Molecular Mechanisms of Bacterial Resistance to Antimicrobial Peptides in the Modern Era: An Updated Review
Abstract
1. Introduction
2. Antimicrobial Peptides
3. Mechanisms of Resistance to AMPs
3.1. Extracellular Proteolytic Degradation
| Bacteria | Mechanisms/Regulatory Pathways of Resistance | Gene(s) Involved | AMPs | Correlation with Pathogenicity/Virulence * | Refs. |
|---|---|---|---|---|---|
| Acinetobacter baumannii | Acylation of lipid A | lpxL | Polymyxin B | Unknown | [132] |
| LPS-full length-mediated protection | lpxA, lpxC, and lpxD | Lysozyme, LL-37, and lactoferrin | Bacteremia (mice) | [133,134,135] | |
| Deacylation of lipid A | naxD (pmrB) | Polymyxin B | Unknown | [136] | |
| Hydroxylation of lipid A | lpxO/pagQ | Polymyxin B, colistin, and HBD-3 | Bacteremia (Galleria mellonella) Antiphagocytic (invertebrates and mammalian cells) | [137] | |
| Addition of PEtN to lipid A | ept, mcr (plasmid-encoded), (pmrAB and stkSR) | Colistin | Increased virulence in G. mellonella | [138,139,140,141,142,143] | |
| Uptake by/binding to porins | ompA ompW | Colistin (uptake) LL-37, BMAP-28 (binding), and colistin | Virulence to the human airway epithelium, adherence to cells, and biofilm formation | [144,145] | |
| Active efflux (MFS and RND-types) | emrB adeABC | Colistin Colistin heteroresistance | Overexpression increased virulence in a pulmonary infection model | [146,147,148] | |
| Manipulation of host AMP production | lpxO | Galiomycin, gallerimycin, and lysozyme | Bacteraemia (G. mellonella) | [137] | |
| Actinobacillus pleuropneumoniae | Outer membrane permeability | ompW (soxS) | Polymyxin B | Unknown | [149] |
| Active efflux (ABC family/K+ dependent) | sap | PR-39 | Respiratory tract infection (mice) | [150] | |
| Bordetella sp. | O-antigen-mediated protection | wlbA and wlbL (bvgAS) | Cecropin, magainin, protamine, and melittin | Tracheal colonization (turkey) | [151,152] |
| Acylation of lipid A | pagP (bvgAS) and lpxL1 | C18G | Respiratory tract infection (mice) Infection of human macrophages | [153,154] | |
| Brucella abortus | Dephosphorylation of lipid A | lpxE | Polymyxin B | Not required | [155] |
| Brucella melitensis | Active efflux (ABC-type) | yejABEF | Protamine, melittin, polymyxins, HBD-1, and HBD-2 | Survival in macrophages (mice) | [156] |
| Burkholderia cenocepacia complex | Degradation by zinc metalloproteases | zmpB and zmpA (cepIR and cciIR) | HBD-1 and LL-37, respectively | Chronic respiratory infection (mice) | [157,158] |
| Inhibition by exopolysaccahrides (mainly cepacian) | - | Cathelicidins | Lung infections of cystic fibrosis (humans) | [159] | |
| Blockage of AMP uptake by the LPS-heptosylated core oligosaccharide | waaF | Polymyxin B, melittin, and HNP-1 | Unknown | [36,160] | |
| Stabilization of the inner membrane lipids | ispH (LytB; isoprenoid synthesis) and hpnJ (encodes hopanoid) | Polymyxin B | Unknown | [36] | |
| Protease-mediated protection (unknown role/mechanism) | mucD (HtrA protease family) | Polymyxin B | Unknown | [36] | |
| Active efflux (MATE-type) | norM | Polymyxin B | Unknown | [161] | |
| Addition of 4AraN to lipid A | ugdBCAL2946 (two ugd in B. caepacia) | Polymyxin B | Unknown (mutants of 4AraN synthesis not viable) | [160] | |
| Alternative sigma factor regulon (37 °C) | rpoE | Polymyxin B | Phagolysosomal fusion in macrophages | [36] | |
| Campylobacter jejuni | LOS-heptosylated core-mediated protection | waaF | Polymyxin B, HNPs, LL-37, and BPI | Invasion of INT407 cells in vitro | [162,163] |
| LOS core-mediated protection | galU | Polymyxin B, colistin, magainin, cecropin, and bacitracin | Unknown | [164] | |
| Active efflux (RND-type) | cme | Polymyxin B | Intracellular survival and multiplication (Acanthamoeba polyphaga) | [165,166] | |
| Capnocytophaga canimorsus | Dephosphorylation of lipid A | lpxE | Polymyxin B | Unknown | [167] |
| Enterobacter cloacae complex | Active efflux (RND-type) | acrAB-tolC (soxSR) kexD/(crrC) | Polymyxin B and colistin | Systemic infection (intraperitoneal mouse model) | [168,169,170] |
| Addition of 4AraN to lipid A | arn (phoPQ and mgrB) | Colistin heteroresistance | Unknown | [171,172] | |
| Addition of PEtN to lipid A | mcr | Colistin | Unknown | [173] | |
| Potential efflux mechanism mediated by an inner membrane protein | dedA (ecl) | Colistin heteroresistance | Unknown | [169] | |
| Erwinia chrysanthemi | Active efflux (ABC family/K+-dependent) | sap | α-thionin and anakin | Unknown | [174] |
| Escherichia coli | Protease-mediated degradation | degP | Lactoferrin | Urovirulence (humans) | [175] |
| ompT and degP | Protamine, C18G, and LL-37 | [131,176,177,178] | |||
| Core oligosaccharide-mediated protection | pmrD | Polymyxin B | Unknown | [179] | |
| Acylation of lipid A | pagP and lpxM (pmrAB and mgrB) | LL-37 | Unknown | [180,181] | |
| Addition of 4AraN to lipid A | arn (phoPQ, pmrAB, and mgrB) | Polymyxin B and colistin | Unknown | [182,183,184] | |
| Addition of PEtN to lipid A | eptA, eptB, eptC, and mcr (phoPQ, pmrAB, and mgrB) | Polymyxin B and colistin | Unknown | [184,185,186] | |
| Decreased entry via porins | ompF | Colicin and P6 | Unknown | [187,188] | |
| Peptidoglycan modification | amiA and amiC (cpxRA and nlpE) | Protamine, magainin, and melittin | Unknown | [189] | |
| Active efflux (ABC-type) | macB | Bacitracin and colistin | Unknown | [190] | |
| Active efflux (RND and MFS-types) | acrAB and emrAB (cpxRA) | Protamine | Unknown | [191] | |
| Transcriptional repression of host’s AMP production (ETEC) | elt (heat-labile toxin-encoding gene) | HBD-1 and LL-37 | Downregulation of kinase A, ERK MAP Kinase, and Cox-2 pathways (intestinal epithelial cells) | [192] | |
| Francisella novicida | Dephosphorylation of lipid A | lpxF | Polymyxin B | Pulmonary and subcutaneous infections (mice) | [193,194] |
| Francisella tularensis | Deacylation of lipid A | naxD | Polymyxin B | Intracellular replication (mice) | [195] |
| Haemophilus ducreyi | Active efflux (ABC family/K+ dependent) | sapA | LL-37 | Chancroid (human) | [196] |
| Active efflux (RND-type) | mtr | LL-37 and β-defensins | Unknown | [197] | |
| Haemophilus influenzae | Acylation of lipid A | lpxL (htrB) | Polymyxin B | Colonization of human airway epithelial cells | [198,199] |
| Active efflux (ABC family/K+-dependent) | sapA | Canine β-defensin-1, HBD-1, -2, -3, LL-37, HNP-1, and melittin | Otitis media (chinchillas) | [200] | |
| Helicobacter pylori | Dephosphorylation of lipid A | lpxE and lpxF | Polymyxin B, LL-37, HBD-2, and P-113 | Gastrointestinal infection (mice) | [201] |
| Addition of PEtN to lipid A | eptA | Polymyxin B | Unknown | [202] | |
| O and N-acetylations of peptidoglycan | patA and pgdA (synergistic) | Lysozyme | Stomach colonization (mice) | [203] | |
| Klebsiella pneumoniae | Capsule-mediated protection | cps | Polymyxin B and lactoferrin | Pulmonary infection (mice) | [204] |
| O-antigen-mediated protection | wcaI, cpsB, wcaJ, and cpsG | Histones | Unknown | [205] | |
| Acylation of lipid A | lpxM lpxL2 and pagP (mgrB and crrab) | Polymyxin B, colistin, CP28, and C18G Polymyxins (lpxL2, pagP, crrab) HNP-1, HBD-1, -2, and -3 (mgrB) | Antiphagocytic, limits the activation of inflammatory responses by macrophages, and survival (G. mellonella); pneumonia (mice) | [143,206,207] | |
| Hydroxylation of lipid A | lpxO (phoPQ) | Polymyxins | Pulmonary infection (mice) | [208] | |
| Addition of 4AraN to lipid A | pmrHFIJKLM (phoPQ, pmrAB, and mgrB) | Colistin | Same phenotypes as for lpxM mutant in G. mellonella | [183,184,209,210] | |
| Addition of PEtN to lipid A | eptA, eptB, eptC, and mcr, (phoPQ, pmrAB, and mgrB) | Colistin | Unknown | [184,209,210,211] | |
| Activation of unknown systems dedicated to ameliorating AMP cytotoxicity | ompA | Polymyxin B and protamine | Pulmonary infection (murine) | [212] | |
| Active efflux (ABC family/K+-dependent) | sapA | LL-37 | Systemic infection (mice) | [213] | |
| Active efflux (RND-type) | acrRAB H239_3064 (crrAB) | Polymyxin B, HNP-1, HBD-1 and HBD-2, and colistin | Pneumonia (mice) | [214,215] | |
| Legionella pneumophila | Acylation of lipid A | rcp pagP (phoPQ) | LL-37 Polymyxin B and C18G | Pulmonary colonization and infection (mice) | [216] |
| Neisseria gonorrhoeae | Inhibition by lactoferrin-binding protein B | lbpB | Lactoferricin | Unknown | [217,218] |
| Inhibition by type IV pili adhesins (Same scenario in N. meningitides, involvement of host cell RhoA and Cdc42 signalling) | pilE | LL-37 | Adherence to human epithelial cells | [219,220] | |
| Active efflux (RND-type) | mtr | LL-37, PG-1, PC-8, polymyxin B, and colistin | Genital tract infection (mice) | [221,222,223,224] | |
| Neisseria meningitidis | Inhibition by lactoferrin-binding protein B | lbpB (nalP) | Lactoferricin | Unknown | [217,218,225] |
| Sequestration/shielding by capsule | cps | HBD-1 and -2, HNP-1 and -2, LL-37, CRAMP, PG-1, and polymyxin B | Meningitis (humans) | [226,227] | |
| Sequestering by blebs from the OM and biofilm formation | - | Cationic AMPs | Unknown | [228] | |
| Addition of PEtN to lipid A (constitutive) (Same scenario in N. gonorrhoeae) | eptA (formerly lptA) (misRS) dsbA | Polymyxin B | Colonization, inflammation, and survival in neutrophils (EptA) | [229,230] | |
| Porin-mediated export | porB | Polymyxin B | Unknown | [229] | |
| Active efflux (RND-type) | mtr (constitutive) | Polymyxin B, PG, and LL-37 | Unknown | [229] | |
| Photorhabdus laumondii | Active efflux (RND-type) | acrAB | Polymyxin B and colistin | Slight effect on virulence (insects) | [231,232] |
| Addition of 4AraN | pbgPE (phoPQ) | Polymyxin B, colistin, cecropins A and C | Septicemia and virulence (insects) | [233,234] | |
| Porphyromonas gingivalis | Dephosphorylation of lipid A | lpxF | Polymyxin B | Unknown | [233,234,235] |
| Outer membrane OmpA-like porins (undefined mechanism) | pgm6 and pgm7 | HBD-1 and -3, and LL-37 | Unknown | [236] | |
| Inactivation by proteases RIA, RIB, and Kgp | prpR1 and kgp | Cecropin B, brevinin, cecropin A 1-7, melittin 2-9, and mastoparan | Unknown | [237] | |
| Proteolytic degradation by gingipains (serine proteases) | rgpAB | Cecropin B | Abscess formation (mice) | ||
| Prevotella sp. | Proteolytic degradation | Unknown | Cecropin B and brevinin | Unknown | [237] |
| Proteus mirabilis | Degradation by metalloprotease | zapA | LL-37 | Urinary tract infection (mice) | [238] |
| Addition of 4AraN to lipid A | pmrAB | Polymyxin B | Unknown | [239] | |
| Active efflux (ABC family/K+-dependent) | sap | Protegrin | Unknown | [239] | |
| Pseudomonas aeruginosa | Degradation by elastase | las | LL-37 | Corneal infection (mice) | [240] |
| Capsule-mediated protection | cps | Polymyxin B | Resistance to neutrophil-mediated killing | [241] | |
| Shedding of host proteoglycans | lasA | LL-37 and human α-defensins | Pulmonary infection (mice) | [242,243] | |
| Hydroxylation of lipid A | lpxO | Polymyxin B | Acquisition of loss-of-function mutations during chronic CF lung infection (mice) | [244,245] | |
| Addition of 4AraN to lipid A | pmrHFIJKLM (pmrAB, phoPQ, parRS, colRS, and cpsRS) | Colistin and polymyxin B | Cystic fibrosis (humans) | [246,247,248] | |
| Addition of PEtN to lipid A | eptA (only by ectopic expression in L-Ara4N-defective mutants) and mcr | Colistin | Unknown | [249,250,251] | |
| Alteration of membrane phospholipid composition | PA0920 | Protamine | Unknown | [252,253] | |
| Active efflux (RND family) | mexAB, mexCD, and mexXY | Colistin and polymyxin B | Controversial and opposing roles since some mutations increase virulence | [254,255,256] | |
| Stimulation of host cathepsins | Unknown | LL-37, HBD-1, and HBD-3 | Cystic fibrosis (humans) | [257,258] | |
| Pseudomonas fluorescens | Alteration of cytoplasmic membrane lipid composition | Unknown | Polymyxin B | Unknown | [259] |
| Rhizobium etli | Dephosphorylation of lipid A | lpxE and lpxF | Polymyxin B | Unknown | [167] |
| Rhizobium leguminosarum | |||||
| Salmonella enterica | Endopeptidase-mediated degradation | pgtE (phoPQ) | LL-37 and C18-G | Unknown | [128] |
| Acylation of lipid A | lpxM (msbB) pagP (phoPQ) | Polymyxin B C18G and PG-1 | Inflammation and septaecemia in mice (msbB), minor effects (pagP), Required for full virulence (phoP) | [182,260] | |
| Dephosphorylation of lipid A | pagL and lpxR (phoPQ) | Polymyxin B | Minor effects in mice (pagL and lpxR) | [261] | |
| Hydroxylation of lipid A | lpxO (phoPQ) | LL-37 | Increased invasion of human epithelial cells and full virulence in animals (lpxO mutant) | [262] | |
| Addition of 4AraN to lipid A | pmrHFIJKLM (phoPQ) | Defensins and polymyxin B | Gastrointestinal infection (mice) | [260,263] | |
| Addition of PEtN to lipid A/core oligosaccharide | eptA, eptBC (core), cptA (core) (pmrAB), mcr | Polymyxin B and colistin | Unknown | [185,264] | |
| Peptidoglycan modification | amiA and amiC (cpxRA and nlpE) | Protamine, magainin, and melittin | Unknown | [189] | |
| Active efflux (ABC transporter) | macAB | C18G | Intracellular survival (macrophages) | [265,266] | |
| Active efflux (ABC family/K+-dependent) | sap yejABEF | Protamine, melittin, and polymyxin B | Gastrointestinal infection (mice) | [48,267,268] | |
| Extracytoplasmic σE factor | rpoE | P2, polymyxin B, and murine α-defensin cryptdin 4 | Gastrointestinal infection (mice) | [269,270] | |
| Shigella dysenteriae | Manipulation of host AMP production | Unknown | LL-37 | Bacillary dysentery (human) | [271] |
| Shigella flexneri | O-antigen-mediated protection | Unknown | Histones | Unknown | [205,272] |
| Alteration of host AMP production | ospF (mxiE) | Rabbit α-defensin NP5 | Repression of NF-kB-responsive genes (Caco-2 and HeLa cells, rabbits) | [273] | |
| Ureaplasma parvum | Host chromatin alterations | Unknown | HBD-1, HNP-6, and LL-37 | Decreased histone H3K9 acetylation (Human THP-1 monocytoid tumor cell line) | [274] |
| Vibrio cholerae | Acylation of lipid A | lpxL | Polymyxin B | Unknown | [275] |
| Hydroxylation of lipid A | lpxN | Polymyxin B | Unknown | [275] | |
| Sensing by OMP/Activation of a DegS-dependent σE factor | ompU/degS/rpoE | Polymyxin B and P2 | Bile resistance in vivo (ompU) | [276,277] | |
| Active efflux (RND-type) | vexAB, vexCD, and vexIJK | Polymyxin B | Small intestine colonization (mice) | [278] | |
| Transcriptional repression of host’s AMP production | ctxA and ctxB (cholera toxin-encoding genes) | LL-37 | Downregulation of kinase A, ERK MAP Kinase, and Cox-2 pathways (intestinal epithelial cells) | [192] | |
| Vibrio vulnificus | K+ uptake transporter system | trkA | Protamine and polymyxin B | Septicemia (mice) | [279] |
| Yersinia enterocolitica | Acylation of lipid A | lpxP and htrB | Polymyxin B | Gastrointestinal infection (mice) | [280] |
| OMP-mediated protection | yadA (pYVe plasmid-encoded) | Lysozyme and defensins from human granulocytes | Adhesion, autoaggregation, resistance to complement-mediated killing | [281] | |
| Active efflux (MFS-type) | rosAB | Polymyxin B, cecropin, and melittin | Unknown | [282] | |
| Yersinia pestis | Degradation by aspartate protease | pla | LL-37, rCRAMP, and rat β-defensin-1 | Plague (mice) | [130] |
| Active efflux (RND-type) | acrAB | Polymyxin B | Not required | [283] | |
| Yersinia pseudotuberculosis | Acylation of lipid A | pagP | Polymyxin B and cecropin | Unknown | [284] |
| Bacteria | Mechanisms/Regulatory Pathways of Resistance | Gene(s) Involved | AMPs | Correlation with Pathogenicity/Virulence * | Refs. |
|---|---|---|---|---|---|
| Bacillus anthracis | Degradation by metalloproteases | clpX | LL-37, α-defensins, and lysozyme | Lethal infection in CRAMP −/− mice | [285,286] |
| Capsule-mediated protection | capA | Defensins, gramicidin, polymyxin B, nisin, protegrin, and melittin | Dissemination of inhalation anthrax infection (guinea pig) | [25,287,288] | |
| D-alanylation of TAs | dlt | Polymyxin B, colistin, nisin, and magainin-2 | Survival in macrophages and full virulence in a mouse model of inhalational infection | [289] | |
| Lysinylation of PG | mprF | Protamine, LL-37, and HNP-1 | Unknown | [290] | |
| Bacillus cereus | D-alanylation of TAs | dlt | Protamine, nisin, polymyxin B, colistin, lysozyme, and cecropin B | Septecaemia and virulence (insects) | [291] |
| Proteolytic degradation by zinc metalloproteases | inhA1 and inhA2 | Cecropin and attacin | Escape from host macrophages Lethal infection by injection into insects | [292,293] | |
| Bacillus subtilis | D-alanylation of TAs | dlt (spoO and abrB) | Nisin | Unknown | [294,295] |
| Alteration of cytoplasmic membrane lipid composition | sigX | Nisin | Unknown | [294] | |
| Clostridium difficile | D-alanylation of TAs | dlt | Nisin, polymyxin B, and gallidermin | Unknown | [296] |
| Enterococcus faecalis | Degradation by gelatinase | gelE | LL-37 and HYL-20 | Peritonitis (mice) | [240,297] |
| Degradation by serine proteases | sprE | HYL-20 | Peritonitis (mice) | [298,299] | |
| Shedding of host proteoglycans and neutralization of AMPs | Undefined (probably gelE) | Neutrophil-derived α-defensins | Unknown | [300] | |
| D-alanylation of TAs | dlt | Colistin, nisin, and polymyxin B | Unknown | [301] | |
| Lysinylation of phospholipids | mprF1 and mprF2 | Defensins and daptomycin | Bacteremia (mice) | [302,303,304] | |
| Alteration of the localization of cardiolipin microdomains | liaR | Daptomycin and telavancin | Unknown | [305] | |
| O-acetylation of peptidoglycan | EF_0783 | Lysozyme | Survival in peritoneal macrophages (mice) | [306] | |
| Group A streptococcus | Degradation by cysteine-proteinases | speB/ideS (covRS also known as csrRS) | LL-37 | SpeB highly expressed in vivo and colocalizes with LL-37 in human tissue samples | [240,307] |
| Capsule (hyaluronic acid)-mediated repelling | hasABC (covRS) | LL-37 | Survival in neutrophil extracellular traps | [308] | |
| Secreted and surface-bound inhibitory proteins | emm1 (Fimbrial M1 proteins) | LL-37 | Skin or systemic infection (mice) | [309] | |
| ska streptokinase | LL-37 and other cationic AMPs | Systemic dissemination and virulence (mice) | [310,311] | ||
| sic | LL-37 and defensins | Skin infection (mice) | [312] | ||
| Shedding of host proteoglycans that bind cationic AMPs | lasA and speB | LL-37 and defensins | Skin infection (mice) | [300,313] | |
| Cleavage by GRAB:SpeB complex | speB grab | LL-37 | Skin infection (mice) | [314] | |
| Regulatory systems sensing and inducing AMP resistance | covRS | LL-37 | In vivo induction by LL-37 | [315,316] | |
| crgR | mCRAMP | Competitive advantage (mice) | [317] | ||
| D-alanylation of TAs | dlt | LL-37, polymyxin B, and lysozyme | Resistance to neutrophil killing, adhesion, and invasion (pharyngeal epithelial cells) | [318] | |
| Active efflux (ABC-type) | salY | SalA and SalA1 lantibiotics | Intramacrophage survival (zebrafish) | [319] | |
| Manipulation of host AMP production | Unknown | Defensins | Unknown | [320] | |
| Group B streptococcus | TCS regulatory pathways | liaR and covRS | Polymyxin B, colistin, nisin, and LL-37 | Sepsis and pneumonia (mice) | [321,322] |
| ciaR | mCRAMP and lyzozyme | Intracellular survival within neutrophils, murine macrophages, and human brain microvascular endothelial cells | [322] | ||
| Competitive binding/inactivation by PBP1a | ponA (liar) | LL-37, CRAMP, and defensins | Antiphacocytic Pulmonary infection and sepsis (rats) | [323,324] | |
| Sequestration by pili | pilB (liar) | LL-37 and mCRAMP | Invasion and paracellular translocation mediating resistance to phagocytic killing and virulence (humans and animal models) | [325,326] | |
| D-alanylation of TAs | dlt (dltSR) | Colistin | Pulmonary or systemic infection (rats) | [327,328] | |
| Listeria monocytogenes | N-deacetylation of peptidoglycan | pgdA | Lysozyme | Virulence after oral and IV inoculations (mice) Survival in macrophages, liver, spleen, and intestinal lumen | [329] |
| O-acetylation of peptidoglycan | oat | Lysozyme | Virulence, survival in macrophages, and control of cytokine production (mice) | [330] | |
| Glycosylation of WTAs | gttA, gltB, and rml | LL-37 | Intestinal epithelium colonization (mice) | [331,332] | |
| D-alanylation of TAs | dlt (virR) | Colistin, polymyxin B, and nisin | Blood infection (mice) | [333,334] | |
| Lysinylation of PG | mprF (virR) | Gallidermin, HNP-1 and -3 | Survival in liver and spleen (mice) | [334,335] | |
| Active efflux (ABC-type) | anrAB (virR and rpoN) | Nisin, bacitracin, and gallidermin | Unknown | [336] | |
| Thermoregulated transcription factor | prfA | Potato defensin (thermo-dependent) | Gastrointestinal infection (mice) | [337] | |
| Mycobacterium tuberculosis | Lysinylation of PG | lysX | HNP-1 and lysozyme | Respiratory infection (mice and pig) | [338] |
| Mycobacterium marinum | Mycolic acid-mediated protection | kasB | HNP-1 and lysozyme | Intramacrophage survival | [339] |
| Staphylococcus aureus | Degradation by metalloprotease aureolysin | aur | Haloganan and LL-37 | Not required | [340,341,342] |
| Degradation by V8 glutamylendopeptidase (serine protease) | sspA | LL-37 | Virulence and in vivo growth (murine abscess models) | [341,343,344] | |
| Inhibition by iron regulated surface determinant A | isdA | Lactoferrin and LL-37 | Initial stage of abscess formation after IV infection (mice) | [345,346,347] | |
| Inhibition by staphylokinase (plasminogen activator protein) | Sak (agr) | Lactoferricin, tritrpticin, and human defensins | Establishment of skin infections (humans and mice models) | [348,349,350,351] | |
| O-acetylation of peptidoglycan | oat | Host lysozyme | Septic arthritis (mice) Anti-inflammation Inhibits the polarization of T-helper cells (mice) | [352,353,354,355,356,357] | |
| WTA-mediated protection | tagO | Group IIA phospholipase A2 and HBD-3 | Induction and progression of endovascular infection (rabbit model of infective endocarditis), adherence to human epithelial cells biofilm formation, colony spreading, and virulence in mammals | [358,359,360,361,362,363] | |
| D-alanylation of TAs | dlt (agr) | HNP1–3, gallidermin, protegrins 3 and 5, tachyplesins-1 and 3, magainin-2, nisin, and tPMP-131 | Sepsis and septic arthritis (mice) | [364,365,366,367,368,369] | |
| Alteration of cytoplasmic membrane lipid composition | pgsA and cls2 | Daptomycin | Unknown | [370] | |
| Lysinylation of PG | mprF/lysS | Defensins | Systemic infection (mice) | [371,372,373] | |
| Active efflux (ABC-type) | pmtABCD | Defensins and LL-37 | Skin infection (mice) | [374] | |
| Plasmid-mediated active efflux (MFS) | qacA | tPMP | Endovascular infections (rabbit) | [375] | |
| TCS inducing AMP resistance | graRS and apsSX/apsR | HBD3, nisin, indolicidin, and LL-37 | Kidney infections (peritoneal infection murine model) | [367,368,376] | |
| Active efflux (ABC-type) | vraFG | HBD3, nisin, indolicidin, and LL-37 | Hemolytic activity, expansion of subcutaneous abscesses | [368,376,377] | |
| Staphylococcus epidermidis | Sequestration/inhibition by EPS: PIA also known as PNAG | ica | HBD-3, LL-37, and anionic dermcidin | Resistance to PMN killing (humans) | [378] |
| AMP-inducible three component systems | apsXRS (via dlt, mprF, and vraFG) | HBD3 | Resistance to PMN killing (humans) | [369,379] | |
| Staphylococcus xylosus | D-alanylation of TAs | dlt | Gallidermin, magainin 2, and nisin | Unknown | [365] |
| Streptococcus iniae | Shielding/ inhibition by polyanionic surface capsule and cell wall structures | pgm (phosphoglucomutase) | Moronecidin and mCRAMP | Meningoencephalitis (hybrid striped bass model) | [380] |
| O-acetylation of peptidoglycan | cpsY and oatA (metR/mtaR) | Lysozyme | Survival in neutrophils | [381] | |
| Streptococcus mutans | Active efflux (ABC-type) | bceABRS (formerly mbrABCD) | Bacitracin and human α- or β-defensins (also induce bce) | Unknown | [382] |
| D-alanylation of TAs (planktonic cells and biofilm) | dlt (ciaR) | HBD-1, HBD-2, HBD-3, and LL37 | Regulation of cariogenic virulence | [383,384] | |
| Streptococcus pneumoniae | Inhibition by pneumococcal surface protein A | pspA | Apolactoferrin | Pneumococcal infection (mice) | [385] |
| Neutralization by free anionic capsular polysaccharide | cps | Polymyxin B and HNP-1 | Unknown | [241] | |
| D-alanylation of TAs | dlt (ciaRH) | Nisin and gallidermin | Competitive advantage in murine model of pneumococcal pneumonia | [386,387,388] | |
| Active efflux (ABC-type) | macAB homolog | Bacitracin, LL-37, and nisin | Unknown | [389,390] | |
| Active efflux (MFS-type) | mefE | Defensins, LL-37, and CRAMP | Unknown | [389,391] | |
| N and O-acetylations of peptidoglycan | pgdA and adr | Lysozyme | Colonization of the upper respiratory tract (mice) | [392] | |
| Streptococcus suis | Degradation by cysteine protease | apdS | LL-37 | Meningitis and sepsis (humans) | [393] |
| O-acetylation of peptidoglycan | oat | Lysozyme | Unknown | [394] |
3.2. Extracellular Trapping and Inactivation
3.2.1. AMP-Inhibitory Proteins Associated with the Bacterial Cell Surface
3.2.2. AMP-Inhibitory Proteins Secreted by Bacteria
3.2.3. AMP-Inhibitory Molecules Released by Host Tissues
3.3. Electrostatic Shielding/Sequestration of AMPs by the Capsule
3.4. AMP Resistance Mechanisms Associated with Bacterial Cell Wall Structures and Their Modifications
3.4.1. Involvement of LPS and Its Modifications in Resistance to AMPs
Resistance to AMPs Due to O-Antigen and Core Oligosaccharide
Roles of the Acylation and Modifications of Lipid A Acyl Chains in Intrinsic and Induced Resistance to AMPs
- Acylation of Lipid A in LPS
- Deacylation of Lipid A in LPS
- Hydroxylation of Lipid A in LPS
Role of Cationic Polar Groups Added to Lipid A in Induced Resistance to AMPs
- Addition of Aminoarabinose to Lipid A in LPS
- Addition of Phosphoethanolamine to Lipid A in LPS
3.4.2. Outer Membrane Proteins and Resistance to AMPs
3.4.3. Peptidoglycan Modifications and AMP Resistance
3.4.4. Modifications of Teichoic Acids and AMP Resistance
3.5. Role of the Cytoplasmic Membrane Phospholipids and Their Modifications in Resistance to AMPs
3.6. Active Efflux and Transport of AMPs
3.7. Alteration of Membrane Energetics and Resistance to AMPs
3.8. Cellular Differentiations and Resistance to AMPs
3.8.1. Small Colony Variants and Niche-Specific Resistance to AMPs
3.8.2. Biofilm Formation and Resistance to AMPs
3.9. Manipulation of Host Cell AMP Production
4. Conclusive Remarks and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. New Report Calls for Urgent Action to Avert Antimicrobial Resistance Crisis. Available online: https://www.who.int/news/item/29-04-2019-new-report-calls-for-urgent-action-to-avert-antimicrobial-resistance-crisis (accessed on 6 April 2024).
