Insights into Chlamydia Development and Host Cells Response
Abstract
1. Introduction
2. The Biphasic Reproductive Cycle of Chlamydia
2.1. The Genomic Characteristics of Chlamydia
2.2. The Reproduction Mode of Chlamydia
2.3. The Morphological and Physiological Differences of EBs and RBs
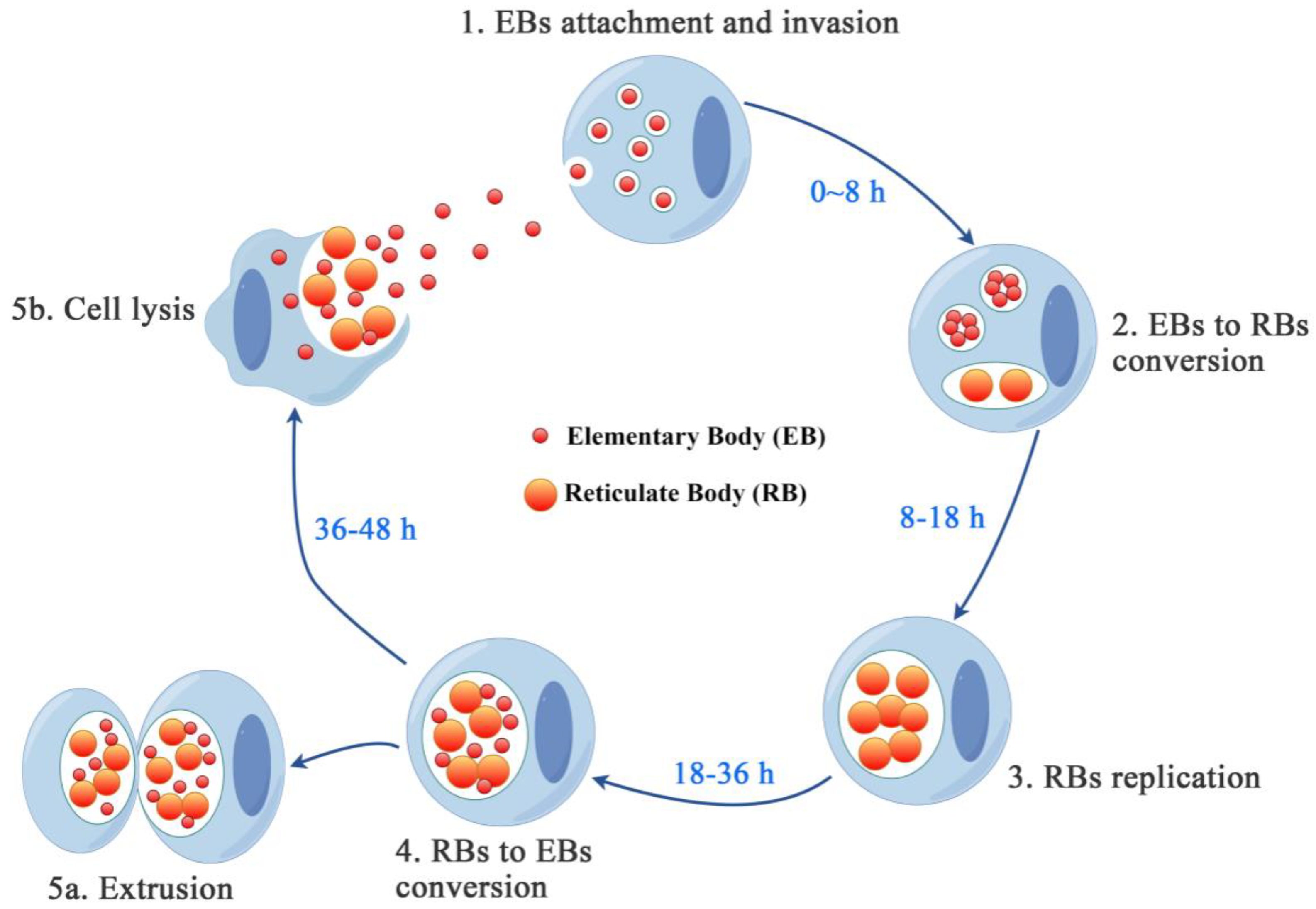
2.4. The Biphasic Developmental Trajectory of Chlamydia
2.4.1. EBs Adhesion
2.4.2. EBs Entry
2.4.3. EBs to RBs Conversion
2.4.4. RBs to EBs Transition
2.4.5. EBs Release
3. Nutrient Acquisition and Intracellular Metabolism of Chlamydia
3.1. Nutrients Acquisition
3.2. Intracellular Metabolism
4. Pro-Death Effects of Chlamydia
4.1. Exit of Host Cells
4.2. Cytotoxicity of Chlamydia
5. Host Cells Defend against Chlamydia
5.1. Cell Fates
5.1.1. Apoptosis [72]
5.1.2. Necroptosis
5.1.3. Autophagy
5.1.4. Pyroptosis [72] (Figure 2)
5.2. Anti-Death Strategies of Host Cells
5.2.1. The Pro-Survival Pathways
5.2.2. The Anti-Apoptosis Pathways
5.3. Anti-Chlamydia Immune Response of Host
5.3.1. IFNgama (IFNγ)
5.3.2. Cell-Autonomous Immunity
5.3.3. Innate Immune Response
5.3.4. Adaptive Immune Response
6. The Prevailing Disease, Treatment, and Prevention of Chlamydia
6.1. The Prevailing Disease of Chlamydia
6.1.1. C. trachomatis and Trachoma
6.1.2. C. psittaci and Psittacosis
6.1.3. C. pneumoniae and Pneumonia
6.2. Anti-Chlamydia Drugs
6.2.1. Tetracycline Antibiotics [129,130]
6.2.2. Macrolide Antibiotics [133,134]
6.2.3. Quinolone Antibiotics [132]
6.2.4. Rifamycin Class of Antibiotics [137]
6.2.5. Aminoglycoside Antibiotic
6.2.6. β-Lactam Antibiotics [138]
6.3. Vaccine
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laroucau, K.; Ortega, N.; Vorimore, F.; Aaziz, R.; Mitura, A.; Szymanska-Czerwinska, M.; Cicerol, M.; Salinas, J.; Sachse, K.; Caro, M.R. Detection of a novel Chlamydia species in captive spur-thighed tortoises (Testudo graeca) in southeastern Spain and proposal of Candidatus Chlamydia testudinis. Syst. Appl. Microbiol. 2020, 43, 126071. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, P.; Hou, J.; Xu, G.; Zhang, J.; Lei, Y.; Lou, Z.; Liang, L.; Wen, Y.; Zhou, J. Detection of Chlamydia psittaci and Chlamydia ibidis in the Endangered Crested Ibis (Nipponia nippon). Epidemiol. Infect. 2020, 148, e1. [Google Scholar] [CrossRef] [PubMed]
- Grieshaber, S.; Grieshaber, N.; Yang, H.; Baxter, B.; Hackstadt, T.; Omsland, A. Impact of Active Metabolism on Chlamydia trachomatis Elementary Body Transcript Profile and Infectivity. J. Bacteriol. 2018, 200, e00065-18. [Google Scholar] [CrossRef] [PubMed]
- Elwell, C.; Mirrashidi, K.; Engel, J. Chlamydia cell biology and pathogenesis. Nat. Rev. Microbiol. 2016, 14, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.R.; Clarke, I.N.; Seth-Smith, H.M.; Solomon, A.W.; Cutcliffe, L.T.; Marsh, P.; Skilton, R.J.; Holland, M.J.; Mabey, D.; Peeling, R.W.; et al. Whole-genome analysis of diverse Chlamydia trachomatis strains identifies phylogenetic relationships masked by current clinical typing. Nat. Genet. 2012, 44, 413–419, S411. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, N.L.; Polkinghorne, A.; Timms, P. Chlamydia genomics: Providing novel insights into Chlamydial biology. Trends Microbiol. 2014, 22, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Stephens, R.S.; Kalman, S.; Lammel, C.; Fan, J.; Marathe, R.; Aravind, L.; Mitchell, W.; Olinger, L.; Tatusov, R.L.; Zhao, Q.; et al. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science 1998, 282, 754–759. [Google Scholar] [CrossRef]
- Hefty, P.S.; Stephens, R.S. Chlamydial type III secretion system is encoded on ten operons preceded by sigma 70-like promoter elements. J. Bacteriol. 2007, 189, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Enciso, G.A.; Boassa, D.; Chander, C.N.; Lou, T.H.; Pairawan, S.S.; Guo, M.C.; Wan, F.Y.M.; Ellisman, M.H.; Sutterlin, C.; et al. Replication-dependent size reduction precedes differentiation in Chlamydia trachomatis. Nat. Commun. 2018, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Sturd, N.; Rucks, E.A. Chlamydia trachomatis. Trends Microbiol. 2023, 31, 535–536. [Google Scholar] [CrossRef] [PubMed]
- Liechti, G.W. Localized Peptidoglycan Biosynthesis in Chlamydia trachomatis Conforms to the Polarized Division and Cell Size Reduction Developmental Models. Front. Microbiol. 2021, 12, 733850. [Google Scholar] [CrossRef] [PubMed]
- Cossé, M.M.; Hayward, R.D.; Subtil, A. One Face of Chlamydia trachomatis: The Infectious Elementary Body. Biol. Chlamydia 2018, 412, 35–58. [Google Scholar] [CrossRef]
