Response of Arugula to Integrated Use of Biological, Inorganic, and Organic Fertilization
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Soil Preparation and Analysis
2.3. Plant Preparation and Analysis
2.4. Statistical Analysis and Graphical Presentation
3. Results
3.1. Soil Physical, Chemical, and Microbiological Properties
3.2. Effect of Fertilization on Soil Microbiological Parameters
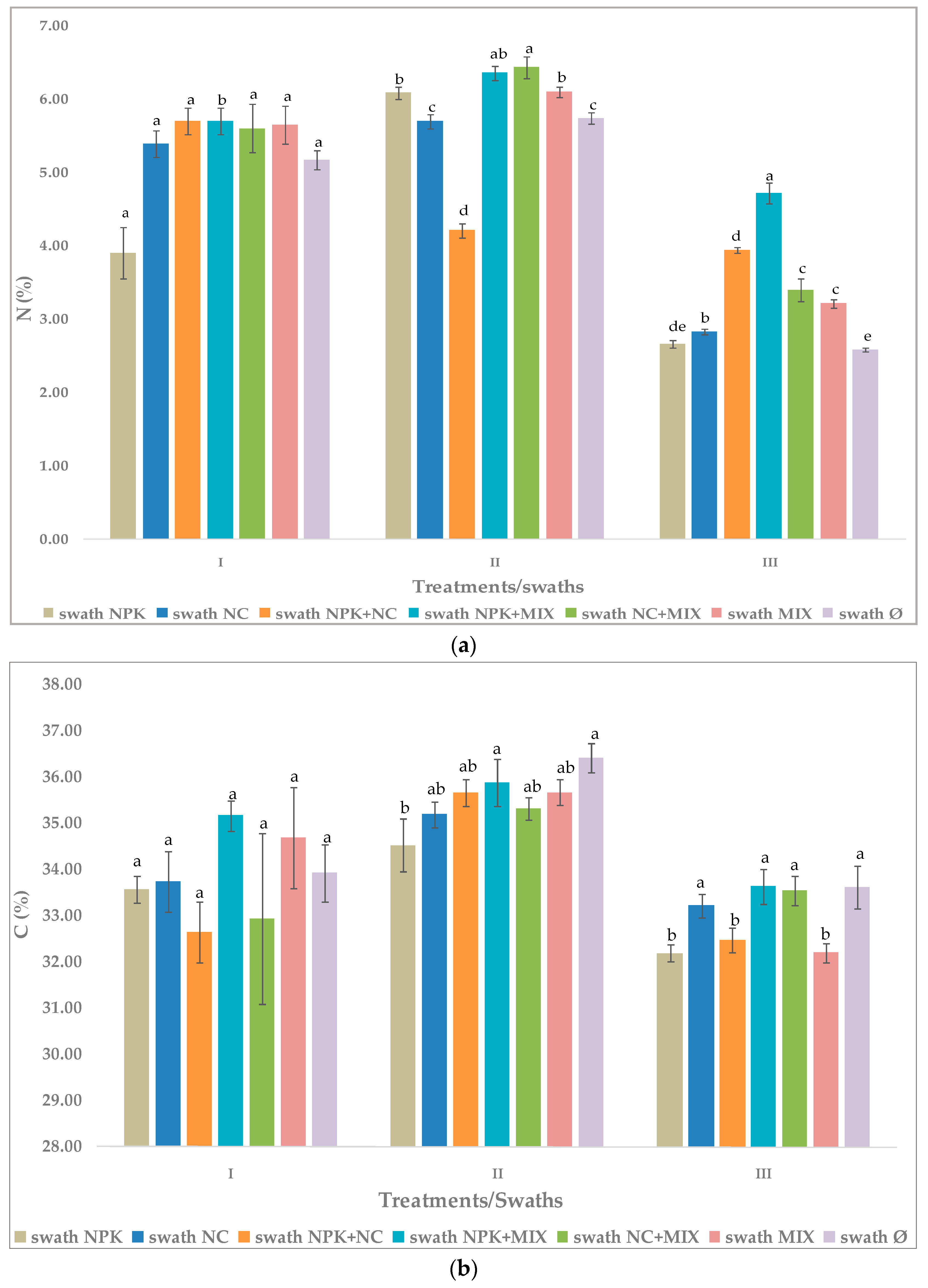
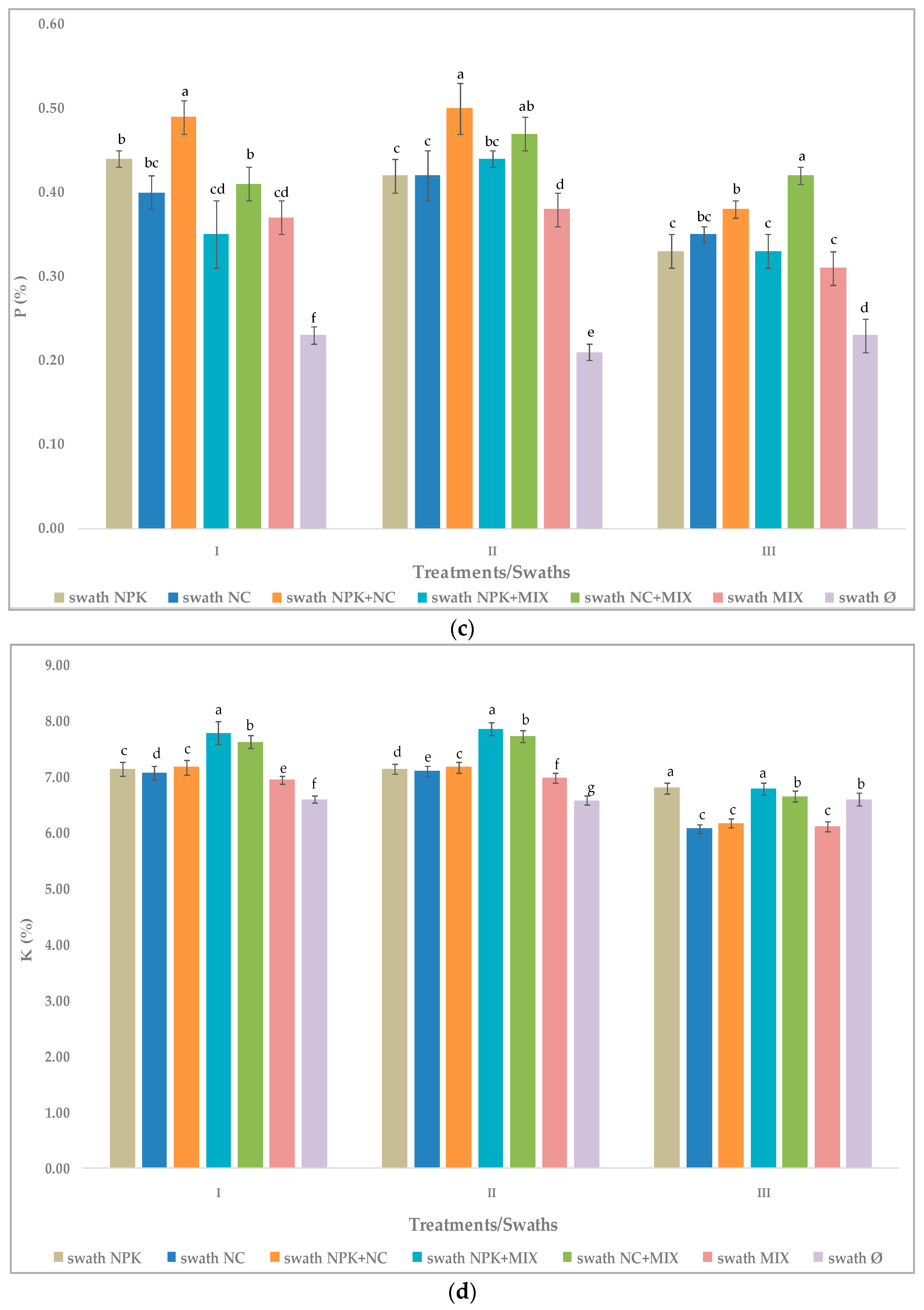
3.3. Effect of Fertilization on Chemical Composition of Arugula
3.3.1. The First Cutting Time—Swath I
3.3.2. The Second Cutting Time—Swath II
3.3.3. The Third Cutting Time—Swath III
3.4. Effect of Cutting Time—Swath and Different Fertilizers on Arugula Chemical Composition
3.5. Effect of Fertilization Treatments on Yield of Arugula
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arugula Is Good for Health, and It Is Grown all Year Round. Available online: https://www.agroklub.rs/povrtarstvo/rukola-dobra-za-zdravlje-a-gaji-se-preko-cele-godine/62687/ (accessed on 24 May 2024).
- Arugula Is a Cure for Many Problems, Food for All Ages. Available online: https://agroinfonet.com/poljoprivreda/povrtarstvo/rukola-lek/ (accessed on 18 June 2024).
- Jaafar, N.S.; Jaafar, I.S. Eruca sativa Linn.: Pharmacognostical and pharmacological properties and pharmaceutical preparations. Asian J. Pharm. Clin. Res. 2019, 12, 39–45. [Google Scholar] [CrossRef]
- Growing of Arugula: It Grows Throughout the Year. Available online: https://www.agroklub.rs/povrtarstvo/gajenje-rukole-raste-tokom-cele-godine/80235/ (accessed on 24 October 2023).