- World Health Organization. Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 7 April 2024).
- O’Neill, J. Review on Antimicrobial Resistance. Tackling Drug-Resistant Infections Globally. 2016. Available online: https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf (accessed on 6 April 2024).
- Ayukekbong, J.A.; Ntemgwa, M.; Atabe, A.N. The Threat of Antimicrobial Resistance in Developing Countries: Causes and Control Strategies. Antimicrob. Resist. Infect. Control 2017, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Pallett, S.J.C.; Boyd, S.E.; O’Shea, M.K.; Martin, J.; Jenkins, D.R.; Hutley, E.J. The Contribution of Human Conflict to the Development of Antimicrobial Resistance. Commun. Med. 2023, 3, 153. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.K. Antibiotic Misuse during COVID-19 Pandemic: A Recipe for Disaster. Indian J. Crit. Care Med. Peer-Rev. Off. Publ. Indian Soc. Crit. Care Med. 2021, 25, 617–619. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Baeder, D.Y.; Regoes, R.R.; Rolff, J. Predicting Drug Resistance Evolution: Insights from Antimicrobial Peptides and Antibiotics. Proc. R. Soc. B Biol. Sci. 2018, 285, 20172687. [Google Scholar] [CrossRef]
- Ciumac, D.; Gong, H.; Hu, X.; Lu, J.R. Membrane Targeting Cationic Antimicrobial Peptides. J. Colloid Interface Sci. 2019, 537, 163–185. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; Yan, Z.-B.; Meng, Y.-M.; Hong, X.-Y.; Shao, G.; Ma, J.-J.; Cheng, X.-R.; Liu, J.; Kang, J.; Fu, C.-Y. Antimicrobial Peptides: Mechanism of Action, Activity and Clinical Potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef]
- Mitra, J.B.; Sharma, V.K.; Kumar, M.; Mukherjee, A. Antimicrobial Peptides: Vestiges of Past or Modern Therapeutics? Mini-Rev. Med. Chem. 2020, 20, 183–195. [Google Scholar] [CrossRef]
- APD3. The Antimicrobial Peptide Database. 2023. Available online: https://aps.unmc.edu (accessed on 7 May 2024).
- Poger, D.; Pöyry, S.; Mark, A.E. Could Cardiolipin Protect Membranes against the Action of Certain Antimicrobial Peptides? Aurein 1.2, a Case Study. ACS Omega 2018, 3, 16453–16464. [Google Scholar] [CrossRef]
- Harrison, P.L.; Heath, G.R.; Johnson, B.R.G.; Abdel-Rahman, M.A.; Strong, P.N.; Evans, S.D.; Miller, K. Phospholipid Dependent Mechanism of Smp24, an α-Helical Antimicrobial Peptide from Scorpion Venom. Biochim. Biophys. Acta BBA—Biomembr. 2016, 1858, 2737–2744. [Google Scholar] [CrossRef]
- von Deuster, C.I.E.; Knecht, V. Antimicrobial Selectivity Based on Zwitterionic Lipids and Underlying Balance of Interactions. Biochim. Biophys. Acta BBA—Biomembr. 2012, 1818, 2192–2201. [Google Scholar] [CrossRef]
- Maturana, P.; Martinez, M.; Noguera, M.E.; Santos, N.C.; Disalvo, E.A.; Semorile, L.; Maffia, P.C.; Hollmann, A. Lipid Selectivity in Novel Antimicrobial Peptides: Implication on Antimicrobial and Hemolytic Activity. Colloids Surf. B Biointerfaces 2017, 153, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Vignoli Muniz, G.S.; De la Torre, L.I.; Duarte, E.L.; Lorenzón, E.N.; Cilli, E.M.; Balan, A.; Lamy, M.T. Interaction of Synthetic Antimicrobial Peptides of the Hylin A1 Family with Models of Eukaryotic Structures: Zwitterionic Membranes and DNA. Biochem. Biophys. Rep. 2020, 24, 100827. [Google Scholar] [CrossRef] [PubMed]
- Schlame, M. Thematic Review Series: Glycerolipids. Cardiolipin Synthesis for the Assembly of Bacterial and Mitochondrial Membranes. J. Lipid Res. 2008, 49, 1607–1620. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, C.; Li, M.; Liu, C.; Wang, J.; Ou, X.; Han, Y. Antimicrobial Peptides Mediate Apoptosis by Changing Mitochondrial Membrane Permeability. Int. J. Mol. Sci. 2022, 23, 12732. [Google Scholar] [CrossRef] [PubMed]
- Magana, M.; Pushpanathan, M.; Santos, A.L.; Leanse, L.; Fernandez, M.; Ioannidis, A.; Giulianotti, M.A.; Apidianakis, Y.; Bradfute, S.; Ferguson, A.L.; et al. The Value of Antimicrobial Peptides in the Age of Resistance. Lancet Infect. Dis. 2020, 20, e216–e230. [Google Scholar] [CrossRef] [PubMed]
- CDC. Are You Using Antibiotics Wisely? Available online: https://www.cdc.gov/antibiotic-use/do-and-dont.html (accessed on 8 April 2024).
- Hassan, S.N.; Ahmad, F. The Relevance of Antibiotic Supplements in Mammalian Cell Cultures: Towards a Paradigm Shift. Gulhane Med. J. 2020, 62, 224–230. [Google Scholar] [CrossRef]
- Peschel, A.; Sahl, H.-G. The Co-Evolution of Host Cationic Antimicrobial Peptides and Microbial Resistance. Nat. Rev. Microbiol. 2006, 4, 529–536. [Google Scholar] [CrossRef]
- Okdeh, N.; Mahfouz, G.; Harb, J.; Sabatier, J.-M.; Roufayel, R.; Gazo Hanna, E.; Kovacic, H.; Fajloun, Z. Protective Role and Functional Engineering of Neuropeptides in Depression and Anxiety: An Overview. Bioengineering 2023, 10, 258. [Google Scholar] [CrossRef]
- Fjell, C.D.; Hiss, J.A.; Hancock, R.E.W.; Schneider, G. Designing Antimicrobial Peptides: Form Follows Function. Nat. Rev. Drug Discov. 2012, 11, 37–51. [Google Scholar] [CrossRef]
- O’Brien, D.K.; Ribot, W.J.; Chabot, D.J.; Scorpio, A.; Tobery, S.A.; Jelacic, T.M.; Wu, Z.; Friedlander, A.M. The Capsule of Bacillus Anthracis Protects It from the Bactericidal Activity of Human Defensins and Other Cationic Antimicrobial Peptides. PLoS Pathog. 2022, 18, e1010851. [Google Scholar] [CrossRef]
- Rima, M.; Rima, M.; Fajloun, Z.; Sabatier, J.-M.; Bechinger, B.; Naas, T. Antimicrobial Peptides: A Potent Alternative to Antibiotics. Antibiotics 2021, 10, 1095. [Google Scholar] [CrossRef] [PubMed]
- Spohn, R.; Daruka, L.; Lázár, V.; Martins, A.; Vidovics, F.; Grézal, G.; Méhi, O.; Kintses, B.; Számel, M.; Jangir, P.K.; et al. Integrated Evolutionary Analysis Reveals Antimicrobial Peptides with Limited Resistance. Nat. Commun. 2019, 10, 4538. [Google Scholar] [CrossRef]
- Jangir, P.K.; Ogunlana, L.; MacLean, R.C. Evolutionary Constraints on the Acquisition of Antimicrobial Peptide Resistance in Bacterial Pathogens. Trends Microbiol. 2021, 29, 1058–1061. [Google Scholar] [CrossRef] [PubMed]
- Kintses, B.; Méhi, O.; Ari, E.; Számel, M.; Györkei, Á.; Jangir, P.K.; Nagy, I.; Pál, F.; Fekete, G.; Tengölics, R.; et al. Phylogenetic Barriers to Horizontal Transfer of Antimicrobial Peptide Resistance Genes in the Human Gut Microbiota. Nat. Microbiol. 2019, 4, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, M.; Spiller, O.B.; Andrey, D.O.; Hinchliffe, P.; Li, H.; MacLean, C.; Niumsup, P.; Powell, L.; Pritchard, M.; et al. Balancing Mcr-1 Expression and Bacterial Survival Is a Delicate Equilibrium between Essential Cellular Defence Mechanisms. Nat. Commun. 2017, 8, 2054. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.I.; Hughes, D.; Kubicek-Sutherland, J.Z. Mechanisms and Consequences of Bacterial Resistance to Antimicrobial Peptides. Drug Resist. Updat. 2016, 26, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Batoni, G.; Maisetta, G.; Lisa Brancatisano, F.; Esin, S.; Campa, M. Use of Antimicrobial Peptides Against Microbial Biofilms: Advantages and Limits. Curr. Med. Chem. 2011, 18, 256–279. [Google Scholar] [CrossRef] [PubMed]
- Nizet, V. Antimicrobial Peptide Resistance Mechanisms of Human Bacterial Pathogens. Curr. Issues Mol. Biol. 2006, 8, 11–26. [Google Scholar] [CrossRef]
- Ghosh, C.; Haldar, J. Membrane-Active Small Molecules: Designs Inspired by Antimicrobial Peptides. ChemMedChem 2015, 10, 1606–1624. [Google Scholar] [CrossRef]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of Antimicrobial Peptide Action and Resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef]
- Loutet, S.A.; Valvano, M.A. Extreme Antimicrobial Peptide and Polymyxin B Resistance in the Genus Burkholderia. Front. Microbiol. 2011, 2, 159. [Google Scholar] [CrossRef]
- Olaitan, A.O.; Morand, S.; Rolain, J.-M. Mechanisms of Polymyxin Resistance: Acquired and Intrinsic Resistance in Bacteria. Front. Microbiol. 2014, 5, 643. [Google Scholar] [CrossRef]
- Ghimire, J.; Guha, S.; Nelson, B.J.; Morici, L.A.; Wimley, W.C. The Remarkable Innate Resistance of Burkholderia Bacteria to Cationic Antimicrobial Peptides: Insights into the Mechanism of AMP Resistance. J. Membr. Biol. 2022, 255, 503–511. [Google Scholar] [CrossRef]
- Malott, R.J.; Steen-Kinnaird, B.R.; Lee, T.D.; Speert, D.P. Identification of Hopanoid Biosynthesis Genes Involved in Polymyxin Resistance in Burkholderia Multivorans. Antimicrob. Agents Chemother. 2012, 56, 464–471. [Google Scholar] [CrossRef]
- La, M.-V.; Raoult, D.; Renesto, P. Regulation of Whole Bacterial Pathogen Transcription within Infected Hosts. FEMS Microbiol. Rev. 2008, 32, 440–460. [Google Scholar] [CrossRef]
- Groisman, E.A. The Pleiotropic Two-Component Regulatory System PhoP-PhoQ. J. Bacteriol. 2001, 183, 1835–1842. [Google Scholar] [CrossRef]
- Erickson, K.D.; Detweiler, C.S. The Rcs Phosphorelay System Is Specific to Enteric Pathogens/Commensals and Activates ydeI, a Gene Important for Persistent Salmonella Infection of Mice. Mol. Microbiol. 2006, 62, 883–894. [Google Scholar] [CrossRef]
- Fernández, L.; Jenssen, H.; Bains, M.; Wiegand, I.; Gooderham, W.J.; Hancock, R.E.W. The Two-Component System CprRS Senses Cationic Peptides and Triggers Adaptive Resistance in Pseudomonas Aeruginosa Independently of ParRS. Antimicrob. Agents Chemother. 2012, 56, 6212–6222. [Google Scholar] [CrossRef]
- Santos, D.E.S.; Pol-Fachin, L.; Lins, R.D.; Soares, T.A. Polymyxin Binding to the Bacterial Outer Membrane Reveals Cation Displacement and Increasing Membrane Curvature in Susceptible but Not in Resistant Lipopolysaccharide Chemotypes. J. Chem. Inf. Model. 2017, 57, 2181–2193. [Google Scholar] [CrossRef]
- Jacob-Dubuisson, F.; Mechaly, A.; Betton, J.-M.; Antoine, R. Structural Insights into the Signalling Mechanisms of Two-Component Systems. Nat. Rev. Microbiol. 2018, 16, 585–593. [Google Scholar] [CrossRef]
- Bader, M.W.; Sanowar, S.; Daley, M.E.; Schneider, A.R.; Cho, U.; Xu, W.; Klevit, R.E.; Le Moual, H.; Miller, S.I. Recognition of Antimicrobial Peptides by a Bacterial Sensor Kinase. Cell 2005, 122, 461–472. [Google Scholar] [CrossRef]
- Vaara, M.; Vaara, T.; Jensen, M.; Helander, I.; Nurminen, M.; Rietschel, E.T.; Mäkelä, P.h. Characterization of the Lipopolysaccharide from the Polymyxin-Resistant pmrA Mutants of Salmonella Typhimurium. FEBS Lett. 1981, 129, 145–149. [Google Scholar] [CrossRef]
- Groisman, E.A.; Parra-Lopez, C.; Salcedo, M.; Lipps, C.J.; Heffron, F. Resistance to Host Antimicrobial Peptides Is Necessary for Salmonella Virulence. Proc. Natl. Acad. Sci. USA 1992, 89, 11939–11943. [Google Scholar] [CrossRef]
- Sun, J.; Xia, Y.; Li, D.; Du, Q.; Liang, D. Relationship between Peptide Structure and Antimicrobial Activity as Studied by de Novo Designed Peptides. Biochim. Biophys. Acta BBA—Biomembr. 2014, 1838, 2985–2993. [Google Scholar] [CrossRef]
- Lyu, Z.; Yang, P.; Lei, J.; Zhao, J. Biological Function of Antimicrobial Peptides on Suppressing Pathogens and Improving Host Immunity. Antibiotics 2023, 12, 1037. [Google Scholar] [CrossRef]
- Iksanova, A.M.; Arzumanian, V.G.; Konanykhina, S.Y.; Samoylikov, P.V. Antimicrobial Peptides and Proteins in Human Biological Fluids. Microbiol. Indep. Res. J. MIR J. 2022, 9, 37–55. [Google Scholar] [CrossRef]
- Wang, G. Unifying the Classification of Antimicrobial Peptides in the Antimicrobial Peptide Database. Methods Enzymol. 2022, 663, 1–18. [Google Scholar] [CrossRef]
- Saint Jean, K.D.; Henderson, K.D.; Chrom, C.L.; Abiuso, L.E.; Renn, L.M.; Caputo, G.A. Effects of Hydrophobic Amino Acid Substitutions on Antimicrobial Peptide Behavior. Probiotics Antimicrob. Proteins 2018, 10, 408–419. [Google Scholar] [CrossRef]
- Ambroggio, E.E.; Separovic, F.; Bowie, J.H.; Fidelio, G.D.; Bagatolli, L.A. Direct Visualization of Membrane Leakage Induced by the Antibiotic Peptides: Maculatin, Citropin, and Aurein. Biophys. J. 2005, 89, 1874–1881. [Google Scholar] [CrossRef]
- Sneideris, T.; Erkamp, N.A.; Ausserwöger, H.; Saar, K.L.; Welsh, T.J.; Qian, D.; Katsuya-Gaviria, K.; Johncock, M.L.L.Y.; Krainer, G.; Borodavka, A.; et al. Targeting Nucleic Acid Phase Transitions as a Mechanism of Action for Antimicrobial Peptides. Nat. Commun. 2023, 14, 7170. [Google Scholar] [CrossRef]
- Runti, G.; Lopez Ruiz, M. del C.; Stoilova, T.; Hussain, R.; Jennions, M.; Choudhury, H.G.; Benincasa, M.; Gennaro, R.; Beis, K.; Scocchi, M. Functional Characterization of SbmA, a Bacterial Inner Membrane Transporter Required for Importing the Antimicrobial Peptide Bac7(1-35). J. Bacteriol. 2013, 195, 5343–5351. [Google Scholar] [CrossRef]
- Akbari, R.; Hakemi Vala, M.; Hashemi, A.; Aghazadeh, H.; Sabatier, J.-M.; Pooshang Bagheri, K. Action Mechanism of Melittin-Derived Antimicrobial Peptides, MDP1 and MDP2, de Novo Designed against Multidrug Resistant Bacteria. Amino Acids 2018, 50, 1231–1243. [Google Scholar] [CrossRef]
- Li, Q.; Montalban-Lopez, M.; Kuipers, O.P. Increasing the Antimicrobial Activity of Nisin-Based Lantibiotics against Gram-Negative Pathogens. Appl. Environ. Microbiol. 2018, 84, e00052-18. [Google Scholar] [CrossRef]
- Majewska, M.; Zamlynny, V.; Pieta, I.S.; Nowakowski, R.; Pieta, P. Interaction of LL-37 Human Cathelicidin Peptide with a Model Microbial-like Lipid Membrane. Bioelectrochemistry 2021, 141, 107842. [Google Scholar] [CrossRef]
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility In Vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, Y.; Hu, J. Intracellular Mechanism of Antimicrobial Peptide HJH-3 against Salmonella pullorum. RSC Adv. 2022, 12, 14485–14491. [Google Scholar] [CrossRef]
- Ruan, Y.; Shen, T.; Wang, Y.; Hou, M.; Li, J.; Sun, T. Antimicrobial Peptide LL-37 Attenuates LTA Induced Inflammatory Effect in Macrophages. Int. Immunopharmacol. 2013, 15, 575–580. [Google Scholar] [CrossRef]
- Starr, C.G.; He, J.; Wimley, W.C. Host Cell Interactions Are a Significant Barrier to the Clinical Utility of Peptide Antibiotics. ACS Chem. Biol. 2016, 11, 3391–3399. [Google Scholar] [CrossRef]
- Inui Kishi, R.N.; Stach-Machado, D.; Singulani, J. de L.; dos Santos, C.T.; Fusco-Almeida, A.M.; Cilli, E.M.; Freitas-Astúa, J.; Picchi, S.C.; Machado, M.A. Evaluation of Cytotoxicity Features of Antimicrobial Peptides with Potential to Control Bacterial Diseases of Citrus. PLoS ONE 2018, 13, e0203451. [Google Scholar] [CrossRef]
- Fazly Bazzaz, B.S.; Seyedi, S.; Hoseini Goki, N.; Khameneh, B. Human Antimicrobial Peptides: Spectrum, Mode of Action and Resistance Mechanisms. Int. J. Pept. Res. Ther. 2021, 27, 801–816. [Google Scholar] [CrossRef]
- Brogden, K.A. Antimicrobial Peptides: Pore Formers or Metabolic Inhibitors in Bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef]
- Yang, D.; Chen, Q.; Schmidt, A.P.; Anderson, G.M.; Wang, J.; Oppenheim, J.J.; Chertov, O. Ll-37, the Neutrophil Granule–And Epithelial Cell–Derived Cathelicidin, Utilizes Formyl Peptide Receptor–Like 1 (Fprl1) as a Receptor to Chemoattract Human Peripheral Blood Neutrophils, Monocytes, and T Cells. J. Exp. Med. 2000, 192, 1069–1074. [Google Scholar]
- Sowers, A.; Wang, G.; Xing, M.; Li, B. Advances in Antimicrobial Peptide Discovery via Machine Learning and Delivery via Nanotechnology. Microorganisms 2023, 11, 1129. [Google Scholar] [CrossRef]
- Heymich, M.-L.; Srirangan, S.; Pischetsrieder, M. Stability and Activity of the Antimicrobial Peptide Leg1 in Solution and on Meat and Its Optimized Generation from Chickpea Storage Protein. Foods 2021, 10, 1192. [Google Scholar] [CrossRef]
- Chu, H.-L.; Yu, H.-Y.; Yip, B.-S.; Chih, Y.-H.; Liang, C.-W.; Cheng, H.-T.; Cheng, J.-W. Boosting Salt Resistance of Short Antimicrobial Peptides. Antimicrob. Agents Chemother. 2013, 57, 4050–4052. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Chau, J.K.; Perry, N.A.; de Boer, L.; Zaat, S.A.J.; Vogel, H.J. Serum Stabilities of Short Tryptophan- and Arginine-Rich Antimicrobial Peptide Analogs. PLoS ONE 2010, 5, e12684. [Google Scholar] [CrossRef]
- Chen, C.H.; Lu, T.K. Development and Challenges of Antimicrobial Peptides for Therapeutic Applications. Antibiotics 2020, 9, 24. [Google Scholar] [CrossRef]
- Schiffter, H.A. The Delivery of Drugs—Peptides and Proteins. In Comprehensive Biotechnology; Elsevier, 2011; pp. 587–604. ISBN 978-0-08-088504-9. Available online: https://linkinghub.elsevier.com/retrieve/pii/B978008088504900338X (accessed on 7 June 2024).