- Saka, H.A.; Thompson, J.W.; Chen, Y.S.; Kumar, Y.; Dubois, L.G.; Moseley, M.A.; Valdivia, R.H. Quantitative proteomics reveals metabolic and pathogenic properties of Chlamydia trachomatis developmental forms. Mol. Microbiol. 2011, 82, 1185–1203. [Google Scholar] [CrossRef] [PubMed]
- Omsland, A.; Sixt, B.S.; Horn, M.; Hackstadt, T. Chlamydial metabolism revisited: Interspecies metabolic variability and developmental stage-specific physiologic activities. FEMS Microbiol. Rev. 2014, 38, 779–801. [Google Scholar] [CrossRef] [PubMed]
- Bastidas, R.J.; Elwell, C.A.; Engel, J.N.; Valdivia, R.H. Chlamydial Intracellular Survival Strategies. Cold Spring Harb. Perspect. Med. 2013, 3, a010256. [Google Scholar] [CrossRef] [PubMed]
- Becker, E.; Hegemann, J.H. All subtypes of the Pmp adhesin family are implicated in Chlamydial virulence and show species-specific function. Microbiologyopen 2014, 3, 544–556. [Google Scholar] [CrossRef] [PubMed]
- Mölleken, K.; Becker, E.; Hegemann, J.H. The Chlamydia pneumoniae Invasin Protein Pmp21 Recruits the EGF Receptor for Host Cell Entry. PLoS Pathog. 2013, 9, e1003325. [Google Scholar] [CrossRef] [PubMed]
- Stallmann, S.; Hegemann, J.H. The Chlamydia trachomatis Ctad1 invasin exploits the human integrin β1 receptor for host cell entry. Cell Microbiol. 2016, 18, 761–775. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jiang, S.; Elwell, C.A.; Engel, J.N. Chlamydia trachomatis co-opts the FGF2 signaling pathway to enhance infection. PLoS Pathog. 2011, 7, e1002285. [Google Scholar] [CrossRef] [PubMed]
- Abromaitis, S.; Stephens, R.S. Attachment and Entry of Chlamydia Have Distinct Requirements for Host Protein Disulfide Isomerase. PLoS Pathog. 2009, 5, e1000357. [Google Scholar] [CrossRef] [PubMed]
- Subbarayal, P.; Karunakaran, K.; Winkler, A.C.; Rother, M.; Gonzalez, E.; Meyer, T.F.; Rudel, T. EphrinA2 Receptor (EphA2) Is an Invasion and Intracellular Signaling Receptor for Chlamydia trachomatis. PLoS Pathog. 2015, 11, e1004846. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.L.; Chen, X.F.; Wood, S.T.; Stuart, E.S.; Arcaro, K.F.; Molina, D.P.; Petrovic, S.; Furdui, C.M.; Tsang, A.W. Activation of epidermal growth factor receptor is required for Chlamydia trachomatis development. BMC Microbiol. 2014, 14, 277. [Google Scholar] [CrossRef] [PubMed]
- Elwell, C.A.; Ceesay, A.; Kim, J.H.; Kalman, D.; Engel, J.N. RNA interference screen identifies Abl kinase and PDGFR signaling in Chlamydia trachomatis entry. PLoS Pathog. 2008, 4, e1000021. [Google Scholar] [CrossRef] [PubMed]
- Gérard, H.C.; Fomicheva, E.; Whittum-Hudson, J.A.; Hudson, A.P. Apolipoprotein E4 enhances attachment of Chlamydophila (Chlamydia) pneumoniae elementary bodies to host cells. Microb. Pathog. 2008, 44, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Mueller, K.E.; Plano, G.V.; Fields, K.A. New Frontiers in Type III Secretion Biology: The Chlamydia Perspective. Infect. Immun. 2014, 82, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Thalmann, J.; Janik, K.; May, M.; Sommer, K.; Ebeling, J.; Hofmann, F.; Genth, H.; Klos, A. Actin Re-Organization Induced by Chlamydia trachomatis Serovar D—Evidence for a Critical Role of the Effector Protein CT166 Targeting Rac. PLoS ONE 2010, 5, e9887. [Google Scholar] [CrossRef] [PubMed]
- Bullock, H.D.; Hower, S.; Fields, K.A. Domain Analyses Reveal That Chlamydia trachomatis CT694 Protein Belongs to the Membrane-localized Family of Type III Effector Proteins. J. Biol. Chem. 2012, 287, 28078–28086. [Google Scholar] [CrossRef] [PubMed]
- Hower, S.; Wolf, K.; Fields, K.A. Evidence that CT694 is a novel Chlamydia trachomatis T3S substrate capable of functioning during invasion or early cycle development. Mol. Microbiol. 2009, 72, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Jiwani, S.; Ohr, R.J.; Fischer, E.R.; Hackstadt, T.; Alvarado, S.; Romero, A.; Jewett, T.J. Chlamydia trachomatis Tarp cooperates with the Arp2/3 complex to increase the rate of actin polymerization. Biochem. Biophys. Res. Commun. 2012, 420, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Bastidas, R.J.; Saka, H.A.; Carpenter, V.K.; Richards, K.L.; Plano, G.V.; Valdivia, R.H. The Chlamydia trachomatis type III secretion chaperone Slc1 engages multiple early effectors, including TepP, a tyrosine-phosphorylated protein required for the recruitment of CrkI-II to nascent inclusions and innate immune signaling. PLoS Pathog. 2014, 10, e1003954. [Google Scholar] [CrossRef]