- Milivojević, J.; Djekić, V.; Jelić, M. Fertility of privately owned plowland used for field crop production in the City of Kragujevac, Serbia. Ratar. Povrt. 2012, 49, 195–201. [Google Scholar]
- Jakovljević, M.; Milivojević, J.; Antić-Mladenović, S.; Jelić, M. Content and availability of copper in Serbian smonitza soils. J. Sci. Agric. Res. 2002, 63, 63–73. [Google Scholar]
- Djekić, V.; Staletić, M.; Milivojević, J.; Popović, V.; Jelić, M. Nutritional value and yield of the oat grain. Agro-know J. 2012, 13, 217–224. [Google Scholar]
- Djukić, D.A.; Emtsev, V.T. Microbiological Biotechnology; Dereta: Belgrade, Republic of Serbia, 2003; pp. 449–455. [Google Scholar]
- Cecílio Filho, A.B.; Nascimento, C.S.; de Pereira, J.B.; Nascimento, C.S. Nitrogen fertilization impacts greenhouse gas emissions, carbon footprint, and agronomic responses of beet intercropped with arugula. J. Environ. Manag. 2022, 307, 114568. [Google Scholar] [CrossRef]
- Du, T.-Y.; He, H.-Y.; Zhang, Q.; Lu, L.; Mao, W.-J.; Zhai, M.-Z. Positive effects of organic fertilizers and biofertilizers on soil microbial community composition and walnut yield. Appl. Soil Ecol. 2022, 175, 104457. [Google Scholar] [CrossRef]
- Zheng, S.; Yin, K.; Yu, L. Factors influencing the farmer’s chemical fertilizer reduction behavior from the perspective of farmer differentiation. Heliyon 2022, 8, e11918. [Google Scholar] [CrossRef] [PubMed]
- Gent, M. Growth and composition of salad greens as affected by organic compared to nitrate fertilizer and by environment in high tunnels. J. Plant Nutr. 2002, 25, 981–998. [Google Scholar] [CrossRef]
- Bloem, E.; Albihn, A.; Elving, J.; Hermann, L.; Lehmann, L.; Sarvi, M.; Schaaf, T.; Schick, J.; Turtola, E.; Ylivainio, K. Contamination of organic nutrient sources with potentially toxic elements, antibiotics and pathogen microorganisms in relation to P fertilizer potential and treatment options for the production of sustainable fertilizers: A review. Sci. Total Environ. 2017, 607–608, 225–242. [Google Scholar] [CrossRef]
- Ren, J.; Liu, X.; Yang, W.; Yang, X.; Li, W.; Xia, Q.; Li, J.; Gao, Z.; Yang, Z. Rhizosphere soil properties, microbial community, and enzyme activities: Short-term responses to partial substitution of chemical fertilizer with organic manure. J. Environ. Manag. 2021, 299, 113650. [Google Scholar] [CrossRef]
- Abou-Sreea, A.I.B.; Rady, M.M.; Roby, M.H.H.; Ahmed, S.M.A.; Majrashi, A.; Ali, E.F. Cattle manure and bio-nourishing royal jelly as alternatives to chemical fertilizers: Potential for sustainable production of organic Hibiscus sabdariffa L. J. Appl. Res. Med. Aromat. Plants 2021, 25, 100334. [Google Scholar] [CrossRef]
- Zhai, L.; Wang, Z.; Zhai, Y.; Zhang, L.; Zheng, M.; Yao, H.; Lv, L.; Shen, H.; Zhang, J.; Yao, Y.; et al. Partial substitution of chemical fertilizer by organic fertilizer benefits grain yield, water use efficiency, and economic return of summer maize. Soil Tillage Res. 2022, 217, 105287. [Google Scholar] [CrossRef]
- Pan, Y.; Guo, J.; Fan, L.; Ji, Y.; Liu, Z.; Wang, F.; Pu, Z.; Ling, N.; Shen, Q.; Guo, S. The source-sink balance during the grain filling period facilitates rice production under organic fertilizer substitution. Eur. J. Agron. 2022, 134, 126468. [Google Scholar] [CrossRef]
- Li, X.; Li, B.; Chen, L.; Liang, J.; Huang, R.; Tang, X.; Zhang, X.; Wang, C. Partial substitution of chemical fertilizer with organic fertilizer over seven years increases yields and restores soil bacterial community diversity in wheat–rice rotation. Eur. J. Agron. 2022, 133, 126445. [Google Scholar] [CrossRef]
- Byrne, M.P.; Tobin, J.T.; Forrestal, P.J.; Danaher, M.; Nkwonta, C.G.; Richards, K.; Cummins, E.; Hogan, S.A.; O’Callaghan, T.F. Urease and nitrification inhibitors—Mitigation tools for greenhouse gas emissions in sustainable dairy systems: A review. Sustainability 2020, 12, 6018. [Google Scholar] [CrossRef]
- Van de Wiel, C.C.M.; van der Linden, C.G.; Scholten, O.E. Improving phosphorus use efficiency in agriculture: Opportunities for breeding. Euphytica 2016, 207, 1–22. [Google Scholar] [CrossRef]