- Gao, Y.; Fang, H.; Fang, L.; Liu, D.; Liu, J.; Su, M.; Fang, Z.; Ren, W.; Jiao, H. The Modification and Design of Antimicrobial Peptide. Curr. Pharm. Des. 2018, 24, 904–910. [Google Scholar] [CrossRef]
- Allen, S.D.; Bobbala, S.; Karabin, N.B.; Scott, E.A. On the Advancement of Polymeric Bicontinuous Nanospheres toward Biomedical Applications. Nanoscale Horiz. 2019, 4, 258–272. [Google Scholar] [CrossRef]
- Di, L. Strategic Approaches to Optimizing Peptide ADME Properties. AAPS J. 2015, 17, 134–143. [Google Scholar] [CrossRef]
- Vlieghe, P.; Lisowski, V.; Martinez, J.; Khrestchatisky, M. Synthetic Therapeutic Peptides: Science and Market. Drug Discov. Today 2010, 15, 40–56. [Google Scholar] [CrossRef]
- Fosgerau, K.; Hoffmann, T. Peptide Therapeutics: Current Status and Future Directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef]
- Larijani, B.; Ozra, T.; Akbar, S.; Eghbal, T.; Mohammad, P.; Mohammad-Hassan, B.; Shahin, A.; Mahmood, M.; Fathemeh, B. Comparison of Desmopressin (Ddavp) Tablet and Intranasal Spray in the Treatment of Central Diabetes Insipidus. DARU J. Pharm. Sci. 2005, 13, 155–159. [Google Scholar]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef]
- Hansen, I.K.Ø.; Lövdahl, T.; Simonovic, D.; Hansen, K.Ø.; Andersen, A.J.C.; Devold, H.; Richard, C.S.M.; Andersen, J.H.; Strøm, M.B.; Haug, T. Antimicrobial Activity of Small Synthetic Peptides Based on the Marine Peptide Turgencin A: Prediction of Antimicrobial Peptide Sequences in a Natural Peptide and Strategy for Optimization of Potency. Int. J. Mol. Sci. 2020, 21, 5460. [Google Scholar] [CrossRef]
- Ito, T.; Matsunaga, N.; Kurashima, M.; Demizu, Y.; Misawa, T. Enhancing Chemical Stability through Structural Modification of Antimicrobial Peptides with Non-Proteinogenic Amino Acids. Antibiotics 2023, 12, 1326. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Reinhardt, A.; Neundorf, I. Design and Application of Antimicrobial Peptide Conjugates. Int. J. Mol. Sci. 2016, 17, 701. [Google Scholar] [CrossRef]
- Kamysz, E.; Sikorska, E.; Jaśkiewicz, M.; Bauer, M.; Neubauer, D.; Bartoszewska, S.; Barańska-Rybak, W.; Kamysz, W. Lipidated Analogs of the LL-37-Derived Peptide Fragment KR12—Structural Analysis, Surface-Active Properties and Antimicrobial Activity. Int. J. Mol. Sci. 2020, 21, 887. [Google Scholar] [CrossRef]
- Wenzel, M.; Schriek, P.; Prochnow, P.; Albada, H.B.; Metzler-Nolte, N.; Bandow, J.E. Influence of Lipidation on the Mode of Action of a Small RW-Rich Antimicrobial Peptide. Biochim. Biophys. Acta BBA—Biomembr. 2016, 1858, 1004–1011. [Google Scholar] [CrossRef]
- Lai, Z.; Chen, H.; Yuan, X.; Tian, J.; Dong, N.; Feng, X.; Shan, A. Designing Double-Site Lipidated Peptide Amphiphiles as Potent Antimicrobial Biomaterials to Combat Multidrug-Resistant Bacteria. Front. Microbiol. 2022, 13, 1074359. [Google Scholar] [CrossRef]
- Makowska, M.; Kosikowska-Adamus, P.; Zdrowowicz, M.; Wyrzykowski, D.; Prahl, A.; Sikorska, E. Lipidation of Naturally Occurring α-Helical Antimicrobial Peptides as a Promising Strategy for Drug Design. Int. J. Mol. Sci. 2023, 24, 3951. [Google Scholar] [CrossRef]
- Lin, B.; Hung, A.; Singleton, W.; Darmawan, K.K.; Moses, R.; Yao, B.; Wu, H.; Barlow, A.; Sani, M.-A.; Sloan, A.J.; et al. The Effect of Tailing Lipidation on the Bioactivity of Antimicrobial Peptides and Their Aggregation Tendency. Aggregate 2023, 4, e329. [Google Scholar] [CrossRef]
- Húmpola, M.V.; Rey, M.C.; Carballeira, N.M.; Simonetta, A.C.; Tonarelli, G.G. Biological and Structural Effects of the Conjugation of an Antimicrobial Decapeptide with Saturated, Unsaturated, Methoxylated and Branched Fatty Acids: Antimicrobial Lipopeptides Containing Unusual Fatty Acids. J. Pept. Sci. 2017, 23, 45–55. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Björn, C.; Ekblom, J. Antimicrobial Peptides as Therapeutic Agents: Opportunities and Challenges. Crit. Rev. Biotechnol. 2020, 40, 978–992. [Google Scholar] [CrossRef]
- Moretta, A.; Salvia, R.; Scieuzo, C.; Di Somma, A.; Vogel, H.; Pucci, P.; Sgambato, A.; Wolff, M.; Falabella, P. A Bioinformatic Study of Antimicrobial Peptides Identified in the Black Soldier Fly (BSF) Hermetia Illucens (Diptera: Stratiomyidae). Sci. Rep. 2020, 10, 16875. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, Y.; Song, Z.; Tan, Z.; Cheng, J. Recent Advances in Design of Antimicrobial Peptides and Polypeptides toward Clinical Translation. Adv. Drug Deliv. Rev. 2021, 170, 261–280. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Babakhani, S.; Moradi, L.; Karami, S.; Shahbandeh, M.; Mirshekar, M.; Mohebi, S.; Moghadam, M.T. Bacteriophage as a Novel Therapeutic Weapon for Killing Colistin-Resistant Multi-Drug-Resistant and Extensively Drug-Resistant Gram-Negative Bacteria. Curr. Microbiol. 2021, 78, 4023–4036. [Google Scholar] [CrossRef]
- Binsker, U.; Käsbohrer, A.; Hammerl, J.A. Global Colistin Use: A Review of the Emergence of Resistant Enterobacterales and the Impact on Their Genetic Basis. FEMS Microbiol. Rev. 2021, 46, fuab049. [Google Scholar] [CrossRef]
- Olaitan, A.O.; Diene, S.M.; Kempf, M.; Berrazeg, M.; Bakour, S.; Gupta, S.K.; Thongmalayvong, B.; Akkhavong, K.; Somphavong, S.; Paboriboune, P.; et al. Worldwide Emergence of Colistin Resistance in Klebsiella Pneumoniae from Healthy Humans and Patients in Lao PDR, Thailand, Israel, Nigeria and France Owing to Inactivation of the PhoP/PhoQ Regulator mgrB: An Epidemiological and Molecular Study. Int. J. Antimicrob. Agents 2014, 44, 500–507. [Google Scholar] [CrossRef]
- Poulikakos, P.; Tansarli, G.S.; Falagas, M.E. Combination Antibiotic Treatment versus Monotherapy for Multidrug-Resistant, Extensively Drug-Resistant, and Pandrug-Resistant Acinetobacter Infections: A Systematic Review. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1675–1685. [Google Scholar] [CrossRef]
- Wang, T.; Liu, H.; Huang, H.; Weng, Y.; Wang, X. Colistin Monotherapy or Combination for the Treatment of Bloodstream Infection Caused by Klebsiella Pneumoniae: A Systematic Review and Meta-Analysis. BMC Infect. Dis. 2024, 24, 161. [Google Scholar] [CrossRef]
- Kalin, G.; Alp, E.; Demiraslan, H.; Doganay, M.; Coskun, R.; Gündogan, K. Use of High-Dose IV and Aerosolized Colistin for the Treatment of Multidrug-Resistant Acinetobacter Baumannii Ventilator-Associated Pneumonia: Do We Really Need This Treatment? J. Infect. Chemother. 2012, 18, 872–877. [Google Scholar] [CrossRef]
- Tsuji, B.T.; Pogue, J.M.; Zavascki, A.P.; Paul, M.; Daikos, G.L.; Forrest, A.; Giacobbe, D.R.; Viscoli, C.; Giamarellou, H.; Karaiskos, I.; et al. International Consensus Guidelines for the Optimal Use of the Polymyxins: Endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-Infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy 2019, 39, 10–39. [Google Scholar] [CrossRef]
- Falagas, M.E.; Kyriakidou, M.; Voulgaris, G.L.; Vokos, F.; Politi, S.; Kechagias, K.S. Clinical Use of Intravenous Polymyxin B for the Treatment of Patients with Multidrug-Resistant Gram-Negative Bacterial Infections: An Evaluation of the Current Evidence. J. Glob. Antimicrob. Resist. 2021, 24, 342–359. [Google Scholar] [CrossRef]
- Rigatto, M.H.; Falci, D.R.; Zavascki, A.P. Clinical Use of Polymyxin B. Adv. Exp. Med. Biol. 2019, 1145, 197–218. [Google Scholar] [CrossRef]
- Rychlíčková, J.; Kubíčková, V.; Suk, P.; Urbánek, K. Challenges of Colistin Use in ICU and Therapeutic Drug Monitoring: A Literature Review. Antibiot. Basel Switz. 2023, 12, 437. [Google Scholar] [CrossRef]
- El-Sayed Ahmed, M.A.E.-G.; Zhong, L.-L.; Shen, C.; Yang, Y.; Doi, Y.; Tian, G.-B. Colistin and Its Role in the Era of Antibiotic Resistance: An Extended Review (2000-2019). Emerg. Microbes Infect. 2020, 9, 868–885. [Google Scholar] [CrossRef]
- Taglialegna, A. Reviving Colistin. Nat. Rev. Microbiol. 2023, 21, 411. [Google Scholar] [CrossRef]
- Shin, J.M.; Gwak, J.W.; Kamarajan, P.; Fenno, J.C.; Rickard, A.H.; Kapila, Y.L. Biomedical Applications of Nisin. J. Appl. Microbiol. 2016, 120, 1449–1465. [Google Scholar] [CrossRef]
- Soltani, S.; Hammami, R.; Cotter, P.D.; Rebuffat, S.; Said, L.B.; Gaudreau, H.; Bédard, F.; Biron, E.; Drider, D.; Fliss, I. Bacteriocins as a New Generation of Antimicrobials: Toxicity Aspects and Regulations. FEMS Microbiol. Rev. 2021, 45, fuaa039. [Google Scholar] [CrossRef]
- Moretta, A.; Scieuzo, C.; Petrone, A.M.; Salvia, R.; Manniello, M.D.; Franco, A.; Lucchetti, D.; Vassallo, A.; Vogel, H.; Sgambato, A.; et al. Antimicrobial Peptides: A New Hope in Biomedical and Pharmaceutical Fields. Front. Cell. Infect. Microbiol. 2021, 11, 668632. [Google Scholar] [CrossRef]
- Taylor, S.D.; Palmer, M. The Action Mechanism of Daptomycin. Bioorg. Med. Chem. 2016, 24, 6253–6268. [Google Scholar] [CrossRef]
- Wang, S.Z.; Hu, J.T.; Zhang, C.; Zhou, W.; Chen, X.F.; Jiang, L.Y.; Tang, Z.H. The Safety and Efficacy of Daptomycin versus Other Antibiotics for Skin and Soft-Tissue Infections: A Meta-Analysis of Randomised Controlled Trials. BMJ Open 2014, 4, e004744. [Google Scholar] [CrossRef]
- David, J.M.; Rajasekaran, A.K. Gramicidin A: A New Mission for an Old Antibiotic. J. Kidney Cancer VHL 2015, 2, 15–24. [Google Scholar] [CrossRef]
- Yang, X.; Yousef, A.E. Antimicrobial Peptides Produced by Brevibacillus Spp.: Structure, Classification and Bioactivity: A Mini Review. World J. Microbiol. Biotechnol. 2018, 34, 57. [Google Scholar] [CrossRef]
- Douglas, E.J.A.; Wulandari, S.W.; Lovell, S.D.; Laabei, M. Novel Antimicrobial Strategies to Treat Multi-Drug Resistant Staphylococcus Aureus Infections. Microb. Biotechnol. 2023, 16, 1456–1474. [Google Scholar] [CrossRef]
- Isaksson, J.; Brandsdal, B.O.; Engqvist, M.; Flaten, G.E.; Svendsen, J.S.M.; Stensen, W. A Synthetic Antimicrobial Peptidomimetic (LTX 109): Stereochemical Impact on Membrane Disruption. J. Med. Chem. 2011, 54, 5786–5795. [Google Scholar] [CrossRef]
- Díez-Aguilar, M.; Hernández-García, M.; Morosini, M.-I.; Fluit, A.; Tunney, M.M.; Huertas, N.; Del Campo, R.; Obrecht, D.; Bernardini, F.; Ekkelenkamp, M.; et al. Murepavadin Antimicrobial Activity against and Resistance Development in Cystic Fibrosis Pseudomonas Aeruginosa Isolates. J. Antimicrob. Chemother. 2021, 76, 984–992. [Google Scholar] [CrossRef]
- Ekkelenkamp, M.B.; Cantón, R.; Díez-Aguilar, M.; Tunney, M.M.; Gilpin, D.F.; Bernardini, F.; Dale, G.E.; Elborn, J.S.; Bayjanov, J.R.; Fluit, A. Susceptibility of Pseudomonas Aeruginosa Recovered from Cystic Fibrosis Patients to Murepavadin and 13 Comparator Antibiotics. Antimicrob. Agents Chemother. 2020, 64, e01541-19. [Google Scholar] [CrossRef]
- Grönberg, A.; Mahlapuu, M.; Ståhle, M.; Whately-Smith, C.; Rollman, O. Treatment with LL-37 Is Safe and Effective in Enhancing Healing of Hard-to-Heal Venous Leg Ulcers: A Randomized, Placebo-Controlled Clinical Trial. Wound Repair Regen. 2014, 22, 613–621. [Google Scholar] [CrossRef]
- Sierra, J.M.; Fusté, E.; Rabanal, F.; Vinuesa, T.; Viñas, M. An Overview of Antimicrobial Peptides and the Latest Advances in Their Development. Expert Opin. Biol. Ther. 2017, 17, 663–676. [Google Scholar] [CrossRef]
- Koo, H.B.; Seo, J. Antimicrobial Peptides under Clinical Investigation. Pept. Sci. 2019, 111, e24122. [Google Scholar] [CrossRef]
- Ge, Y.; MacDonald, D.L.; Holroyd, K.J.; Thornsberry, C.; Wexler, H.; Zasloff, M. In Vitro Antibacterial Properties of Pexiganan, an Analog of Magainin. Antimicrob. Agents Chemother. 1999, 43, 782–788. [Google Scholar] [CrossRef]
- Gottler, L.M.; Ramamoorthy, A. Structure, Membrane Orientation, Mechanism, and Function of Pexiganan — A Highly Potent Antimicrobial Peptide Designed from Magainin. Biochim. Biophys. Acta BBA—Biomembr. 2009, 1788, 1680–1686. [Google Scholar] [CrossRef]
- Willcox, M.D.P.; Hume, E.B.H.; Aliwarga, Y.; Kumar, N.; Cole, N. A Novel Cationic-peptide Coating for the Prevention of Microbial Colonization on Contact Lenses. J. Appl. Microbiol. 2008, 105, 1817–1825. [Google Scholar] [CrossRef]
- Rubinchik, E.; Dugourd, D.; Algara, T.; Pasetka, C.; Friedland, H.D. Antimicrobial and Antifungal Activities of a Novel Cationic Antimicrobial Peptide, Omiganan, in Experimental Skin Colonisation Models. Int. J. Antimicrob. Agents 2009, 34, 457–461. [Google Scholar] [CrossRef]
- Sinha, M.; Kaushik, S.; Kaur, P.; Sharma, S.; Singh, T.P. Antimicrobial Lactoferrin Peptides: The Hidden Players in the Protective Function of a Multifunctional Protein. Int. J. Pept. 2013, 2013, 390230. [Google Scholar] [CrossRef]
- Mensa, B.; Howell, G.L.; Scott, R.; DeGrado, W.F. Comparative Mechanistic Studies of Brilacidin, Daptomycin, and the Antimicrobial Peptide LL16. Antimicrob. Agents Chemother. 2014, 58, 5136–5145. [Google Scholar] [CrossRef]
- Cole, J.N.; Nizet, V. Bacterial Evasion of Host Antimicrobial Peptide Defenses. Microbiol. Spectr. 2016, 4, 4.1.04. [Google Scholar] [CrossRef]
- Hooper, N.M. Proteases: A Primer. Essays Biochem. 2002, 38, 1–8. [Google Scholar] [CrossRef]
- Guina, T.; Yi, E.C.; Wang, H.; Hackett, M.; Miller, S.I. A PhoP-Regulated Outer Membrane Protease of Salmonella Enterica Serovar Typhimurium Promotes Resistance to Alpha-Helical Antimicrobial Peptides. J. Bacteriol. 2000, 182, 4077–4086. [Google Scholar] [CrossRef]
- Sugimura, K.; Nishihara, T. Purification, Characterization, and Primary Structure of Escherichia Coli Protease VII with Specificity for Paired Basic Residues: Identity of Protease VII and OmpT. J. Bacteriol. 1988, 170, 5625–5632. [Google Scholar] [CrossRef]
- Sodeinde, O.A.; Subrahmanyam, Y.V.B.K.; Stark, K.; Quan, T.; Bao, Y.; Goguen, J.D. A Surface Protease and the Invasive Character of Plague. Science 1992, 258, 1004–1007. [Google Scholar] [CrossRef]
- Stumpe, S.; Bakker, E.P. Requirement of a Large K+-Uptake Capacity and of Extracytoplasmic Protease Activity for Protamine Resistance of Escherichia Coli. Arch. Microbiol. 1997, 167, 126–136. [Google Scholar] [CrossRef]
- Boll, J.M.; Tucker, A.T.; Klein, D.R.; Beltran, A.M.; Brodbelt, J.S.; Davies, B.W.; Trent, M.S. Reinforcing Lipid A Acylation on the Cell Surface of Acinetobacter Baumannii Promotes Cationic Antimicrobial Peptide Resistance and Desiccation Survival. mBio 2015, 6, e00478-15. [Google Scholar] [CrossRef]
- Kamoshida, G.; Akaji, T.; Takemoto, N.; Suzuki, Y.; Sato, Y.; Kai, D.; Hibino, T.; Yamaguchi, D.; Kikuchi-Ueda, T.; Nishida, S.; et al. Lipopolysaccharide-Deficient Acinetobacter Baumannii Due to Colistin Resistance Is Killed by Neutrophil-Produced Lysozyme. Front. Microbiol. 2020, 11, 573. [Google Scholar] [CrossRef]
- Moffatt, J.H.; Harper, M.; Mansell, A.; Crane, B.; Fitzsimons, T.C.; Nation, R.L.; Li, J.; Adler, B.; Boyce, J.D. Lipopolysaccharide-Deficient Acinetobacter Baumannii Shows Altered Signaling through Host Toll-Like Receptors and Increased Susceptibility to the Host Antimicrobial Peptide LL-37. Infect. Immun. 2013, 81, 684–689. [Google Scholar] [CrossRef]
- Vila-Farrés, X.; Ferrer-Navarro, M.; Callarisa, A.E.; Martí, S.; Espinal, P.; Gupta, S.; Rolain, J.-M.; Giralt, E.; Vila, J. Loss of LPS Is Involved in the Virulence and Resistance to Colistin of Colistin-Resistant Acinetobacter Nosocomialis Mutants Selected In Vitro. J. Antimicrob. Chemother. 2015, 70, 2981–2986. [Google Scholar] [CrossRef][Green Version]
- Chin, C.-Y.; Gregg, K.A.; Napier, B.A.; Ernst, R.K.; Weiss, D.S. A PmrB-Regulated Deacetylase Required for Lipid A Modification and Polymyxin Resistance in Acinetobacter Baumannii. Antimicrob. Agents Chemother. 2015, 59, 7911–7914. [Google Scholar] [CrossRef]
- Bartholomew, T.L.; Kidd, T.J.; Sá Pessoa, J.; Conde Álvarez, R.; Bengoechea, J.A. 2-Hydroxylation of Acinetobacter Baumannii Lipid A Contributes to Virulence. Infect. Immun. 2019, 87, e00066-19. [Google Scholar] [CrossRef]
- Srisakul, S.; Wannigama, D.L.; Higgins, P.G.; Hurst, C.; Abe, S.; Hongsing, P.; Saethang, T.; Luk-in, S.; Liao, T.; Kueakulpattana, N.; et al. Overcoming Addition of Phosphoethanolamine to Lipid A Mediated Colistin Resistance in Acinetobacter Baumannii Clinical Isolates with Colistin–Sulbactam Combination Therapy. Sci. Rep. 2022, 12, 11390. [Google Scholar] [CrossRef]
- Giles, S.K.; Stroeher, U.H.; Papudeshi, B.; Edwards, R.A.; Carlson-Jones, J.A.; Roach, M.; Brown, M.H. The StkSR Two-Component System Influences Colistin Resistance in Acinetobacter Baumannii. Microorganisms 2022, 10, 985. [Google Scholar] [CrossRef]
- Trebosc, V.; Gartenmann, S.; Tötzl, M.; Lucchini, V.; Schellhorn, B.; Pieren, M.; Lociuro, S.; Gitzinger, M.; Tigges, M.; Bumann, D.; et al. Dissecting Colistin Resistance Mechanisms in Extensively Drug-Resistant Acinetobacter Baumannii Clinical Isolates. mBio 2019, 10, e01083-19. [Google Scholar] [CrossRef]
- Adams, M.D.; Nickel, G.C.; Bajaksouzian, S.; Lavender, H.; Murthy, A.R.; Jacobs, M.R.; Bonomo, R.A. Resistance to Colistin in Acinetobacter Baumannii Associated with Mutations in the PmrAB Two-Component System. Antimicrob. Agents Chemother. 2009, 53, 3628–3634. [Google Scholar] [CrossRef]
- Martins-Sorenson, N.; Snesrud, E.; Xavier, D.E.; Cacci, L.C.; Iavarone, A.T.; McGann, P.; Riley, L.W.; Moreira, B.M. A Novel Plasmid-Encoded Mcr-4.3 Gene in a Colistin-Resistant Acinetobacter Baumannii Clinical Strain. J. Antimicrob. Chemother. 2020, 75, 60–64. [Google Scholar] [CrossRef]
- Mills, G.; Dumigan, A.; Kidd, T.; Hobley, L.; Bengoechea, J.A. Identification and Characterization of Two Klebsiella Pneumoniae lpxL Lipid A Late Acyltransferases and Their Role in Virulence. Infect. Immun. 2017, 85, e00068-17. [Google Scholar] [CrossRef]
- Tsai, Y.-K.; Liou, C.-H.; Lin, J.-C.; Fung, C.-P.; Chang, F.-Y.; Siu, L.K. Effects of Different Resistance Mechanisms on Antimicrobial Resistance in Acinetobacter Baumannii: A Strategic System for Screening and Activity Testing of New Antibiotics. Int. J. Antimicrob. Agents 2020, 55, 105918. [Google Scholar] [CrossRef]
- Uppalapati, S.R.; Sett, A.; Pathania, R. The Outer Membrane Proteins OmpA, CarO, and OprD of Acinetobacter Baumannii Confer a Two-Pronged Defense in Facilitating Its Success as a Potent Human Pathogen. Front. Microbiol. 2020, 11, 589234. [Google Scholar] [CrossRef]
- Lin, M.-F.; Lin, Y.-Y.; Lan, C.-Y. Contribution of EmrAB Efflux Pumps to Colistin Resistance in Acinetobacter Baumannii. J. Microbiol. Seoul Korea 2017, 55, 130–136. [Google Scholar] [CrossRef]
- Machado, D.; Antunes, J.; Simões, A.; Perdigão, J.; Couto, I.; McCusker, M.; Martins, M.; Portugal, I.; Pacheco, T.; Batista, J.; et al. Contribution of Efflux to Colistin Heteroresistance in a Multidrug Resistant Acinetobacter Baumannii Clinical Isolate. J. Med. Microbiol. 2018, 67, 740–749. [Google Scholar] [CrossRef]
- Yoon, E.-J.; Balloy, V.; Fiette, L.; Chignard, M.; Courvalin, P.; Grillot-Courvalin, C. Contribution of the Ade Resistance-Nodulation-Cell Division-Type Efflux Pumps to Fitness and Pathogenesis of Acinetobacter Baumannii. mBio 2016, 7, e00697-16. [Google Scholar] [CrossRef]
- Chen, X.; Shao, Z.; Wu, L.; He, B.; Yang, W.; Chen, J.; Jin, E.; Huang, Q.; Lei, L.; Xu, J.; et al. Involvement of the Actinobacillus Pleuropneumoniae ompW Gene in Confrontation of Environmental Pressure. Front. Vet. Sci. 2022, 9, 846322. [Google Scholar] [CrossRef]
- Xie, F.; Wang, Y.; Li, G.; Liu, S.; Cui, N.; Liu, S.; Langford, P.R.; Wang, C. The SapA Protein Is Involved in Resistance to Antimicrobial Peptide PR-39 and Virulence of Actinobacillus Pleuropneumoniae. Front. Microbiol. 2017, 8, 811. [Google Scholar] [CrossRef]
- Banemann, A.; Deppisch, H.; Gross, R. The Lipopolysaccharide of Bordetella Bronchiseptica Acts as a Protective Shield against Antimicrobial Peptides. Infect. Immun. 1998, 66, 5607–5612. [Google Scholar] [CrossRef]
- Spears, P.A.; Temple, L.M.; Orndorff, P.E. A Role for Lipopolysaccharide in Turkey Tracheal Colonization by Bordetella Avium as Demonstrated in Vivo and in Vitro. Mol. Microbiol. 2000, 36, 1425–1435. [Google Scholar] [CrossRef]
- Hittle, L.E.; Jones, J.W.; Hajjar, A.M.; Ernst, R.K.; Preston, A. Bordetella Parapertussis PagP Mediates the Addition of Two Palmitates to the Lipopolysaccharide Lipid A. J. Bacteriol. 2015, 197, 572–580. [Google Scholar] [CrossRef]
- Geurtsen, J.; Angevaare, E.; Janssen, M.; Hamstra, H.-J.; ten Hove, J.; de Haan, A.; Kuipers, B.; Tommassen, J.; van der Ley, P. A Novel Secondary Acyl Chain in the Lipopolysaccharide of Bordetella Pertussis Required for Efficient Infection of Human Macrophages. J. Biol. Chem. 2007, 282, 37875–37884. [Google Scholar] [CrossRef]
- Conde-Álvarez, R.; Palacios-Chaves, L.; Gil-Ramírez, Y.; Salvador-Bescós, M.; Bárcena-Varela, M.; Aragón-Aranda, B.; Martínez-Gómez, E.; Zúñiga-Ripa, A.; De Miguel, M.J.; Bartholomew, T.L.; et al. Identification of lptA, lpxE, and lpxO, Three Genes Involved in the Remodeling of Brucella Cell Envelope. Front. Microbiol. 2018, 8, 2657. [Google Scholar] [CrossRef]
- Wang, Z.; Bie, P.; Cheng, J.; Lu, L.; Cui, B.; Wu, Q. The ABC Transporter YejABEF Is Required for Resistance to Antimicrobial Peptides and the Virulence of Brucella Melitensis. Sci. Rep. 2016, 6, 31876. [Google Scholar] [CrossRef]
- Kooi, C.; Subsin, B.; Chen, R.; Pohorelic, B.; Sokol, P.A. Burkholderia Cenocepacia ZmpB Is a Broad-Specificity Zinc Metalloprotease Involved in Virulence. Infect. Immun. 2006, 74, 4083–4093. [Google Scholar] [CrossRef] [PubMed]
- Kooi, C.; Sokol, P.A. Burkholderia Cenocepacia Zinc Metalloproteases Influence Resistance to Antimicrobial Peptides. Microbiology 2009, 155, 2818–2825. [Google Scholar] [CrossRef] [PubMed]
- Foschiatti, M.; Cescutti, P.; Tossi, A.; Rizzo, R. Inhibition of Cathelicidin Activity by Bacterial Exopolysaccharides. Mol. Microbiol. 2009, 72, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Loutet, S.A.; Flannagan, R.S.; Kooi, C.; Sokol, P.A.; Valvano, M.A. A Complete Lipopolysaccharide Inner Core Oligosaccharide Is Required for Resistance of Burkholderia Cenocepacia to Antimicrobial Peptides and Bacterial Survival in Vivo. J. Bacteriol. 2006, 188, 2073–2080. [Google Scholar] [CrossRef] [PubMed]