- Pais, S.V.; Milho, C.; Almeida, F.; Mota, L.J. Identification of novel type III secretion chaperone-substrate complexes of Chlamydia trachomatis. PLoS ONE 2013, 8, e56292. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, J.T.; Puolakkainen, M.; Haveri, A.; Tammiruusu, A.; Sarvas, M.; Lahesmaa, R. Chlamydia pneumoniae entry into epithelial cells by clathrin-independent endocytosis. Microb. Pathog. 2012, 52, 157–164. [Google Scholar] [CrossRef]
- Ford, C.; Nans, A.; Boucrot, E.; Hayward, R.D. Chlamydia exploits filopodial capture and a macropinocytosis-like pathway for host cell entry. PLoS Pathog. 2018, 14, e1007051. [Google Scholar] [CrossRef] [PubMed]
- Mehlitz, A.; Rudel, T. Modulation of host signaling and cellular responses by Chlamydia. Cell Commun. Signal. 2013, 11, 90. [Google Scholar] [CrossRef] [PubMed]
- Ridderhof, J.C.; Barnes, R.C. Fusion of inclusions following superinfection of HeLa cells by two serovars of Chlamydia trachomatis. Infect. Immun. 1989, 57, 3189–3193. [Google Scholar] [CrossRef]
- Rajeeve, K.; Vollmuth, N.; Janaki-Raman, S.; Wulff, T.F.; Baluapuri, A.; Dejure, F.R.; Huber, C.; Fink, J.; Schmalhofer, M.; Schmitz, W.; et al. Reprogramming of host glutamine metabolism during Chlamydia trachomatis infection and its key role in peptidoglycan synthesis. Nat. Microbiol. 2020, 5, 1390–1402. [Google Scholar] [CrossRef] [PubMed]
- Hackstadt, T.; Baehr, W.; Ying, Y. Chlamydia trachomatis developmentally regulated protein is homologous to eukaryotic histone H1. Proc. Natl. Acad. Sci. USA 1991, 88, 3937–3941. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.; McMahon, R.M.; Martin, J.L.; Huston, W.M. Life inside and out: Making and breaking protein disulfide bonds in Chlamydia. Crit. Rev. Microbiol. 2019, 45, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.R.; Baidya, A.K.; Mamou, G.; Bhattacharya, S.; Socol, Y.; Kobi, S.; Katsowich, N.; Ben-Yehuda, S.; Rosenshine, I. Pathogenic E. coli Extracts Nutrients from Infected Host Cells Utilizing Injectisome Components. Cell 2019, 177, 683–696.e18. [Google Scholar] [CrossRef] [PubMed]
- Stelzner, K.; Vollmuth, N.; Rudel, T. Intracellular lifestyle of Chlamydia trachomatis and host-pathogen interactions. Nat. Rev. Microbiol. 2023, 21, 448–462. [Google Scholar] [CrossRef]
- Hybiske, K.; Stephens, R.S. Mechanisms of host cell exit by the intracellular bacterium Chlamydia. Proc. Natl. Acad. Sci. USA 2007, 104, 11430–11435. [Google Scholar] [CrossRef] [PubMed]
- Omsland, A.; Sager, J.; Nair, V.; Sturdevant, D.E.; Hackstadt, T. Developmental stage-specific metabolic and transcriptional activity of in an axenic medium. Proc. Natl. Acad. Sci. USA 2012, 109, 19781–19785, Corrected in Proc. Natl. Acad. Sci. USA 2013, 110, 1970. [Google Scholar] [CrossRef] [PubMed]
- Saka, H.A.; Valdivia, R.H. Acquisition of nutrients by Chlamydiae: Unique challenges of living in an intracellular compartment. Curr. Opin. Microbiol. 2010, 13, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Larson, C.L.; Heinzen, R.A. High-Content Imaging Reveals Expansion of the Endosomal Compartment during Coxiella burnetii Parasitophorous Vacuole Maturation. Front. Cell. Infect. Microbiol. 2017, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Pokorzynski, N.D.; Thompson, C.C.; Carabeo, R.A. Ironing Out the Unconventional Mechanisms of Iron Acquisition and Gene Regulation in Chlamydia. Front. Cell. Infect. Microbiol. 2017, 7, 394. [Google Scholar] [CrossRef] [PubMed]
- Gurumurthy, R.K.; Chumduri, C.; Karlas, A.; Kimmig, S.; Gonzalez, E.; Machuy, N.; Rudel, T.; Meyer, T.F. Dynamin-mediated lipid acquisition is essential for Chlamydia trachomatis development. Mol. Microbiol. 2014, 94, 186–201. [Google Scholar] [CrossRef] [PubMed]
- Agaisse, H.; Derré, I. STIM1 Is a Novel Component of ER-Chlamydia trachomatis Inclusion Membrane Contact Sites. PLoS ONE 2015, 10, e0125671. [Google Scholar] [CrossRef] [PubMed]
- Beatty, W.L. Trafficking from CD63-positive late endocytic multivesicular bodies is essential for intracellular development of Chlamydia trachomatis. J. Cell Sci. 2006, 119, 350–359. [Google Scholar] [CrossRef]
- Lutter, E.I.; Barger, A.C.; Nair, V.; Hackstadt, T. Chlamydia trachomatis inclusion membrane protein CT228 recruits elements of the myosin phosphatase pathway to regulate release mechanisms. Cell Rep. 2013, 3, 1921–1931. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.M.; Mathews, S.A.; Theodoropoulos, C.; Timms, P. In vitro characterisation of koala Chlamydia pneumoniae: Morphology, inclusion development and doubling time. Vet. Microbiol. 2009, 136, 91–99. [Google Scholar] [CrossRef]
- Mehlitz, A.; Eylert, E.; Huber, C.; Lindner, B.; Vollmuth, N.; Karunakaran, K.; Goebel, W.; Eisenreich, W.; Rudel, T. Metabolic adaptation of to mammalian host cells. Mol. Microbiol. 2017, 103, 1004–1019. [Google Scholar] [CrossRef] [PubMed]
