Varietal Susceptibility of Olive to Pseudomonas savastanoi pv. savastanoi and the Antibacterial Potential of Plant-Based Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Bacterial Inoculum
2.2. Evaluation of Olive Knot Disease Susceptibility in Greenhouse Experiment
2.3. Preparation of Antibacterial Treatments
2.4. Determination of Total Phenolic Content
2.5. Qualitative Determination of Antibacterial Effect on Bacterial Inoculum
2.6. Quantitative Determination of Antibacterial Effect
2.7. Disease Evaluation and Data Analysis
3. Results
3.1. Greenhouse Experiment
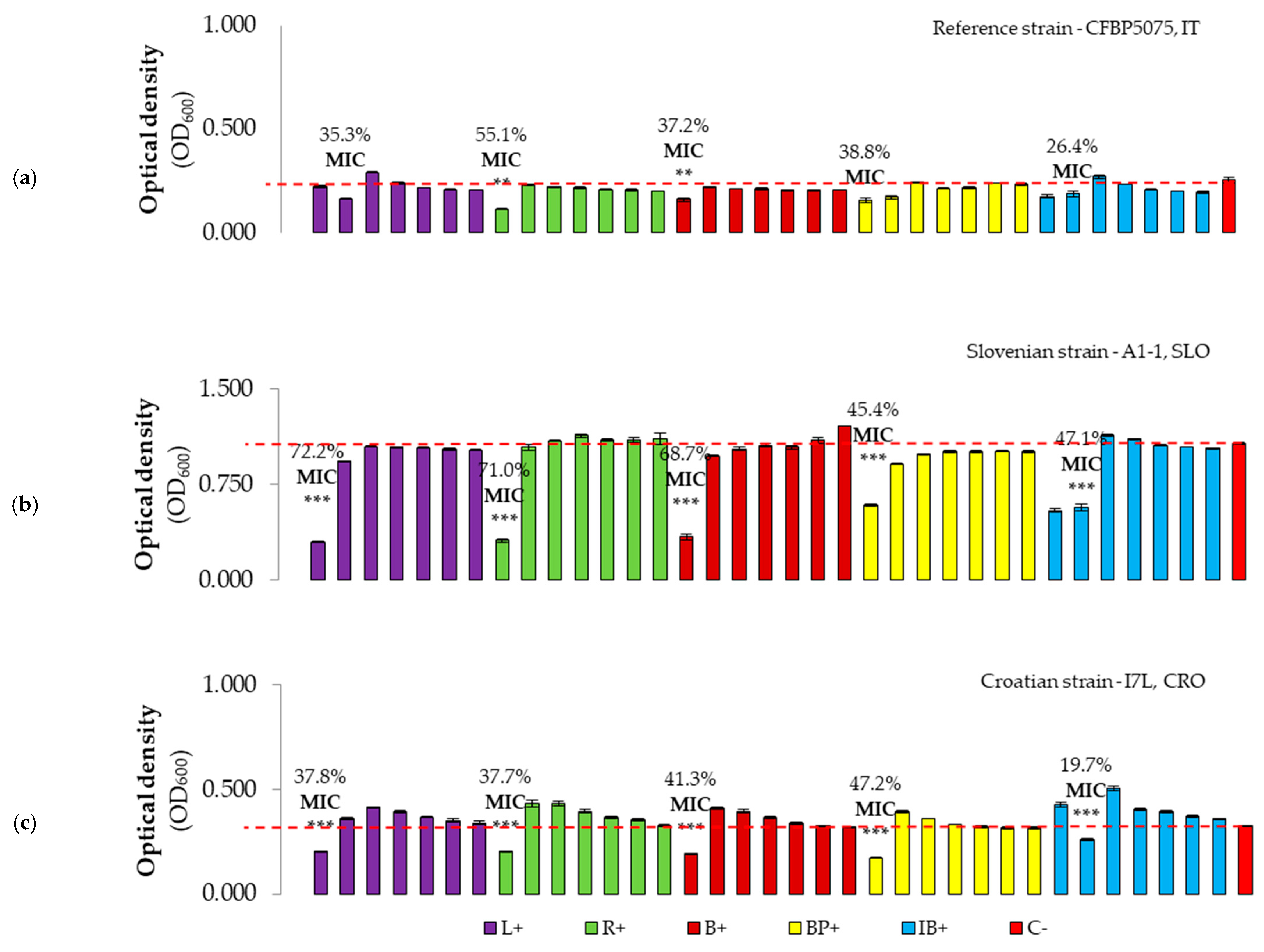
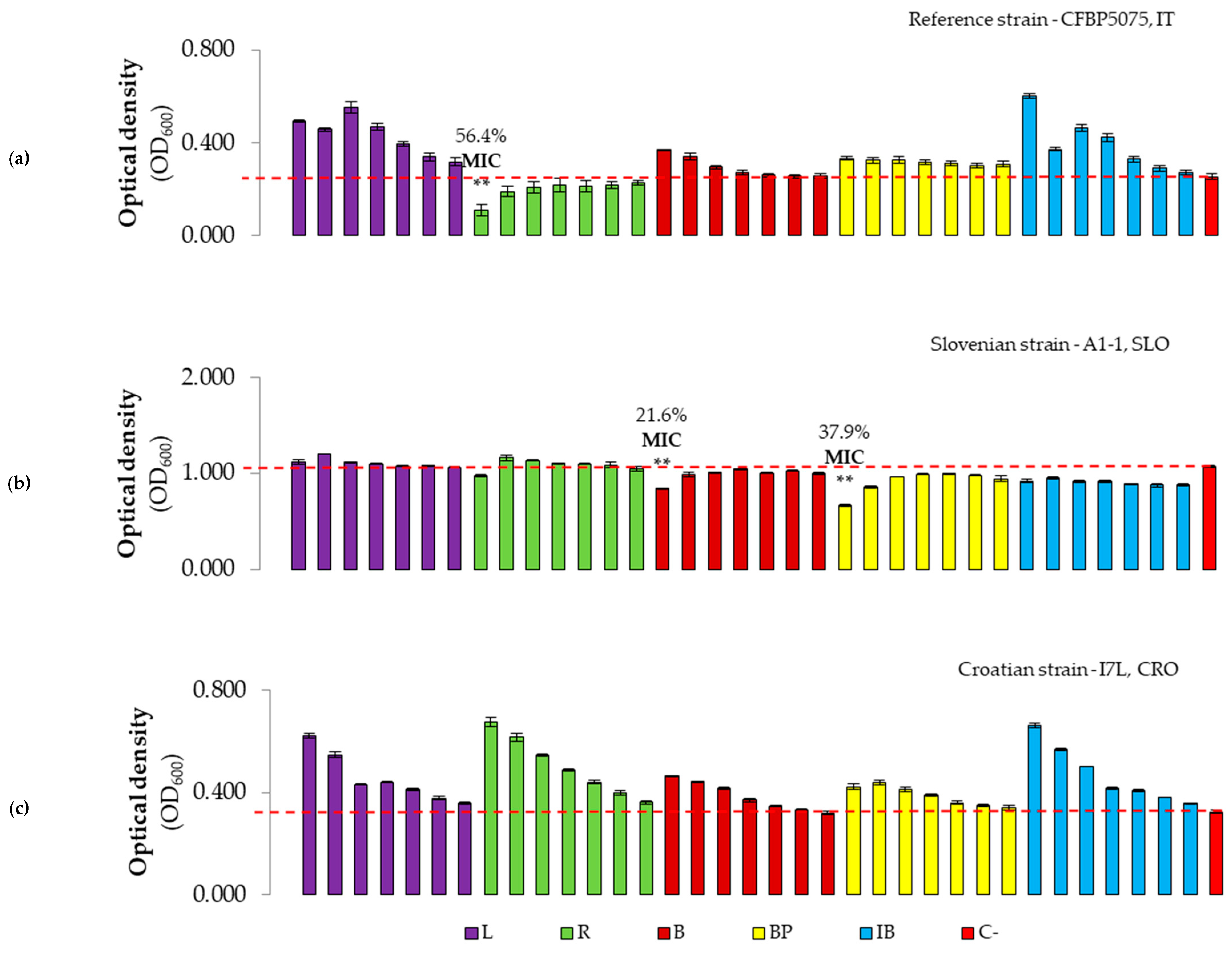
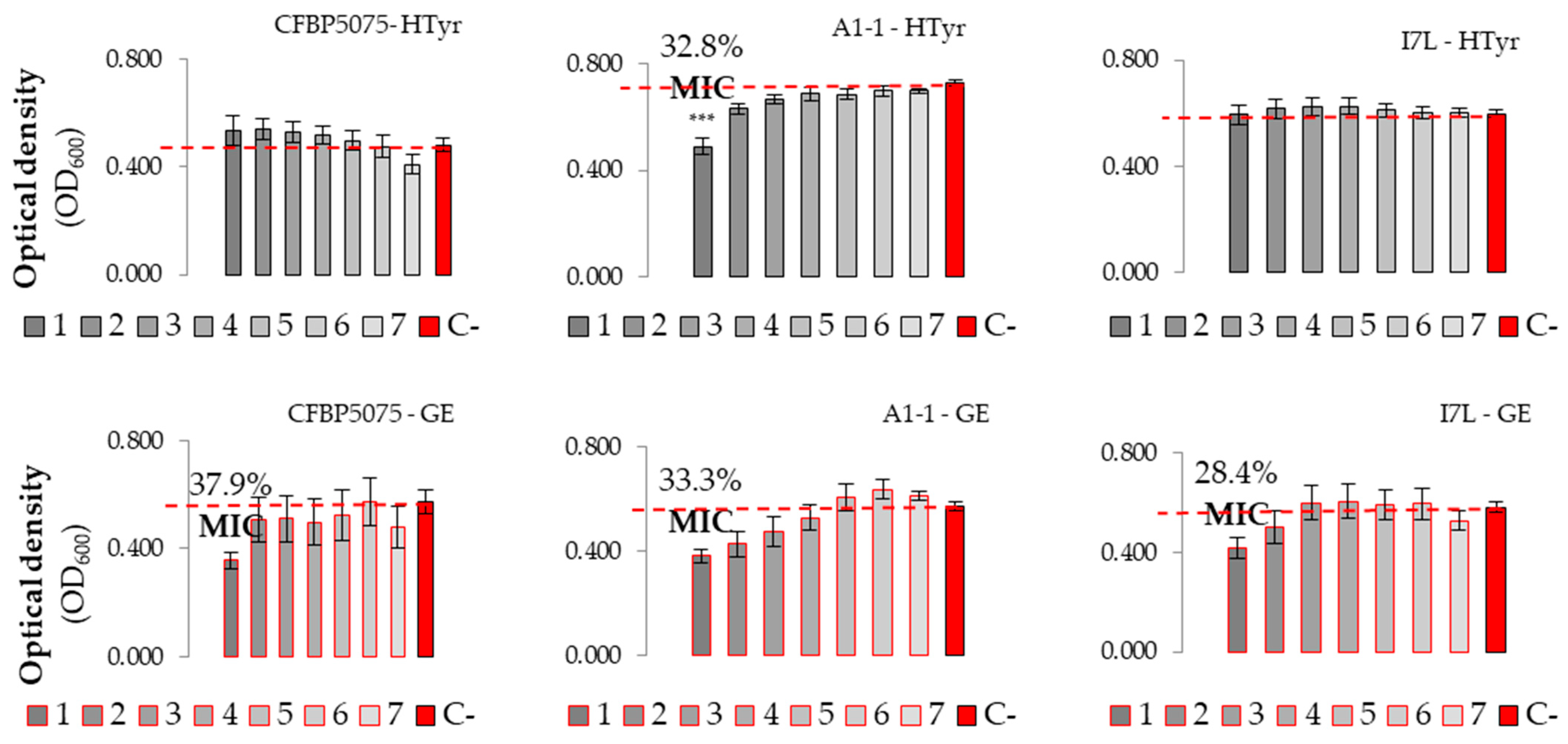
3.2. Antibacterial Efficacy of Plant-Based Antimicrobials
3.2.1. Qualitative Characterization
3.2.2. Evaluation of Antibacterial Effect and MICs of Plant-Based Preparations
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Abbassi, A.; Saadaoui, N.; Kiai, H.; Raiti, J.; Hafidi, A. Potential application of olive mill wastewater as biopesticide for crops protection. Sci. Total Environ. 2017, 576, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Medina, E.; Romero, C.; de los Santos, B.; de Castro, A.; García, A.; Romero, F.; Brenes, M. Antimicrobial Activity of Olive Solutions from Stored Alpeorujo against Plant Pathogenic Microorganisms. J. Agric. Food Chem. 2011, 59, 6927–6932. [Google Scholar] [CrossRef] [PubMed]
- Tegli, S.; Carboneschi, M.; Fatmi, M. Detection of Pseudomonas savastanoi pv. savastanoi in Asymptomatic Olive Plants. In Detection of Plant-Pathogenic Bacteria in Seed and Other Planting Material; Fatmi, M., Walcott, R.R., Schaad, N.W., Eds.; APS Press: St. Paul, MN, USA, 2017; pp. 311–319. [Google Scholar] [CrossRef]
- Mirik, M.; Aysan, Y.; Sahin, F. Characterization of Pseudomonas savastanoi pv. savastanoi strains isolated from several host plants in Turkey and report of fontanesia as a new host. J. Plant Pathol. 2011, 93, 263–270. [Google Scholar]
- Cesari, E.; Marocchi, F.; L’Aurora, A.; Pucci, N.; Scala, V.; Loreti, S.; Scortichini, M. Occurrence of copper-resistant Pseudomonas syringae pv. actinidiae strains in kiwifruit orchards of Central Italy. J. Phytopahol. 2023, 171, 768–774. [Google Scholar] [CrossRef]