- Fehlner-Gardiner, C.C.; Valvano, M.A. Cloning and Characterization of the Burkholderia Vietnamiensis norM Gene Encoding a Multi-Drug Efflux Protein. FEMS Microbiol. Lett. 2002, 215, 279–283. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Keo, T.; Collins, J.; Kunwar, P.; Blaser, M.J.; Iovine, N.M. Campylobacter Capsule and Lipooligosaccharide Confer Resistance to Serum and Cationic Antimicrobials. Virulence 2011, 2, 30–40. [Google Scholar] [CrossRef]
- Kanipes, M.I.; Holder, L.C.; Corcoran, A.T.; Moran, A.P.; Guerry, P. A Deep-Rough Mutant of Campylobacter Jejuni 81-176 Is Noninvasive for Intestinal Epithelial Cells. Infect. Immun. 2004, 72, 2452–2455. [Google Scholar] [CrossRef]
- Lin, J.; Wang, Y.; Van Hoang, K. Systematic Identification of Genetic Loci Required for Polymyxin Resistance in Campylobacter jejuni Using an Efficient In Vivo Transposon Mutagenesis System. Foodborne Pathog. Dis. 2009, 6, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Akiba, M.; Lin, J.; Barton, Y.-W.; Zhang, Q. Interaction of CmeABC and CmeDEF in Conferring Antimicrobial Resistance and Maintaining Cell Viability in Campylobacter jejuni. J. Antimicrob. Chemother. 2006, 57, 52–60. [Google Scholar] [CrossRef]
- Vieira, A.; Ramesh, A.; Seddon, A.M.; Karlyshev, A.V. CmeABC Multidrug Efflux Pump Contributes to Antibiotic Resistance and Promotes Campylobacter jejuni Survival and Multiplication in Acanthamoeba Polyphaga. Appl. Environ. Microbiol. 2017, 83, e01600-17. [Google Scholar] [CrossRef]
- Renzi, F.; Zähringer, U.; Chandler, C.E.; Ernst, R.K.; Cornelis, G.R.; Ittig, S.J. Modification of the 1-Phosphate Group during Biosynthesis of Capnocytophaga Canimorsus Lipid A. Infect. Immun. 2016, 84, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Telke, A.A.; Olaitan, A.O.; Morand, S.; Rolain, J.-M. soxRS Induces Colistin Hetero-Resistance in Enterobacter Asburiae and Enterobacter Cloacae by Regulating the acrAB-tolC Efflux Pump. J. Antimicrob. Chemother. 2017, 72, 2715–2721. [Google Scholar] [CrossRef]
- Huang, L.; Feng, Y.; Zong, Z. Heterogeneous Resistance to Colistin in Enterobacter Cloacae Complex Due to a New Small Transmembrane Protein. J. Antimicrob. Chemother. 2019, 74, 2551–2558. [Google Scholar] [CrossRef]
- Pérez, A.; Poza, M.; Fernández, A.; del Carmen Fernández, M.; Mallo, S.; Merino, M.; Rumbo-Feal, S.; Cabral, M.P.; Bou, G. Involvement of the AcrAB-TolC Efflux Pump in the Resistance, Fitness, and Virulence of Enterobacter Cloacae. Antimicrob. Agents Chemother. 2012, 56, 2084–2090. [Google Scholar] [CrossRef]
- Kang, K.N.; Klein, D.R.; Kazi, M.I.; Guérin, F.; Cattoir, V.; Brodbelt, J.S.; Boll, J.M. Colistin Heteroresistance in Enterobacter Cloacae Is Regulated by PhoPQ-Dependent 4-Amino-4-Deoxy-l-Arabinose Addition to Lipid A. Mol. Microbiol. 2019, 111, 1604–1616. [Google Scholar] [CrossRef]
- Doijad, S.P.; Gisch, N.; Frantz, R.; Kumbhar, B.V.; Falgenhauer, J.; Imirzalioglu, C.; Falgenhauer, L.; Mischnik, A.; Rupp, J.; Behnke, M.; et al. Resolving Colistin Resistance and Heteroresistance in Enterobacter Species. Nat. Commun. 2023, 14, 140. [Google Scholar] [CrossRef] [PubMed]
- Xedzro, C.; Shimamoto, T.; Yu, L.; Zuo, H.; Sugawara, Y.; Sugai, M.; Shimamoto, T. Emergence of Colistin-Resistant Enterobacter Cloacae and Raoultella Ornithinolytica Carrying the Phosphoethanolamine Transferase Gene, Mcr-9, Derived from Vegetables in Japan. Microbiol. Spectr. 2023, 11, e0106323. [Google Scholar] [CrossRef] [PubMed]
- López-Solanilla, E.; García-Olmedo, F.; Rodríguez-Palenzuela, P. Inactivation of the sapA to sapF Locus of Erwinia Chrysanthemi Reveals Common Features in Plant and Animal Bacterial Pathogenesis. Plant Cell 1998, 10, 917–924. [Google Scholar] [CrossRef]
- Ulvatne, H.; Haukland, H.H.; Samuelsen, Ø.; Krämer, M.; Vorland, L.H. Proteases in Escherichia Coli and Staphylococcus Aureus Confer Reduced Susceptibility to Lactoferricin B. J. Antimicrob. Chemother. 2002, 50, 461–467. [Google Scholar] [CrossRef]
- Stumpe, S.; Schmid, R.; Stephens, D.L.; Georgiou, G.; Bakker, E.P. Identification of OmpT as the Protease That Hydrolyzes the Antimicrobial Peptide Protamine before It Enters Growing Cells of Escherichia coli. J. Bacteriol. 1998, 180, 4002–4006. [Google Scholar] [CrossRef]
- Thomassin, J.-L.; Brannon, J.R.; Gibbs, B.F.; Gruenheid, S.; Le Moual, H. OmpT Outer Membrane Proteases of Enterohemorrhagic and Enteropathogenic Escherichia coli Contribute Differently to the Degradation of Human LL-37. Infect. Immun. 2012, 80, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Desloges, I.; Taylor, J.A.; Leclerc, J.-M.; Brannon, J.R.; Portt, A.; Spencer, J.D.; Dewar, K.; Marczynski, G.T.; Manges, A.; Gruenheid, S.; et al. Identification and Characterization of OmpT-like Proteases in Uropathogenic Escherichia coli Clinical Isolates. MicrobiologyOpen 2019, 8, e915. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, W.; Fang, Y.; Liang, H.; Yang, H.; Wang, Y.; Dong, X.; Zhan, Y.; Wang, X. Core Oligosaccharide Portion of Lipopolysaccharide Plays Important Roles in Multiple Antibiotic Resistance in Escherichia coli. Antimicrob. Agents Chemother. 2021, 65, e00341-21. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.E.; Kim, S.-H.; Liu, F.; Jia, W.; Vinogradov, E.; Gyles, C.L.; Bishop, R.E. PagP Activation in the Outer Membrane Triggers R3 Core Oligosaccharide Truncation in the Cytoplasm of Escherichia coli O157:H7*. J. Biol. Chem. 2008, 283, 4332–4343. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Fu, J.; Zhao, Y.; Shi, H.; Hu, H.; Wang, H. Escherichia Coli PagP Enzyme-Based De Novo Design and In Vitro Activity of Antibacterial Peptide LL-37. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2017, 23, 2558–2564. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.X.; Lester, M.E.; Stead, C.M.; Raetz, C.R.H.; Maskell, D.J.; McGrath, S.C.; Cotter, R.J.; Trent, M.S. Resistance to the Antimicrobial Peptide Polymyxin Requires Myristoylation of Escherichia coli and Salmonella typhimurium Lipid A*. J. Biol. Chem. 2005, 280, 28186–28194. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.D.; Groisman, E.A. The PmrA/PmrB Two-Component System: The Major Regulator of LPS Modifications. Annu. Rev. Microbiol. 2013, 67, 83–112. [Google Scholar] [CrossRef] [PubMed]
- Lippa, A.M.; Goulian, M. Feedback Inhibition in the PhoQ/PhoP Signaling System by a Membrane Peptide. PLoS Genet. 2009, 5, e1000788. [Google Scholar] [CrossRef] [PubMed]
- Gerster, T.; Wröbel, M.; Hofstaedter, C.E.; Schwudke, D.; Ernst, R.K.; Ranf, S.; Gisch, N. Remodeling of Lipid A in Pseudomonas Syringae Pv. Phaseolicola In Vitro. Int. J. Mol. Sci. 2022, 23, 1996. [Google Scholar] [CrossRef]
- Salazar, J.; Alarcón, M.; Huerta, J.; Navarro, B.; Aguayo, D. Phosphoethanolamine Addition to the Heptose I of the Lipopolysaccharide Modifies the Inner Core Structure and Has an Impact on the Binding of Polymyxin B to the Escherichia coli Outer Membrane. Arch. Biochem. Biophys. 2017, 620, 28–34. [Google Scholar] [CrossRef]
- Bredin, J.; Simonet, V.; Iyer, R.; Delcour, A.H.; Pagès, J.-M. Colicins, Spermine and Cephalosporins: A Competitive Interaction with the OmpF Eyelet. Biochem. J. 2003, 376, 245–252. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Necula, G.; Bacalum, M.; Radu, M. Interaction of Tryptophan- and Arginine-Rich Antimicrobial Peptide with E. Coli Outer Membrane—A Molecular Simulation Approach. Int. J. Mol. Sci. 2023, 24, 2005. [Google Scholar] [CrossRef] [PubMed]
- Weatherspoon-Griffin, N.; Zhao, G.; Kong, W.; Kong, Y.; Morigen; Andrews-Polymenis, H.; McClelland, M.; Shi, Y. The CpxR/CpxA Two-Component System Up-Regulates Two Tat-Dependent Peptidoglycan Amidases to Confer Bacterial Resistance to Antimicrobial Peptide *. J. Biol. Chem. 2011, 286, 5529–5539. [Google Scholar] [CrossRef] [PubMed]
- Crow, A.; Greene, N.P.; Kaplan, E.; Koronakis, V. Structure and Mechanotransmission Mechanism of the MacB ABC Transporter Superfamily. Proc. Natl. Acad. Sci. USA 2017, 114, 12572–12577. [Google Scholar] [CrossRef] [PubMed]
- Weatherspoon-Griffin, N.; Yang, D.; Kong, W.; Hua, Z.; Shi, Y. The CpxR/CpxA Two-Component Regulatory System up-Regulates the Multidrug Resistance Cascade to Facilitate Escherichia coli Resistance to a Model Antimicrobial Peptide. J. Biol. Chem. 2014, 289, 32571–32582. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, K.; Ghosh, S.; Koley, H.; Mukhopadhyay, A.K.; Ramamurthy, T.; Saha, D.R.; Mukhopadhyay, D.; Roychowdhury, S.; Hamabata, T.; Takeda, Y.; et al. Bacterial Exotoxins Downregulate Cathelicidin (hCAP-18/LL-37) and Human Beta-Defensin 1 (HBD-1) Expression in the Intestinal Epithelial Cells. Cell. Microbiol. 2008, 10, 2520–2537. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; McGrath, S.C.; Cotter, R.J.; Raetz, C.R.H. Expression Cloning and Periplasmic Orientation of the Francisella novicida Lipid A 4′-Phosphatase LpxF*. J. Biol. Chem. 2006, 281, 9321–9330. [Google Scholar] [CrossRef] [PubMed]
- Kanistanon, D.; Powell, D.A.; Hajjar, A.M.; Pelletier, M.R.; Cohen, I.E.; Way, S.S.; Skerrett, S.J.; Wang, X.; Raetz, C.R.H.; Ernst, R.K. Role of Francisella Lipid A Phosphate Modification in Virulence and Long-Term Protective Immune Responses. Infect. Immun. 2012, 80, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, A.C.; Zhao, J.; Song, F.; Parvathareddy, J.; Xu, Q.; Napier, B.A.; Laroui, H.; Merlin, D.; Bina, J.E.; Cotter, P.A.; et al. NaxD Is a Deacetylase Required for Lipid A Modification and F Rancisella Pathogenesis. Mol. Microbiol. 2012, 86, 611–627. [Google Scholar] [CrossRef]
- Mount, K.L.B.; Townsend, C.A.; Rinker, S.D.; Gu, X.; Fortney, K.R.; Zwickl, B.W.; Janowicz, D.M.; Spinola, S.M.; Katz, B.P.; Bauer, M.E. Haemophilus Ducreyi SapA Contributes to Cathelicidin Resistance and Virulence in Humans. Infect. Immun. 2010, 78, 1176–1184. [Google Scholar] [CrossRef]
- Rinker, S.D.; Gu, X.; Fortney, K.R.; Zwickl, B.W.; Katz, B.P.; Janowicz, D.M.; Spinola, S.M.; Bauer, M.E. Permeases of the Sap Transporter Are Required for Cathelicidin Resistance and Virulence of Haemophilus ducreyi in Humans. J. Infect. Dis. 2012, 206, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Starner, T.D.; Swords, W.E.; Apicella, M.A.; McCray, P.B. Susceptibility of Nontypeable Haemophilus Influenzae to Human β-Defensins Is Influenced by Lipooligosaccharide Acylation. Infect. Immun. 2002, 70, 5287–5289. [Google Scholar] [CrossRef] [PubMed]
- Swords, W.E.; Chance, D.L.; Cohn, L.A.; Shao, J.; Apicella, M.A.; Smith, A.L. Acylation of the Lipooligosaccharide of Haemophilus Influenzae and Colonization: An htrB Mutation Diminishes the Colonization of Human Airway Epithelial Cells. Infect. Immun. 2002, 70, 4661–4668. [Google Scholar] [CrossRef] [PubMed]
- Mason, K.M.; Munson, R.S.; Bakaletz, L.O. A Mutation in the Sap Operon Attenuates Survival of Nontypeable Haemophilus Influenzae in a Chinchilla Model of Otitis Media. Infect. Immun. 2005, 73, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Cullen, T.W.; Giles, D.K.; Wolf, L.N.; Ecobichon, C.; Boneca, I.G.; Trent, M.S. Helicobacter Pylori versus the Host: Remodeling of the Bacterial Outer Membrane Is Required for Survival in the Gastric Mucosa. PLoS Pathog. 2011, 7, e1002454. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.X.; Whittimore, J.D.; Wyrick, P.B.; McGrath, S.C.; Cotter, R.J.; Trent, M.S. The Lipid A 1-Phosphatase of Helicobacter Pylori Is Required for Resistance to the Antimicrobial Peptide Polymyxin. J. Bacteriol. 2006, 188, 4531–4541. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Lo, L.F.; Forsberg, L.S.; Maier, R.J. Helicobacter Pylori Peptidoglycan Modifications Confer Lysozyme Resistance and Contribute to Survival in the Host. mBio 2012, 3, e00409-12. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.A.; Vargas, M.A.; Regueiro, V.; Llompart, C.M.; Albertí, S.; Bengoechea, J.A. Capsule Polysaccharide Mediates Bacterial Resistance to Antimicrobial Peptides. Infect. Immun. 2004, 72, 7107–7114. [Google Scholar] [CrossRef] [PubMed]
- Chaput, C.; Spindler, E.; Gill, R.T.; Zychlinsky, A. O-Antigen Protects Gram-Negative Bacteria from Histone Killing. PLoS ONE 2013, 8, e71097. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Clements, A.; Tull, D.; Jenney, A.W.; Farn, J.L.; Kim, S.-H.; Bishop, R.E.; McPhee, J.B.; Hancock, R.E.W.; Hartland, E.L.; Pearse, M.J.; et al. Secondary Acylation of Klebsiella Pneumoniae Lipopolysaccharide Contributes to Sensitivity to Antibacterial Peptides. J. Biol. Chem. 2007, 282, 15569–15577. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Y.; Cai, T.; Li, X.; Li, N.; Xie, Z.; Yang, F.; You, X. CrrAB Regulates PagP-Mediated Glycerophosphoglycerol Palmitoylation in the Outer Membrane of Klebsiella Pneumoniae. J. Lipid Res. 2022, 63, 100251. [Google Scholar] [CrossRef]
- Llobet, E.; Martínez-Moliner, V.; Moranta, D.; Dahlström, K.M.; Regueiro, V.; Tomás, A.; Cano, V.; Pérez-Gutiérrez, C.; Frank, C.G.; Fernández-Carrasco, H.; et al. Deciphering Tissue-Induced Klebsiella Pneumoniae Lipid A Structure. Proc. Natl. Acad. Sci. USA 2015, 112, E6369–E6378. [Google Scholar] [CrossRef] [PubMed]
- Cannatelli, A.; D’Andrea, M.M.; Giani, T.; Di Pilato, V.; Arena, F.; Ambretti, S.; Gaibani, P.; Rossolini, G.M. In Vivo Emergence of Colistin Resistance in Klebsiella Pneumoniae Producing KPC-Type Carbapenemases Mediated by Insertional Inactivation of the PhoQ/PhoP mgrB Regulator. Antimicrob. Agents Chemother. 2013, 57, 5521–5526. [Google Scholar] [CrossRef] [PubMed]
- Mirshekar, M.; Zadeh, R.G.; Moghadam, M.T.; Shahbazi, S.; Masjedian Jazi, F. Upregulation of pmrA, pmrB, pmrC, phoQ, phoP, and arnT Genes Contributing to Resistance to Colistin in Superbug Klebsiella Pneumoniae Isolates from Human Clinical Samples in Tehran, Iran. New Microbes New Infect. 2024, 59, 101275. [Google Scholar] [CrossRef]
- Singh, S.; Pathak, A.; Kumar, A.; Rahman, M.; Singh, A.; Gonzalez-Zorn, B.; Prasad, K.N. Emergence of Chromosome-Borne Colistin Resistance Gene Mcr-1 in Clinical Isolates of Klebsiella Pneumoniae from India. Antimicrob. Agents Chemother. 2018, 62, e01885-17. [Google Scholar] [CrossRef] [PubMed]
- Llobet, E.; March, C.; Giménez, P.; Bengoechea, J.A. Klebsiella Pneumoniae OmpA Confers Resistance to Antimicrobial Peptides. Antimicrob. Agents Chemother. 2009, 53, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-R.; Chang, I.-W.; Hsieh, P.-F.; Lin, T.-L.; Liu, P.-Y.; Huang, C.-H.; Li, K.-T.; Wang, J.-T. A Novel Role for the Klebsiella Pneumoniae Sap (Sensitivity to Antimicrobial Peptides) Transporter in Intestinal Cell Interactions, Innate Immune Responses, Liver Abscess, and Virulence. J. Infect. Dis. 2019, 219, 1294–1306. [Google Scholar] [CrossRef] [PubMed]
- Padilla, E.; Llobet, E.; Doménech-Sánchez, A.; Martínez-Martínez, L.; Bengoechea, J.A.; Albertí, S. Klebsiella Pneumoniae AcrAB Efflux Pump Contributes to Antimicrobial Resistance and Virulence. Antimicrob. Agents Chemother. 2010, 54, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-H.; Lin, T.-L.; Lin, Y.-T.; Wang, J.-T. A Putative RND-Type Efflux Pump, H239_3064, Contributes to Colistin Resistance through CrrB in Klebsiella Pneumoniae. J. Antimicrob. Chemother. 2018, 73, 1509–1516. [Google Scholar] [CrossRef]
- Robey, M.; O’Connell, W.; Cianciotto, N.P. Identification of Legionella Pneumophila Rcp, a pagP-Like Gene That Confers Resistance to Cationic Antimicrobial Peptides and Promotes Intracellular Infection. Infect. Immun. 2001, 69, 4276–4286. [Google Scholar] [CrossRef]
- Morgenthau, A.; Livingstone, M.; Adamiak, P.; Schryvers, A.B. The Role of Lactoferrin Binding Protein B in Mediating Protection against Human Lactoferricin. Biochem. Cell Biol. 2012, 90, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Morgenthau, A.; Beddek, A.; Schryvers, A.B. The Negatively Charged Regions of Lactoferrin Binding Protein B, an Adaptation against Anti-Microbial Peptides. PloS ONE 2014, 9, e86243. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.I.; Stohl, E.A.; Seifert, H.S. The Neisseria Gonorrhoeae Type IV Pilus Promotes Resistance to Hydrogen Peroxide- and LL-37-Mediated Killing by Modulating the Availability of Intracellular, Labile Iron. PLoS Pathog. 2022, 18, e1010561. [Google Scholar] [CrossRef] [PubMed]
- Rudel, T.; van Putten, J.P.; Gibbs, C.P.; Haas, R.; Meyer, T.F. Interaction of Two Variable Proteins (PilE and PilC) Required for Pilus-Mediated Adherence of Neisseria Gonorrhoeae to Human Epithelial Cells. Mol. Microbiol. 1992, 6, 3439–3450. [Google Scholar] [CrossRef] [PubMed]
- Warner, D.M.; Shafer, W.M.; Jerse, A.E. Clinically Relevant Mutations That Cause Derepression of the Neisseria Gonorrhoeae MtrC-MtrD-MtrE Efflux Pump System Confer Different Levels of Antimicrobial Resistance and in Vivo Fitness. Mol. Microbiol. 2008, 70, 462–478. [Google Scholar] [CrossRef] [PubMed]
- Chitsaz, M.; Booth, L.; Blyth, M.T.; O’Mara, M.L.; Brown, M.H. Multidrug Resistance in Neisseria Gonorrhoeae: Identification of Functionally Important Residues in the MtrD Efflux Protein. mBio 2019, 10, e02277-19. [Google Scholar] [CrossRef]
- Shafer, W.M.; Qu, X.-D.; Waring, A.J.; Lehrer, R.I. Modulation of Neisseria Gonorrhoeae Susceptibility to Vertebrate Antibacterial Peptides Due to a Member of the Resistance/Nodulation/Division Efflux Pump Family. Proc. Natl. Acad. Sci. USA 1998, 95, 1829–1833. [Google Scholar] [CrossRef] [PubMed]
- Handing, J.W.; Ragland, S.A.; Bharathan, U.V.; Criss, A.K. The MtrCDE Efflux Pump Contributes to Survival of Neisseria Gonorrhoeae From Human Neutrophils and Their Antimicrobial Components. Front. Microbiol. 2018, 9, 2688. [Google Scholar] [CrossRef] [PubMed]
- Roussel-Jazédé, V.; Jongerius, I.; Bos, M.P.; Tommassen, J.; van Ulsen, P. NalP-Mediated Proteolytic Release of Lactoferrin-Binding Protein B from the Meningococcal Cell Surface. Infect. Immun. 2010, 78, 3083–3089. [Google Scholar] [CrossRef] [PubMed]
- Spinosa, M.R.; Progida, C.; Talà, A.; Cogli, L.; Alifano, P.; Bucci, C. The Neisseria Meningitidis Capsule Is Important for Intracellular Survival in Human Cells. Infect. Immun. 2007, 75, 3594–3603. [Google Scholar] [CrossRef]
- Jones, A.; Geörg, M.; Maudsdotter, L.; Jonsson, A.-B. Endotoxin, Capsule, and Bacterial Attachment Contribute to Neisseria meningitidis Resistance to the Human Antimicrobial Peptide LL-37. J. Bacteriol. 2009, 191, 3861–3868. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, Y.-L.; Stephens, D.S. Antimicrobial Peptide Resistance in Neisseria Meningitidis. Biochim. Biophys. Acta 2015, 1848, 3026–3031. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, Y.-L.; Ambrose, K.D.; Zughaier, S.; Zhou, X.; Miller, Y.K.; Shafer, W.M.; Stephens, D.S. Cationic Antimicrobial Peptide Resistance in Neisseria Meningitidis. J. Bacteriol. 2005, 187, 5387–5396. [Google Scholar] [CrossRef]
- Piek, S.; Wang, Z.; Ganguly, J.; Lakey, A.M.; Bartley, S.N.; Mowlaboccus, S.; Anandan, A.; Stubbs, K.A.; Scanlon, M.J.; Vrielink, A.; et al. The Role of Oxidoreductases in Determining the Function of the Neisserial Lipid A Phosphoethanolamine Transferase Required for Resistance to Polymyxin. PLoS ONE 2014, 9, e106513. [Google Scholar] [CrossRef]
- Abi Khattar, Z.; Lanois, A.; Hadchity, L.; Gaudriault, S.; Givaudan, A. Spatiotemporal Expression of the Putative MdtABC Efflux Pump of Phtotorhabdus Luminescens Occurs in a Protease-Dependent Manner during Insect Infection. PLoS ONE 2019, 14, e0212077. [Google Scholar] [CrossRef] [PubMed]
- Hadchity, L.; Lanois, A.; Kiwan, P.; Nassar, F.; Givaudan, A.; Khattar, Z.A. AcrAB, the Major RND-Type Efflux Pump of Photorhabdus Laumondii, Confers Intrinsic Multidrug-Resistance and Contributes to Virulence in Insects. Environ. Microbiol. Rep. 2021, 13, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Derzelle, S.; Turlin, E.; Duchaud, E.; Pages, S.; Kunst, F.; Givaudan, A.; Danchin, A. The PhoP-PhoQ Two-Component Regulatory System of Photorhabdus Luminescens Is Essential for Virulence in Insects. J. Bacteriol. 2004, 186, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Bennett, H.P.J.; Clarke, D.J. The pbgPE Operon in Photorhabdus Luminescens Is Required for Pathogenicity and Symbiosis. J. Bacteriol. 2005, 187, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Coats, S.R.; Jones, J.W.; Do, C.T.; Braham, P.H.; Bainbridge, B.W.; To, T.T.; Goodlett, D.R.; Ernst, R.K.; Darveau, R.P. Human Toll-like Receptor 4 Responses to P. Gingivalis Are Regulated by Lipid A 1- and 4′-Phosphatase Activities. Cell. Microbiol. 2009, 11, 1587–1599. [Google Scholar] [CrossRef]
- Horie, T.; Inomata, M.; Into, T. OmpA-Like Proteins of Porphyromonas Gingivalis Mediate Resistance to the Antimicrobial Peptide LL-37. J. Pathog. 2018, 2018, 2068435. [Google Scholar] [CrossRef]
- Devine, D.A.; Marsh, P.D.; Percival, R.S.; Rangarajan, M.; Curtis, M.A. Modulation of Antibacterial Peptide Activity by Products of Porphyromonas Gingivalis and Prevotella Spp. Microbiology 1999, 145, 965–971. [Google Scholar] [CrossRef]
- Belas, R.; Manos, J.; Suvanasuthi, R. Proteus Mirabilis ZapA Metalloprotease Degrades a Broad Spectrum of Substrates, Including Antimicrobial Peptides. Infect. Immun. 2004, 72, 5159–5167. [Google Scholar] [CrossRef]
- McCoy, A.J.; Liu, H.; Falla, T.J.; Gunn, J.S. Identification of Proteus Mirabilis Mutants with Increased Sensitivity to Antimicrobial Peptides. Antimicrob. Agents Chemother. 2001, 45, 2030–2037. [Google Scholar] [CrossRef] [PubMed]
- Schmidtchen, A.; Frick, I.-M.; Andersson, E.; Tapper, H.; Björck, L. Proteinases of Common Pathogenic Bacteria Degrade and Inactivate the Antibacterial Peptide LL-37. Mol. Microbiol. 2002, 46, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Llobet, E.; Tomás, J.M.; Bengoechea, J.A. Capsule Polysaccharide Is a Bacterial Decoy for Antimicrobial Peptides. Microbiol. Read. Engl. 2008, 154, 3877–3886. [Google Scholar] [CrossRef] [PubMed]
