Structure and Diversity of Endophytic Bacteria in Maize Seeds and Germinating Roots
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pretreatment in Seeds and Roots
2.3. Extraction of Endophytic Bacterial DNA from Seeds, Illumina MiSeq Sequencing, and Bioinformatics Analysis
2.4. Data Processing
3. Results
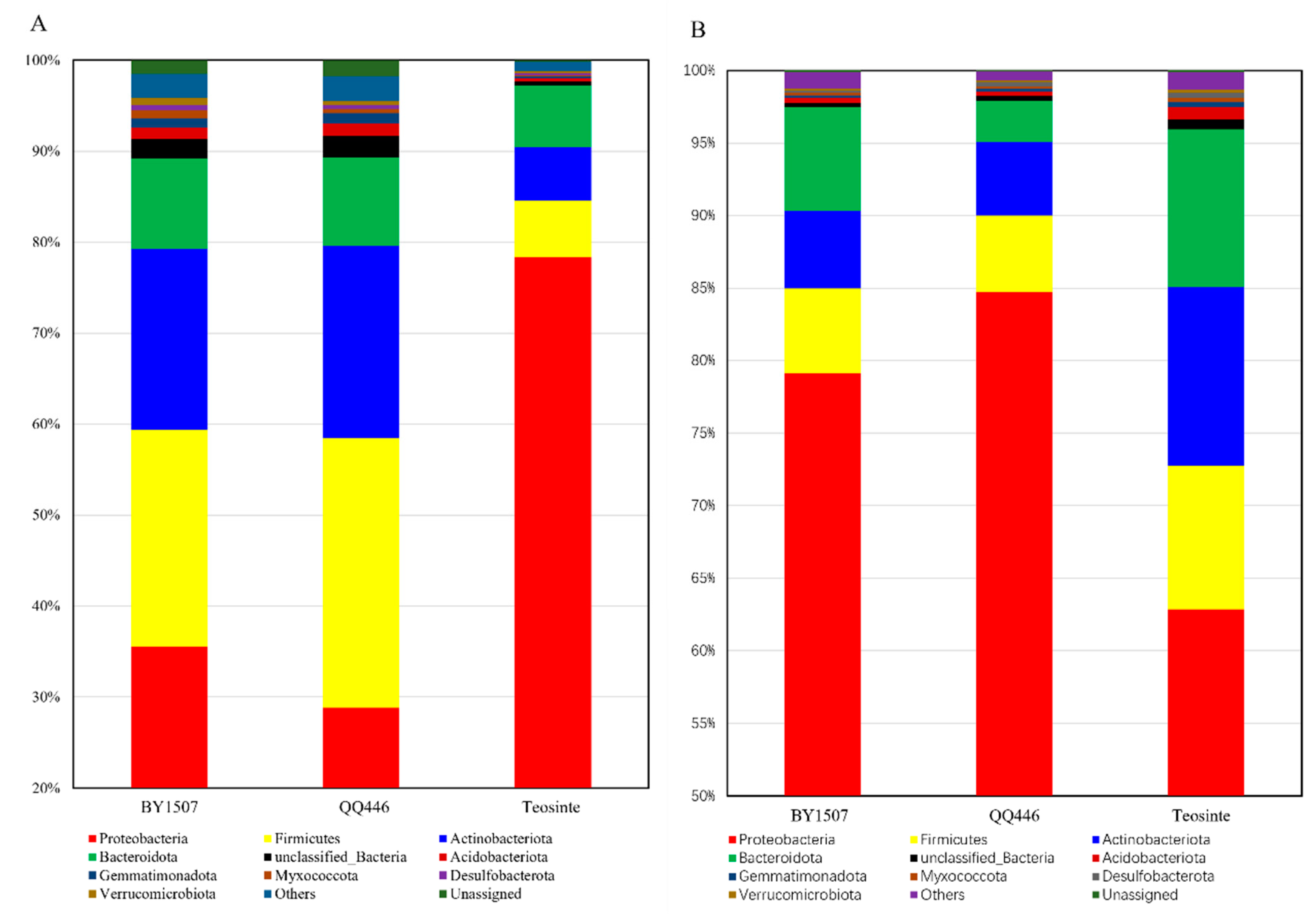
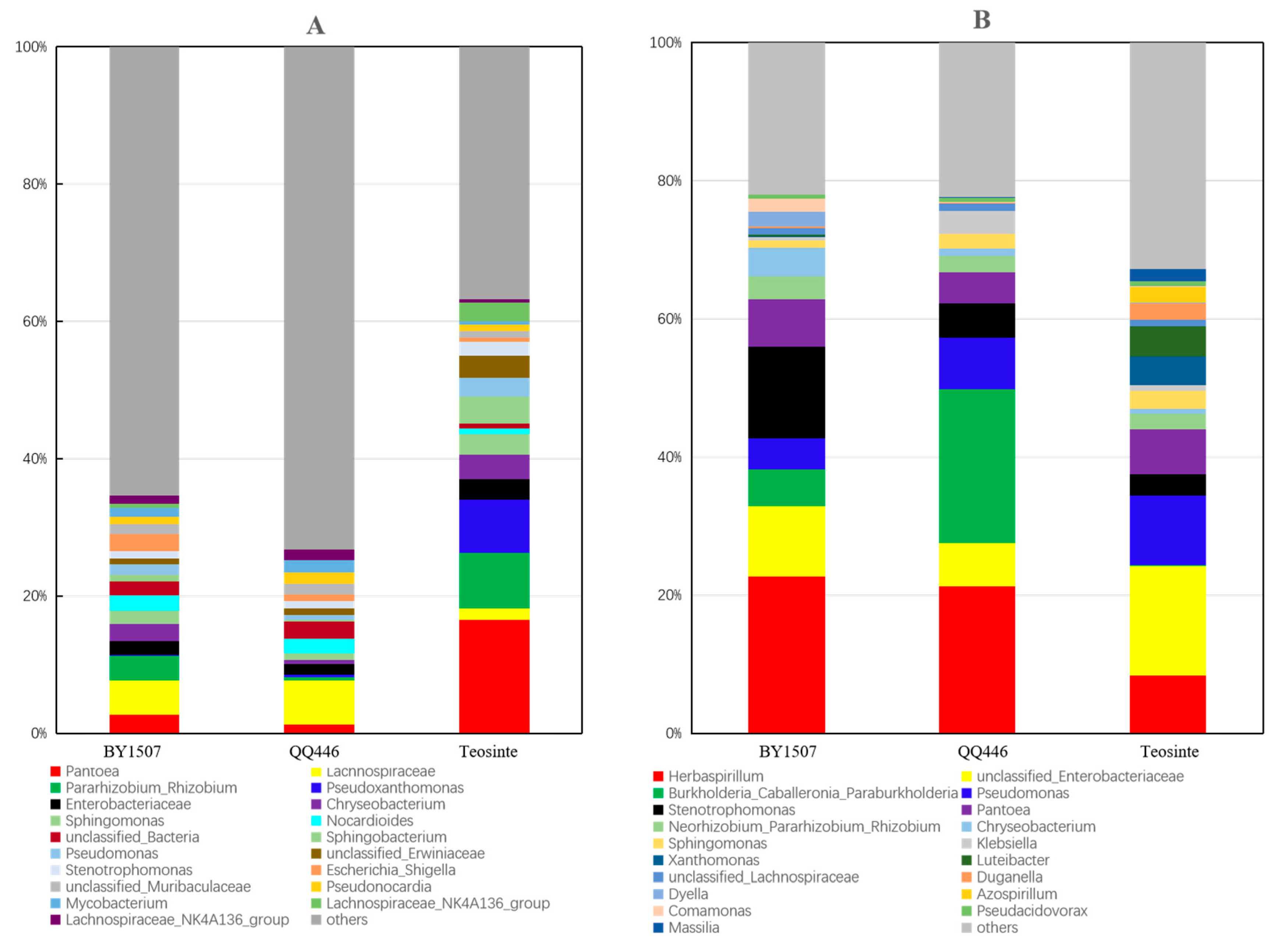