- Roychowdhury, D.; Paul, M.; Banerjee, S.K. A review of the effects of biofertilizers and biopesticides on rice and tea cultivation and productivity. Int. J. Eng. Sci. Technol. 2014, 2, 96–105. [Google Scholar]
- Shaheen, A.; Fatma, M.; Rizk, A.; Singer, S.M. Growing onion plants without chemical fertilization. Res. J. Agr. Biol. Sci. 2007, 3, 95–104. [Google Scholar]
- Kawa, A.A.; Hussain, H.H.; Shorsh, K.Q. Effect of bio and chemical fertilizers on some physiological traits and yield of arugula. J. Zankoy Sulaimani Part A 2020, 22, 99–108. [Google Scholar]
- Naghdi, A.A.; Piri, S.; Khaligi, A.; Moradi, P. Enhancing the qualitative and quantitative traits of potato by biological, organic, and chemical fertilizers. J. Saudi Soc. Agric. Sci. 2022, 21, 87–92. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Djurić, N.; Kresović, B.; Glamočlija, D. Systems of Conventional and Organic Production of Field Crops; Grafos Internacional: Pančevo, Republic of Serbia, 2015; pp. 335–336. [Google Scholar]
- Shimoia, E.P.; Da-Silva, C.J.; Posso, D.A.; da Silva Martins, T.; Agualongo, D.A.P.; de Oliveira, A.C.B.; do Amarante, L. Co-inoculation of seeds with Bradyrhizobium, Azospirillum, and Rhizophagus improves nitrogen assimilation and growth in soybean plants subjected to waterlogging. Russ. J. Plant Physiol. 2023, 70, 146. [Google Scholar] [CrossRef]
- Stajković-Srbinović, O.; Delić, D.; Rasulić, N.; Kuzmanović, D.; Houšková, B.; Sikirić, B.; Mrvić, V. Microorganisms in soils with high nickel and chromium concentrations in Western Serbia. Pol. J. Environ. Stud. 2017, 26, 1663–1671. [Google Scholar] [CrossRef] [PubMed]
- Stanojković-Sebić, A.; Dinić, Z.; Iličić, R.; Pivić, R.; Jošić, D. Effect of indigenous Pseudomonas chlororaphis strains on morphological and main chemical growth parameters of basil (Ocimum basilicum L.). Ratar. Povrt. 2017, 54, 42–47. [Google Scholar] [CrossRef]
- SRPS ISO 11464:2004; Soil Quality—Pretreatment of Samples for Physico-Chemical Analysis. Institute for Standardization: Belgrade, Republic of Serbia, 2004.
- ISO 18400-206:2018; Soil Quality—Sampling—Part 206: Collection, Handling and Storage of Soil under Aerobic Conditions for the Assessment of Microbiological Processes, Biomass and Diversity in the Laboratory. International Organization for Standardization: Geneva, Switzerland, 2018.
- ISO 11277:2020; Soil Quality—Determination of Particle Size Distribution in Mineral Soil Material—Method by Sieving and Sedimentation. International Organization for Standardization: Geneva, Switzerland, 2020.
- Moeys, J. The Soil Texture Wizard: R Functions for Plotting, Classifying, Transforming and Exploring Soil Texture Data. 2018. Available online: https://cran.r-project.org/web/packages/soiltexture/vignettes/soiltexture_vignette.pdf (accessed on 21 October 2020).
- SRPS EN ISO 10390:2022; Soil, Treated Biowaste and Sludge—Determination of pH. Institute for Standardization: Belgrade, Republic of Serbia, 2022.
- Milivojević, J.Ž.; Djalović, I.G.; Jelić, M.Ž.; Trifunović, S.R.; Bogdanović, D.M.; Milošev, D.S.; Nedeljković, B.D.; Bjelić, D.D. Distribution and forms of manganese in vertisols of Serbia. J. Serb. Chem. Soc. 2011, 76, 1177–1190. [Google Scholar] [CrossRef]
- SRPS ISO 13878:2005; Soil Quality—Determination of Total Nitrogen Content by Dry Combustion (“Elemental Analysis”). Institute for Standardization: Belgrade, Republic of Serbia, 2005.
- Latković, D.; Maksimović, J.; Dinić, Z.; Pivić, R.; Stanojković, A.; Stanojković-Sebić, A. Case study upon foliar application of biofertilizers affecting microbial biomass and enzyme activity in soil and yield related properties of maize and wheat grains. Biology 2020, 9, 452. [Google Scholar] [CrossRef] [PubMed]
- Laid, B.; Kamel, K.; Mouloud, G.; Manel, S.; Walid, S.; Amar, B.; Hamenna, B.; Faiçal, B. Effects of plant growth promoting rhizobacteria (PGPR) on in vitro bread wheat (Triticum aestivum L.) growth parameters and biological control mechanisms. Adv. Microbiol. 2016, 6, 677–690. [Google Scholar] [CrossRef][Green Version]
- SRPS ISO 11465:2002; Soil Quality—Determination of Dry Matter and Water Content on a Mass Basis—Gravimetric Method. Institute for Standardization: Belgrade, Republic of Serbia, 2002.