- Park, P.W.; Pier, G.B.; Hinkes, M.T.; Bernfield, M. Exploitation of Syndecan-1 Shedding by Pseudomonas Aeruginosa Enhances Virulence. Nature 2001, 411, 98–102. [Google Scholar] [CrossRef]
- Park, P.W.; Pier, G.B.; Preston, M.J.; Goldberger, O.; Fitzgerald, M.L.; Bernfield, M. Syndecan-1 Shedding Is Enhanced by LasA, a Secreted Virulence Factor of Pseudomonas Aeruginosa*. J. Biol. Chem. 2000, 275, 3057–3064. [Google Scholar] [CrossRef]
- Lo Sciuto, A.; Cervoni, M.; Stefanelli, R.; Spinnato, M.C.; Di Giamberardino, A.; Mancone, C.; Imperi, F. Genetic Basis and Physiological Effects of Lipid A Hydroxylation in Pseudomonas Aeruginosa PAO1. Pathogens 2019, 8, 291. [Google Scholar] [CrossRef]
- Hofstaedter, C.E.; Chandler, C.E.; Met, C.M.; Gillespie, J.J.; Harro, J.M.; Goodlett, D.R.; Rasko, D.A.; Ernst, R.K. Divergent Pseudomonas Aeruginosa LpxO Enzymes Perform Site-Specific Lipid A 2-Hydroxylation. mBio 2023, 15, e02823-23. [Google Scholar] [CrossRef]
- Poirel, L.; Jayol, A.; Nordmann, P. Polymyxins: Antibacterial Activity, Susceptibility Testing, and Resistance Mechanisms Encoded by Plasmids or Chromosomes. Clin. Microbiol. Rev. 2017, 30, 557–596. [Google Scholar] [CrossRef]
- Ernst, R.K.; Yi, E.C.; Guo, L.; Lim, K.B.; Burns, J.L.; Hackett, M.; Miller, S.I. Specific Lipopolysaccharide Found in Cystic Fibrosis Airway Pseudomonas Aeruginosa. Science 1999, 286, 1561–1565. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, S.M.; Ernst, R.K.; Miller, S.I. PmrAB, a Two-Component Regulatory System of Pseudomonas Aeruginosa That Modulates Resistance to Cationic Antimicrobial Peptides and Addition of Aminoarabinose to Lipid A. J. Bacteriol. 2004, 186, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Snesrud, E.; Maybank, R.; Kwak, Y.I.; Jones, A.R.; Hinkle, M.K.; McGann, P. Chromosomally Encoded Mcr-5 in Colistin-Nonsusceptible Pseudomonas Aeruginosa. Antimicrob. Agents Chemother. 2018, 62, e00679-18. [Google Scholar] [CrossRef] [PubMed]
- Cervoni, M.; Sposato, D.; Lo Sciuto, A.; Imperi, F. Regulatory Landscape of the Pseudomonas Aeruginosa Phosphoethanolamine Transferase Gene eptA in the Context of Colistin Resistance. Antibiotics 2023, 12, 200. [Google Scholar] [CrossRef] [PubMed]
- Cervoni, M.; Lo Sciuto, A.; Bianchini, C.; Mancone, C.; Imperi, F. Exogenous and Endogenous Phosphoethanolamine Transferases Differently Affect Colistin Resistance and Fitness in Pseudomonas Aeruginosa. Front. Microbiol. 2021, 12, 778968. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Lorenzo, C.; Hoffmann, S.; Walther, J.M.; Storbeck, S.; Piekarski, T.; Tindall, B.J.; Wray, V.; Nimtz, M.; Moser, J. Adaptation of Pseudomonas Aeruginosa to Various Conditions Includes tRNA-Dependent Formation of Alanyl-Phosphatidylglycerol. Mol. Microbiol. 2009, 71, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Roy, H.; Dare, K.; Ibba, M. Adaptation of the Bacterial Membrane to Changing Environments Using Aminoacylated Phospholipids. Mol. Microbiol. 2009, 71, 547–550. [Google Scholar] [CrossRef]
- Pamp, S.J.; Gjermansen, M.; Johansen, H.K.; Tolker-Nielsen, T. Tolerance to the Antimicrobial Peptide Colistin in Pseudomonas Aeruginosa Biofilms Is Linked to Metabolically Active Cells, and Depends on the Pmr and mexAB-oprM Genes. Mol. Microbiol. 2008, 68, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Masuda, N.; Sakagawa, E.; Ohya, S.; Gotoh, N.; Tsujimoto, H.; Nishino, T. Substrate Specificities of MexAB-OprM, MexCD-OprJ, and MexXY-OprM Efflux Pumps in Pseudomonas Aeruginosa. Antimicrob. Agents Chemother. 2000, 44, 3322–3327. [Google Scholar] [CrossRef]
- Fernandes, S.E.; Jorth, P. A Brief Update on the Controversial and Opposing Roles of Pseudomonas Aeruginosa Efflux Pumps in Virulence Regulation. Front. Bacteriol. 2023, 2, 1231657. [Google Scholar] [CrossRef]
- Taggart, C.C.; Greene, C.M.; Smith, S.G.; Levine, R.L.; McCray, P.B., Jr.; O’Neill, S.; McElvaney, N.G. Inactivation of Human β-Defensins 2 and 3 by Elastolytic Cathepsins1. J. Immunol. 2003, 171, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Andrault, P.-M.; Samsonov, S.A.; Weber, G.; Coquet, L.; Nazmi, K.; Bolscher, J.G.M.; Lalmanach, A.-C.; Jouenne, T.; Brömme, D.; Pisabarro, M.T.; et al. Antimicrobial Peptide LL-37 Is Both a Substrate of Cathepsins S and K and a Selective Inhibitor of Cathepsin L. Biochemistry 2015, 54, 2785–2798. [Google Scholar] [CrossRef] [PubMed]
- Dorrer, E.; Teuber, M. Induction of Polymyxin Resistance in Pseudomonas Fluorescens by Phosphate Limitation. Arch. Microbiol. 1977, 114, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Lim, K.B.; Gunn, J.S.; Bainbridge, B.; Darveau, R.P.; Hackett, M.; Miller, S.I. Regulation of Lipid A Modifications by Salmonella Typhimurium Virulence Genes phoP-phoQ. Science 1997, 276, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, K.; China, K.; Nishijima, M. Release of the Lipopolysaccharide Deacylase PagL from Latency Compensates for a Lack of Lipopolysaccharide Aminoarabinose Modification-Dependent Resistance to the Antimicrobial Peptide Polymyxin B in Salmonella Enterica. J. Bacteriol. 2007, 189, 4911–4919. [Google Scholar] [CrossRef] [PubMed]
- Strandberg, K.L.; Richards, S.M.; Gunn, J.S. Cathelicidin Antimicrobial Peptide Expression Is Not Induced or Required for Bacterial Clearance during Salmonella Enterica Infection of Human Monocyte-Derived Macrophages. Infect. Immun. 2012, 80, 3930–3938. [Google Scholar] [CrossRef]
- Otton, L.M.; da Silva Campos, M.; Meneghetti, K.L.; Corção, G. Influence of Twitching and Swarming Motilities on Biofilm Formation in Pseudomonas Strains. Arch. Microbiol. 2017, 199, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, R.; Choudhury, B.; Septer, A.; Merighi, M.; Carlson, R.; Gunn, J.S. Identification of cptA, a PmrA-Regulated Locus Required for Phosphoethanolamine Modification of the Salmonella Enterica Serovar Typhimurium Lipopolysaccharide Core. J. Bacteriol. 2005, 187, 3391–3399. [Google Scholar] [CrossRef]
- Bogomolnaya, L.M.; Andrews, K.D.; Talamantes, M.; Maple, A.; Ragoza, Y.; Vazquez-Torres, A.; Andrews-Polymenis, H. The ABC-Type Efflux Pump MacAB Protects Salmonella Enterica Serovar Typhimurium from Oxidative Stress. mBio 2013, 4, e00630-13. [Google Scholar] [CrossRef]
- Honeycutt, J.D.; Wenner, N.; Li, Y.; Brewer, S.M.; Massis, L.M.; Brubaker, S.W.; Chairatana, P.; Owen, S.V.; Canals, R.; Hinton, J.C.D.; et al. Genetic Variation in the MacAB-TolC Efflux Pump Influences Pathogenesis of Invasive Salmonella Isolates from Africa. PLOS Pathog. 2020, 16, e1008763. [Google Scholar] [CrossRef]
- Parra-Lopez, C.; Baer, M.T.; Groisman, E.A. Molecular Genetic Analysis of a Locus Required for Resistance to Antimicrobial Peptides in Salmonella Typhimurium. EMBO J. 1993, 12, 4053–4062. [Google Scholar] [CrossRef] [PubMed]
- Eswarappa, S.M.; Panguluri, K.K.; Hensel, M.; Chakravortty, D. The yejABEF Operon of Salmonella Confers Resistance to Antimicrobial Peptides and Contributes to Its Virulence. Microbiology 2008, 154, 666–678. [Google Scholar] [CrossRef] [PubMed]
- Crouch, M.-L.; Becker, L.A.; Bang, I.-S.; Tanabe, H.; Ouellette, A.J.; Fang, F.C. The Alternative Sigma Factor Sigma Is Required for Resistance of Salmonella Enterica Serovar Typhimurium to Anti-Microbial Peptides. Mol. Microbiol. 2005, 56, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, S.; Stevenson, A.; Bacon, A.; Weinhardt, A.B.; Roberts, M. The Alternative Sigma Factor, sigmaE, Is Critically Important for the Virulence of Salmonella Typhimurium. Infect. Immun. 1999, 67, 1560–1568. [Google Scholar] [CrossRef] [PubMed]
- Islam, D.; Bandholtz, L.; Nilsson, J.; Wigzell, H.; Christensson, B.; Agerberth, B.; Gudmundsson, G. Downregulation of Bactericidal Peptides in Enteric Infections: A Novel Immune Escape Mechanism with Bacterial DNA as a Potential Regulator. Nat. Med. 2001, 7, 180–185. [Google Scholar] [CrossRef]
- West, N.P.; Sansonetti, P.; Mounier, J.; Exley, R.M.; Parsot, C.; Guadagnini, S.; Prévost, M.-C.; Prochnicka-Chalufour, A.; Delepierre, M.; Tanguy, M.; et al. Optimization of Virulence Functions Through Glucosylation of Shigella LPS. Science 2005, 307, 1313–1317. [Google Scholar] [CrossRef]
- Arbibe, L.; Kim, D.W.; Batsche, E.; Pedron, T.; Mateescu, B.; Muchardt, C.; Parsot, C.; Sansonetti, P.J. An Injected Bacterial Effector Targets Chromatin Access for Transcription Factor NF-kappaB to Alter Transcription of Host Genes Involved in Immune Responses. Nat. Immunol. 2007, 8, 47–56. [Google Scholar] [CrossRef]
- Xiao, L.; Crabb, D.M.; Dai, Y.; Chen, Y.; Waites, K.B.; Atkinson, T.P. Suppression of Antimicrobial Peptide Expression by Ureaplasma Species. Infect. Immun. 2014, 82, 1657–1665. [Google Scholar] [CrossRef]
- Hankins, J.V.; Madsen, J.A.; Giles, D.K.; Childers, B.M.; Klose, K.E.; Brodbelt, J.S.; Trent, M.S. Elucidation of a Novel Vibrio Cholerae Lipid A Secondary Hydroxy-Acyltransferase and Its Role in Innate Immune Recognition. Mol. Microbiol. 2011, 81, 1313–1329. [Google Scholar] [CrossRef]
- Mathur, J.; Waldor, M.K. The Vibrio Cholerae ToxR-Regulated Porin OmpU Confers Resistance to Antimicrobial Peptides. Infect. Immun. 2004, 72, 3577–3583. [Google Scholar] [CrossRef]
- Mathur, J.; Davis, B.M.; Waldor, M.K. Antimicrobial Peptides Activate the Vibrio Cholerae sigmaE Regulon through an OmpU-Dependent Signalling Pathway. Mol. Microbiol. 2007, 63, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Bina, X.R.; Provenzano, D.; Nguyen, N.; Bina, J.E. Vibrio Cholerae RND Family Efflux Systems Are Required for Antimicrobial Resistance, Optimal Virulence Factor Production, and Colonization of the Infant Mouse Small Intestine. Infect. Immun. 2008, 76, 3595–3605. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Chuang, Y.-C.; Chang, C.-C.; Jeang, C.-L.; Chang, M.-C. A K+ Yptake Protein, TrkA, Is Required for Serum, Protamine, and Polymyxin B Resistance in Vibrio Vulnificus. Infect. Immun. 2004, 72, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gutiérrez, C.; Llobet, E.; Llompart, C.M.; Reinés, M.; Bengoechea, J.A. Role of Lipid A Acylation in Yersinia enterocolitica Virulence. Infect. Immun. 2010, 78, 2768–2781. [Google Scholar] [CrossRef] [PubMed]
- Visser, L.G.; Hiemstra, P.S.; van den Barselaar, M.T.; Ballieux, P.A.; van Furth, R. Role of YadA in Resistance to Killing of Yersinia enterocolitica by Antimicrobial Polypeptides of Human Granulocytes. Infect. Immun. 1996, 64, 1653–1658. [Google Scholar] [CrossRef] [PubMed]
- Bengoechea, J.A.; Skurnik, M. Temperature-Regulated Efflux Pump/Potassium Antiporter System Mediates Resistance to Cationic Antimicrobial Peptides in Yersinia. Mol. Microbiol. 2000, 37, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Lister, I.M.; Raftery, C.; Mecsas, J.; Levy, S.B. Yersinia Pestis acrAB-tolC in Antibiotic Resistance and Virulence. Antimicrob. Agents Chemother. 2012, 56, 1120–1123. [Google Scholar] [CrossRef] [PubMed]
- Rebeil, R.; Ernst, R.K.; Gowen, B.B.; Miller, S.I.; Hinnebusch, B.J. Variation in Lipid A Structure in the Pathogenic Yersiniae. Mol. Microbiol. 2004, 52, 1363–1373. [Google Scholar] [CrossRef]
- Thwaite, J.E.; Hibbs, S.; Titball, R.W.; Atkins, T.P. Proteolytic Degradation of Human Antimicrobial Peptide LL-37 by Bacillus Anthracis May Contribute to Virulence. Antimicrob. Agents Chemother. 2006, 50, 2316–2322. [Google Scholar] [CrossRef] [PubMed]
- McGillivray, S.M.; Ebrahimi, C.M.; Fisher, N.; Sabet, M.; Zhang, D.X.; Chen, Y.; Haste, N.M.; Aroian, R.V.; Gallo, R.L.; Guiney, D.G.; et al. ClpX Contributes to Innate Defense Peptide Resistance and Virulence Phenotypes of Bacillus Anthracis. J. Innate Immun. 2009, 1, 494–506. [Google Scholar] [CrossRef]
- Drysdale, M.; Bourgogne, A.; Hilsenbeck, S.G.; Koehler, T.M. atxA Controls Bacillus Anthracis Capsule Synthesis via acpA and a Newly Discovered Regulator, acpB. J. Bacteriol. 2004, 186, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Richter, S.; Anderson, V.J.; Garufi, G.; Lu, L.; Budzik, J.M.; Joachimiak, A.; He, C.; Schneewind, O.; Missiakas, D. Capsule Anchoring in Bacillus Anthracis Occurs by a Transpeptidation Reaction That Is Inhibited by Capsidin. Mol. Microbiol. 2009, 71, 404–420. [Google Scholar] [CrossRef] [PubMed]
- Fisher, N.; Shetron-Rama, L.; Herring-Palmer, A.; Heffernan, B.; Bergman, N.; Hanna, P. The dltABCD Operon of Bacillus Anthracis Sterne Is Required for Virulence and Resistance to Peptide, Enzymatic, and Cellular Mediators of Innate Immunity. J. Bacteriol. 2006, 188, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Samant, S.; Hsu, F.-F.; Neyfakh, A.A.; Lee, H. The Bacillus Anthracis Protein MprF Is Required for Synthesis of Lysylphosphatidylglycerols and for Resistance to Cationic Antimicrobial Peptides. J. Bacteriol. 2009, 191, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Abi Khattar, Z.; Rejasse, A.; Destoumieux-Garzón, D.; Escoubas, J.M.; Sanchis, V.; Lereclus, D.; Givaudan, A.; Kallassy, M.; Nielsen-Leroux, C.; Gaudriault, S. The Dlt Operon of Bacillus Cereus Is Required for Resistance to Cationic Antimicrobial Peptides and for Virulence in Insects. J. Bacteriol. 2009, 191, 7063–7073. [Google Scholar] [CrossRef] [PubMed]
- Haydar, A.; Tran, S.-L.; Guillemet, E.; Darrigo, C.; Perchat, S.; Lereclus, D.; Coquet, L.; Jouenne, T.; Ramarao, N. InhA1-Mediated Cleavage of the Metalloprotease NprA Allows Bacillus Cereus to Escape From Macrophages. Front. Microbiol. 2018, 9, 1063. [Google Scholar] [CrossRef] [PubMed]
- Guillemet, E.; Cadot, C.; Tran, S.-L.; Guinebretière, M.-H.; Lereclus, D.; Ramarao, N. The InhA Metalloproteases of Bacillus Cereus Contribute Concomitantly to Virulence. J. Bacteriol. 2010, 192, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Helmann, J.D. The Bacillus Subtilis Extracytoplasmic-Function σX Factor Regulates Modification of the Cell Envelope and Resistance to Cationic Antimicrobial Peptides. J. Bacteriol. 2004, 186, 1136–1146. [Google Scholar] [CrossRef]
- Perego, M.; Glaser, P.; Minutello, A.; Strauch, M.A.; Leopold, K.; Fischer, W. Incorporation of D-Alanine into Lipoteichoic Acid and Wall Teichoic Acid in Bacillus Subtilis. Identification of Genes and Regulation. J. Biol. Chem. 1995, 270, 15598–15606. [Google Scholar] [CrossRef]
- McBride, S.M.; Sonenshein, A.L. The Dlt Operon Confers Resistance to Cationic Antimicrobial Peptides in Clostridium Difficile. Microbiology 2011, 157, 1457–1465. [Google Scholar] [CrossRef]
- Singh, K.V.; Coque, T.M.; Weinstock, G.M.; Murray, B.E. In Vivo Testing of an Enterococcus Faecalis efaA Mutant and Use of efaA Homologs for Species Identification. FEMS Immunol. Med. Microbiol. 1998, 21, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Nešuta, O.; Buděšínský, M.; Hadravová, R.; Monincová, L.; Humpoličková, J.; Čeřovský, V. How Proteases from Enterococcus Faecalis Contribute to Its Resistance to Short α-Helical Antimicrobial Peptides. Pathog. Dis. 2017, 75, ftx091. [Google Scholar] [CrossRef] [PubMed]
- Engelbert, M.; Mylonakis, E.; Ausubel, F.M.; Calderwood, S.B.; Gilmore, M.S. Contribution of Gelatinase, Serine Protease, and Fsr to the Pathogenesis of Enterococcus Faecalis Endophthalmitis. Infect. Immun. 2004, 72, 3628–3633. [Google Scholar] [CrossRef] [PubMed]
- Schmidtchen, A.; Frick, I.-M.; Björck, L. Dermatan Sulphate Is Released by Proteinases of Common Pathogenic Bacteria and Inactivates Antibacterial α-Defensin. Mol. Microbiol. 2001, 39, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Fabretti, F.; Theilacker, C.; Baldassarri, L.; Kaczynski, Z.; Kropec, A.; Holst, O.; Huebner, J. Alanine Esters of Enterococcal Lipoteichoic Acid Play a Role in Biofilm Formation and Resistance to Antimicrobial Peptides. Infect. Immun. 2006, 74, 4164–4171. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Sakinc, T.; Laverde, D.; Wobser, D.; Benachour, A.; Theilacker, C.; Hartke, A.; Huebner, J. Role of mprF1 and mprF2 in the Pathogenicity of Enterococcus Faecalis. PLoS ONE 2012, 7, e38458. [Google Scholar] [CrossRef]
- Kandaswamy, K.; Liew, T.H.; Wang, C.Y.; Huston-Warren, E.; Meyer-Hoffert, U.; Hultenby, K.; Schröder, J.M.; Caparon, M.G.; Normark, S.; Henriques-Normark, B.; et al. Focal Targeting by Human β-Defensin 2 Disrupts Localized Virulence Factor Assembly Sites in Enterococcus Faecalis. Proc. Natl. Acad. Sci. USA 2013, 110, 20230–20235. [Google Scholar] [CrossRef] [PubMed]
- Nair, Z.J.; Gao, I.H.; Firras, A.; Chong, K.K.L.; Hill, E.D.; Choo, P.Y.; Colomer-Winter, C.; Chen, Q.; Manzano, C.; Pethe, K.; et al. An Essential Protease, FtsH, Influences Daptomycin Resistance Acquisition in Enterococcus faecalis. Mol. Microbiol. 2024, 121, 1021–1038. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.; Panesso, D.; Tran, T.T.; Mishra, N.N.; Cruz, M.R.; Munita, J.M.; Singh, K.V.; Yeaman, M.R.; Murray, B.E.; Shamoo, Y.; et al. A liaR Deletion Restores Susceptibility to Daptomycin and Antimicrobial Peptides in Multidrug-Resistant Enterococcus Faecalis. J. Infect. Dis. 2015, 211, 1317–1325. [Google Scholar] [CrossRef]
- Hébert, L.; Courtin, P.; Torelli, R.; Sanguinetti, M.; Chapot-Chartier, M.-P.; Auffray, Y.; Benachour, A. Enterococcus Faecalis Constitutes an Unusual Bacterial Model in Lysozyme Resistance. Infect. Immun. 2007, 75, 5390–5398. [Google Scholar] [CrossRef]
- Johansson, L.; Thulin, P.; Sendi, P.; Hertzén, E.; Linder, A.; Åkesson, P.; Low, D.E.; Agerberth, B.; Norrby-Teglund, A. Cathelicidin LL-37 in Severe Streptococcus Pyogenes Soft Tissue Infections in Humans. Infect. Immun. 2008, 76, 3399–3404. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.N.; Pence, M.A.; von Köckritz-Blickwede, M.; Hollands, A.; Gallo, R.L.; Walker, M.J.; Nizet, V. M Protein and Hyaluronic Acid Capsule Are Essential for in Vivo Selection of covRS Mutations Characteristic of Invasive Serotype M1T1 Group A Streptococcus. mBio 2010, 1, e00191-10. [Google Scholar] [CrossRef] [PubMed]
- Lauth, X.; von Köckritz-Blickwede, M.; McNamara, C.W.; Myskowski, S.; Zinkernagel, A.S.; Beall, B.; Ghosh, P.; Gallo, R.L.; Nizet, V. M1 Protein Allows Group A Streptococcal Survival in Phagocyte Extracellular Traps through Cathelicidin Inhibition. J. Innate Immun. 2009, 1, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Hollands, A.; Gonzalez, D.; Leire, E.; Donald, C.; Gallo, R.L.; Sanderson-Smith, M.; Dorrestein, P.C.; Nizet, V. A Bacterial Pathogen Co-Opts Host Plasmin to Resist Killing by Cathelicidin Antimicrobial Peptides. J. Biol. Chem. 2012, 287, 40891–40897. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Xu, Y.; Sitkiewicz, I.; Ma, Y.; Wang, X.; Yestrepsky, B.D.; Huang, Y.; Lapadatescu, M.C.; Larsen, M.J.; Larsen, S.D.; et al. Inhibitor of Streptokinase Gene Expression Improves Survival after Group A Streptococcus Infection in Mice. Proc. Natl. Acad. Sci. USA 2012, 109, 3469–3474. [Google Scholar] [CrossRef] [PubMed]
- Frick, I.-M.; Åkesson, P.; Rasmussen, M.; Schmidtchen, A.; Björck, L. SIC, a Secreted Protein of Streptococcus pyogenesThat Inactivates Antibacterial Peptides *. J. Biol. Chem. 2003, 278, 16561–16566. [Google Scholar] [CrossRef]
- Barańska-Rybak, W.; Sonesson, A.; Nowicki, R.; Schmidtchen, A. Glycosaminoglycans Inhibit the Antibacterial Activity of LL-37 in Biological Fluids. J. Antimicrob. Chemother. 2006, 57, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, P.; Rasmussen, M.; Björck, L. A2-Macroglobulin-Proteinase Complexes Protect Streptococcus Pyogenes from Killing by the Antimicrobial Peptide LL-37 *. J. Biol. Chem. 2004, 279, 52820–52823. [Google Scholar] [CrossRef] [PubMed]
- Froehlich, B.J.; Bates, C.; Scott, J.R. Streptococcus Pyogenes CovRS Mediates Growth in Iron Starvation and in the Presence of the Human Cationic Antimicrobial Peptide LL-37. J. Bacteriol. 2009, 191, 673–677. [Google Scholar] [CrossRef]
- Tran-Winkler, H.J.; Love, J.F.; Gryllos, I.; Wessels, M.R. Signal Transduction through CsrRS Confers an Invasive Phenotype in Group A Streptococcus. PLoS Pathog. 2011, 7, e1002361. [Google Scholar] [CrossRef]
- Nizet, V.; Ohtake, T.; Lauth, X.; Trowbridge, J.; Rudisill, J.; Dorschner, R.A.; Pestonjamasp, V.; Piraino, J.; Huttner, K.; Gallo, R.L. Innate Antimicrobial Peptide Protects the Skin from Invasive Bacterial Infection. Nature 2001, 414, 454–457. [Google Scholar] [CrossRef]
- Kristian, S.A.; Datta, V.; Weidenmaier, C.; Kansal, R.; Fedtke, I.; Peschel, A.; Gallo, R.L.; Nizet, V. D-Alanylation of Teichoic Acids Promotes Group a Streptococcus Antimicrobial Peptide Resistance, Neutrophil Survival, and Epithelial Cell Invasion. J. Bacteriol. 2005, 187, 6719–6725. [Google Scholar] [CrossRef] [PubMed]
- Phelps, H.A.; Neely, M.N. SalY of the Streptococcus Pyogenes Lantibiotic Locus Is Required for Full Virulence and Intracellular Survival in Macrophages. Infect. Immun. 2007, 75, 4541–4551. [Google Scholar] [CrossRef] [PubMed]
- Dinulos, J.G.H.; Mentele, L.; Fredericks, L.P.; Dale, B.A.; Darmstadt, G.L. Keratinocyte Expression of Human β Defensin 2 Following Bacterial Infection: Role in Cutaneous Host Defense. Clin. Diagn. Lab. Immunol. 2003, 10, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Klinzing, D.C.; Ishmael, N.; Hotopp, J.C.D.; Tettelin, H.; Shields, K.R.; Madoff, L.C.; Puopolo, K.M. The Two-Component Response Regulator LiaR Regulates Cell Wall Stress Responses, Pili Expression and Virulence in Group B Streptococcus. Microbiology 2013, 159, 1521–1534. [Google Scholar] [CrossRef] [PubMed]