- Tjaden, J.; Winkler, H.H.; Schwoppe, C.; Van Der Laan, M.; Mohlmann, T.; Neuhaus, H.E. Two nucleotide transport proteins in Chlamydia trachomatis, one for net nucleoside triphosphate uptake and the other for transport of energy. J. Bacteriol. 1999, 181, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Al’perina, E.L. Involvement of thedopaminergic system inthe mechanisms ofimmunomodulation. Usp. Fiziol. Nauk. 2014, 45, 45–56. [Google Scholar] [PubMed]
- Eisenreich, W.; Heesemann, J.; Rudel, T.; Goebel, W. Metabolic Adaptations of Intracellullar Bacterial Pathogens and their Mammalian Host Cells during Infection (“Pathometabolism”). Microbiol. Spectr. 2015, 3, 27–58. [Google Scholar] [CrossRef] [PubMed]
- Al-Nedawi, K.N.; Czyz, M.; Bednarek, R.; Szemraj, J.; Swiatkowska, M.; Cierniewska-Cieslak, A.; Wyczolkowska, J.; Cierniewski, C.S. Thymosin beta 4 induces the synthesis of plasminogen activator inhibitor 1 in cultured endothelial cells and increases its extracellular expression. Blood 2004, 103, 1319–1324. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Iliffe-Lee, E.R.; McClarty, G. Regulation of carbon metabolism in. Mol. Microbiol. 2000, 38, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Read, T.D.; Brunham, R.C.; Shen, C.; Gill, S.R.; Heidelberg, J.F.; White, O.; Hickey, E.K.; Peterson, J.; Utterback, T.; Berry, K.; et al. Genome sequences of Chlamydia trachomatis MoPn and Chlamydia pneumoniae AR39. Nucleic Acids Res. 2000, 28, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Read, T.D.; Myers, G.S.; Brunham, R.C.; Nelson, W.C.; Paulsen, I.T.; Heidelberg, J.; Holtzapple, E.; Khouri, H.; Federova, N.B.; Carty, H.A.; et al. Genome sequence of Chlamydophila caviae (Chlamydia psittaci GPIC): Examining the role of niche-specific genes in the evolution of the Chlamydiaceae. Nucleic Acids Res. 2003, 31, 2134–2147. [Google Scholar] [CrossRef] [PubMed]
- Rother, M.; Teixeira da Costa, A.R.; Zietlow, R.; Meyer, T.F.; Rudel, T. Modulation of Host Cell Metabolism by Chlamydia trachomatis. Microbiol. Spectr. 2019, 7, 110–128. [Google Scholar] [CrossRef] [PubMed]
- Juul, N.; Jensen, H.; Hvid, M.; Christiansen, G.; Birkelund, S. Characterization of in vitro Chlamydial cultures in low-oxygen atmospheres. J. Bacteriol. 2007, 189, 6723–6726. [Google Scholar] [CrossRef] [PubMed]
- Dietz, I.; Jerchel, S.; Szaszák, M.; Shima, K.; Rupp, J. When oxygen runs short: The microenvironment drives host-pathogen interactions. Microbes Infect. 2012, 14, 311–316. [Google Scholar] [CrossRef]
- Iliffe-Lee, E.R.; McClarty, G. Glucose metabolism in Chlamydia trachomatis: The ‘energy parasite’ hypothesis revisited. Mol. Microbiol. 1999, 33, 177–187. [Google Scholar] [CrossRef]
- Yang, C.F.; Starr, T.; Song, L.H.; Carlson, J.H.; Sturdevant, G.L.; Beare, P.A.; Whitmire, W.M.; Caldwell, H.D. Chlamydial Lytic Exit from Host Cells Is Plasmid Regulated. Mbio 2015, 6, e01648-15. [Google Scholar] [CrossRef]
- Kerr, M.C.; Gomez, G.A.; Ferguson, C.; Tanzer, M.C.; Murphy, J.M.; Yap, A.S.; Parton, R.G.; Huston, W.M.; Teasdale, R.D. Laser-mediated rupture of Chlamydial inclusions triggers pathogen egress and host cell necrosis. Nat. Commun. 2017, 8, 14729. [Google Scholar] [CrossRef]
- Rake, G.; Jones, H.P. Studies on Lymphogranuloma Venereum: II. The Association of Specific Toxins with Agents of the Lymphogranuloma-Psittacosis Group. J. Exp. Med. 1944, 79, 463–486. [Google Scholar] [CrossRef]
- Schoborg, R.V. Chlamydia persistence—A tool to dissect Chlamydia-host interactions. Microbes Infect. 2011, 13, 649–662. [Google Scholar] [CrossRef]
- Pospischil, A.; Borel, N.; Chowdhury, E.H.; Guscetti, F. Aberrant Chlamydial developmental forms in the gastrointestinal tract of pigs spontaneously and experimentally infected with Chlamydia suis. Vet. Microbiol. 2009, 135, 147–156. [Google Scholar] [CrossRef]
- Moulder, J.W.; Hatch, T.P.; Byrne, G.I.; Kellogg, K.R. Immediate toxicity of high multiplicities of Chlamydia psittaci for mouse fibroblasts (L cells). Infect. Immun. 1976, 14, 277–289. [Google Scholar] [CrossRef]
- Chang, M.C. Development of the oral contraceptives. Am. J. Obstet. Gynecol. 1978, 132, 217–219. [Google Scholar] [CrossRef]
- Perfettini, J.L.; Darville, T.; Gachelin, G.; Souque, P.; Huerre, M.; Dautry-Varsat, A.; Ojcius, D.M. Effect of Chlamydia trachomatis infection and subsequent tumor necrosis factor alpha secretion on apoptosis in the murine genital tract. Infect. Immun. 2000, 68, 2237–2244. [Google Scholar] [CrossRef]