3.1. Composition of Endophytic Bacterial Communities in Seeds and Germinating Roots
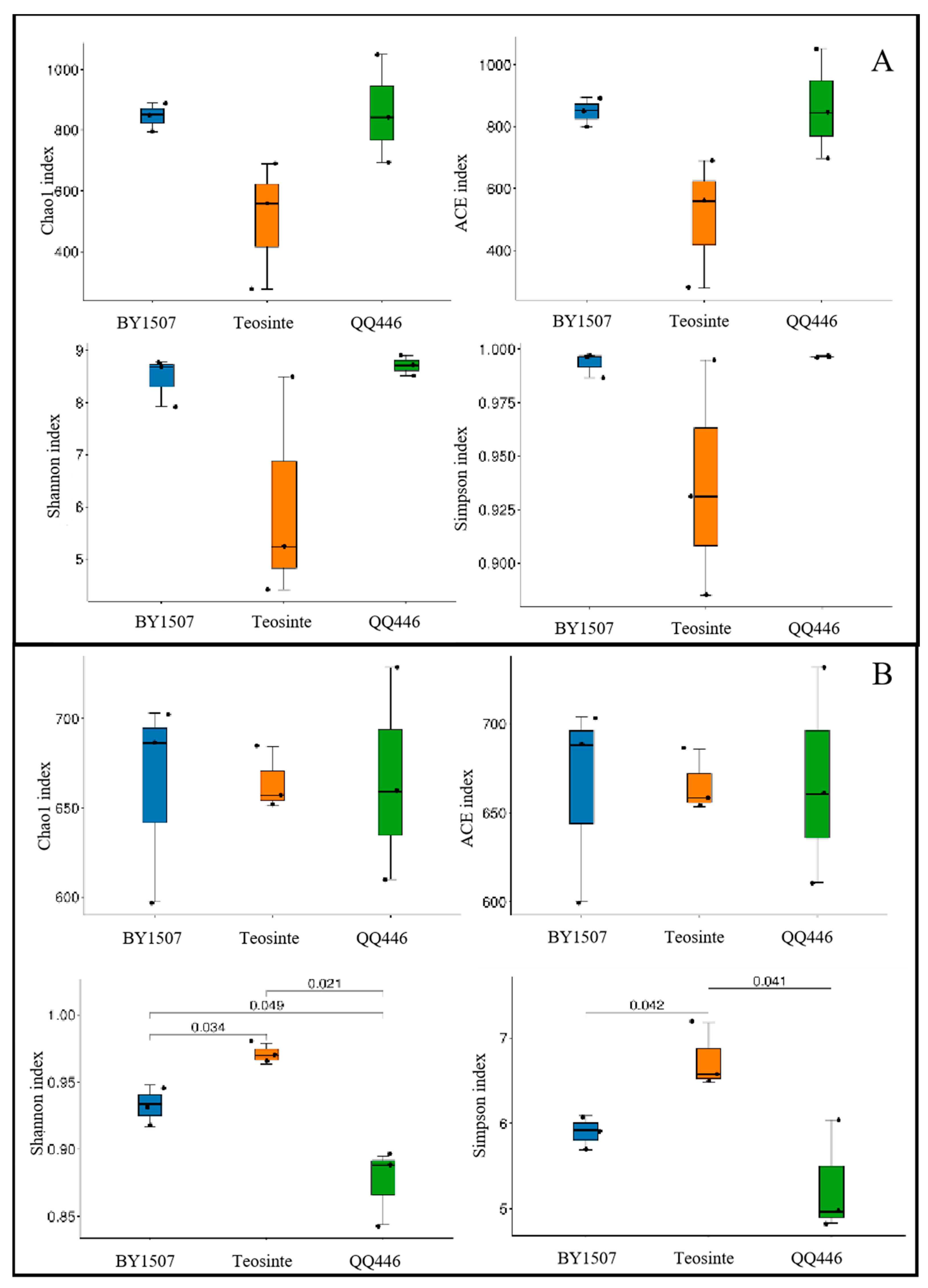