- Quach, D.; van Sorge, N.M.; Kristian, S.A.; Bryan, J.D.; Shelver, D.W.; Doran, K.S. The CiaR Response Regulator in Group B Streptococcus Promotes Intracellular Survival and Resistance to Innate Immune Defenses. J. Bacteriol. 2009, 191, 2023–2032. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A.; Popham, D.L.; Carl, D.J.; Lauth, X.; Nizet, V.; Jones, A.L. Penicillin-Binding Protein 1a Promotes Resistance of Group B Streptococcus to Antimicrobial Peptides. Infect. Immun. 2006, 74, 6179–6187. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.L.; Mertz, R.H.; Carl, D.J.; Rubens, C.E. A Streptococcal Penicillin-Binding Protein Is Critical for Resisting Innate Airway Defenses in the Neonatal Lung1. J. Immunol. 2007, 179, 3196–3202. [Google Scholar] [CrossRef] [PubMed]
- Maisey, H.C.; Doran, K.S.; Nizet, V. Recent Advances in Understanding the Molecular Basis of Group B Streptococcus Virulence. Expert Rev. Mol. Med. 2008, 10, e27. [Google Scholar] [CrossRef]
- Shabayek, S.; Spellerberg, B. Group B Streptococcal Colonization, Molecular Characteristics, and Epidemiology. Front. Microbiol. 2018, 9, 437. [Google Scholar] [CrossRef]
- Poyart, C.; Lamy, M.C.; Boumaila, C.; Fiedler, F.; Trieu-Cuot, P. Regulation of D-Alanyl-Lipoteichoic Acid Biosynthesis in Streptococcus Agalactiae Involves a Novel Two-Component Regulatory System. J. Bacteriol. 2001, 183, 6324–6334. [Google Scholar] [CrossRef] [PubMed]
- Saar-Dover, R.; Bitler, A.; Nezer, R.; Shmuel-Galia, L.; Firon, A.; Shimoni, E.; Trieu-Cuot, P.; Shai, Y. D-Alanylation of Lipoteichoic Acids Confers Resistance to Cationic Peptides in Group B Streptococcus by Increasing the Cell Wall Density. PLoS Pathog. 2012, 8, e1002891. [Google Scholar] [CrossRef] [PubMed]
- Boneca, I.G.; Dussurget, O.; Cabanes, D.; Nahori, M.-A.; Sousa, S.; Lecuit, M.; Psylinakis, E.; Bouriotis, V.; Hugot, J.-P.; Giovannini, M.; et al. A Critical Role for Peptidoglycan N-Deacetylation in Listeria Evasion from the Host Innate Immune System. Proc. Natl. Acad. Sci. USA 2007, 104, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Aubry, C.; Goulard, C.; Nahori, M.-A.; Cayet, N.; Decalf, J.; Sachse, M.; Boneca, I.G.; Cossart, P.; Dussurget, O. OatA, a Peptidoglycan O-Acetyltransferase Involved in Listeria Monocytogenes Immune Escape, Is Critical for Virulence. J. Infect. Dis. 2011, 204, 731–740. [Google Scholar] [CrossRef]
- Carvalho, F.; Atilano, M.L.; Pombinho, R.; Covas, G.; Gallo, R.L.; Filipe, S.R.; Sousa, S.; Cabanes, D. L-Rhamnosylation of Listeria Monocytogenes Wall Teichoic Acids Promotes Resistance to Antimicrobial Peptides by Delaying Interaction with the Membrane. PLoS Pathog. 2015, 11, e1004919. [Google Scholar] [CrossRef] [PubMed]
- Meireles, D.; Pombinho, R.; Carvalho, F.; Sousa, S.; Cabanes, D. Listeria Monocytogenes Wall Teichoic Acid Glycosylation Promotes Surface Anchoring of Virulence Factors, Resistance to Antimicrobial Peptides, and Decreased Susceptibility to Antibiotics. Pathogens 2020, 9, 290. [Google Scholar] [CrossRef]
- Abachin, E.; Poyart, C.; Pellegrini, E.; Milohanic, E.; Fiedler, F.; Berche, P.; Trieu-Cuot, P. Formation of D-Alanyl-Lipoteichoic Acid Is Required for Adhesion and Virulence of Listeria Monocytogenes. Mol. Microbiol. 2002, 43, 1–14. [Google Scholar] [CrossRef]
- Mandin, P.; Fsihi, H.; Dussurget, O.; Vergassola, M.; Milohanic, E.; Toledo-Arana, A.; Lasa, I.; Johansson, J.; Cossart, P. VirR, a Response Regulator Critical for Listeria Monocytogenes Virulence. Mol. Microbiol. 2005, 57, 1367–1380. [Google Scholar] [CrossRef]
- Thedieck, K.; Hain, T.; Mohamed, W.; Tindall, B.J.; Nimtz, M.; Chakraborty, T.; Wehland, J.; Jänsch, L. The MprF Protein Is Required for Lysinylation of Phospholipids in Listerial Membranes and Confers Resistance to Cationic Antimicrobial Peptides (CAMPs) on Listeria Monocytogenes. Mol. Microbiol. 2006, 62, 1325–1339. [Google Scholar] [CrossRef]
- Collins, B.; Curtis, N.; Cotter, P.D.; Hill, C.; Ross, R.P. The ABC Transporter AnrAB Contributes to the Innate Resistance of Listeria Monocytogenes to Nisin, Bacitracin, and Various β-Lactam Antibiotics. Antimicrob. Agents Chemother. 2010, 54, 4416–4423. [Google Scholar] [CrossRef]
- López-Solanilla, E.; González-Zorn, B.; Novella, S.; Vázquez-Boland, J.A.; Rodríguez-Palenzuela, P. Susceptibility of Listeria Monocytogenes to Antimicrobial Peptides. FEMS Microbiol. Lett. 2003, 226, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Maloney, E.; Stankowska, D.; Zhang, J.; Fol, M.; Cheng, Q.-J.; Lun, S.; Bishai, W.R.; Rajagopalan, M.; Chatterjee, D.; Madiraju, M.V. The Two-Domain LysX Protein of Mycobacterium Tuberculosis Is Required for Production of Lysinylated Phosphatidylglycerol and Resistance to Cationic Antimicrobial Peptides. PLoS Pathog. 2009, 5, e1000534. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.-Y.; Laval, F.; Lawson, E.H.; Groger, R.K.; Woodruff, A.; Morisaki, J.H.; Cox, J.S.; Daffe, M.; Brown, E.J. Requirement for kasB in Mycobacterium Mycolic Acid Biosynthesis, Cell Wall Impermeability and Intracellular Survival: Implications for Therapy. Mol. Microbiol. 2003, 49, 1547–1563. [Google Scholar] [CrossRef] [PubMed]
- Sieprawska-Lupa, M.; Mydel, P.; Krawczyk, K.; Wójcik, K.; Puklo, M.; Lupa, B.; Suder, P.; Silberring, J.; Reed, M.; Pohl, J.; et al. Degradation of Human Antimicrobial Peptide LL-37 by Staphylococcus Aureus-Derived Proteinases. Antimicrob. Agents Chemother. 2004, 48, 4673–4679. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Jung, J.; Kim, C.Y.; Kang, H.; Lee, I.H. Antimicrobial Peptides Encounter Resistance of Aureolysin during Their Action on Staphylococcus Aureus Biofilm. Biotechnol. Bioprocess Eng. 2021, 26, 216–222. [Google Scholar] [CrossRef]
- Frey, A.M.; Chaput, D.; Shaw, L.N. Insight into the Human Pathodegradome of the V8 Protease from Staphylococcus Aureus. Cell Rep. 2021, 35, 108930. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.; Golonka, E.; Potempa, J.; Foster, S.J. The Role and Regulation of the Extracellular Proteases of Staphylococcus Aureus. Microbiol. Read. Engl. 2004, 150, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Coulter, S.N.; Schwan, W.R.; Ng, E.Y.; Langhorne, M.H.; Ritchie, H.D.; Westbrock-Wadman, S.; Hufnagle, W.O.; Folger, K.R.; Bayer, A.S.; Stover, C.K. Staphylococcus Aureus Genetic Loci Impacting Growth and Survival in Multiple Infection Environments. Mol. Microbiol. 1998, 30, 393–404. [Google Scholar] [CrossRef]
- Clarke, S.R.; Foster, S.J. IsdA Protects Staphylococcus Aureus against the Bactericidal Protease Activity of Apolactoferrin. Infect. Immun. 2008, 76, 1518–1526. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.R.; Mohamed, R.; Bian, L.; Routh, A.F.; Kokai-Kun, J.F.; Mond, J.J.; Tarkowski, A.; Foster, S.J. The Staphylococcus Aureus Surface Protein IsdA Mediates Resistance to Innate Defenses of Human Skin. Cell Host Microbe 2007, 1, 199–212. [Google Scholar] [CrossRef]
- Cheng, A.G.; Kim, H.K.; Burts, M.L.; Krausz, T.; Schneewind, O.; Missiakas, D.M. Genetic Requirements for Staphylococcus Aureus Abscess Formation and Persistence in Host Tissues. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2009, 23, 3393–3404. [Google Scholar] [CrossRef] [PubMed]
- Recsei, P.; Kreiswirth, B.; O’Reilly, M.; Schlievert, P.; Gruss, A.; Novick, R.P. Regulation of Exoprotein Gene Expression in Staphylococcus Aureus by Agr. Mol. Gen. Genet. MGG 1986, 202, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Bokarewa, M.; Foster, T.; Mitchell, J.; Higgins, J.; Tarkowski, A. Staphylococcus Aureus Resists Human Defensins by Production of Staphylokinase, a Novel Bacterial Evasion Mechanism1. J. Immunol. 2004, 172, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Vogel, H.J. Staphylokinase Has Distinct Modes of Interaction with Antimicrobial Peptides, Modulating Its Plasminogen-Activation Properties. Sci. Rep. 2016, 6, 31817. [Google Scholar] [CrossRef] [PubMed]
- Kwiecinski, J.; Jacobsson, G.; Karlsson, M.; Zhu, X.; Wang, W.; Bremell, T.; Josefsson, E.; Jin, T. Staphylokinase Promotes the Establishment of Staphylococcus Aureus Skin Infections While Decreasing Disease Severity. J. Infect. Dis. 2013, 208, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.S.; Anderson, A.C.; Clarke, A.J. Mechanism of Staphylococcus Aureus Peptidoglycan O-Acetyltransferase A as an O-Acyltransferase. Proc. Natl. Acad. Sci. USA 2021, 118, e2103602118. [Google Scholar] [CrossRef] [PubMed]
- Bera, A.; Herbert, S.; Jakob, A.; Vollmer, W.; Götz, F. Why Are Pathogenic Staphylococci so Lysozyme Resistant? The Peptidoglycan O-Acetyltransferase OatA Is the Major Determinant for Lysozyme Resistance of Staphylococcus Aureus. Mol. Microbiol. 2005, 55, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Bera, A.; Biswas, R.; Herbert, S.; Götz, F. The Presence of Peptidoglycan O-Acetyltransferase in Various Staphylococcal Species Correlates with Lysozyme Resistance and Pathogenicity. Infect. Immun. 2006, 74, 4598–4604. [Google Scholar] [CrossRef]
- Baranwal, G.; Mohammad, M.; Jarneborn, A.; Reddy, B.R.; Golla, A.; Chakravarty, S.; Biswas, L.; Götz, F.; Shankarappa, S.; Jin, T.; et al. Impact of Cell Wall Peptidoglycan O-Acetylation on the Pathogenesis of Staphylococcus aureus in Septic Arthritis. Int. J. Med. Microbiol. 2017, 307, 388–397. [Google Scholar] [CrossRef]
- Shimada, T.; Park, B.G.; Wolf, A.J.; Brikos, C.; Goodridge, H.S.; Becker, C.A.; Reyes, C.N.; Miao, E.A.; Aderem, A.; Götz, F.; et al. Staphylococcus Aureus Evades the Lysozyme-Based Digestion of Peptidoglycan That Links Phagocytosis and Macrophage IL-1β Secretion. Cell Host Microbe 2010, 7, 38. [Google Scholar] [CrossRef]
- Sanchez, M.; Kolar, S.L.; Müller, S.; Reyes, C.N.; Wolf, A.J.; Ogawa, C.; Singhania, R.; Carvalho, D.D.; Arditi, M.; Underhill, D.M.; et al. O-Acetylation of Peptidoglycan Limits Helper T Cell Priming and Permits Staphylococcus aureus Reinfection. Cell Host Microbe 2017, 22, 543–551.e4. [Google Scholar] [CrossRef] [PubMed]
- Koprivnjak, T.; Weidenmaier, C.; Peschel, A.; Weiss, J.P. Wall Teichoic Acid Deficiency in Staphylococcus Aureus Confers Selective Resistance to Mammalian Group IIA Phospholipase A2 and Human β-Defensin 3. Infect. Immun. 2008, 76, 2169–2176. [Google Scholar] [CrossRef] [PubMed]
- Weidenmaier, C.; Peschel, A.; Kempf, V.A.J.; Lucindo, N.; Yeaman, M.R.; Bayer, A.S. DltABCD- and MprF-Mediated Cell Envelope Modifications of Staphylococcus Aureus Confer Resistance to Platelet Microbicidal Proteins and Contribute to Virulence in a Rabbit Endocarditis Model. Infect. Immun. 2005, 73, 8033–8038. [Google Scholar] [CrossRef] [PubMed]
- Weidenmaier, C.; Peschel, A.; Xiong, Y.-Q.; Kristian, S.A.; Dietz, K.; Yeaman, M.R.; Bayer, A.S. Lack of Wall Teichoic Acids in Staphylococcus Aureus Leads to Reduced Interactions with Endothelial Cells and to Attenuated Virulence in a Rabbit Model of Endocarditis. J. Infect. Dis. 2005, 191, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Weidenmaier, C.; Kokai-Kun, J.F.; Kristian, S.A.; Chanturiya, T.; Kalbacher, H.; Gross, M.; Nicholson, G.; Neumeister, B.; Mond, J.J.; Peschel, A. Role of Teichoic Acids in Staphylococcus Aureus Nasal Colonization, a Major Risk Factor in Nosocomial Infections. Nat. Med. 2004, 10, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Gross, M.; Cramton, S.E.; Götz, F.; Peschel, A. Key Role of Teichoic Acid Net Charge in Staphylococcus Aureus Colonization of Artificial Surfaces. Infect. Immun. 2001, 69, 3423–3426. [Google Scholar] [CrossRef] [PubMed]
- Kaito, C.; Sekimizu, K. Colony Spreading in Staphylococcus Aureus. J. Bacteriol. 2007, 189, 2553–2557. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.V.; Kristian, S.A.; Weidenmaier, C.; Faigle, M.; Van Kessel, K.P.M.; Van Strijp, J.A.G.; Götz, F.; Neumeister, B.; Peschel, A. Staphylococcus Aureus Strains Lacking D-Alanine Modifications of Teichoic Acids Are Highly Susceptible to Human Neutrophil Killing and Are Virulence Attenuated in Mice. J. Infect. Dis. 2002, 186, 214–219. [Google Scholar] [CrossRef]
- Peschel, A.; Otto, M.; Jack, R.W.; Kalbacher, H.; Jung, G.; Götz, F. Inactivation of the Dlt Operon in Staphylococcus Aureus Confers Sensitivity to Defensins, Protegrins, and Other Antimicrobial Peptides. J. Biol. Chem. 1999, 274, 8405–8410. [Google Scholar] [CrossRef]
- Dunman, P.M.; Murphy, E.; Haney, S.; Palacios, D.; Tucker-Kellogg, G.; Wu, S.; Brown, E.L.; Zagursky, R.J.; Shlaes, D.; Projan, S.J. Transcription Profiling-Based Identification of Staphylococcus aureus Genes Regulated by the Agr and/or sarA Loci. J. Bacteriol. 2001, 183, 7341–7353. [Google Scholar] [CrossRef]
- Kraus, D.; Herbert, S.; Kristian, S.A.; Khosravi, A.; Nizet, V.; Götz, F.; Peschel, A. The GraRS Regulatory System Controls Staphylococcus Aureus Susceptibility to Antimicrobial Host Defenses. BMC Microbiol. 2008, 8, 85. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cha, D.J.; Lai, Y.; Villaruz, A.E.; Sturdevant, D.E.; Otto, M. The Antimicrobial Peptide-Sensing System Aps of Staphylococcus Aureus. Mol. Microbiol. 2007, 66, 1136–1147. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lai, Y.; Villaruz, A.E.; Cha, D.J.; Sturdevant, D.E.; Otto, M. Gram-Positive Three-Component Antimicrobial Peptide-Sensing System. Proc. Natl. Acad. Sci. USA 2007, 104, 9469–9474. [Google Scholar] [CrossRef] [PubMed]
- Peleg, A.Y.; Miyakis, S.; Ward, D.V.; Earl, A.M.; Rubio, A.; Cameron, D.R.; Pillai, S.; Moellering, R.C.; Eliopoulos, G.M. Whole Genome Characterization of the Mechanisms of Daptomycin Resistance in Clinical and Laboratory Derived Isolates of Staphylococcus Aureus. PLoS ONE 2012, 7, e28316. [Google Scholar] [CrossRef] [PubMed]
- Peschel, A.; Jack, R.W.; Otto, M.; Collins, L.V.; Staubitz, P.; Nicholson, G.; Kalbacher, H.; Nieuwenhuizen, W.F.; Jung, G.; Tarkowski, A.; et al. Staphylococcus Aureus Resistance to Human Defensins and Evasion of Neutrophil Killing via the Novel Virulence Factor MprF Is Based on Modification of Membrane Lipids with L-Lysine. J. Exp. Med. 2001, 193, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Shireen, T.; Singh, M.; Das, T.; Mukhopadhyay, K. Differential Adaptive Responses of Staphylococcus Aureus to In Vitro Selection with Different Antimicrobial Peptides. Antimicrob. Agents Chemother. 2013, 57, 5134–5137. [Google Scholar] [CrossRef] [PubMed]
- Nishi, H.; Komatsuzawa, H.; Fujiwara, T.; McCallum, N.; Sugai, M. Reduced Content of Lysyl-Phosphatidylglycerol in the Cytoplasmic Membrane Affects Susceptibility to Moenomycin, as Well as Vancomycin, Gentamicin, and Antimicrobial Peptides, in Staphylococcus Aureus. Antimicrob. Agents Chemother. 2004, 48, 4800–4807. [Google Scholar] [CrossRef] [PubMed]
- Cheung, G.Y.C.; Fisher, E.L.; McCausland, J.W.; Choi, J.; Collins, J.W.M.; Dickey, S.W.; Otto, M. Antimicrobial Peptide Resistance Mechanism Contributes to Staphylococcus Aureus Infection. J. Infect. Dis. 2018, 217, 1153–1159. [Google Scholar] [CrossRef]
- Kupferwasser, L.I.; Skurray, R.A.; Brown, M.H.; Firth, N.; Yeaman, M.R.; Bayer, A.S. Plasmid-Mediated Resistance to Thrombin-Induced Platelet Microbicidal Protein in Staphylococci: Role of the qacA Locus. Antimicrob. Agents Chemother. 1999, 43, 2395–2399. [Google Scholar] [CrossRef]
- Falord, M.; Karimova, G.; Hiron, A.; Msadek, T. GraXSR Proteins Interact with the VraFG ABC Transporter to Form a Five-Component System Required for Cationic Antimicrobial Peptide Sensing and Resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 2012, 56, 1047–1058. [Google Scholar] [CrossRef]
- Cao, R.; Su, H.; Wei, Z.; He, Z.; Pan, T.; Li, Y.; Sun, B. An Induced Mutation of ABC-Transporter Component VraF(K84E) Contributes to Vancomycin Resistance and Virulence in Staphylococcus Aureus Strain MW2. Int. J. Med. Microbiol. IJMM 2024, 315, 151624. [Google Scholar] [CrossRef] [PubMed]
- Vuong, C.; Voyich, J.M.; Fischer, E.R.; Braughton, K.R.; Whitney, A.R.; DeLeo, F.R.; Otto, M. Polysaccharide Intercellular Adhesin (PIA) Protects Staphylococcus Epidermidis against Major Components of the Human Innate Immune System. Cell. Microbiol. 2004, 6, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Cheung, G.Y.C.; Otto, M. Understanding the Significance of Staphylococcus Epidermidis Bacteremia in Babies and Children. Curr. Opin. Infect. Dis. 2010, 23, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, J.T.; Stannard, J.A.; Lauth, X.; Ostland, V.E.; Powell, H.C.; Westerman, M.E.; Nizet, V. Streptococcus Iniae Phosphoglucomutase Is a Virulence Factor and a Target for Vaccine Development. Infect. Immun. 2005, 73, 6935–6944. [Google Scholar] [CrossRef]
- Allen, J.P.; Neely, M.N. CpsY Influences Streptococcus Iniae Cell Wall Adaptations Important for Neutrophil Intracellular Survival. Infect. Immun. 2012, 80, 1707–1715. [Google Scholar] [CrossRef]
- Ouyang, J.; Tian, X.-L.; Versey, J.; Wishart, A.; Li, Y.-H. The BceABRS Four-Component System Regulates the Bacitracin-Induced Cell Envelope Stress Response in Streptococcus Mutans. Antimicrob. Agents Chemother. 2010, 54, 3895–3906. [Google Scholar] [CrossRef]
- Mazda, Y.; Kawada-Matsuo, M.; Kanbara, K.; Oogai, Y.; Shibata, Y.; Yamashita, Y.; Miyawaki, S.; Komatsuzawa, H. Association of CiaRH with Resistance of Streptococcus Mutans to Antimicrobial Peptides in Biofilms. Mol. Oral Microbiol. 2012, 27, 124–135. [Google Scholar] [CrossRef]
- Du, J.; Huang, S.; Wu, M.; Chen, S.; Zhou, W.; Zhan, L.; Huang, X. Dlt Operon Regulates Physiological Function and Cariogenic Virulence in Streptococcus Mutans. Future Microbiol. 2023, 18, 225–233. [Google Scholar] [CrossRef]
- Shaper, M.; Hollingshead, S.K.; Benjamin, W.H.; Briles, D.E. PspA Protects Streptococcus Pneumoniae from Killing by Apolactoferrin, and Antibody to PspA Enhances Killing of Pneumococci by Apolactoferrin. Infect. Immun. 2004, 72, 5031–5040. [Google Scholar] [CrossRef]
- Kovács, M.; Halfmann, A.; Fedtke, I.; Heintz, M.; Peschel, A.; Vollmer, W.; Hakenbeck, R.; Brückner, R. A Functional Dlt Operon, Encoding Proteins Required for Incorporation of d-Alanine in Teichoic Acids in Gram-Positive Bacteria, Confers Resistance to Cationic Antimicrobial Peptides in Streptococcus Pneumoniae. J. Bacteriol. 2006, 188, 5797–5805. [Google Scholar] [CrossRef]
- Zafar, M.A.; Hammond, A.J.; Hamaguchi, S.; Wu, W.; Kono, M.; Zhao, L.; Weiser, J.N. Identification of Pneumococcal Factors Affecting Pneumococcal Shedding Shows That the Dlt Locus Promotes Inflammation and Transmission. mBio 2019, 10, e01032-19. [Google Scholar] [CrossRef] [PubMed]
- Wartha, F.; Beiter, K.; Albiger, B.; Fernebro, J.; Zychlinsky, A.; Normark, S.; Henriques-Normark, B. Capsule and D-Alanylated Lipoteichoic Acids Protect Streptococcus Pneumoniae against Neutrophil Extracellular Traps. Cell. Microbiol. 2007, 9, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Majchrzykiewicz, J.A.; Kuipers, O.P.; Bijlsma, J.J.E. Generic and Specific Adaptive Responses of Streptococcus Pneumoniae to Challenge with Three Distinct Antimicrobial Peptides, Bacitracin, LL-37, and Nisin. Antimicrob. Agents Chemother. 2010, 54, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-B.; Hou, W.-T.; Cheng, M.-T.; Jiang, Y.-L.; Chen, Y.; Zhou, C.-Z. Structure of a MacAB-like Efflux Pump from Streptococcus Pneumoniae—Nature Communications. Nat. Commun. 2018, 9, 196. [Google Scholar] [CrossRef] [PubMed]
- Zähner, D.; Zhou, X.; Chancey, S.T.; Pohl, J.; Shafer, W.M.; Stephens, D.S. Human Antimicrobial Peptide LL-37 Induces MefE/Mel-Mediated Macrolide Resistance in Streptococcus Pneumoniae. Antimicrob. Agents Chemother. 2010, 54, 3516–3519. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.M.; Akinbi, H.T.; Standish, A.J.; Weiser, J.N. Resistance to Mucosal Lysozyme Compensates for the Fitness Deficit of Peptidoglycan Modifications by Streptococcus Pneumoniae. PLoS Pathog. 2008, 4, e1000241. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Zan, Y.; Zhang, Y.; Zheng, N.; Yan, Q.; Zhang, W.; Zhang, H.; Jin, M.; Chen, F.; Zhang, X.; et al. The Cysteine Protease ApdS from Streptococcus Suis Promotes Evasion of Innate Immune Defenses by Cleaving the Antimicrobial Peptide Cathelicidin LL-37. J. Biol. Chem. 2019, 294, 17962–17977. [Google Scholar] [CrossRef] [PubMed]
- Wichgers Schreur, P.J.; van Weeghel, C.; Rebel, J.M.J.; Smits, M.A.; van Putten, J.P.M.; Smith, H.E. Lysozyme Resistance in Streptococcus Suis Is Highly Variable and Multifactorial. PLoS ONE 2012, 7, e36281. [Google Scholar] [CrossRef] [PubMed]
- LaRock, C.N.; Döhrmann, S.; Todd, J.; Corriden, R.; Olson, J.; Johannssen, T.; Lepenies, B.; Gallo, R.L.; Ghosh, P.; Nizet, V. Group A Streptococcal M1 Protein Sequesters Cathelicidin to Evade Innate Immune Killing. Cell Host Microbe 2015, 18, 471–477. [Google Scholar] [CrossRef]
- Yadav, R.; Govindan, S.; Daczkowski, C.; Mesecar, A.; Chakravarthy, S.; Noinaj, N. Structural Insight into the Dual Function of LbpB in Mediating Neisserial Pathogenesis. eLife 2021, 10, e71683. [Google Scholar] [CrossRef]
- Morgenthau, A.; Partha, S.K.; Adamiak, P.; Schryvers, A.B. The Specificity of Protection against Cationic Antimicrobial Peptides by Lactoferrin Binding Protein B. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2014, 27, 923–933. [Google Scholar] [CrossRef]
- Ostan, N.K.H.; Cole, G.B.; Wang, F.Z.; Reichheld, S.E.; Moore, G.; Pan, C.; Yu, R.; Lai, C.C.-L.; Sharpe, S.; Lee, H.O.; et al. A Secreted Bacterial Protein Protects Bacteria from Cationic Antimicrobial Peptides by Entrapment in Phase-Separated Droplets. PNAS Nexus 2024, 3, pgae139. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, X.; Wang, X.; Wang, L.; Zhang, J.; Yin, Y. ComE, an Essential Response Regulator, Negatively Regulates the Expression of the Capsular Polysaccharide Locus and Attenuates the Bacterial Virulence in Streptococcus Pneumoniae. Front. Microbiol. 2017, 8, 277. [Google Scholar] [CrossRef] [PubMed]
- Cress, B.F.; Englaender, J.A.; He, W.; Kasper, D.; Linhardt, R.J.; Koffas, M.A.G. Masquerading Microbial Pathogens: Capsular Polysaccharides Mimic Host-Tissue Molecules. FEMS Microbiol. Rev. 2014, 38, 660–697. [Google Scholar] [CrossRef]