- Jendro, M.C.; Fingerle, F.; Deutsch, T.; Liese, A.; Kohler, L.; Kuipers, J.G.; Raum, E.; Martin, M.; Zeidler, H. Chlamydia trachomatis-infected macrophages induce apoptosis of activated T cells by secretion of tumor necrosis factor-alpha in vitro. Med. Microbiol. Immunol. 2004, 193, 45–52. [Google Scholar] [CrossRef]
- Sixt, B.S. Host cell death during infection with Chlamydia: A double-edged sword. FEMS Microbiol. Rev. 2021, 45, fuaa043. [Google Scholar] [CrossRef]
- Ojcius, D.M.; Souque, P.; Perfettini, J.L.; Dautry-Varsat, A. Apoptosis of epithelial cells and macrophages due to infection with the obligate intracellular pathogen Chlamydia psittaci. J. Immunol. 1998, 161, 4220–4226. [Google Scholar] [CrossRef]
- Dadsena, S.; Cuevas Arenas, R.; Vieira, G.; Brodesser, S.; Melo, M.N.; Garcia-Saez, A.J. Lipid unsaturation promotes BAX and BAK pore activity during apoptosis. Nat. Commun. 2024, 15, 4700. [Google Scholar] [CrossRef]
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat. Rev. Mol. Cell Bio 2014, 15, 49–63. [Google Scholar] [CrossRef]
- Dumoux, M.; Nans, A.; Saibil, H.R.; Hayward, R.D. Making connections: Snapshots of Chlamydial type III secretion systems in contact with host membranes. Curr. Opin. Microbiol. 2015, 23, 1–7. [Google Scholar] [CrossRef]
- Sixt, B.S.; Nunez-Otero, C.; Kepp, O.; Valdivia, R.H.; Kroemer, G. Chlamydia trachomatis fails to protect its growth niche against pro-apoptotic insults. Cell Death Differ. 2019, 26, 1485–1500. [Google Scholar] [CrossRef]
- Al-Zeer, M.A.; Al-Younes, H.M.; Braun, P.R.; Zerrahn, J.; Meyer, T.F. IFN-γ-Inducible Irga6 Mediates Host Resistance against Chlamydia trachomatis via Autophagy. PLoS ONE 2009, 4, e4588. [Google Scholar] [CrossRef]
- Yasir, M.; Pachikara, N.D.; Bao, X.F.; Pan, Z.; Fan, H.Z. Regulation of Chlamydial Infection by Host Autophagy and Vacuolar ATPase-Bearing Organelles. Infect. Immun. 2011, 79, 4019–4028. [Google Scholar] [CrossRef]
- Witkin, S.S.; Minis, E.; Athanasiou, A.; Leizer, J.; Linhares, I.M. Chlamydia trachomatis: The Persistent Pathogen. Clin. Vaccine Immunol. 2017, 24, e00203-17. [Google Scholar] [CrossRef]
- Finethy, R.; Jorgensen, I.; Haldar, A.K.; de Zoete, M.R.; Strowig, T.; Flavell, R.A.; Yamamoto, M.; Nagarajan, U.M.; Miao, E.A.; Coers, J. Guanylate Binding Proteins Enable Rapid Activation of Canonical and Noncanonical Inflammasomes in Chlamydia-Infected Macrophages. Infect. Immun. 2015, 83, 4740–4749. [Google Scholar] [CrossRef]
- Weber, M.M.; Lam, J.L.; Dooley, C.A.; Noriea, N.F.; Hansen, B.T.; Hoyt, F.H.; Carmody, A.B.; Sturdevant, G.L.; Hackstadt, T. Absence of Specific Chlamydia trachomatis Inclusion Membrane Proteins Triggers Premature Inclusion Membrane Lysis and Host Cell Death. Cell Rep. 2017, 19, 1406–1417. [Google Scholar] [CrossRef]
- Abdul-Sater, A.A.; Koo, E.; Häcker, G.; Ojcius, D.M. Inflammasome-dependent Caspase-1 Activation in Cervical Epithelial Cells Stimulates Growth of the Intracellular Pathogen Chlamydia trachomatis. J. Biol. Chem. 2009, 284, 26789–26796. [Google Scholar] [CrossRef]
- Christian, J.G.; Heymann, J.; Paschen, S.A.; Vier, J.; Schauenburg, L.; Rupp, J.; Meyer, T.F.; Häcker, G.; Heuer, D. Targeting of a Chlamydial Protease Impedes Intracellular Bacterial Growth. PLoS Pathog. 2011, 7, e1002283. [Google Scholar] [CrossRef]
- Itoh, R.; Murakami, I.; Chou, B.; Ishii, K.; Soejima, T.; Suzuki, T.; Hiromatsu, K. Chlamydia pneumoniae harness host NLRP3 inflammasome-mediated caspase-1 activation for optimal intracellular growth in murine macrophages. Biochem. Biophys. Res. Commun. 2014, 452, 689–694. [Google Scholar] [CrossRef]
- Suchland, R.J.; Dimond, Z.E.; Putman, T.E.; Rockey, D.D. Demonstration of Persistent Infections and Genome Stability by Whole-Genome Sequencing of Repeat-Positive, Same-Serovar Chlamydia trachomatis Collected From the Female Genital Tract. J. Infect. Dis. 2017, 215, 1657–1665. [Google Scholar] [CrossRef]
- Roth, A.; Konig, P.; van Zandbergen, G.; Klinger, M.; Hellwig-Burgel, T.; Daubener, W.; Bohlmann, M.K.; Rupp, J. Hypoxia abrogates antiChlamydial properties of IFN-gamma in human fallopian tube cells in vitro and ex vivo. Proc. Natl. Acad. Sci. USA 2010, 107, 19502–19507. [Google Scholar] [CrossRef]
- Cocchiaro, J.L.; Valdivia, R.H. New insights into Chlamydia intracellular survival mechanisms. Cell Microbiol. 2009, 11, 1571–1578. [Google Scholar] [CrossRef]
- Su, H.; McClarty, G.; Dong, F.; Hatch, G.M.; Pan, Z.X.K.; Zhong, G.M. Activation of Raf/MEK/ERK/cPLA2 signaling pathway is essential for Chlamydial acquisition of host glycerophospholipids. J. Biol. Chem. 2004, 279, 9409–9416. [Google Scholar] [CrossRef]