- Sarić, Z. Manual in Microbiology; Science book: Belgrade, Republic of Serbia, 1989. [Google Scholar]
- SRPS EN ISO 23753-1:2019; Soil Quality—Determination of Dehydrogenase Activity in Soil—Part 1: Method Using Triphenyltetrazolium Chloride (TTC). Institute for Standardization: Belgrade, Republic of Serbia, 2019.
- Miller, R.O. Determination of dry matter content of plant tissue: Gravimetric moisture. In Handbook of Reference Methods for Plant Analysis; Kalra, Y., Ed.; CRC Press, Taylor & Francis Group: Boca Raton, Florida, 1998; pp. 51–52. [Google Scholar]
- Djurdjević, B. Spectrophotometric determination of phosphorus. In Manual in Plant Nutrition; Miklavčić, D., Ed.; Faculty of Agriculture: Osijek, Croatia, 2014; pp. 60–61. [Google Scholar]
- Djurdjević, B. Determination of K, Ca, Mg, and Na. In Manual in Plant Nutrition; Miklavčić, D., Ed.; Faculty of Agriculture: Osijek, Croatia, 2014; pp. 62–63. [Google Scholar]
- Antonious, G.F. The impact of organic, inorganic fertilizers, and biochar on phytochemicals content of three Brassicaceae vegetables. Appl. Sci. 2023, 13, 8801. [Google Scholar] [CrossRef]
- Gonçalves, J.; Freitas, J.; Fernandes, I.; Silva, P. Microalgae as biofertilizers: A sustainable way to improve soil fertility and plant growth. Sustainability 2023, 15, 12413. [Google Scholar] [CrossRef]
- Biberdžić, O.; Barać, S.; Lalević, D.; Stojiljković, J.; Knežević, B.; Beković, D. Influence of soil type and compaction on maize yield. J. Agric. Sci. 2018, 63, 323–334. [Google Scholar]
- Ma, G.; Cheng, S.; He, W.; Dong, Y.; Qi, S.; Tu, N.; Tao, W. Effects of organic and inorganic fertilizers on soil nutrient conditions in rice fields with varying soil fertility. Land 2023, 12, 1026. [Google Scholar] [CrossRef]
- Jin, N.; Jin, L.; Wang, S.; Li, J.; Liu, F.; Liu, Z.; Luo, S.; Wu, Y.; Lyu, J.; Yu, J. Reduced chemical fertilizer combined with bio-organic fertilizer affects the soil microbial community and yield and quality of lettuce. Front. Microbiol. 2022, 13, 863325. [Google Scholar] [CrossRef] [PubMed]
- Mandić, L. Microbiological Activity and Productivity of Vertisol under Maize in Conditions of Different Fertilizers Application. Ph.D. Thesis, University of Kragujevac, Faculty of Agriculture, Čačak, Republic of Serbia, 2002. [Google Scholar]
- Suliasih; Widawati, S. The effect of biofertilizer combined with organic or inorganic fertilizer on growth of Caesalpinia pulcherrima and bacterial population in soil. IOP Conf. Ser. Earth Environ. Sci. 2018, 166, 012024. [Google Scholar]
- Brar, A.; Gosal, S.K.; Walia, S.S. Effect of biofertilizer and farmyard manure on microbial dynamics and soil health in maize (Zea mays L.) rhizosphere. Chem. Sci. Rev. Lett. 2017, 6, 1524–1529. [Google Scholar]
- Angulo, J.; Martínez-Salgado, M.M.; Ortega-Blu, R.; Fincheira, P. Combined effects of chemical fertilization and microbial inoculant on nutrient use efficiency and soil quality indicators. Sci. Agropecu. 2020, 11, 375–380. [Google Scholar] [CrossRef]
- Dincǎ, L.C.; Grenni, P.; Onet, C.; Onet, A. Fertilization and soil microbial community: A review. Appl. Sci. 2022, 12, 1198. [Google Scholar] [CrossRef]
- Tao, R.; Liang, Y.; Wakelin, S.A.; Chu, G. Supplementing chemical fertilizer with an organic component increases soil biological function and quality. Appl. Soil Ecol. 2015, 96, 42–51. [Google Scholar] [CrossRef]