- Cameron, A.; Sarojini, V. Pseudomonas syringae pv. actinidiae: Chemical control, resistance mechanisms and possible alternatives. Plant Pathol. 2014, 63, 1–11. [Google Scholar] [CrossRef]
- Caballo-Ponce, E.; Meng, X.; Uzelac, G.; Halliday, N.; Cámara, M.; Licastro, D.; da Silva, D.P.; Ramos, C.; Venturi, V. Quorum Sensing in Pseudomonas savastanoi pv. savastanoi and Erwinia toletana: Role in Virulence and Interspecies Interactions in the Olive Knot. Appl. Environ. Microbiol. 2018, 84, e00950-18. [Google Scholar] [CrossRef] [PubMed]
- Scortichini, M. Predisposing Factors for “Olive Quick Decline Syndrome” in Salento (Apulia, Italy). Agronomy 2020, 10, 1445. [Google Scholar] [CrossRef]
- Moretti, C.; Vinatzer, B.A.; Onofri, A.; Valentini, F.; Buonaurio, R. Genetic and phenotypic diversity of Mediterranean populations of the olive knot pathogen, Pseudomonas savastanoi pv. savastanoi. Plant Pathol 2017, 66, 595–605. [Google Scholar] [CrossRef]
- Ramos, C.; Matas, I.; Bardaji, L.; Aragón, I.M.; Murillo, J. Pseudomonas savastanoi pv. savastanoi: Some like it knot. Mol. Plant Pathol. 2012, 13, 998–1009. [Google Scholar] [CrossRef]
- Sisto, A.; Cipriani, M.G.; Morea, M. Knot Formation Caused by Pseudomonas syringae subsp. savastanoi on Olive Plants Is hrp-Dependent. Phytopathology 2004, 94, 484–489. [Google Scholar] [CrossRef]
- Abuamsha, R.; Kluepfel, D.; McClean, A.; Salman, M. Evaluation of Commercial Olive Accessions for Resistance to the Olive Knot Disease Caused by Pseudomonas savastanoi pv. savastanoi. Arab. J. Sci. Eng. 2023, 49, 87–95. [Google Scholar] [CrossRef]
- Zucchini, M.; Maoloni, A.; Lodolini, E.M.; Ferrocino, I.; Aquilanti, L.; Neri, D. Knot formation and spread along the shoot stem in 13 olive cultivars inoculated with and indigenous pathobiome of 7 species of Pseudomonas including Pseudomonas savastanoi. PLoS ONE 2023, 18, e0289875. [Google Scholar] [CrossRef] [PubMed]
- Licciardello, G.; Mosca, A.; Di Silvestro, S.; Puglisi, D.; Russo, M.P.; Catara, V.; Caruso, P. Cultivar Susceptibility to Olive Knot Disease and Association with Endophytic Microbiota Community. Agronomy 2023, 13, 468. [Google Scholar] [CrossRef]
- Vuletin Selak, G.; Raboteg Božiković, M.; Abrouk, D.; Bolčić, M.; Žanić, K.; Perica, S.; Normand, P.; Pujić, P. Pseudomonas ST1 and Pantoea Paga Strains Cohabit in Olive Knots. Microorganisms 2022, 10, 1528. [Google Scholar] [CrossRef] [PubMed]