- Buchholz, K.R.; Stephens, R.S. The extracellular signal-regulated kinase/mitogen-activated protein kinase pathway induces the inflammatory factor interleukin-8 following Chlamydia trachomatis infection. Infect. Immun. 2007, 75, 5924–5929. [Google Scholar] [CrossRef]
- Rajalingam, K.; Sharma, M.; Lohmann, C.; Oswald, M.; Thieck, O.; Froelich, C.J.; Rudel, T. Mcl-1 Is a Key Regulator of Apoptosis Resistance in Chlamydia trachomatis-Infected Cells. PLoS ONE 2008, 3, e3102. [Google Scholar] [CrossRef]
- Verbeke, P.; Welter-Stahl, L.; Ying, S.; Hansen, J.; Hacker, G.; Darville, T.; Ojcius, D.M. Recruitment of BAD by the Chlamydia trachomatis vacuole correlates with host-cell survival. PLoS Pathog. 2006, 2, e45. [Google Scholar] [CrossRef]
- Mehlitz, A.; Banhart, S.; Maurer, A.P.; Kaushansky, A.; Gordus, A.G.; Zielecki, J.; Macbeath, G.; Meyer, T.F. Tarp regulates early Chlamydia-induced host cell survival through interactions with the human adaptor protein SHC1. J. Cell Biol. 2010, 190, 143–157. [Google Scholar] [CrossRef]
- Du, K.; Zheng, Q.; Zhou, M.; Zhu, L.; Ai, B.; Zhou, L. Chlamydial antiapoptotic activity involves activation of the Raf/MEK/ERK survival pathway. Curr. Microbiol. 2011, 63, 341–346. [Google Scholar] [CrossRef]
- Kun, D.; Xiang-Lin, C.; Ming, Z.; Qi, L. Chlamydia inhibit host cell apoptosis by inducing Bag-1 via the MAPK/ERK survival pathway. Apoptosis 2013, 18, 1083–1092. [Google Scholar] [CrossRef]
- Siegl, C.; Prusty, B.K.; Karunakaran, K.; Wischhusen, J.; Rudel, T. Tumor suppressor p53 alters host cell metabolism to limit Chlamydia trachomatis infection. Cell Rep. 2014, 9, 918–929. [Google Scholar] [CrossRef]
- Li, L.; Wang, C.; Wen, Y.T.; Hu, Y.M.; Xie, Y.F.; Xu, M.; Liang, M.X.; Liu, W.; Liu, L.Z.; Wu, Y.M. ERK1/2 and the Bcl-2 Family Proteins Mcl-1, tBid, and Bim Are Involved in Inhibition of Apoptosis During Persistent Chlamydia psittaci Infection. Inflammation 2018, 41, 1372–1383. [Google Scholar] [CrossRef]
- Paland, N.; Rajalingam, K.; Machuy, N.; Szczepek, A.; Wehrl, W.; Rudel, T. NF-kappaB and inhibitor of apoptosis proteins are required for apoptosis resistance of epithelial cells persistently infected with Chlamydophila pneumoniae. Cell Microbiol. 2006, 8, 1643–1655. [Google Scholar] [CrossRef]
- Wahl, C.; Oswald, F.; Simnacher, U.; Weiss, S.; Marre, R.; Essig, A. Survival of Chlamydia pneumoniae-Infected Mono Mac 6 cells is dependent on NFκB binding activity. Infect. Immun. 2001, 69, 7039–7045. [Google Scholar] [CrossRef]
- Wahl, C.; Maier, S.; Marre, R.; Essig, A. Chlamydia pneumoniae induces the expression of inhibitor of apoptosis 2 (c-IAP2) in a human monocytic cell line by an NF-kappaB-dependent pathway. Int. J. Med. Microbiol. 2003, 293, 377–381. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, P.; Chen, S.; Hu, C.; Bai, Q.; Wu, H.; Chen, Y.; Zhou, P.; Zeng, X.; Liu, Z.; et al. The JAK/STAT3 signaling pathway mediates inhibition of host cell apoptosis by Chlamydia psittaci infection. Pathog. Dis. 2017, 75, ftx088. [Google Scholar] [CrossRef]
- Byrne, G.I.; Ojcius, D.M. Chlamydia and apoptosis: Life and death decisions of an intracellular pathogen. Nat. Rev. Microbiol. 2004, 2, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbacher, R.; Stenner-Liewen, F.; Liewen, H.; Robinson, H.; Hua, Y.A.; Bossy-Wetzel, E.; Reed, J.C.; Liddington, R.C. Structure of the Chlamydia protein CADD reveals a redox enzyme that modulates host cell apoptosis. J. Biol. Chem. 2004, 279, 29320–29324. [Google Scholar] [CrossRef]
- Dong, F.; Pirbhai, M.; Xiao, Y.; Zhong, Y.; Wu, Y.; Zhong, G. Degradation of the proapoptotic proteins Bik, Puma, and Bim with Bcl-2 domain 3 homology in Chlamydia trachomatis-infected cells. Infect. Immun. 2005, 73, 1861–1864. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.; Seiffert, B.M.; Hacker, G.; Fischer, S.F. Broad degradation of proapoptotic proteins with the conserved Bcl-2 homology domain 3 during infection with Chlamydia trachomatis. Infect. Immun. 2005, 73, 1399–1403. [Google Scholar] [CrossRef] [PubMed]
- Bohme, L.; Albrecht, M.; Riede, O.; Rudel, T. Chlamydia trachomatis-infected host cells resist dsRNA-induced apoptosis. Cell Microbiol. 2010, 12, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.F.; Harlander, T.; Vier, J.; Häcker, G. Protection against CD95-induced apoptosis by Chlamydial infection at a mitochondrial step. Infect. Immun. 2004, 72, 1107–1115. [Google Scholar] [CrossRef]
- Pirbhai, M.; Dong, F.; Zhong, Y.M.; Pan, K.Z.; Zhong, G.M. The secreted protease factor CPAF is responsible for degrading pro-apoptotic BH3-only proteins in Chlamydia trachomatis-infected cells. J. Biol. Chem. 2006, 281, 31495–31501. [Google Scholar] [CrossRef] [PubMed]