- Li, J.; Zhao, B.; Li, X.; Jiang, R.; Bing, S.H. Effects of long-term combined application of organic and mineral fertilizers on microbial biomass, soil enzyme activities, and soil fertility. Agric. Sci. China 2008, 7, 336–343. [Google Scholar] [CrossRef]
- Stanojković-Sebić, A.; Djukić, A.D.; Mandić, L.; Pivić, R.; Stanojković, A. Evaluation of mineral and bacterial fertilization influence on the number of microorganisms from the nitrogen cycle in soil under maize. Commun. Soil Sci. Plant Anal. 2012, 43, 2777–2788. [Google Scholar] [CrossRef]
- Jarak, M.; Milošević, N.; Milić, V.; Mrkovački, N.; Djurić, S.; Marinković, J. Microbiological activities—Fertility and soil degradation indicators. Econ. Agric. 2005, 52, 483–493. [Google Scholar]
- Cassman, N.A.; Leite, M.F.A.; Pan, Y.; de Hollander, M.; van Veen, J.A.; Kuramae, E.E. Plant and soil fungal but not soil bacterial communities are linked in long-term fertilized grassland. Sci. Rep. 2016, 6, 23680. [Google Scholar] [CrossRef]
- Semenov, M.V.; Krasnov, G.S.; Semenov, V.M.; van Bruggen, A. Mineral and organic fertilizers distinctly affect fungal communities in the crop rhizosphere. J. Fungi 2022, 8, 251. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, S.; Dubey, Y.P. Effect of biofertilizers and inorganic fertilizers on yield attributes, yield and quality of Triticum aestivum and Zea mays in an acid Alfisol. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 2594–2603. [Google Scholar] [CrossRef][Green Version]
- Mantovani, J.R.; Carrera, M.; Alvarenga, M.J.L.; José, M.D.; Bortolotti Da Silva, A. Fertility properties and leafy vegetable production in soils fertilized with cattle manure. Rev. Caatinga Mossoró 2017, 30, 825–836. [Google Scholar] [CrossRef][Green Version]
- Barlas, N.T.; Irget, M.E.; Tepecik, M. Mineral content of the rocket plant (Eruca sativa). Afr. J. Biotechnol. 2011, 10, 14080–14082. [Google Scholar]
- Demir, H.; Sönmez, İ.; Uçan, U.; Akgün, İ.H. Biofertilizers improve the plant growth, yield, and mineral concentration of lettuce and broccoli. Agronomy 2023, 13, 2031. [Google Scholar] [CrossRef]
- de Morais, E.G.; Freire, M.M.; de Santos, A.Y.O.; da Silva, G.G.C.; Oliveira, E.M.M.; Franco, L.B.; de Souza, G.L.F. Growth and accumulation of macronutrients in arugula. Int. J. Adv. Eng. Res. Sci. 2020, 7, 176–183. [Google Scholar] [CrossRef]
- Bantis, F.; Kaponas, C.; Charalambous, C.; Koukounaras, A. Strategic successive harvesting of rocket and spinach baby leaves enhanced their quality and production efficiency. Agriculture 2021, 11, 465. [Google Scholar] [CrossRef]
- Sharma, A.; Chetani, R. A review on the effect of organic and chemical fertilizers on plants. Int. J. Res. Appl. Sci. Eng. Technol. 2017, 5, 677–680. [Google Scholar] [CrossRef]
- Al Methyeb, M.; Ruppel, S.; Eichler-Löbermann, B.; Vassilev, N. The combined applications of microbial inoculants and organic fertilizer improve plant growth under unfavourable soil conditions. Microorganisms 2023, 11, 1721. [Google Scholar] [CrossRef]

| Treatment | Abbreviation |
|---|---|
| Inorganic Fertilizer (alone) | NPK |
| Organic Fertilizer (alone) | NC |
| Inorganic Fertilizer + Organic Fertilizer | NPK+NC |
| Inorganic Fertilizer + Biological Fertilizer | NPK+MIX |
| Organic Fertilizer + Biological Fertilizer | NC+MIX |
| Biological Fertilizer (alone) | MIX |
| Control | Ø, non-fertilized soil |