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential Oils as Antimicrobial Agents—Myth or Real Alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef] [PubMed]
- Magryś, A.; Olender, A.; Tchórzewska, D. Antibacterial properties of Allium sativum L. against the most emerging multiidrug-resistant bacteria and its synergy with antibiotics. Arch. Microbiol. 2021, 203, 2257–2268. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Ma, X.; Deng, L.; Zhao, X.; Wei, Y.; Gao, Z.; Jia, J.; Xu, J.; Sun, C. Fresh Garlic Extract Enhances the Antimicrobial Activities of Antibiotics on Resistant Strains In Vitro. Jundishapur J. Microbiol. 2015, 8, e14814. [Google Scholar] [CrossRef] [PubMed]
- Kshirsagar, M.M.; Dodamani, A.S.; Karibasappa, G.N.; Vishwakarma, P.K.; Vathar, J.B.; Sonawane, K.R.; Jadhav, H.C.; Khobragade, V.R. Antibacterial activity of garlic extract on cariogenic bacteria: An in vitro study. Ayu 2018, 39, 165–168. [Google Scholar] [CrossRef]
- Morillo, J.A.; Antizar-Ladislao, B.; Monteoliva-Sánchez, M.; Ramos-Cormenzana, A.; Russell, N.J. Bioremediation and biovalorisation of olive-mill wastes. Appl. Microbiol. Biotechnol. 2009, 82, 25–39. [Google Scholar] [CrossRef]
- Obied, H.K.; Bedgood, D.R., Jr.; Prenzler, P.D.; Robards, K. Bioscreening of Australian olive mill waste extracts: Biophenol content, antioxidant, antimicrobial and molluscicidal activities. Food Chem. Toxicol. 2007, 45, 1238–1248. [Google Scholar] [CrossRef]
- Xie, P.; Cecchi, L.; Bellumori, M.; Balli, D.; Giovannelli, L.; Huang, L.; Mulinacci, N. Phenolic Compounds and Triterpenes in Different Olive Tissues and Olive Oil By-Products, and Cytotoxicity on Human Colorectal Cancer Cells: The Case of Frantoio, Moraiolo and Leccino Cultivars (Olea europaea L.). Foods 2021, 10, 2823. [Google Scholar] [CrossRef] [PubMed]
- Brenes, M.; García, A.; Santos, B.; Medina, E.; Romero, C.; de Castro, A.; Romero, F. Olive glutaraldehyde-like compounds against plant pathogenic bacteria and fungi. Food Chem. 2011, 125, 1262–1266. [Google Scholar] [CrossRef]
- Yakhlef, W.; Arhab, R.; Romero, C.; Brenes, M.; de Castro, A.; Medina, E. Phenolic composition and antimicrobial activity of Algerian olive products and by-products. Food Sci. Technol. 2018, 98, 323–328. [Google Scholar] [CrossRef]