- Dockterman, J.; Coers, J. Immunopathogenesis of genital Chlamydia infection: Insights from mouse models. Pathog. Dis. 2021, 79, ftab012. [Google Scholar] [CrossRef] [PubMed]
- Schairer, D.O.; Chouake, J.S.; Nosanchuk, J.D.; Friedman, A.J. The potential of nitric oxide releasing therapies as antimicrobial agents. Virulence 2012, 3, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, K.H.; Miranpuri, G.S.; Sigar, I.M.; Ouellette, S.; Byrne, G.I. Chlamydia trachomatis persistence in the female mouse genital tract: Inducible nitric oxide synthase and infection outcome. Infect. Immun. 2001, 69, 5131–5137. [Google Scholar] [CrossRef] [PubMed]
- Brunham, R.C.; Rey-Ladino, J. Immunology of Chlamydia infection: Implications for a Chlamydia trachomatis vaccine. Nat. Rev. Immunol. 2005, 5, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Helble, J.D.; Starnbach, M.N. T cell responses to Chlamydia. Pathog. Dis. 2021, 79, ftab014. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.C.; Xie, G.; Bonner, C.A.; Jensen, R.A. The alternative translational profile that underlies the immune-evasive state of persistence in Chlamydiaceae exploits differential tryptophan contents of the protein repertoire. Microbiol. Mol. Biol. Rev. 2012, 76, 405–443. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Randow, F.; MacMicking, J.D.; James, L.C. Cellular self-defense: How cell-autonomous immunity protects against pathogens. Science 2013, 340, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Finethy, R.; Coers, J. Sensing the enemy, containing the threat: Cell-autonomous immunity to Chlamydia trachomatis. FEMS Microbiol. Rev. 2016, 40, 875–893. [Google Scholar] [CrossRef] [PubMed]
- Labuda, J.C.; McSorley, S.J. Diversity in the T cell response to Chlamydia-sum are better than one. Immunol. Lett. 2018, 202, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Al-Zeer, M.A.; Al-Younes, H.M.; Lauster, D.; Abu Lubad, M.; Meyer, T.F. Autophagy restricts Chlamydia trachomatis growth in human macrophages via IFNG-inducible guanylate binding proteins. Autophagy 2013, 9, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Bulir, D.; Kaushic, C.; Mahony, J. Considerations for the rational design of a Chlamydia vaccine. Hum. Vaccin. Immunother. 2017, 13, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Panzetta, M.E.; Valdivia, R.H.; Saka, H.A. Chlamydia Persistence: A Survival Strategy to Evade Antimicrobial Effects in-vitro and in-vivo. Front. Microbiol. 2018, 9, 3101. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.R.; Burton, M.J.; Haddad, D.; West, S.; Wright, H. Trachoma. Lancet 2014, 384, 2142–2152. [Google Scholar] [CrossRef] [PubMed]
- Ramadhani, A.M.; Derrick, T.; Macleod, D.; Holland, M.J.; Burton, M.J. The Relationship between Active Trachoma and Ocular Chlamydia trachomatis Infection before and after Mass Antibiotic Treatment. PLoS Negl. Trop. Dis. 2016, 10, e0005080. [Google Scholar] [CrossRef] [PubMed]
- Renneker, K.K.; Mtuy, T.B.; Kabona, G.; Mbwambo, S.G.; Mosha, P.; Mollel, J.M.; Hooper, P.J.; Emerson, P.M.; Hollingsworth, T.D.; Butcher, R.; et al. Acceptability and feasibility of tests for infection, serological testing, and photography to define need for interventions against trachoma. PLoS Negl. Trop. Dis. 2024, 18, e0011941. [Google Scholar] [CrossRef] [PubMed]
- Garin, N.; Marti, C.; Skali Lami, A.; Prendki, V. Atypical Pathogens in Adult Community-Acquired Pneumonia and Implications for Empiric Antibiotic Treatment: A Narrative Review. Microorganisms 2022, 10, 2326. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, H.; Xu, Y.; Chen, Y.; Liu, B.; Chen, J.; Nie, W.; Zhong, S.; Ma, J.; Liu, C. Venous-arterial extracorporeal membrane oxygenation for psittacosis pneumonia complicated with cardiogenic shock: Case report and literature review. BMC Cardiovasc. Disord. 2024, 24, 6. [Google Scholar] [CrossRef] [PubMed]
- Stewardson, A.J.; Grayson, M.L. Psittacosis. Infect. Dis. Clin. N. Am. 2010, 24, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Premachandra, N.M.; Jayaweera, J. Chlamydia pneumoniae infections and development of lung cancer: Systematic review. Infect. Agent. Cancer 2022, 17, 11. [Google Scholar] [CrossRef] [PubMed]
- Covvey, J.R.; Guarascio, A.J. Clinical use of lefamulin: A first-in-class semisynthetic pleuromutilin antibiotic. J. Intern. Med. 2022, 291, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Suchland, R.J.; Geisler, W.M.; Stamm, W.E. Methodologies and cell lines used for antimicrobial susceptibility testing of Chlamydia spp. Antimicrob. Agents Chemother. 2003, 47, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Suchland, R.J.; Sandoz, K.M.; Jeffrey, B.M.; Stamm, W.E.; Rockey, D.D. Horizontal transfer of tetracycline resistance among Chlamydia spp. in vitro. Antimicrob. Agents Chemother. 2009, 53, 4604–4611. [Google Scholar] [CrossRef] [PubMed]