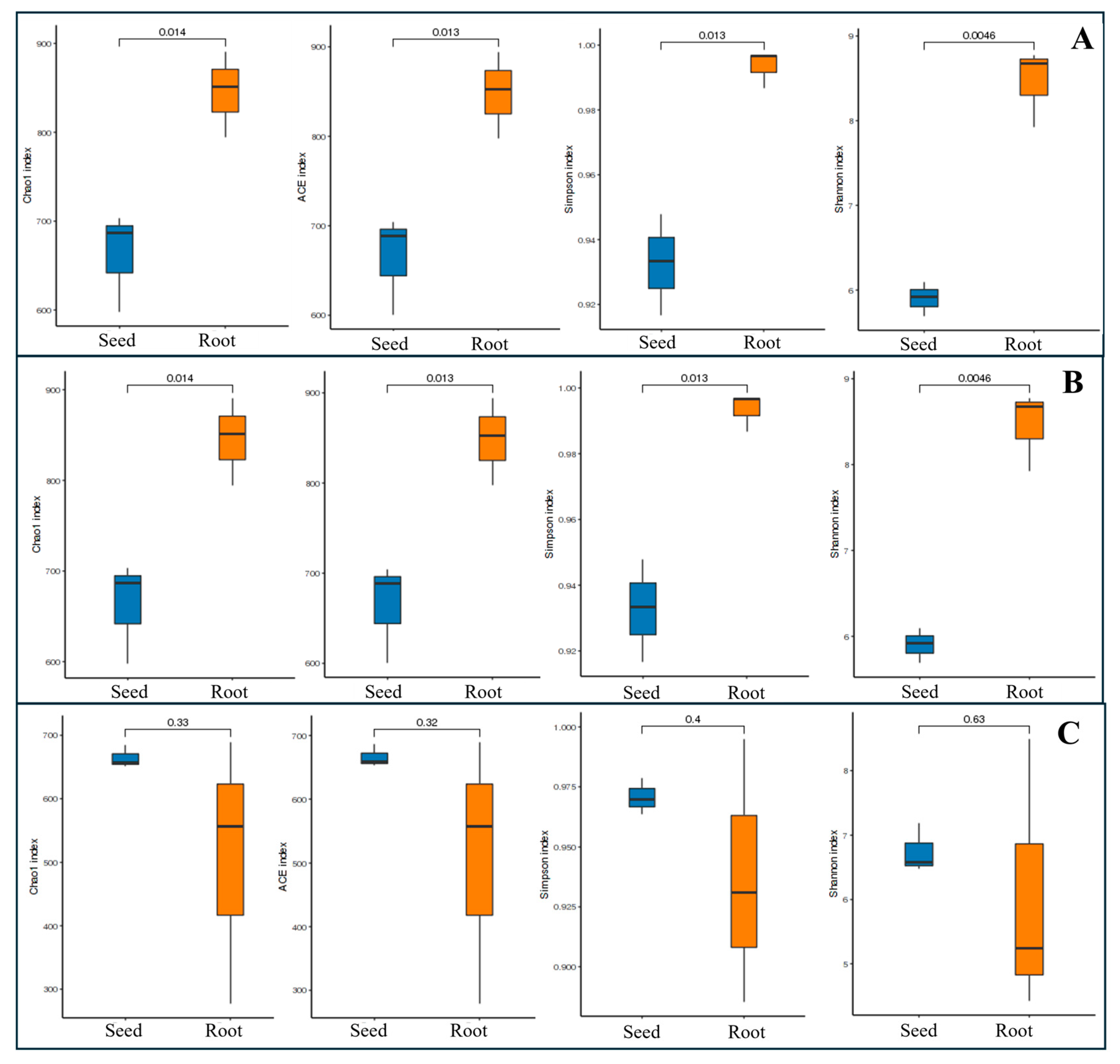
3.2. Endophytic Bacterial Community Diversity in Seeds and Germinating Roots