- Ciafardini, G.; Zullo, B.A. Antimicrobial activity of oil-mill waste water polyphenols on the phytopathogen Xanthomonas campestris spp. Ann. Microbiol. 2003, 53, 283–290. [Google Scholar]
- Hahlbrock, K.; Scheel, D. Physiology and molecular biology of phenylpropanoid metabolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 347–369. [Google Scholar] [CrossRef]
- Medina-Martínez, M.S.; Truchado, P.; Castro-Ibáñez, I.; Allende, A. Antimicrobial activity of hydroxytyrosol: A current controversy. Biosci. Bitech. Biochem. 2016, 80, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Košćak, L.; Lamovšek, J.; Đermić, E.; Tegli, S.; Gruntar, I.; Godena, S. Identification and Characterisation of Pseudomonas savastanoi pv. savastanoi as the Causal Agent of Olive Knot Disease in Croatian, Slovenian and Portuguese Olive (Olea europaea L.) Orchards. Plants 2023, 12, 307. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Methods for Determining Bactericidal Activity of Antimicrobial Agents; Approved Guideline. CLSI Document M26-A; Clinical Laboratory Standards Institute: Wayne, PA, USA, 1999. [Google Scholar]
- Russo, E.; Spallarossa, A.; Comite, A.; Pagliero, M.; Guida, V.; Belotti, V.; Caviglia, D.; Schito, A.M. Valorization and Potential Antimicrobial Use of Olive Mill Wastewater (OMW) from Italian Olive Oil Production. Antioxidants 2022, 11, 903. [Google Scholar] [CrossRef] [PubMed]
- Bryan-Thomas, J.; McClear, T.; Omoregie, S. Antimicrobial potential of unstressed and heat stressed Allium sativum. Saudi J. Biol. Sci. 2023, 30, 103749. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Hudzicki, J. Kirby-Bauer Disk Diffusion Susceptibility Test Protocol; American Society for Microbiology: Washington, DC, USA, 2009; pp. 1–23. [Google Scholar]
- Aires, A.; Mota, V.R.; Saavedra, M.J.; Monteiro, A.A.; Simões, M.; Rosa, E.A.S.; Bennett, R.N. Initial in vitro evaluations of the antibacterial activities of glucosinolate enzymatic hydrolysis products against plant pathogenic bacteria. J. Appl. Microbiol. 2009, 106, 2096–2105. [Google Scholar] [CrossRef] [PubMed]
- Pasković, I.; Lukić, I.; Žurga, P.; Majetić Germek, V.; Brkljača, M.; Koprivnjak, O.; Major, N.; Grozić, K.; Franić, M.; Ban, D.; et al. Temporal Variation of Phenolic and Mineral Composition in Olive Leaves Is Cultivar Dependent. Plants 2020, 9, 1099. [Google Scholar] [CrossRef] [PubMed]