| Tested Parameters | |
|---|---|
| Textural class | Light clay |
| Granulometric and chemical parameters | Values (mean ± standard deviation) |
| Silt (%), fractions 0.02–0.002 mm | 33.1 ± 0.45 |
| Clay (%), fractions < 0.002 mm | 36.6 ± 0.67 |
| Sand (%), fractions > 0.02 mm | 30.3 ± 0.26 |
| pH in H2O | 5.80 ± 0.08 |
| pH in 1M KCl | 4.72 ± 0.03 |
| Total N (%) | 0.17 ± 0.002 |
| Soil organic matter (SOM, %) | 3.30 ± 0.04 |
| Available P (mg 100 g−1) | 0.20 ± 0.00 |
| Available K (mg 100 g−1) | 26.70 ± 0.30 |
| Microbiological parameters | |
| Total number of microorganisms (×106) 1 | 7.04 ± 1.88 |
| Fungi (×104) 1 | 7.09 ± 1.55 |
| Actinomycetes (×104) 1 | 7.56 ± 1.92 |
| Azotobacter spp. 2 | 22.67 ± 0.00 |
| Ammonifiers (×105) 2 | 115.50 ± 48.69 |
| DHA (µg TPF g−1) 3 | 39.97 ± 4.18 |
| Fertilization Treatment 1 | Tested Parameters (Average ± Standard Deviation) | |||||
|---|---|---|---|---|---|---|
| Total Number of Microorganisms (×106) 2 | Fungi (×104) 2 | Actinomycetes (×104) 2 | Azotobacter spp. 3 | Ammonifiers (×105) 3 | DHA (µg TPF g−1) 4 | |
| NPK | 6.93 ± 1.27 c | 13.15 ± 1.40 a | 8.70 ± 0.64 b | 30.67 ± 8.08 c | 121.67 ± 12.58 a | 86.91 ± 1.02 e |
| NC | 10.75 ± 1.46 bc | 12.61 ± 1.46 a | 12.57 ± 1.71 b | 24.00 ± 5.29 c | 121.83 ± 3.18 a | 88.21 ± 1.53 e |
| NPK+NC | 14.61 ± 1.20 ab | 10.79 ± 1.72 ab | 12.18 ± 2.11 b | 31.00 ± 6.56 c | 120.33 ± 10.00 a | 104.37 ± 0.51 c |
| NPK+MIX | 14.76 ± 1.08 ab | 8.71 ± 1.36 ab | 13.82 ± 3.39 b | 49.33 ± 9.45 b | 133.67 ± 2.75 a | 127.11 ± 0.40 b |
| NC+MIX | 17.96 ± 4.78 a | 10.15 ± 1.36 ab | 19.30 ± 4.27 a | 62.00 ± 10.00 a | 137.00 ± 2.60 a | 134.76 ± 2.15 a |
| MIX | 11.96 ± 0.93 b | 7.11 ± 2.04 b | 9.26 ± 0.65 b | 31.67 ± 5.77 c | 123.67 ± 10.61 a | 96.71 ± 1.19 d |
| Ø, non-fertilized | 9.53 ± 0.70 bc | 7.16 ± 2.16 b | 7.92 ± 1.92 b | 25.00 ± 0.00 c | 118.50 ± 6.06 a | 54.17 ± 1.16 f |
| Statistical analyses | Source of variation | |||||
| Fertilization treatment | ||||||
| p value | *** | ** | *** | *** | NSD | *** |
| LSD (0.05) | 3.56 | 3.28 | 4.20 | 12.56 | 13.75 | 2.25 |
| LSD (0.01) | 5.07 | 4.55 | 5.83 | 17.44 | 19.09 | 3.13 |
| Fertilization Treatment 1 | Chemical Composition (%, Dry Biomass) 2 | |||
|---|---|---|---|---|
| N | C | P | K | |
| NPK | 3.90 ± 0.35 a | 33.57 ± 0.29 a | 0.44 ± 0.01 b | 7.15 ± 0.02 c |
| NC | 5.39 ± 0.99 a | 33.74 ± 0.47 a | 0.40 ± 0.01 bc | 7.08 ± 0.03 d |
| NPK+NC | 5.66 ± 0.58 a | 32.65 ± 0.66 a | 0.49 ± 0.02 a | 7.18 ± 0.01 c |
| NPK+MIX | 5.70 ± 0.18 b | 35.17 ± 0.32 a | 0.35 ± 0.04 cd | 7.80 ± 0.02 a |
| NC+MIX | 5.61 ± 0.33 a | 32.93 ± 1.85 a | 0.41 ± 0.02 b | 7.64 ± 0.01 b |
| MIX | 5.65 ± 0.26 a | 34.69 ± 1.10 a | 0.37 ± 0.02 cd | 6.95 ± 0.04 e |
| Ø, non-fertilized | 5.18 ± 0.13 a | 33.93 ± 0.62 a | 0.23 ± 0.01 f | 6.61 ± 0.01 f |
| Statistical analyses | Source of variation | |||
| Fertilization treatment | ||||
| p value | * | NSD | *** | *** |
| LSD (0.05) | 1.046 | 1.960 | 0.037 | 0.039 |
| LSD (0.01) | 1.452 | 2.721 | 0.051 | 0.054 |
| Fertilization Treatment 1 | Chemical Composition (%, Dry Biomass) 2 | |||
|---|---|---|---|---|
| N | C | P | K | |
| NPK | 6.09 ± 0.08 b | 34.53 ± 0.58 b | 0.42 ± 0.02 c | 7.15 ± 0.02 d |
| NC | 5.70 ± 0.01 c | 35.19 ± 0.31 ab | 0.42 ± 0.03 c | 7.11 ± 0.01 e |
| NPK+NC | 4.21 ± 0.10 d | 35.66 ± 0.29 ab | 0.50 ± 0.03 a | 7.18 ± 0.01 c |
