220. [Google Scholar] [CrossRef]
- Puccio, G.; Ingraffia, R.; Mercati, F.; Amato, G.; Giambalvo, D.; Martinelli, F.; Sunseri, F.; Frenda, A.S. Transcriptome changes induced by Arbuscular mycorrhizal symbiosis in leaves of durum wheat (Triticum durum Desf.) promote higher salt tolerance. Sci. Rep. 2023, 13, 116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hu, W.; Xie, X.; Wu, Y.; Liang, F.; Tang, M. Arbuscular mycorrhizal fungi promote lead immobilization by increasing the polysaccharide content within pectin and inducing cell wall peroxidase activity. Chemosphere 2021, 267, 128924. [Google Scholar] [CrossRef] [PubMed]



| AMF Species | Water Treatment Systems | Aquatic Plants | Pollutants | Reference |
|---|---|---|---|---|
| Funneliformis mosseae | Vertical Flow Constructed Wetland (VFCW) | Phragmites australis | Pb, Zn, Cu, and Cd | [65] |
| F. mosseae | VFCW | P. australis | Copper oxide nanoparticles (CuO-NPs) | [68] |
| F. mosseae | VFCW | P. australis | COD, TN, and CuO-NPs | [62] |
| F. mosseae | VFCW | Canna indica | Tetracycline and Cu | [66] |
| F. mosseae | VFCW | C. indica | Sulfamethoxazole, Cu, and Cd | [69] |
| F. mosseae | VFCW | Pteris vittata | As | [63] |
| F. mosseae | EFB | Zea mays | Pb | [70] |
| Glomus etunicatum | EFB | Cyperus alternifolius | TDS, COD, TN, TP, and salt ions | [71] |
| Rhizophagus irregularis | VFCW | Glyceria maxima | PPCPs (Ibuprofen and diclofenac) | [67] |
| R. irregularis | VFCW | G. maxima | PPCPs (Hydrochlorothiazide, chloramphenicol, furosemide, gemfibrozil triclosan and triclocarban) | [64] |
| Commercially available mycorrhizal inoculant | Stormwater biofilter | Ficinia nodosa Juncus australis Carex appressa | TN, TP, phosphate, and Cd | [72] |
| Native AMF communities | Horizontal Subsurface Flow Constructed Wetland (HFCW) | Canna flaccida C. indica Watsonia borbonica Agapanthus africanus Zantedeschia aethiopica | TSS, BOD5, COD, PO43−, and NH4+ | [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Wang, Z.; Lu, Y.; Wei, J.; Qi, S.; Wu, B.; Cheng, S. Sustainable Remediation of Soil and Water Utilizing Arbuscular Mycorrhizal Fungi: A Review. Microorganisms 2024, 12, 1255. https://doi.org/10.3390/microorganisms12071255
Zhang X, Wang Z, Lu Y, Wei J, Qi S, Wu B, Cheng S. Sustainable Remediation of Soil and Water Utilizing Arbuscular Mycorrhizal Fungi: A Review. Microorganisms. 2024; 12(7):1255. https://doi.org/10.3390/microorganisms12071255
Chicago/Turabian StyleZhang, Xueqi, Zongcheng Wang, Yebin Lu, Jun Wei, Shiying Qi, Boran Wu, and Shuiping Cheng. 2024. "Sustainable Remediation of Soil and Water Utilizing Arbuscular Mycorrhizal Fungi: A Review" Microorganisms 12, no. 7: 1255. https://doi.org/10.3390/microorganisms12071255