- Godena, S.; Dminić, I.; Đermić, E. Differential Susceptibility of olive varieties to olive knot disease in Istria. J. Cent. Eur. Agric. 2012, 13, 85–94. [Google Scholar] [CrossRef]
- Hanani, A.; Valentini, F.; Sanzai, S.M.; Santoro, F.; Minutillo, S.A.; Gallo, M.; Cavallo, G.; Mourou, M.; El Moujabber, M.; D’Onghia, A.M.; et al. Community analysis of culturable sapwood endophytes from Apulian olive varieties with different susceptibility to Xylella fastidiosa. Agronomy 2021, 12, 9. [Google Scholar] [CrossRef]
- Petrović, E.; Vrandečić, K.; Ćosić, J.; Kanižai Šarić, G.; Godena, S. First Report of Phaeoacremonium iranianum Causing Olive Twig and Branch Dieback. Plants 2022, 11, 3578. [Google Scholar] [CrossRef]
- Ivić, D.; Petrović, E.; Godena, S. Fungi associated with canker diseases on olive in Istria (Croatia). J. Cent. Eur. Agric. 2023, 24, 470–475. [Google Scholar] [CrossRef]
- Pérez, M.; Dominguez-López, I.; Lamuela-Raventós, R.M. The Chemistry Behind the Folin-Ciocalteu Method for the Estimation of (Poly)phenol Content in Food: Total Phenolic Intake in a Mediterranean Dietary Pattern. J. Agric. Food Chem. 2023, 71, 17543–17553. [Google Scholar] [CrossRef] [PubMed]
- Krid, S.; Bouaziz, M.; Triki, M.A.; Gargouri, A.; Rhouma, A. Inhibition of olive knot disease by polyphenols extracted from olive mill waste waters. J. Plant Pathol. 2011, 93, 561–568. [Google Scholar]
- Mandal, S.M.; Chakraborty, D.; Dey, S. Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal. Behavior. 2010, 5, 359–368. [Google Scholar] [CrossRef]
- Eloff, J.N. Avoiding pitfalls in determining antimicrobial activity of plant extracts and publishing the results. BMC Complement. Altern. Med. 2019, 22, 106. [Google Scholar] [CrossRef]
- Bubonja-Šonje, M.; Knežević, M.; Abram, M. Challenges to antimicrobial susceptibility testing of plant-derived polyphenolic compounds. Arch. Ind. Hyg. Toxicol. 2020, 71, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Novy, P.; Kloucek, P.; Rondevaldova, J.; Havlik, J.; Kourimska, L.; Kokoska, L. Thymoquinone vapor significantly affects the results of Staphylococcus aureus sensitivity tests using the standard broth microdilution method. Fitoterapia 2014, 94, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Reiter, J.; Levina, N.; van der Linden, M.; Gruhlke, M.; Martin, C.; Slusarenko, A.J. Diallylthiosulfinate (Allicin), a Volatile Antimicrobial from Garlic (Allium sativum), Kills Human Lung Pathogenic Bacteria, Including MDR Strains, as a Vapor. Molecules 2017, 22, 1711. [Google Scholar] [CrossRef] [PubMed]