- Gao, S.; Jin, W.; Quan, Y.; Li, Y.; Shen, Y.; Yuan, S.; Yi, L.; Wang, Y.; Wang, Y. Bacterial Capsules: Occurrence, Mechanism, and Function. Npj Biofilms Microbiomes 2024, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Fleeman, R.M.; Macias, L.A.; Brodbelt, J.S.; Davies, B.W. Defining Principles That Influence Antimicrobial Peptide Activity against Capsulated Klebsiella Pneumoniae. Proc. Natl. Acad. Sci. USA 2020, 117, 27620–27626. [Google Scholar] [CrossRef]
- Claessen, D.; Errington, J. Cell Wall Deficiency as a Coping Strategy for Stress. Trends Microbiol. 2019, 27, 1025–1033. [Google Scholar] [CrossRef]
- de Tejada, G.M.; Sánchez-Gómez, S.; Kowalski, I.; Kaconis, Y.; Andrä, J.; Schürholz, T.; Hornef, M.; Dupont, A.; Garidel, P.; Gutsmann, T.; et al. Bacterial Cell Wall Compounds as Promising Targets of Antimicrobial Agents I. Antimicrobial Peptides and Lipopolyamines. Curr. Drug Targets 2012, 13, 1121–1130. [Google Scholar] [CrossRef]
- Nikaido, H. Molecular Basis of Bacterial Outer Membrane Permeability Revisited. Microbiol. Mol. Biol. Rev. MMBR 2003, 67, 593–656. [Google Scholar] [CrossRef]
- Papo, N.; Shai, Y. A Molecular Mechanism for Lipopolysaccharide Protection of Gram-Negative Bacteria from Antimicrobial Peptides*. J. Biol. Chem. 2005, 280, 10378–10387. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Rozek, A. Role of Membranes in the Activities of Antimicrobial Cationic Peptides. FEMS Microbiol. Lett. 2002, 206, 143–149. [Google Scholar] [CrossRef]
- Caroff, M.; Novikov, A. Lipopolysaccharides: Structure, Function and Bacterial Identifications. OCL 2020, 27, 31. [Google Scholar] [CrossRef]
- Som, N.; Reddy, M. Cross-Talk between Phospholipid Synthesis and Peptidoglycan Expansion by a Cell Wall Hydrolase. Proc. Natl. Acad. Sci. USA 2023, 120, e2300784120. [Google Scholar] [CrossRef]
- Thomanek, N.; Arends, J.; Lindemann, C.; Barkovits, K.; Meyer, H.E.; Marcus, K.; Narberhaus, F. Intricate Crosstalk Between Lipopolysaccharide, Phospholipid and Fatty Acid Metabolism in Escherichia Coli Modulates Proteolysis of LpxC. Front. Microbiol. 2019, 9, 3285. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, W.; Wang, X. Insights into the Structure of Escherichia Coli Outer Membrane as the Target for Engineering Microbial Cell Factories. Microb. Cell Factories 2021, 20, 73. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.; Eom, H.; Cho, Y.; Rho, M.; Song, W.J. Discovery and Characterization of Polymyxin-Resistance Genes pmrE and pmrF from Sediment and Seawater Microbiome. Microbiol. Spectr. 2023, 11, e02736-22. [Google Scholar] [CrossRef] [PubMed]
- Moran, A.P.; Prendergast, M.M.; Appelmelk, B.J. Molecular Mimicry of Host Structures by Bacterial Lipopolysaccharides and Its Contribution to Disease. FEMS Immunol. Med. Microbiol. 1996, 16, 105–115. [Google Scholar] [CrossRef]
- Peterson, A.A.; Haug, A.; McGroarty, E.J. Physical Properties of Short- and Long-O-Antigen-Containing Fractions of Lipopolysaccharide from Escherichia Coli 0111:B4. J. Bacteriol. 1986, 165, 116–122. [Google Scholar] [CrossRef]
- Stinavage, P.; Martin, L.E.; Spitznagel, J.K. O Antigen and Lipid A Phosphoryl Groups in Resistance of Salmonella Typhimurium LT-2 to Nonoxidative Killing in Human Polymorphonuclear Neutrophils. Infect. Immun. 1989, 57, 3894–3900. [Google Scholar] [CrossRef]
- Capodici, C.; Chen, S.; Sidorczyk, Z.; Elsbach, P.; Weiss, J. Effect of Lipopolysaccharide (LPS) Chain Length on Interactions of Bactericidal/Permeability-Increasing Protein and Its Bioactive 23-Kilodalton NH2-Terminal Fragment with Isolated LPS and Intact Proteus Mirabilis and Escherichia Coli. Infect. Immun. 1994, 62, 259–265. [Google Scholar] [CrossRef]
- Allen, C.A.; Adams, L.G.; Ficht, T.A. Transposon-Derived Brucella Abortus Rough Mutants Are Attenuated and Exhibit Reduced Intracellular Survival. Infect. Immun. 1998, 66, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Clifton, L.A.; Ciesielski, F.; Skoda, M.W.A.; Paracini, N.; Holt, S.A.; Lakey, J.H. The Effect of Lipopolysaccharide Core Oligosaccharide Size on the Electrostatic Binding of Antimicrobial Proteins to Models of the Gram Negative Bacterial Outer Membrane. Langmuir 2016, 32, 3485–3494. [Google Scholar] [CrossRef] [PubMed]
- Adams, P.G.; Lamoureux, L.; Swingle, K.L.; Mukundan, H.; Montaño, G.A. Lipopolysaccharide-Induced Dynamic Lipid Membrane Reorganization: Tubules, Perforations, and Stacks. Biophys. J. 2014, 106, 2395–2407. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.G. Bacterial Lipopolysaccharides—Themes and Variations. Prog. Lipid Res. 1996, 35, 283–343. [Google Scholar] [CrossRef] [PubMed]
- Papahadjopoulos, D.; Portis, A.; Pangborn, W. Calcium-Induced Lipid Phase Transitions and Membrane Fusion. Ann. N. Y. Acad. Sci. 1978, 308, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, K.; Koch, M.H.J.; Seydel, U. Phase Diagram of Lipid A from Salmonella Minnesota and Escherichia Coli Rough Mutant Lipopolysaccharide. J. Struct. Biol. 1990, 105, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Seydel, U.; Koch, M.H.; Brandenburg, K. Structural Polymorphisms of Rough Mutant Lipopolysaccharides Rd to Ra from Salmonella Minnesota. J. Struct. Biol. 1993, 110, 232–243. [Google Scholar] [CrossRef]
- Jia, W.; Zoeiby, A.E.; Petruzziello, T.N.; Jayabalasingham, B.; Seyedirashti, S.; Bishop, R.E. Lipid Trafficking Controls Endotoxin Acylation in Outer Membranes of Escherichia coli*. J. Biol. Chem. 2004, 279, 44966–44975. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.W.; Scott, M.G. The Role of Antimicrobial Peptides in Animal Defenses. Proc. Natl. Acad. Sci. USA 2000, 97, 8856–8861. [Google Scholar] [CrossRef]
- Morrison, D.C.; Leive, L. Fractions of Lipopolysaccharide from Escherichia Coli O111:B4 Prepared by Two Extraction Procedures. J. Biol. Chem. 1975, 250, 2911–2919. [Google Scholar] [CrossRef]
- Brandenburg, K.; Seydel, U. Physical Aspects of Structure and Function of Membranes Made from Lipopolysaccharides and Free Lipid A. Biochim. Biophys. Acta BBA—Biomembr. 1984, 775, 225–238. [Google Scholar] [CrossRef]
- Yeh, H.Y.; Jacobs, D.M. Characterization of Lipopolysaccharide Fractions and Their Interactions with Cells and Model Membranes. J. Bacteriol. 1992, 174, 336–341. [Google Scholar] [CrossRef]
- Rana, F.R.; Blazyk, J. Interactions between the Antimicrobial Peptide, Magainin 2, and Salmonella Typhimurium Lipopolysaccharides. FEBS Lett. 1991, 293, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Ginez, L.D.; Osorio, A.; Vázquez-Ramírez, R.; Arenas, T.; Mendoza, L.; Camarena, L.; Poggio, S. Changes in Fluidity of the E. Coli Outer Membrane in Response to Temperature, Divalent Cations and Polymyxin-B Show Two Different Mechanisms of Membrane Fluidity Adaptation. FEBS J. 2022, 289, 3550–3567. [Google Scholar] [CrossRef]
- Hac-Wydro, K.; Wydro, P. The Influence of Fatty Acids on Model Cholesterol/Phospholipid Membranes. Chem. Phys. Lipids 2007, 150, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Vaara, M. Agents That Increase the Permeability of the Outer Membrane. Microbiol. Rev. 1992, 56, 395–411. [Google Scholar] [CrossRef]
- Seydel, U.; Labischinski, H.; Kastowsky, M.; Brandenburg, K. Phase Behavior, Supramolecular Structure, and Molecular Conformation of Lipopolysaccharide. Immunobiology 1993, 187, 191–211. [Google Scholar] [CrossRef]
- Cullis, P.R.; Hope, M.J. Physical Properties and Functional Roles of Lipids in Membranes. In Biochemistry of Lipids and Membranes; Vance, D.E., Vance, J.E., Eds.; Benjamin-Cummings: Menlo Park, CA, USA, 1985. [Google Scholar]
- Kim, S.; Patel, D.S.; Park, S.; Slusky, J.; Klauda, J.B.; Widmalm, G.; Im, W. Bilayer Properties of Lipid A from Various Gram-Negative Bacteria. Biophys. J. 2016, 111, 1750–1760. [Google Scholar] [CrossRef]
- Emiola, A.; George, J.; Andrews, S.S. A Complete Pathway Model for Lipid A Biosynthesis in Escherichia Coli. PLoS ONE 2015, 10, e0121216. [Google Scholar] [CrossRef]
- Clementz, T.; Bednarski, J.J.; Raetz, C.R. Function of the htrB High Temperature Requirement Gene of Escherichia Coli in the Acylation of Lipid A: HtrB Catalyzed Incorporation of Laurate. J. Biol. Chem. 1996, 271, 12095–12102. [Google Scholar] [CrossRef]
- Schilling, B.; Hunt, J.; Gibson, B.W.; Apicella, M.A. Site-Specific Acylation Changes in the Lipid A of Escherichia Coli lpxL Mutants Grown at High Temperatures. Innate Immun. 2014, 20, 269–282. [Google Scholar] [CrossRef]
- Steeghs, L.; de Cock, H.; Evers, E.; Zomer, B.; Tommassen, J.; Ley, P. van der Outer Membrane Composition of a Lipopolysaccharide-Deficient Neisseria Meningitidis Mutant. EMBO J. 2001, 20, 6937–6945. [Google Scholar] [CrossRef] [PubMed]
- Somerville, J.E.; Cassiano, L.; Bainbridge, B.; Cunningham, M.D.; Darveau, R.P. A Novel Escherichia Coli Lipid A Mutant That Produces an Antiinflammatory Lipopolysaccharide. J. Clin. Invest. 1996, 97, 359–365. [Google Scholar] [CrossRef]
- Khan, S.A.; Everest, P.; Servos, S.; Foxwell, N.; Zähringer, U.; Brade, H.; Rietschel, E.T.; Dougan, G.; Charles, I.G.; Maskell, D.J. A Lethal Role for Lipid A in Salmonella Infections. Mol. Microbiol. 1998, 29, 571–579. [Google Scholar] [CrossRef] [PubMed]
- d’Hauteville, H.; Khan, S.; Maskell, D.J.; Kussak, A.; Weintraub, A.; Mathison, J.; Ulevitch, R.J.; Wuscher, N.; Parsot, C.; Sansonetti, P.J. Two msbB Genes Encoding Maximal Acylation of Lipid A Are Required for Invasive Shigella Flexneri to Mediate Inflammatory Rupture and Destruction of the Intestinal Epithelium1. J. Immunol. 2002, 168, 5240–5251. [Google Scholar] [CrossRef]
- Murray, S.R.; Bermudes, D.; de Felipe, K.S.; Low, K.B. Extragenic Suppressors of Growth Defects in msbB Salmonella. J. Bacteriol. 2001, 183, 5554–5561. [Google Scholar] [CrossRef]
- Karow, M.; Fayet, O.; Georgopoulos, C. The Lethal Phenotype Caused by Null Mutations in the Escherichia Coli htrB Gene Is Suppressed by Mutations in the accBC Operon, Encoding Two Subunits of Acetyl Coenzyme A Carboxylase. J. Bacteriol. 1992, 174, 7407–7418. [Google Scholar] [CrossRef] [PubMed]
- Karow, M.; Georgopoulos, C. Isolation and Characterization of the Escherichia Coli msbB Gene, a Multicopy Suppressor of Null Mutations in the High-Temperature Requirement Gene htrB. J. Bacteriol. 1992, 174, 702–710. [Google Scholar] [CrossRef]
- Somerville, J.E.; Cassiano, L.; Darveau, R.P. Escherichia Coli msbB Gene as a Virulence Factor and a Therapeutic Target. Infect. Immun. 1999, 67, 6583–6590. [Google Scholar] [CrossRef]
- Vorachek-Warren, M.K.; Ramirez, S.; Cotter, R.J.; Raetz, C.R.H. A Triple Mutant of Escherichia Coli Lacking Secondary Acyl Chains on Lipid A. J. Biol. Chem. 2002, 277, 14194–14205. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, K.; Han, M.-L.; Yuan, B.; Li, J.; Gong, B.; Velkov, T.; Schreiber, F.; Wang, L.; Li, J. Outer Membranes of Polymyxin-Resistant Acinetobacter Baumannii with Phosphoethanolamine-Modified Lipid A and Lipopolysaccharide Loss Display Different Atomic-Scale Interactions with Polymyxins. ACS Infect. Dis. 2020, 6, 2698–2708. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ribeiro, A.A.; Guan, Z.; McGrath, S.C.; Cotter, R.J.; Raetz, C.R.H. Structure and Biosynthesis of Free Lipid A Molecules That Replace Lipopolysaccharide in Francisella Novicida. Biochemistry 2006, 45, 14427–14440. [Google Scholar] [CrossRef]
- Akira, S. TLR Signaling. Curr. Top. Microbiol. Immunol. 2006, 311, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Dalebroux, Z.D.; Matamouros, S.; Whittington, D.; Bishop, R.E.; Miller, S.I. PhoPQ Regulates Acidic Glycerophospholipid Content of the Salmonella Typhimurium Outer Membrane. Proc. Natl. Acad. Sci. USA 2014, 111, 1963–1968. [Google Scholar] [CrossRef] [PubMed]
- Pilione, M.R.; Pishko, E.J.; Preston, A.; Maskell, D.J.; Harvill, E.T. pagP Is Required for Resistance to Antibody-Mediated Complement Lysis during Bordetella Bronchiseptica Respiratory Infection. Infect. Immun. 2004, 72, 2837–2842. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, K.; Ernst, R.K.; Miller, S.I. Deacylation and Palmitoylation of Lipid A by Salmonellae Outer Membrane Enzymes Modulate Host Signaling through Toll-like Receptor 4. J. Endotoxin Res. 2004, 10, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Lipid A Acylation and Bacterial Resistance against Vertebrate Antimicrobial Peptides. Cell 1998, 95, 189–198. [CrossRef] [PubMed]
- Preston, A.; Maxim, E.; Toland, E.; Pishko, E.J.; Harvill, E.T.; Caroff, M.; Maskell, D.J. Bordetella Bronchiseptica PagP Is a Bvg-Regulated Lipid A Palmitoyl Transferase That Is Required for Persistent Colonization of the Mouse Respiratory Tract. Mol. Microbiol. 2003, 48, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Trent, M.S.; Pabich, W.; Raetz, C.R.H.; Miller, S.I. A PhoP/PhoQ-Induced Lipase (PagL) That Catalyzes 3-O-Deacylation of Lipid A Precursors in Membranes ofSalmonella Typhimurium *. J. Biol. Chem. 2001, 276, 9083–9092. [Google Scholar] [CrossRef]
- Rutten, L.; Mannie, J.-P.B.A.; Stead, C.M.; Raetz, C.R.H.; Reynolds, C.M.; Bonvin, A.M.J.J.; Tommassen, J.P.; Egmond, M.R.; Trent, M.S.; Gros, P. Active-Site Architecture and Catalytic Mechanism of the Lipid A Deacylase LpxR of Salmonella Typhimurium. Proc. Natl. Acad. Sci. USA 2009, 106, 1960–1964. [Google Scholar] [CrossRef]
- Gibbons, H.S.; Kalb, S.R.; Cotter, R.J.; Raetz, C.R.H. Role of Mg2+ and pH in the Modification of Salmonella Lipid A after Endocytosis by Macrophage Tumour Cells. Mol. Microbiol. 2005, 55, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, H.S.; Lin, S.; Cotter, R.J.; Raetz, C.R. Oxygen Requirement for the Biosynthesis of the S-2-Hydroxymyristate Moiety in Salmonella Typhimurium Lipid A. Function of LpxO, A New Fe2+/Alpha-Ketoglutarate-Dependent Dioxygenase Homologue. J. Biol. Chem. 2000, 275, 32940–32949. [Google Scholar] [CrossRef]
- MacArthur, I.; Jones, J.W.; Goodlett, D.R.; Ernst, R.K.; Preston, A. Role of pagL and lpxO in Bordetella Bronchiseptica Lipid A Biosynthesis. J. Bacteriol. 2011, 193, 4726–4735. [Google Scholar] [CrossRef] [PubMed]
- Ogunlana, L.; Kaur, D.; Shaw, L.P.; Jangir, P.; Walsh, T.; Uphoff, S.; MacLean, R.C. Regulatory Fine-Tuning of Mcr-1 Increases Bacterial Fitness and Stabilises Antibiotic Resistance in Agricultural Settings. ISME J. 2023, 17, 2058–2069. [Google Scholar] [CrossRef] [PubMed]
- Nang, S.C.; Li, J.; Velkov, T. The Rise and Spread of Mcr Plasmid-Mediated Polymyxin Resistance. Crit. Rev. Microbiol. 2019, 45, 131–161. [Google Scholar] [CrossRef] [PubMed]
- Shprung, T.; Wani, N.A.; Wilmes, M.; Mangoni, M.L.; Bitler, A.; Shimoni, E.; Sahl, H.-G.; Shai, Y. Opposing Effects of PhoPQ and PmrAB on the Properties of Salmonella Enterica Serovar Typhimurium: Implications on Resistance to Antimicrobial Peptides. Biochemistry 2021, 60, 2943–2955. [Google Scholar] [CrossRef]
- Rubin, E.J.; Herrera, C.M.; Crofts, A.A.; Trent, M.S. PmrD Is Required for Modifications to Escherichia Coli Endotoxin That Promote Antimicrobial Resistance. Antimicrob. Agents Chemother. 2015, 59, 2051–2061. [Google Scholar] [CrossRef]
- Huang, J.; Li, C.; Song, J.; Velkov, T.; Wang, L.; Zhu, Y.; Li, J. Regulating Polymyxin Resistance in Gram-Negative Bacteria: Roles of Two-Component Systems PhoPQ and PmrAB. Future Microbiol. 2020, 15, 445–459. [Google Scholar] [CrossRef]
- Gunn, J.S. Bacterial Modification of LPS and Resistance to Antimicrobial Peptides. J. Endotoxin Res. 2001, 7, 57–62. [Google Scholar] [CrossRef]
- Raetz, C.R.H.; Reynolds, C.M.; Trent, M.S.; Bishop, R.E. Lipid A Modification Systems in Gram-Negative Bacteria. Annu. Rev. Biochem. 2007, 76, 295–329. [Google Scholar] [CrossRef]
- Yan, A.; Guan, Z.; Raetz, C.R.H. An Undecaprenyl Phosphate-Aminoarabinose Flippase Required for Polymyxin Resistance in Escherichia Coli*. J. Biol. Chem. 2007, 282, 36077–36089. [Google Scholar] [CrossRef] [PubMed]
- Murray, S.R.; Ernst, R.K.; Bermudes, D.; Miller, S.I.; Low, K.B. PmrA(Con) Confers pmrHFIJKL-Dependent EGTA and Polymyxin Resistance on msbB Salmonella by Decorating Lipid A with Phosphoethanolamine. J. Bacteriol. 2007, 189, 5161–5169. [Google Scholar] [CrossRef] [PubMed]
- Shearer, J.; Jefferies, D.; Khalid, S. Outer Membrane Proteins OmpA, FhuA, OmpF, EstA, BtuB, and OmpX Have Unique Lipopolysaccharide Fingerprints. J. Chem. Theory Comput. 2019, 15, 2608–2619. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.M.A.; Zeth, K.; Bavro, V.N.; Sancho-Vaello, E. The Role of Bacterial Transport Systems in the Removal of Host Antimicrobial Peptides in Gram-Negative Bacteria. FEMS Microbiol. Rev. 2022, 46, fuac032. [Google Scholar] [CrossRef] [PubMed]
- Zgurskaya, H.I.; Krishnamoorthy, G.; Ntreh, A.; Lu, S. Mechanism and Function of the Outer Membrane Channel TolC in Multidrug Resistance and Physiology of Enterobacteria. Front. Microbiol. 2011, 2, 189. [Google Scholar] [CrossRef] [PubMed]
- Housden, N.G.; Webby, M.N.; Lowe, E.D.; El-Baba, T.J.; Kaminska, R.; Redfield, C.; Robinson, C.V.; Kleanthous, C. Toxin Import through the Antibiotic Efflux Channel TolC. Nat. Commun. 2021, 12, 4625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Ye, Z.; Ye, C.; Zou, H.; Gao, Z.; Pan, J. OmpW Is Positively Regulated by Iron via Fur, and Negatively Regulated by SoxS Contribution to Oxidative Stress Resistance in Escherichia coli. Microb. Pathog. 2020, 138, 103808. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Yong, D.; Jeong, S.H.; Chong, Y. Multidrug-Resistant Acinetobacter Spp.: Increasingly Problematic Nosocomial Pathogens. Yonsei Med. J. 2011, 52, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, M.; Walker, S. Envelope Structures of Gram-Positive Bacteria. Curr. Top. Microbiol. Immunol. 2017, 404, 1–44. [Google Scholar] [CrossRef]
- Kim, S.J.; Chang, J.; Singh, M. Peptidoglycan Architecture of Gram-Positive Bacteria by Solid-State NMR. Biochim. Biophys. Acta 2015, 1848, 350–362. [Google Scholar] [CrossRef]
- Silhavy, T.J.; Kahne, D.; Walker, S. The Bacterial Cell Envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, F.C.; Baddiley, J. A Continuum of Anionic Charge: Structures and Functions of d-Alanyl-Teichoic Acids in Gram-Positive Bacteria. Microbiol. Mol. Biol. Rev. MMBR 2003, 67, 686–723. [Google Scholar] [CrossRef] [PubMed]
- Grangette, C.; Müller-Alouf, H.; Hols, P.; Goudercourt, D.; Delcour, J.; Turneer, M.; Mercenier, A. Enhanced Mucosal Delivery of Antigen with Cell Wall Mutants of Lactic Acid Bacteria. Infect. Immun. 2004, 72, 2731–2737. [Google Scholar] [CrossRef] [PubMed]
- de Vos, W.M. Lipotechoic Acid in Lactobacilli: D-Alanine Makes the Difference. Proc. Natl. Acad. Sci. USA 2005, 102, 10763–10764. [Google Scholar] [CrossRef] [PubMed]
- Kristian, S.A.; Lauth, X.; Nizet, V.; Goetz, F.; Neumeister, B.; Peschel, A.; Landmann, R. Alanylation of Teichoic Acids Protects Staphylococcus Aureus against Toll-like Receptor 2-Dependent Host Defense in a Mouse Tissue Cage Infection Model. J. Infect. Dis. 2003, 188, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Fischer, W. Phosphocholine of Pneumococcal Teichoic Acids: Role in Bacterial Physiology and Pneumococcal Infection. Res. Microbiol. 2000, 151, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Baltz, R.H.; Norris, F.H.; Matsushima, P.; DeHoff, B.S.; Rockey, P.; Porter, G.; Burgett, S.; Peery, R.; Hoskins, J.; Braverman, L.; et al. DNA Sequence Sampling of the Streptococcus Pneumoniae Genome to Identify Novel Targets for Antibiotic Development. Microb. Drug Resist. Larchmt. N 1998, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wartha, F.; Beiter, K.; Normark, S.; Henriques-Normark, B. Neutrophil Extracellular Traps: Casting the NET over Pathogenesis. Curr. Opin. Microbiol. 2007, 10, 52–56. [Google Scholar] [CrossRef]
- Walter, J.; Loach, D.M.; Alqumber, M.; Rockel, C.; Hermann, C.; Pfitzenmaier, M.; Tannock, G.W. D-Alanyl Ester Depletion of Teichoic Acids in Lactobacillus Reuteri 100-23 Results in Impaired Colonization of the Mouse Gastrointestinal Tract. Environ. Microbiol. 2007, 9, 1750–1760. [Google Scholar] [CrossRef] [PubMed]
- Epand, R.F.; Savage, P.B.; Epand, R.M. Bacterial Lipid Composition and the Antimicrobial Efficacy of Cationic Steroid Compounds (Ceragenins). Biochim. Biophys. Acta BBA—Biomembr. 2007, 1768, 2500–2509. [Google Scholar] [CrossRef]
- Rashid, R.; Veleba, M.; Kline, K.A. Focal Targeting of the Bacterial Envelope by Antimicrobial Peptides. Front. Cell Dev. Biol. 2016, 4, 55. [Google Scholar] [CrossRef] [PubMed]
- Strahl, H.; Errington, J. Bacterial Membranes: Structure, Domains, and Function. Annu. Rev. Microbiol. 2017, 71, 519–538. [Google Scholar] [CrossRef]
- Short, S.A.; White, D.C. Metabolism of Phosphatidylglycerol, Lysylphosphatidylglycerol, and Cardiolipin of Staphylococcus Aureus. J. Bacteriol. 1971, 108, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Nahaie, M.R.; Goodfellow, M.; Minnikin, D.E.; Hájek, V. Polar Lipid and Isoprenoid Quinone Composition in the Classification of Staphylococcus. J. Gen. Microbiol. 1984, 130, 2427–2437. [Google Scholar] [CrossRef]
- Kilelee, E.; Pokorny, A.; Yeaman, M.R.; Bayer, A.S. Lysyl-Phosphatidylglycerol Attenuates Membrane Perturbation Rather than Surface Association of the Cationic Antimicrobial Peptide 6W-RP-1 in a Model Membrane System: Implications for Daptomycin Resistance. Antimicrob. Agents Chemother. 2010, 54, 4476–4479. [Google Scholar] [CrossRef]
- Zhang, T.; Muraih, J.K.; Tishbi, N.; Herskowitz, J.; Victor, R.L.; Silverman, J.; Uwumarenogie, S.; Taylor, S.D.; Palmer, M.; Mintzer, E. Cardiolipin Prevents Membrane Translocation and Permeabilization by Daptomycin. J. Biol. Chem. 2014, 289, 11584–11591. [Google Scholar] [CrossRef]
- Bayer, A.S.; Prasad, R.; Chandra, J.; Koul, A.; Smriti, M.; Varma, A.; Skurray, R.A.; Firth, N.; Brown, M.H.; Koo, S.P.; et al. In Vitro Resistance of Staphylococcus Aureus to Thrombin-Induced Platelet Microbicidal Protein Is Associated with Alterations in Cytoplasmic Membrane Fluidity. Infect. Immun. 2000, 68, 3548–3553. [Google Scholar] [CrossRef] [PubMed]