- Donati, M.; Di Francesco, A.; D’Antuono, A.; Delucca, F.; Shurdhi, A.; Moroni, A.; Baldelli, R.; Cevenini, R. In Vitro Activities of Several Antimicrobial Agents against Recently Isolated and Genotyped Chlamydia trachomatis Urogenital Serovars D through K. Antimicrob. Agents Chemother. 2010, 54, 5379–5380. [Google Scholar] [CrossRef] [PubMed]
- Hammerschlag, M.R.; Kohlhoff, S.A. Treatment of Chlamydial infections. Expert. Opin. Pharmacother. 2012, 13, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Binet, R.; Bowlin, A.K.; Maurelli, A.T.; Rank, R.G. Impact of azithromycin resistance mutations on the virulence and fitness of Chlamydia caviae in guinea pigs. Antimicrob. Agents Chemother. 2010, 54, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Riska, P.F.; Kutlin, A.; Ajiboye, P.; Cua, A.; Roblin, P.M.; Hammerschlag, M.R. Genetic and culture-based approaches for detecting macrolide resistance in Chlamydia pneumoniae. Antimicrob. Agents Chemother. 2004, 48, 3586–3590. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rupp, J.; Gebert, A.; Solbach, W.; Maass, M. Serine-to-asparagine substitution in the GyrA gene leads to quinolone resistance in moxifloxacin-exposed Chlamydia pneumoniae. Antimicrob. Agents Chemother. 2005, 49, 406–407. [Google Scholar] [CrossRef] [PubMed]
- Rupp, J.; Solbach, W.; Gieffers, J. Variation in the mutation frequency determining quinolone resistance in Chlamydia trachomatis serovars L2 and D. J. Antimicrob. Chemother. 2008, 61, 91–94. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kutlin, A.; Kohlhoff, S.; Roblin, P.; Hammerschlag, M.R.; Riska, P. Emergence of resistance to rifampin and rifalazil in Chlamydophila pneumoniae and Chlamydia trachomatis. Antimicrob. Agents Chemother. 2005, 49, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Storey, C.; Chopra, I. Affinities of beta-lactams for penicillin binding proteins of Chlamydia trachomatis and their antiChlamydial activities. Antimicrob. Agents Chemother. 2001, 45, 303–305. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Puolakkainen, M. Innate immunity and vaccines in Chlamydial infection with special emphasis on Chlamydia pneumoniae. FEMS Immunol. Med. Microbiol. 2009, 55, 167–177. [Google Scholar] [CrossRef]
- Hafner, L.; Beagley, K.; Timms, P. Chlamydia trachomatis infection: Host immune responses and potential vaccines. Mucosal Immunol. 2008, 1, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, M.; Armitage, C.W.; O’Meara, C.P.; Beagley, K.W. Towards a Chlamydia trachomatis vaccine: How close are we? Future Microbiol. 2010, 5, 1833–1856. [Google Scholar] [CrossRef] [PubMed]
- Murray, S.M.; McKay, P.F. Chlamydia trachomatis: Cell biology, immunology and vaccination. Vaccine 2021, 39, 2965–2975. [Google Scholar] [CrossRef] [PubMed]
- Olsen, A.W.; Follmann, F.; Erneholm, K.; Rosenkrands, I.; Andersen, P. Protection Against Chlamydia trachomatis Infection and Upper Genital Tract Pathological Changes by Vaccine-Promoted Neutralizing Antibodies Directed to the VD4 of the Major Outer Membrane Protein. J. Infect. Dis. 2015, 212, 978–989. [Google Scholar] [CrossRef] [PubMed]
- de la Maza, L.M.; Zhong, G.; Brunham, R.C. Update on Chlamydia trachomatis Vaccinology. Clin. Vaccine Immunol. 2017, 24, e00543-16. [Google Scholar] [CrossRef] [PubMed]
- Rey-Ladino, J.; Ross, A.G.; Cripps, A.W. Immunity, immunopathology, and human vaccine development against sexually transmitted Chlamydia trachomatis. Hum. Vaccin. Immunother. 2014, 10, 2664–2673. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Zeng, J.; Yu, J.; Sun, R.; Tuo, Y.; Bai, H. Insights into Chlamydia Development and Host Cells Response. Microorganisms 2024, 12, 1302. https://doi.org/10.3390/microorganisms12071302
Yang S, Zeng J, Yu J, Sun R, Tuo Y, Bai H. Insights into Chlamydia Development and Host Cells Response. Microorganisms. 2024; 12(7):1302. https://doi.org/10.3390/microorganisms12071302
Chicago/Turabian StyleYang, Shuaini, Jiajia Zeng, Jinxi Yu, Ruoyuan Sun, Yuqing Tuo, and Hong Bai. 2024. "Insights into Chlamydia Development and Host Cells Response" Microorganisms 12, no. 7: 1302. https://doi.org/10.3390/microorganisms12071302
APA StyleYang, S., Zeng, J., Yu, J., Sun, R., Tuo, Y., & Bai, H. (2024). Insights into Chlamydia Development and Host Cells Response. Microorganisms, 12(7), 1302. https://doi.org/10.3390/microorganisms12071302