- Pannucci, E.; Caracciolo, R.; Romani, A.; Cacciola, F.; Dugo, P.; Bernini, R.; Varvaro, L.; Santi, L. An hydroxytyrosol enriched extract from olive mill wastewater exerts antioxidant activity and antimicrobial activity on Pseudomonas savastanoi pv. savastanoi and Agrobacterium tumefaciens. Nat. Prod. Res. 2019, 35, 2677–2684. [Google Scholar] [CrossRef] [PubMed]
- Capasso, R.; Evidente, A.; Schivo, L.; Orru’, G.; Marcialis, M.A.; Cristinzio, G. Antibacterial polyphenols from olive oil mill waste waters. J. Appl. Bacteriol. 1995, 79, 393–398. [Google Scholar] [CrossRef]
- Ashraf, S.; Chatha, M.A.; Ejaz, W.; Janjua, H.A.; Hussain, I. Lysozyme-coated silver nanoparticles for differentiating bacterial strains on the basis of antibacterial activity. Nanoscale Res. Lett. 2014, 9, 565. [Google Scholar] [CrossRef]

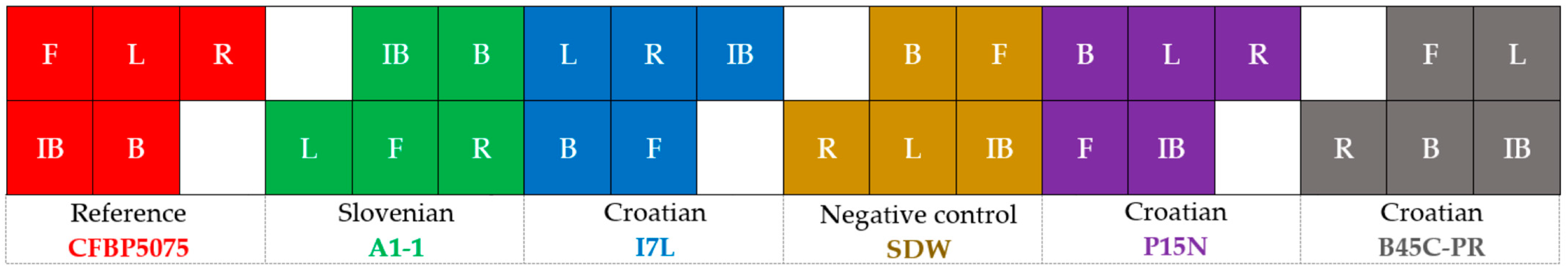



| Type of Antimicrobial | Antimicrobial | Code | pH | TPC, mg GAE/mL | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial Concentration * | Dilutions ** | ||||||||||
| 1:2 (C1) | 1:4 (C2) | 1:8 (C3) | 1:16 (C4) | 1:32 (C5) | 1:64 (C6) | 1:128 (C7) | |||||
| OMWW | Istarska bjelica | IB | 5.26 | 0.757 ± 0.013 | 0.379 | 0.189 | 0.095 | 0.047 | 0.024 | 0.012 | 0.006 |
| Leccino | L | 6.06 | 0.327 ± 0.000 | 0.164 | 0.082 | 0.041 | 0.020 | 0.010 | 0.005 | 0.003 | |
| Rosinjola | R | 6.66 | 0.220 ± 0.014 | 0.110 | 0.055 | 0.028 | 0.014 | 0.007 | 0.003 | 0.002 | |
| Buža | C | 6.00 | 0.140 ± 0.007 | 0.070 | 0.035 | 0.018 | 0.009 | 0.004 | 0.002 | 0.001 | |
| Buža puntoža | BP | 7.17 | 0.046 ± 0.002 | 0.023 | 0.012 | 0.006 | 0.003 | 0.001 | 0.001 | 0.000 | |
| OMWW acidified to pH = 2.0 | Istarska bjelica | IB+ | 2.00 | 0.744 ± 0.017 | 0.372 | 0.186 | 0.093 | 0.047 | 0.023 | 0.012 | 0.006 |
| Leccino | L+ | 2.00 | 0.252 ± 0.003 | 0.126 | 0.063 | 0.032 | 0.016 | 0.008 | 0.004 | 0.002 | |
| Rosinjola | R+ | 2.00 | 0.156 ± 0.001 | 0.078 | 0.039 | 0.020 | 0.010 | 0.005 | 0.002 | 0.001 | |
| Buža | B+ | 2.00 | 0.101 ± 0.005 | 0.051 | 0.025 | 0.013 | 0.006 | 0.003 | 0.002 | 0.001 | |
| Buža puntoža | BP+ | 2.00 | 0.035 ± 0.000 | 0.018 | 0.009 | 0.004 | 0.002 | 0.001 | 0.001 | 0.000 | |