- Ernst, C.M.; Staubitz, P.; Mishra, N.N.; Yang, S.-J.; Hornig, G.; Kalbacher, H.; Bayer, A.S.; Kraus, D.; Peschel, A. The Bacterial Defensin Resistance Protein MprF Consists of Separable Domains for Lipid Lysinylation and Antimicrobial Peptide Repulsion. PLoS Pathog. 2009, 5, e1000660. [Google Scholar] [CrossRef]
- Bayer, A.S.; Mishra, N.N.; Chen, L.; Kreiswirth, B.N.; Rubio, A.; Yang, S.-J. Frequency and Distribution of Single-Nucleotide Polymorphisms within mprF in Methicillin-Resistant Staphylococcus Aureus Clinical Isolates and Their Role in Cross-Resistance to Daptomycin and Host Defense Antimicrobial Peptides. Antimicrob. Agents Chemother. 2015, 59, 4930–4937. [Google Scholar] [CrossRef]
- Yang, S.-J.; Mishra, N.N.; Rubio, A.; Bayer, A.S. Causal Role of Single Nucleotide Polymorphisms within the mprF Gene of Staphylococcus Aureus in Daptomycin Resistance. Antimicrob. Agents Chemother. 2013, 57, 5658–5664. [Google Scholar] [CrossRef]
- Rubio, A.; Conrad, M.; Haselbeck, R.J.; Kedar, G.C.; Brown-Driver, V.; Finn, J.; Silverman, J.A. Regulation of mprF by Antisense RNA Restores Daptomycin Susceptibility to Daptomycin-Resistant Isolates of Staphylococcus Aureus. Antimicrob. Agents Chemother. 2011, 55, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Koprivnjak, T.; Peschel, A.; Gelb, M.H.; Liang, N.S.; Weiss, J.P. Role of Charge Properties of Bacterial Envelope in Bactericidal Action of Human Group IIA Phospholipase A2 against Staphylococcus Aureus. J. Biol. Chem. 2002, 277, 47636–47644. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Jiao, H.; Liu, Z. Phospholipid Translocation Captured in a Bifunctional Membrane Protein MprF. Nat. Commun. 2021, 12, 2927. [Google Scholar] [CrossRef] [PubMed]
- Rashid, R.; Nair, Z.J.; Chia, D.M.H.; Chong, K.K.L.; Cazenave Gassiot, A.; Morley, S.A.; Allen, D.K.; Chen, S.L.; Chng, S.S.; Wenk, M.R.; et al. Depleting Cationic Lipids Involved in Antimicrobial Resistance Drives Adaptive Lipid Remodeling in Enterococcus Faecalis. mBio 2023, 14, e0307322. [Google Scholar] [CrossRef] [PubMed]
- Boldrin, F.; Cioetto Mazzabò, L.; Lanéelle, M.-A.; Rindi, L.; Segafreddo, G.; Lemassu, A.; Etienne, G.; Conflitti, M.; Daffé, M.; Garzino Demo, A.; et al. LysX2 Is a Mycobacterium Tuberculosis Membrane Protein with an Extracytoplasmic MprF-like Domain. BMC Microbiol. 2022, 22, 85. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.N.; Ferre, R.; Castanho, M.A.R.B. Antimicrobial Peptides: Linking Partition, Activity and High Membrane-Bound Concentrations. Nat. Rev. Microbiol. 2009, 7, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, H. Multidrug Efflux Pumps of Gram-Negative Bacteria. J. Bacteriol. 1996, 178, 5853–5859. [Google Scholar] [CrossRef]
- Paulsen, I.T.; Brown, M.H.; Skurray, R.A. Proton-Dependent Multidrug Efflux Systems. Microbiol. Rev. 1996, 60, 575–608. [Google Scholar] [CrossRef]
- Li, X.-Z.; Nikaido, H. Efflux-Mediated Drug Resistance in Bacteria. Drugs 2004, 64, 159–204. [Google Scholar] [CrossRef]
- Clemens, R.; Zaschke-Kriesche, J.; Khosa, S.; Smits, S.H.J. Insight into Two ABC Transporter Families Involved in Lantibiotic Resistance. Front. Mol. Biosci. 2017, 4, 91. [Google Scholar] [CrossRef]
- Feng, Y.; Pan, X.; Huang, N.; Feng, Y.; Wu, Q.; Wang, B. The human beta-defensins expression in female genital tract and pregnancy-related tissues. Sichuan Da Xue Xue Bao Yi Xue Ban 2003, 34, 217–219. [Google Scholar] [PubMed]
- Colclough, A.L.; Alav, I.; Whittle, E.E.; Pugh, H.L.; Darby, E.M.; Legood, S.W.; McNeil, H.E.; Blair, J.M. RND Efflux Pumps in Gram-Negative Bacteria; Regulation, Structure and Role in Antibiotic Resistance. Future Microbiol. 2020, 15, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Gaurav, A.; Bakht, P.; Saini, M.; Pandey, S.; Pathania, R. Role of Bacterial Efflux Pumps in Antibiotic Resistance, Virulence, and Strategies to Discover Novel Efflux Pump Inhibitors. Microbiology 2023, 169, 001333. [Google Scholar] [CrossRef] [PubMed]
- Alav, I.; Kobylka, J.; Kuth, M.S.; Pos, K.M.; Picard, M.; Blair, J.M.A.; Bavro, V.N. Structure, Assembly, and Function of Tripartite Efflux and Type 1 Secretion Systems in Gram-Negative Bacteria. Chem. Rev. 2021, 121, 5479–5596. [Google Scholar] [CrossRef] [PubMed]
- Beis, K.; Rebuffat, S. Multifaceted ABC Transporters Associated to Microcin and Bacteriocin Export. Res. Microbiol. 2019, 170, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Nishino, K.; Yamaguchi, A. Novel Macrolide-Specific ABC-Type Efflux Transporter in Escherichia coli. J. Bacteriol. 2001, 183, 5639–5644. [Google Scholar] [CrossRef] [PubMed]
- Mason, K.M.; Bruggeman, M.E.; Munson, R.S.; Bakaletz, L.O. The Non-Typeable Haemophilus Influenzae Sap Transporter Provides a Mechanism of Antimicrobial Peptide Resistance and SapD-Dependent Potassium Acquisition. Mol. Microbiol. 2006, 62, 1357–1372. [Google Scholar] [CrossRef] [PubMed]
- Mason, K.M.; Raffel, F.K.; Ray, W.C.; Bakaletz, L.O. Heme Utilization by Nontypeable Haemophilus Influenzae Is Essential and Dependent on Sap Transporter Function. J. Bacteriol. 2011, 193, 2527–2535. [Google Scholar] [CrossRef]
- Shelton, C.L.; Raffel, F.K.; Beatty, W.L.; Johnson, S.M.; Mason, K.M. Sap Transporter Mediated Import and Subsequent Degradation of Antimicrobial Peptides in Haemophilus. PLoS Pathog. 2011, 7, e1002360. [Google Scholar] [CrossRef]
- Yang, S.-J.; Bayer, A.S.; Mishra, N.N.; Meehl, M.; Ledala, N.; Yeaman, M.R.; Xiong, Y.Q.; Cheung, A.L. The Staphylococcus Aureus Two-Component Regulatory System, GraRS, Senses and Confers Resistance to Selected Cationic Antimicrobial Peptides. Infect. Immun. 2012, 80, 74–81. [Google Scholar] [CrossRef]
- Kobylka, J.; Kuth, M.S.; Müller, R.T.; Geertsma, E.R.; Pos, K.M. AcrB: A Mean, Keen, Drug Efflux Machine. Ann. N. Y. Acad. Sci. 2020, 1459, 38–68. [Google Scholar] [CrossRef] [PubMed]
- Lyu, M.; Moseng, M.A.; Reimche, J.L.; Holley, C.L.; Dhulipala, V.; Su, C.-C.; Shafer, W.M.; Yu, E.W. Cryo-EM Structures of a Gonococcal Multidrug Efflux Pump Illuminate a Mechanism of Drug Recognition and Resistance. mBio 2020, 11, e00996-20. [Google Scholar] [CrossRef] [PubMed]
- Rieg, S.; Huth, A.; Kalbacher, H.; Kern, W.V. Resistance against Antimicrobial Peptides Is Independent of Escherichia Coli AcrAB, Pseudomonas Aeruginosa MexAB and Staphylococcus Aureus NorA Efflux Pumps. Int. J. Antimicrob. Agents 2009, 33, 174–176. [Google Scholar] [CrossRef] [PubMed]
- Warner, D.M.; Levy, S.B. Different Effects of Transcriptional Regulators MarA, SoxS and Rob on Susceptibility of Escherichia Coli to Cationic Antimicrobial Peptides (CAMPs): Rob-Dependent CAMP Induction of the marRAB Operon. Microbiol. Read. Engl. 2010, 156, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Dorschner, R.A.; Lopez-Garcia, B.; Peschel, A.; Kraus, D.; Morikawa, K.; Nizet, V.; Gallo, R.L. The Mammalian Ionic Environment Dictates Microbial Susceptibility to Antimicrobial Defense Peptides. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2006, 20, 35–42. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, R.F.; Thornsberry, C.; Baker, C.N.; Kirven, L.A. Effect of Calcium and Magnesium Ions on the Susceptibility of Pseudomonas Species to Tetracycline, Gentamicin Polymyxin B, and Carbenicillin. Antimicrob. Agents Chemother. 1975, 7, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.L.; Fernando, S.; King, M.D. Escherichia Coli Resistance Mechanism AcrAB-TolC Efflux Pump Interactions with Commonly Used Antibiotics: A Molecular Dynamics Study. Sci. Rep. 2024, 14, 2742. [Google Scholar] [CrossRef] [PubMed]
- Trampari, E.; Prischi, F.; Vargiu, A.V.; Abi-Assaf, J.; Bavro, V.N.; Webber, M.A. Functionally Distinct Mutations within AcrB Underpin Antibiotic Resistance in Different Lifestyles. npj Antimicrob. Resist. 2023, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Ababou, A.; Koronakis, V. Structures of Gate Loop Variants of the AcrB Drug Efflux Pump Bound by Erythromycin Substrate. PloS One 2016, 11, e0159154. [Google Scholar] [CrossRef] [PubMed]
- Tamer, Y.T.; Gaszek, I.; Rodrigues, M.; Coskun, F.S.; Farid, M.; Koh, A.Y.; Russ, W.; Toprak, E. The Antibiotic Efflux Protein TolC Is a Highly Evolvable Target under Colicin E1 or TLS Phage Selection. Mol. Biol. Evol. 2021, 38, 4493–4504. [Google Scholar] [CrossRef]
- Saw, H.T.H.; Webber, M.A.; Mushtaq, S.; Woodford, N.; Piddock, L.J.V. Inactivation or Inhibition of AcrAB-TolC Increases Resistance of Carbapenemase-Producing Enterobacteriaceae to Carbapenems. J. Antimicrob. Chemother. 2016, 71, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Pantel, L.; Guérin, F.; Serri, M.; Gravey, F.; Houard, J.; Maurent, K.; Attwood, M.; Noel, A.; MacGowan, A.; Racine, E.; et al. Exploring Cluster-Dependent Antibacterial Activities and Resistance Pathways of NOSO-502 and Colistin against Enterobacter Cloacae Complex Species. Antimicrob. Agents Chemother. 2022, 66, e00776-22. [Google Scholar] [CrossRef] [PubMed]
- García-Romero, I.; Srivastava, M.; Monjarás-Feria, J.; Korankye, S.O.; MacDonald, L.; Scott, N.E.; Valvano, M.A. Drug Efflux and Lipid A Modification by 4-L-Aminoarabinose Are Key Mechanisms of Polymyxin B Resistance in the Sepsis Pathogen Enterobacter Bugandensis. J. Glob. Antimicrob. Resist. 2024, 37, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Peschel, A.; Vincent Collins, L. Staphylococcal Resistance to Antimicrobial Peptides of Mammalian and Bacterial Origin. Peptides 2001, 22, 1651–1659. [Google Scholar] [CrossRef]
- Durell, S.R.; Guy, H.R. Structural Models of the KtrB, TrkH, and Trk1,2 Symporters Based on the Structure of the KcsA K(+) Channel. Biophys. J. 1999, 77, 789–807. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, A.-B.; Wenzel, M. A How-To Guide for Mode of Action Analysis of Antimicrobial Peptides. Front. Cell. Infect. Microbiol. 2020, 10, 540898. [Google Scholar] [CrossRef] [PubMed]
- Yeaman, M.R.; Bayer, A.S.; Koo, S.P.; Foss, W.; Sullam, P.M. Platelet Microbicidal Proteins and Neutrophil Defensin Disrupt the Staphylococcus Aureus Cytoplasmic Membrane by Distinct Mechanisms of Action. J. Clin. Investig. 1998, 101, 178–187. [Google Scholar] [CrossRef]
- Turcot, I.; Ponnampalam, T.V.; Bouwman, C.W.; Martin, N.L. Isolation and Characterization of a Chromosomally Encoded Disulphide Oxidoreductase from Salmonella Enterica Serovar Typhimurium. Can. J. Microbiol. 2001, 47, 711–721. [Google Scholar] [CrossRef]
- Johns, B.E.; Purdy, K.J.; Tucker, N.P.; Maddocks, S.E. Phenotypic and Genotypic Characteristics of Small Colony Variants and Their Role in Chronic Infection. Microbiol. Insights 2015, 8, 15–23. [Google Scholar] [CrossRef]
- Vesga, O.; Groeschel, M.C.; Otten, M.F.; Brar, D.W.; Vann, J.M.; Proctor, R.A. Staphylococcus Aureus Small Colony Variants Are Induced by the Endothelial Cell Intracellular Milieu. J. Infect. Dis. 1996, 173, 739–742. [Google Scholar] [CrossRef]
- Proctor, R.A.; Peters, G. Small Colony Variants in Staphylococcal Infections: Diagnostic and Therapeutic Implications. Clin. Infect. Dis. 1998, 27, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Proctor, R.A.; Balwit, J.M.; Vesga, O. Variant Subpopulations of Staphylococcus Aureus as Cause of Persistent and Recurrent Infections. Infect. Agents Dis. 1994, 3, 302–312. [Google Scholar] [PubMed]
- Samuelsen, Ø.; Haukland, H.H.; Kahl, B.C.; von Eiff, C.; Proctor, R.A.; Ulvatne, H.; Sandvik, K.; Vorland, L.H. Staphylococcus Aureus Small Colony Variants Are Resistant to the Antimicrobial Peptide Lactoferricin B. J. Antimicrob. Chemother. 2005, 56, 1126–1129. [Google Scholar] [CrossRef] [PubMed]
- Gläser, R.; Becker, K.; von Eiff, C.; Meyer-Hoffert, U.; Harder, J. Decreased Susceptibility of Staphylococcus Aureus Small-Colony Variants toward Human Antimicrobial Peptides. J. Invest. Dermatol. 2014, 134, 2347–2350. [Google Scholar] [CrossRef]
- de la Fuente-Núñez, C.; Korolik, V.; Bains, M.; Nguyen, U.; Breidenstein, E.B.M.; Horsman, S.; Lewenza, S.; Burrows, L.; Hancock, R.E.W. Inhibition of Bacterial Biofilm Formation and Swarming Motility by a Small Synthetic Cationic Peptide. Antimicrob. Agents Chemother. 2012, 56, 2696–2704. [Google Scholar] [CrossRef] [PubMed]
- Thöming, J.G.; Häussler, S. Pseudomonas Aeruginosa Is More Tolerant Under Biofilm Than Under Planktonic Growth Conditions: A Multi-Isolate Survey. Front. Cell. Infect. Microbiol. 2022, 12, 851784. [Google Scholar] [CrossRef] [PubMed]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and Its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef]
- George, J.; Halami, P.M. Presence of Extracellular DNA & Protein in Biofilm Formation by Gentamicin-Resistant Lactobacillus Plantarum. Indian J. Med. Res. 2019, 149, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Datta, S.; Narayanan, K.B.; Rajnish, K.N. Bacterial Exo-Polysaccharides in Biofilms: Role in Antimicrobial Resistance and Treatments. J. Genet. Eng. Biotechnol. 2021, 19, 140. [Google Scholar] [CrossRef]
- Yasir, M.; Willcox, M.D.P.; Dutta, D. Action of Antimicrobial Peptides against Bacterial Biofilms. Materials 2018, 11, 2468. [Google Scholar] [CrossRef]
- Wang, M.; Deng, Z.; Li, Y.; Xu, K.; Ma, Y.; Yang, S.-T.; Wang, J. Antibiofilm Property and Multiple Action of Peptide PEW300 against Pseudomonas Aeruginosa. Front. Microbiol. 2022, 13, 963292. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, L.V.; White, R.L.; Bosso, J.A. Impact of Use of Multiple Antimicrobials on Changes in Susceptibility of Gram-Negative Aerobes. Clin. Infect. Dis. 1999, 28, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Govan, J.R.; Nelson, J.W. Microbiology of Lung Infection in Cystic Fibrosis. Br. Med. Bull. 1992, 48, 912–930. [Google Scholar] [CrossRef] [PubMed]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial Biofilms: From the Natural Environment to Infectious Diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Begun, J.; Gaiani, J.M.; Rohde, H.; Mack, D.; Calderwood, S.B.; Ausubel, F.M.; Sifri, C.D. Staphylococcal Biofilm Exopolysaccharide Protects against Caenorhabditis Elegans Immune Defenses. PLoS Pathog. 2007, 3, e57. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.G.; O’Toole, G.A. Innate and Induced Resistance Mechanisms of Bacterial Biofilms. Curr. Top. Microbiol. Immunol. 2008, 322, 85–105. [Google Scholar] [CrossRef] [PubMed]
- Cloete, T.E.; Westaard, D.; van Vuuren, S.J. Dynamic Response of Biofilm to Pipe Surface and Fluid Velocity. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2003, 47, 57–59. [Google Scholar] [CrossRef][Green Version]
- Gillis, R.J.; White, K.G.; Choi, K.-H.; Wagner, V.E.; Schweizer, H.P.; Iglewski, B.H. Molecular Basis of Azithromycin-Resistant Pseudomonas Aeruginosa Biofilms. Antimicrob. Agents Chemother. 2005, 49, 3858–3867. [Google Scholar] [CrossRef]
- Kvist, M.; Hancock, V.; Klemm, P. Inactivation of Efflux Pumps Abolishes Bacterial Biofilm Formation. Appl. Environ. Microbiol. 2008, 74, 7376–7382. [Google Scholar] [CrossRef]
- Folkesson, A.; Haagensen, J.A.J.; Zampaloni, C.; Sternberg, C.; Molin, S. Biofilm Induced Tolerance towards Antimicrobial Peptides. PLoS ONE 2008, 3, e1891. [Google Scholar] [CrossRef]
- Allesen-Holm, M.; Barken, K.B.; Yang, L.; Klausen, M.; Webb, J.S.; Kjelleberg, S.; Molin, S.; Givskov, M.; Tolker-Nielsen, T. A Characterization of DNA Release in Pseudomonas Aeruginosa Cultures and Biofilms. Mol. Microbiol. 2006, 59, 1114–1128. [Google Scholar] [CrossRef] [PubMed]
- Chiang, W.-C.; Nilsson, M.; Jensen, P.Ø.; Høiby, N.; Nielsen, T.E.; Givskov, M.; Tolker-Nielsen, T. Extracellular DNA Shields against Aminoglycosides in Pseudomonas Aeruginosa Biofilms. Antimicrob. Agents Chemother. 2013, 57, 2352–2361. [Google Scholar] [CrossRef]
- Mulcahy, H.; Charron-Mazenod, L.; Lewenza, S. Extracellular DNA Chelates Cations and Induces Antibiotic Resistance in Pseudomonas Aeruginosa Biofilms. PLoS Pathog. 2008, 4, e1000213. [Google Scholar] [CrossRef]
- Verstraeten, N.; Braeken, K.; Debkumari, B.; Fauvart, M.; Fransaer, J.; Vermant, J.; Michiels, J. Living on a Surface: Swarming and Biofilm Formation. Trends Microbiol. 2008, 16, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Surette, M.G. Swarming Populations of Salmonella Represent a Unique Physiological State Coupled to Multiple Mechanisms of Antibiotic Resistance. Biol. Proced. Online 2003, 5, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Birchler, T.; Seibl, R.; Büchner, K.; Loeliger, S.; Seger, R.; Hossle, J.P.; Aguzzi, A.; Lauener, R.P. Human Toll-like Receptor 2 Mediates Induction of the Antimicrobial Peptide Human Beta-Defensin 2 in Response to Bacterial Lipoprotein. Eur. J. Immunol. 2001, 31, 3131–3137. [Google Scholar] [CrossRef]
- Tomasinsig, L.; Scocchi, M.; Di Loreto, C.; Artico, D.; Zanetti, M. Inducible Expression of an Antimicrobial Peptide of the Innate Immunity in Polymorphonuclear Leukocytes. J. Leukoc. Biol. 2002, 72, 1003–1010. [Google Scholar] [CrossRef]
- Lauth, X.; Babon, J.J.; Stannard, J.A.; Singh, S.; Nizet, V.; Carlberg, J.M.; Ostland, V.E.; Pennington, M.W.; Norton, R.S.; Westerman, M.E. Bass Hepcidin Synthesis, Solution Structure, Antimicrobial Activities and Synergism, and in Vivo Hepatic Response to Bacterial Infections. J. Biol. Chem. 2005, 280, 9272–9282. [Google Scholar] [CrossRef]
- Lindmark, H.; Johansson, K.C.; Stöven, S.; Hultmark, D.; Engström, Y.; Söderhäll, K. Enteric Bacteria Counteract Lipopolysaccharide Induction of Antimicrobial Peptide Genes. J. Immunol. 2001, 167, 6920–6923. [Google Scholar] [CrossRef]
- Sperandio, B.; Regnault, B.; Guo, J.; Zhang, Z.; Stanley, S.L.; Sansonetti, P.J.; Pédron, T. Virulent Shigella Flexneri Subverts the Host Innate Immune Response through Manipulation of Antimicrobial Peptide Gene Expression. J. Exp. Med. 2008, 205, 1121–1132. [Google Scholar] [CrossRef]
- Hamon, M.A.; Batsché, E.; Régnault, B.; Tham, T.N.; Seveau, S.; Muchardt, C.; Cossart, P. Histone Modifications Induced by a Family of Bacterial Toxins. Proc. Natl. Acad. Sci. USA 2007, 104, 13467–13472. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wen, Y.; Li, Z. Clear Victory for Chlamydia: The Subversion of Host Innate Immunity. Front. Microbiol. 2019, 10, 1412. [Google Scholar] [CrossRef] [PubMed]
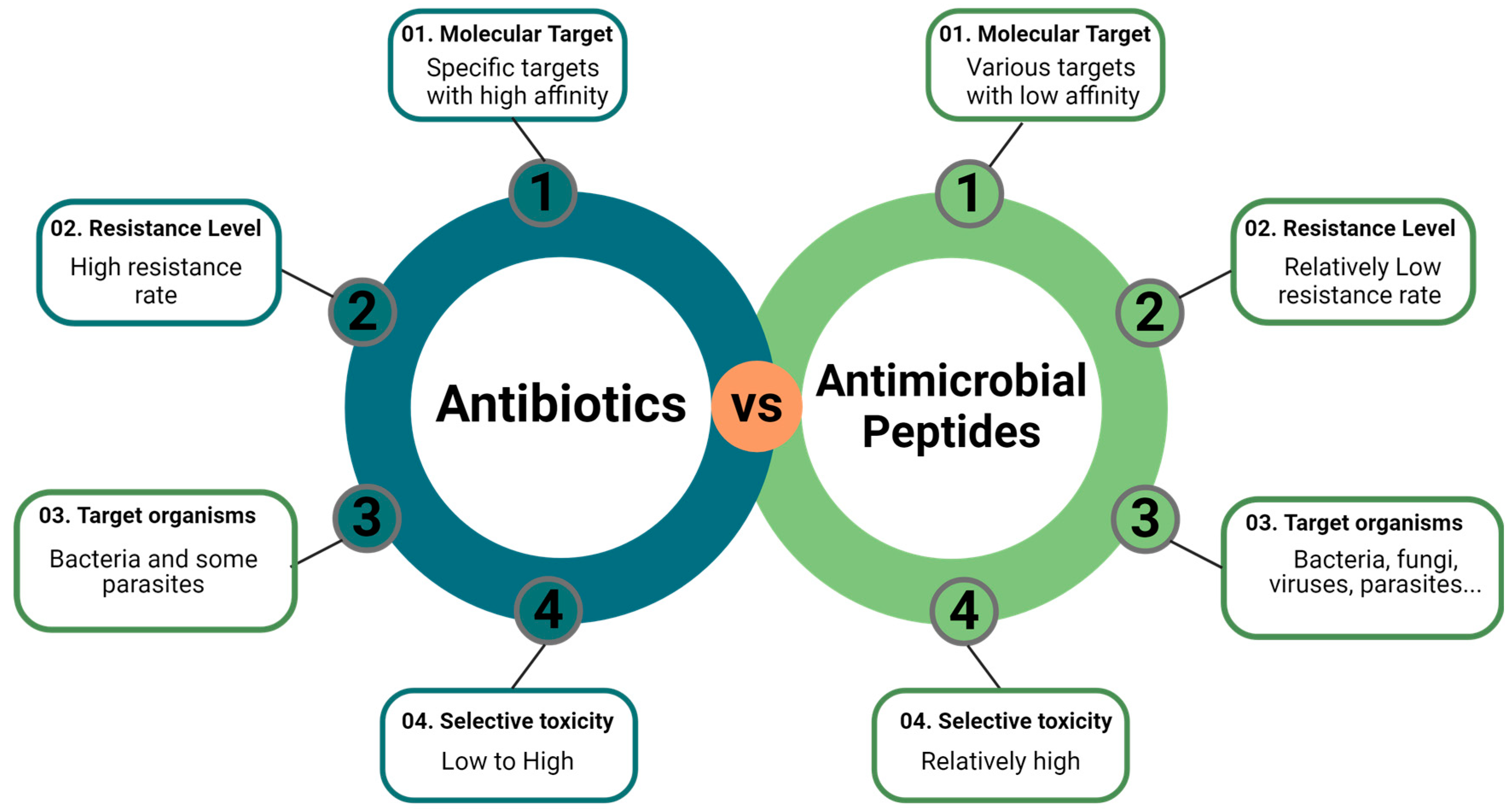

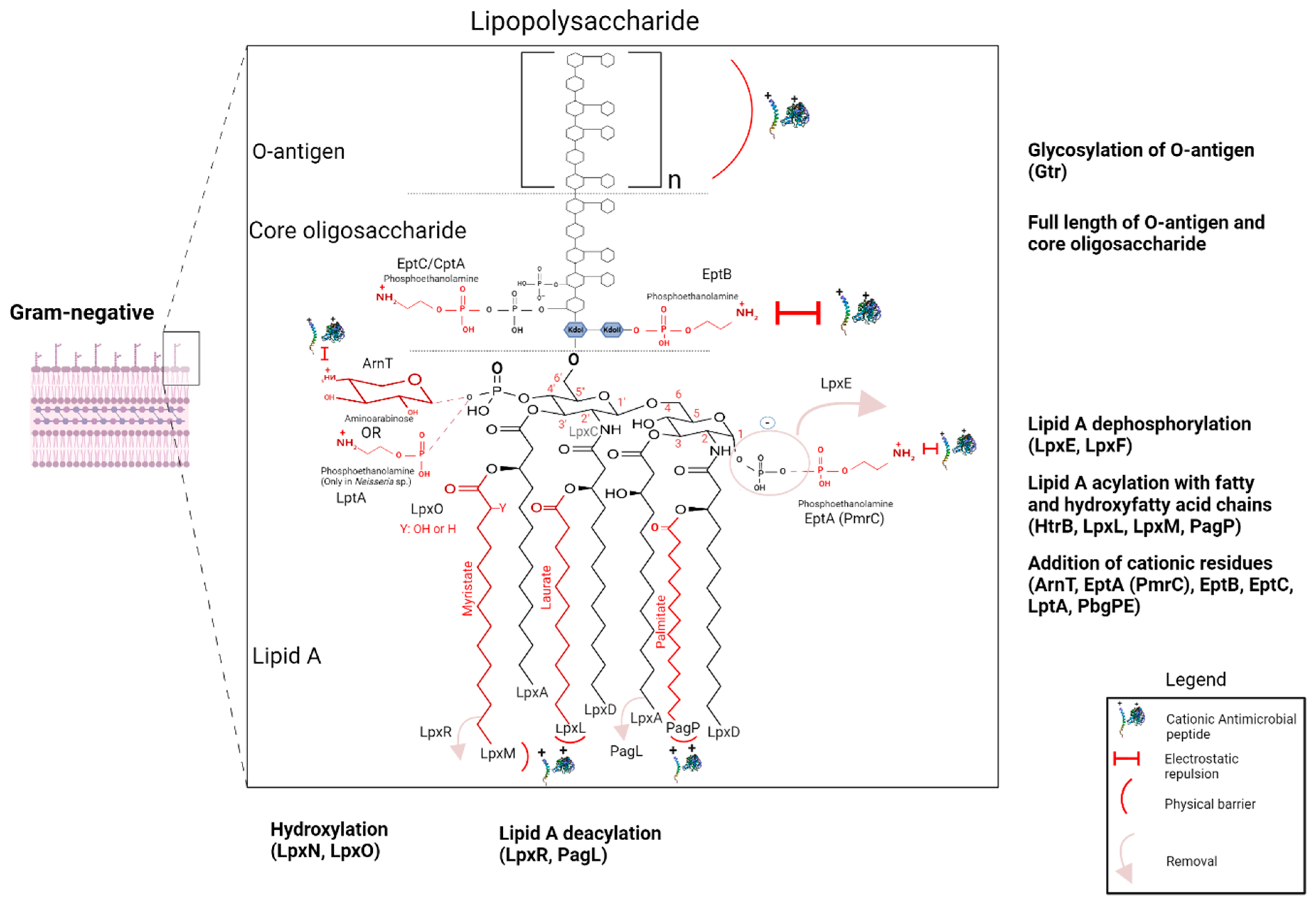

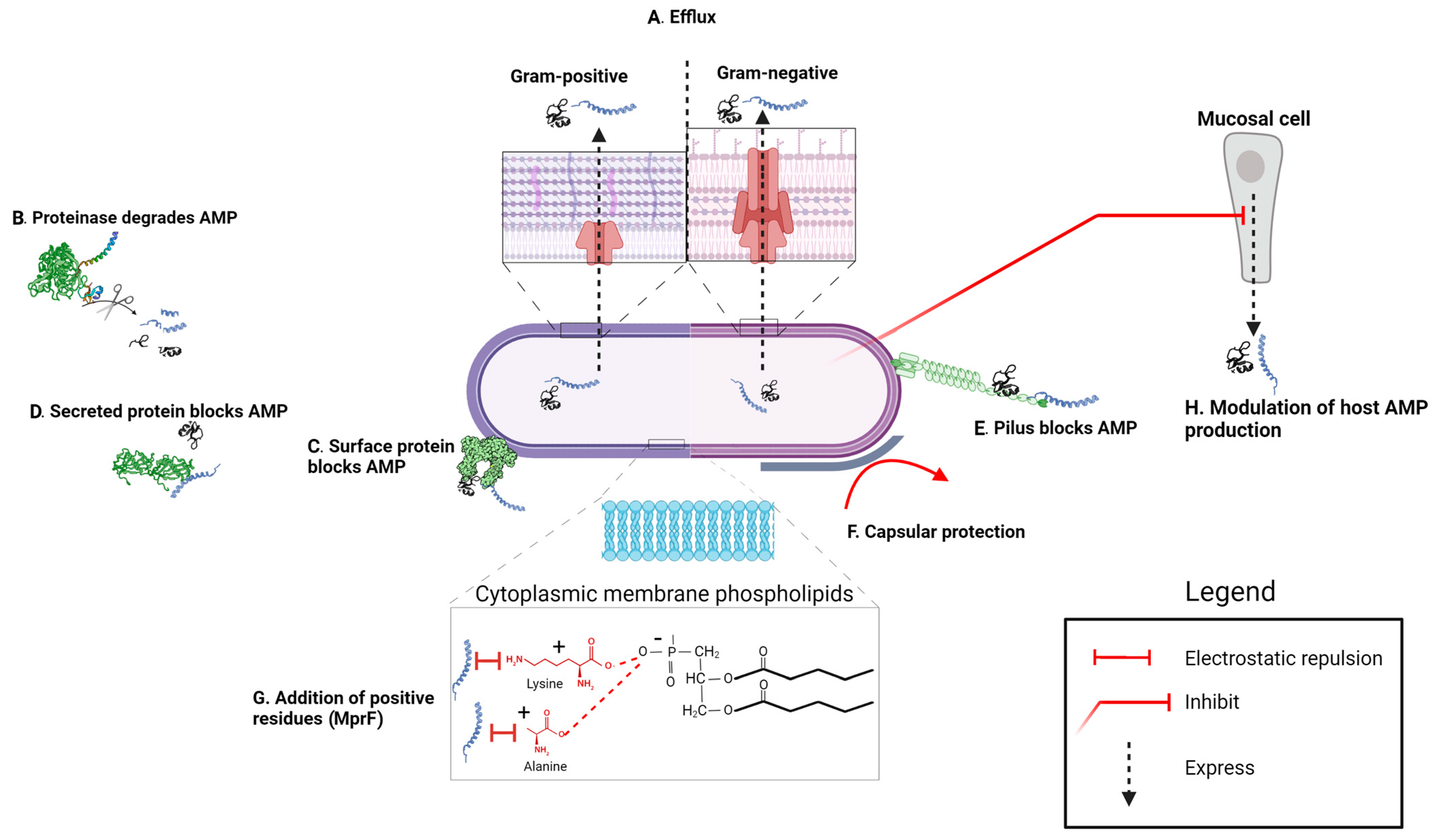
| AMPs | Structure/Charge | Source | Amino Acid Sequence a | Target Bacteria/Indications | Clinical Phase b | Administration | Refs. |
|---|---|---|---|---|---|---|---|
| Polymyxin B | Cyclic/+5 | Bacillus polymyxa | 6-mo-DabTDabDab[γDablLDabDabT] (Polymyxin B1) | MDR G-infections | IV | Topical, oral, IV, ophthalmic, aerosolized | [101,102] |
| Colistin | Cyclic/+5 | Bacillus colistinus | 6-mh-DabTDab[γDablLDabDabT] (Colistin A) | MDR G-infections | IV | Topical, oral, IV | [103,104,105] |
| Nisin | Cyclic/+4 | Lactococcus lactis | ITSISLCTPGCKTGALMGCNMKTATCHCSIHVSK (Nisin A) | Broad spectrum, food preservative | High clinical potential | Undefined; oral or IP in animal models | [106,107,108] |
| Daptomycin | Cyclic/-3 | Streptomyces roseosporus | WNDTGKDADGSEY | G+ skin infections, endocarditis, & bacteremia | IV | IV | [109,110] |
| Gramicidin S | Cyclic/+2 | Brevibacillus brevis | VKLFPVKLFP | Broad spectrum; wound infections, conjunctivitis, genital ulcers | In market | Ophthalmic and topical preparations | [111,112] |
| LTX-109 (Lytixar) | Cyclic/+3 | Synthetic | R-Tbt-R-NH-EtPh | G+ skin infections, anti-MRSA and VRSA | II/III | Topical or nasal | [113,114] |
| Murepavadin (POL708) | Cyclic/+5 | Synthetic | Ala-Ser-D-Pro-Pro-Thr-Trp-Ile-Dab-Orn-D-Dab-Dab-Trp-Dab-Dab | Pseudomonas in cystic fibrosis | III | IV and eFlow® nebulizer system | [115,116] |
| LL-37 (hCAP18) | α-helical/+6 | Human | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | Hard-to-heal venous leg ulcers | II | Wound bed preparations | [117,118,119] |
| Pexiganan (MSI-78) | α-helical/+9 | Synthetic | GIGKFLKKAKKFGKAFVKILKK | Infected diabetic foot ulcers | III | Topical | [119,120,121] |
| Melimine | Random coil c/+15 | Synthetic | TLISWIKNKRKQRPRVSRRRRRRGGRRRR | Contact lens colonizers, anti-biofilm | II/III | Ocular | [122] |
| Omiganan (CLS001 or MBI-226) | Linear c/+5 | Synthetic | ILRWPWWPWRRK | Skin and catheter infections, antisepsis | III | Topical | [123] |
| hLF1-11 | Linear c/+4 | Synthetic | GRRRSVQWCAV | MDR A. baumannii MRSA, Lm, E. coli, and Kp | I/II | IV | [124] |
|
Brilacidin (PMX-30063) | Linear/+4 | Synthetic | Not available since it is a non-peptide arylamide oligomer | Staphylococcus spp. skin infections | II | Topical and mouth rinse | [125] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tajer, L.; Paillart, J.-C.; Dib, H.; Sabatier, J.-M.; Fajloun, Z.; Abi Khattar, Z. Molecular Mechanisms of Bacterial Resistance to Antimicrobial Peptides in the Modern Era: An Updated Review. Microorganisms 2024, 12, 1259. https://doi.org/10.3390/microorganisms12071259
Tajer L, Paillart J-C, Dib H, Sabatier J-M, Fajloun Z, Abi Khattar Z. Molecular Mechanisms of Bacterial Resistance to Antimicrobial Peptides in the Modern Era: An Updated Review. Microorganisms. 2024; 12(7):1259. https://doi.org/10.3390/microorganisms12071259
Chicago/Turabian StyleTajer, Layla, Jean-Christophe Paillart, Hanna Dib, Jean-Marc Sabatier, Ziad Fajloun, and Ziad Abi Khattar. 2024. "Molecular Mechanisms of Bacterial Resistance to Antimicrobial Peptides in the Modern Era: An Updated Review" Microorganisms 12, no. 7: 1259. https://doi.org/10.3390/microorganisms12071259
APA StyleTajer, L., Paillart, J.-C., Dib, H., Sabatier, J.-M., Fajloun, Z., & Abi Khattar, Z. (2024). Molecular Mechanisms of Bacterial Resistance to Antimicrobial Peptides in the Modern Era: An Updated Review. Microorganisms, 12(7), 1259. https://doi.org/10.3390/microorganisms12071259