| NPK+MIX | 6.36 ± 0.17 ab | 35.89 ± 0.51 a | 0.44 ± 0.01 bc | 7.87 ± 0.02 a |
| NC+MIX | 6.44 ± 0.15 a | 35.33 ± 0.24 ab | 0.47 ± 0.02 ab | 7.74 ± 0.01 b |
| MIX | 6.10 ± 0.07 b | 35.67 ± 0.27 ab | 0.38 ± 0.02 d | 6.99 ± 0.01 f |
| Ø, non-fertilized | 5.74 ± 0.08 c | 36.42 ± 0.32 a | 0.21 ± 0.01 e | 6.59 ± 0.01 g |
| Statistical analyses | Source of variation | |||
| Fertilization treatment | ||||
| p value | *** | ** | *** | *** |
| LSD (0.05) | 0.229 | 0.814 | 0.034 | 0.022 |
| LSD (0.01) | 0.319 | 1.129 | 0.047 | 0.031 |
| Fertilization Treatment 1 | Chemical Composition (%, Dry Biomass) 2 | |||
|---|---|---|---|---|
| N | C | P | K | |
| NPK | 2.66 ± 0.05 de | 32.19 ± 0.18 b | 0.33 ± 0.02 c | 6.81 ± 0.10 a |
| NC | 3.94 ± 0.04 b | 33.22 ± 0.23 a | 0.35 ± 0.04 bc | 6.08 ± 0.04 c |
| NPK+NC | 2.83 ± 0.05 d | 32.47 ± 0.26 b | 0.38 ± 0.01 b | 6.18 ± 0.01 c |
| NPK+MIX | 4.72 ± 0.15 a | 33.63 ± 0.37 a | 0.33 ± 0.02 c | 6.80 ± 0.11 a |
| NC+MIX | 3.40 ± 0.15 c | 33.55 ± 0.32 a | 0.42 ± 0.01 a | 6.66 ± 0.06 b |
| MIX | 3.21 ± 0.06 c | 32.20 ± 0.20 b | 0.31 ± 0.02 c | 6.12 ± 0.03 c |
| Ø, non-fertilized | 2.58 ± 0.03 e | 33.62 ± 0.46 a | 0.23 ± 0.02 d | 6.61 ± 0.02 b |
| Statistical analyses | Source of variation | |||
| Fertilization treatment | ||||
| p value | *** | *** | *** | *** |
| LSD (0.05) | 0.191 | 0.652 | 0.037 | 0.113 |
| LSD (0.01) | 0.266 | 0.905 | 0.051 | 0.156 |
| Fertilization Treatment 1 | Yield (g pot−1, Air Dry Biomass) 2 | |||
|---|---|---|---|---|
| Swath I | Swath II | Swath III | Total Biomass | |
| NPK | 4.52 ± 0.99 b | 5.29 ± 2.55 a | 6.47 ± 1.41 a | 16.28 ± 4.38 b |
| NC | 4.59 ± 2.06 b | 5.99 ± 0.87 a | 6.65 ± 1.93 a | 17.23 ± 1.74 b |
| NPK+NC | 4.71 ± 0.72 b | 5.42 ± 1.62 a | 5.10 ± 2.75 a | 15.23 ± 1.60 b |
| NPK+MIX | 8.60 ± 0.52 a | 7.05 ± 0.55 a | 6.68 ± 2.08 a | 22.33 ± 3.35 a |
| NC+MIX | 4.83 ± 0.54 b | 6.81 ± 1.73 a | 7.25 ± 0.54 a | 18.89 ± 3.48 b |
| MIX | 4.48 ± 1.76 b | 4.31 ± 0.50 a | 4.76 ± 0.82 a | 13.55 ± 1.88 b |
| Ø, non-fertilized | 3.23 ± 0.97 b | 3.59 ± 1.39 a | 3.27 ± 1.86 a | 10.09 ± 1.43 b |
| Statistical analyses | Source of variation | |||
| Fertilization treatment | ||||
| p value | *** | NSD | NSD | * |
| LSD (0.05) | 2.176 | 3.294 | 3.969 | 5.937 |
| LSD (0.01) | 3.020 | 4.496 | 5.509 | 8.239 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanojković-Sebić, A.; Miladinović, V.; Stajković-Srbinović, O.; Pivić, R. Response of Arugula to Integrated Use of Biological, Inorganic, and Organic Fertilization. Microorganisms 2024, 12, 1334. https://doi.org/10.3390/microorganisms12071334
Stanojković-Sebić A, Miladinović V, Stajković-Srbinović O, Pivić R. Response of Arugula to Integrated Use of Biological, Inorganic, and Organic Fertilization. Microorganisms. 2024; 12(7):1334. https://doi.org/10.3390/microorganisms12071334
Chicago/Turabian StyleStanojković-Sebić, Aleksandra, Vladimir Miladinović, Olivera Stajković-Srbinović, and Radmila Pivić. 2024. "Response of Arugula to Integrated Use of Biological, Inorganic, and Organic Fertilization" Microorganisms 12, no. 7: 1334. https://doi.org/10.3390/microorganisms12071334
APA StyleStanojković-Sebić, A., Miladinović, V., Stajković-Srbinović, O., & Pivić, R. (2024). Response of Arugula to Integrated Use of Biological, Inorganic, and Organic Fertilization. Microorganisms, 12(7), 1334. https://doi.org/10.3390/microorganisms12071334