| Garlic extract | Aqueous extract of Garlic | GE | n.d. | 0.064 ± 0.001 | 0.032 | 0.016 | 0.008 | 0.004 | 0.002 | 0.001 | 0.001 |
| Pure phenol | Solution of Hydroxytyrosol in water | HTyr | n.d. | 2.50 | 2.500 | 1.250 | 0.625 | 0.313 | 0.156 | 0.078 | 0.039 |
| 1.25 | |||||||||||
| Standard antibiotic | Tetracycline | AB | n.d. | 0.50 | |||||||
| Cu-based preparation | Copper (I) oxide (Nordox 75 WG) | Cu2O | n.d. | 2.00 | |||||||
| P. savastanoi pv. savastanoi (S) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Olive Cultivar (C) | REF CFBP5075 | SLO A1-1 | CRO I7L | CRO P15N | CRO B45C-PR | Average per Olive Cultivar | Cluster * | C × S | |
| Width (mm) | Frantoio | 7.14 ± 0.44 h–j | 5.53 ± 0.50 ij | 13.85 ± 0.54 a–c | 10.68 ± 0.54 d–g | 6.98 ± 0.57 h–j | 8.84 ± 0.29 | 2 | *** |
| Leccino | 9.50 ± 0.64 f–h | 9.58 ± 0.74 e–h | 11.47 ± 0.72 c–f | 11.53 ± 0.44 c–f | 7.02 ± 0.65 h–j | 9.82 ± 0.30 | 2 | ||
| Rosinjola | 12.41 ± 0.95 b–f | 14.73 ± 0.71 ab | 16.11 ± 0.75 a | 13.05 ± 0.74 a–d | 12.65 ± 0.83 b–e | 13.79 ± 0.36 | 3 | ||
| Buža | 7.78 ± 0.57 g–j | 7.30 ± 0.49 h–j | 10.59 ± 0.52 d–g | 7.47 ± 0.53 h–j | 7.04 ± 0.55 h–j | 8.04 ± 0.25 | 2 | ||
| Istarska bjelica | 7.21 ± 0.46 h–j | 5.13 ± 0.36 j | 8.34 ± 0.36 g–i | 6.13 ± 0.44 ij | 6.91 ± 0.38 h–j | 6.74 ± 0.19 | 2 | ||
| Average per Pss strain | 8.81 ± 0.31 | 8.45 ± 0.33 | 12.07 ± 0.31 | 9.77 ± 0.29 | 8.12 ± 0.30 | ||||
| Length (mm) | Frantoio | 14.73 ± 0.85 c–f | 10.22 ± 0.94 f–j | 21.44 ± 0.59 ab | 16.75 ± 0.85 c–e | 8.21 ± 0.74 j | 14.27 ± 0.45 | 3 | *** |
| Leccino | 14.11 ± 0.95 d–g | 15.71 ± 1.17 c–e | 16.60 ± 1.06 c–e | 17.17 ± 0.65 b–e | 8.94 ± 0.93 ij | 14.51 ± 0.46 | 3 | ||
| Rosinjola | 17.49 ± 1.30 b–e | 22.87 ± 1.05 a | 22.57 ± 0.97 a | 19.39 ± 0.88 a–c | 14.43 ± 1.08 d–f | 19.35 ± 0.51 | 4 | ||
| Buža | 13.80 ± 0.94 d–h | 14.06 ± 0.92 d–h | 17.90 ± 0.81 b–d | 10.88 ± 0.92 f–j | 8.18 ± 0.75 j | 12.96 ± 0.43 | 3 | ||
| Istarska bjelica | 13.03 ± 0.85 e–i | 9.62 ± 0.87 g–j | 14.89 ± 0.64 c–f | 8.65 ± 0.77 ij | 9.43 ± 0.68 h–j | 11.12 ± 0.37 | 3 | ||
| Average per Pss strain | 14.63 ± 0.45 | 14.50 ± 0.52 | 18.68 ± 0.41 | 14.57 ± 0.43 | 9.84 ± 0.40 | ||||
| Surface area (mm2) | Frantoio | 124.49 ± 9.59 e–g | 82.04 ± 10.19 g–i | 309.99 ± 17.53 b | 203.26 ± 15.65 cd | 79.37 ± 9.14 hi | 159.83 ± 7.64 | *** | |
| Leccino | 166.34 ± 15.43 de | 197.96 ± 19.13 cd | 232.56 ± 17.62 c | 212.39 ± 12.63 c | 95.21 ± 12.16 f–i | 180.89 ± 7.48 | |||
| Rosinjola | 285.65 ± 25.65 b | 377.97 ± 23.07 a | 401.56 ± 23.38 a | 287.29 ± 22.48 b | 230.67 ± 25.94 c | 316.63 ± 11.34 | |||
| Buža | 134.54 ± 11.42 ef | 126.45 ± 11.43 ef | 211.68 ± 11.12 c | 105.89 ± 11.25 f–i | 78.79 ± 9.26 hi | 131.47 ± 5.49 | |||
| Istarska bjelica | 115.02 ± 9.16 f–h | 65.42 ± 6.98 i | 136.15 ± 8.35 ef | 70.65 ± 7.33 i | 77.91 ± 7.49 hi | 93.03 ± 3.86 | |||
| Average per Pss strain | 165.21 ± 7.78 | 169.97 ± 9.48 | 258.39 ± 9.00 | 175.90 ± 7.96 | 112.39 ± 7.29 | ||||
| Group of Treatments | Treatment | Growth Inhibition of P. savastanoi pv. savastanoi Strains (Clearing Zone, mm) | ||
|---|---|---|---|---|
| REF CFBP5075 | SLO A1-1 | CRO I7L | ||
| OMWWs | non-acidified and acidified | 0.00 ± 0.00 x | 0.00 ± 0.00 x | 0.00 ± 0.00 x |
| Phenol | Hydroxytyrosol (HTyr): 2.5 mg/mL | 11.64 ± 3.15 + | 10.13 ± 1.12 + | 11.26 ± 1.60 + |
| 1.25 mg/mL | 7.03 ± 2.32 + | 9.19 ± 0.92 + | 9.57 ± 1.14 + | |
| Aqueous extract | Garlic (GE) | 16.17 ± 2.36 + | 16.30 ± 1.85 + | 17.80 ± 1.71 + |
| Copper-based Treatment | Copper (I) oxide | 19.00 ± 0.85 | 18.81 ± 1.03 | 17.84 ± 2.93 |
| Antibiotic | Tetracycline | 25.49 ± 0.84 | 25.45 ± 1.17 | 25.57 ± 0.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Košćak, L.; Lamovšek, J.; Lukić, M.; Kovačević, T.K.; Đermić, E.; Goreta Ban, S.; Major, N.; Godena, S. Varietal Susceptibility of Olive to Pseudomonas savastanoi pv. savastanoi and the Antibacterial Potential of Plant-Based Agents. Microorganisms 2024, 12, 1301. https://doi.org/10.3390/microorganisms12071301
Košćak L, Lamovšek J, Lukić M, Kovačević TK, Đermić E, Goreta Ban S, Major N, Godena S. Varietal Susceptibility of Olive to Pseudomonas savastanoi pv. savastanoi and the Antibacterial Potential of Plant-Based Agents. Microorganisms. 2024; 12(7):1301. https://doi.org/10.3390/microorganisms12071301
Chicago/Turabian StyleKošćak, Laura, Janja Lamovšek, Marina Lukić, Tvrtko Karlo Kovačević, Edyta Đermić, Smiljana Goreta Ban, Nikola Major, and Sara Godena. 2024. "Varietal Susceptibility of Olive to Pseudomonas savastanoi pv. savastanoi and the Antibacterial Potential of Plant-Based Agents" Microorganisms 12, no. 7: 1301. https://doi.org/10.3390/microorganisms12071301