3.2.1. Alpha Diversity
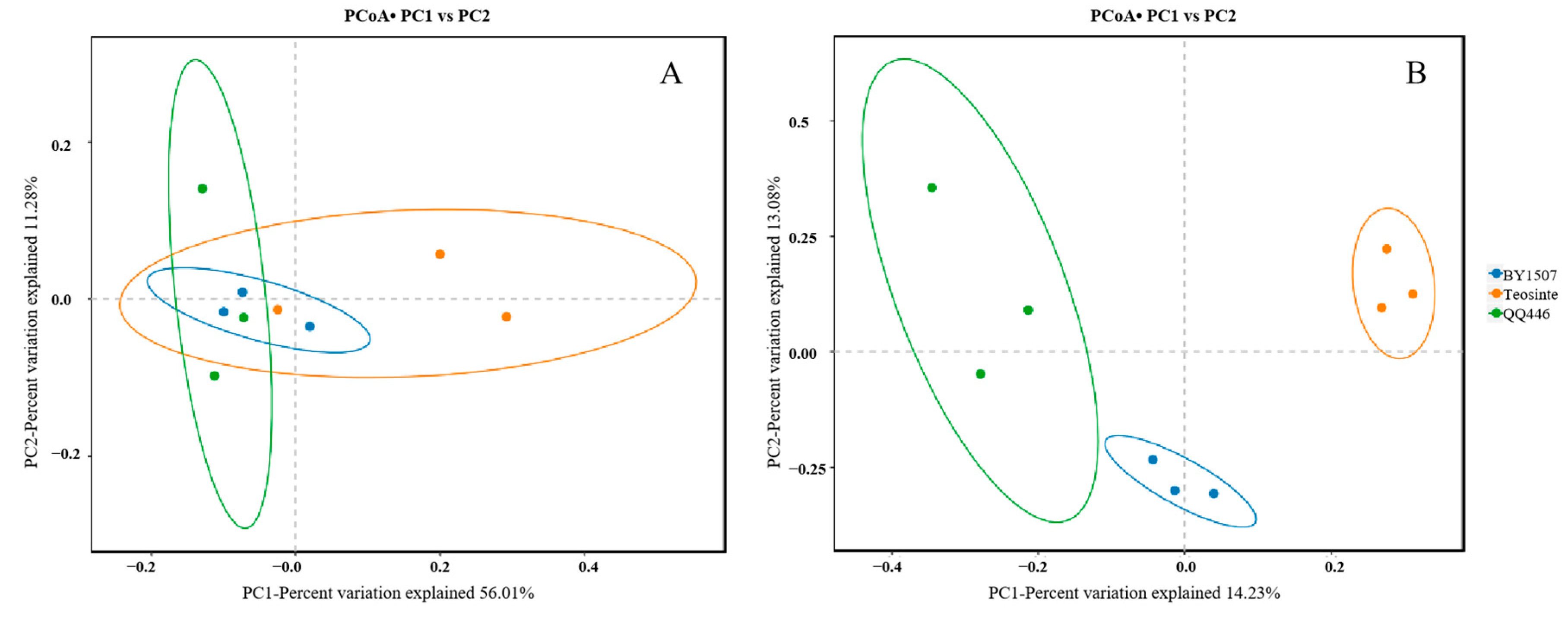
3.2.2. PCoA Analysis
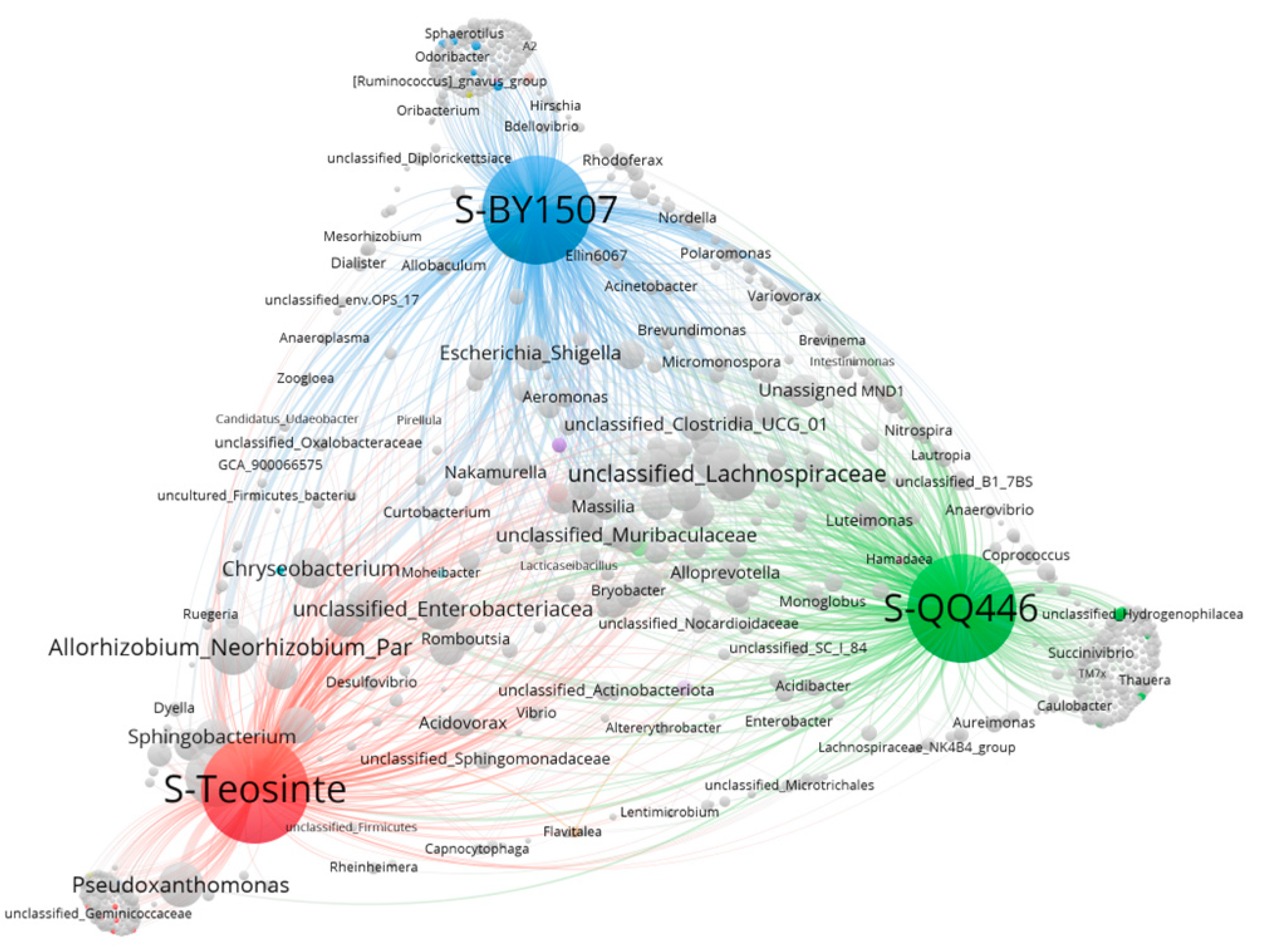
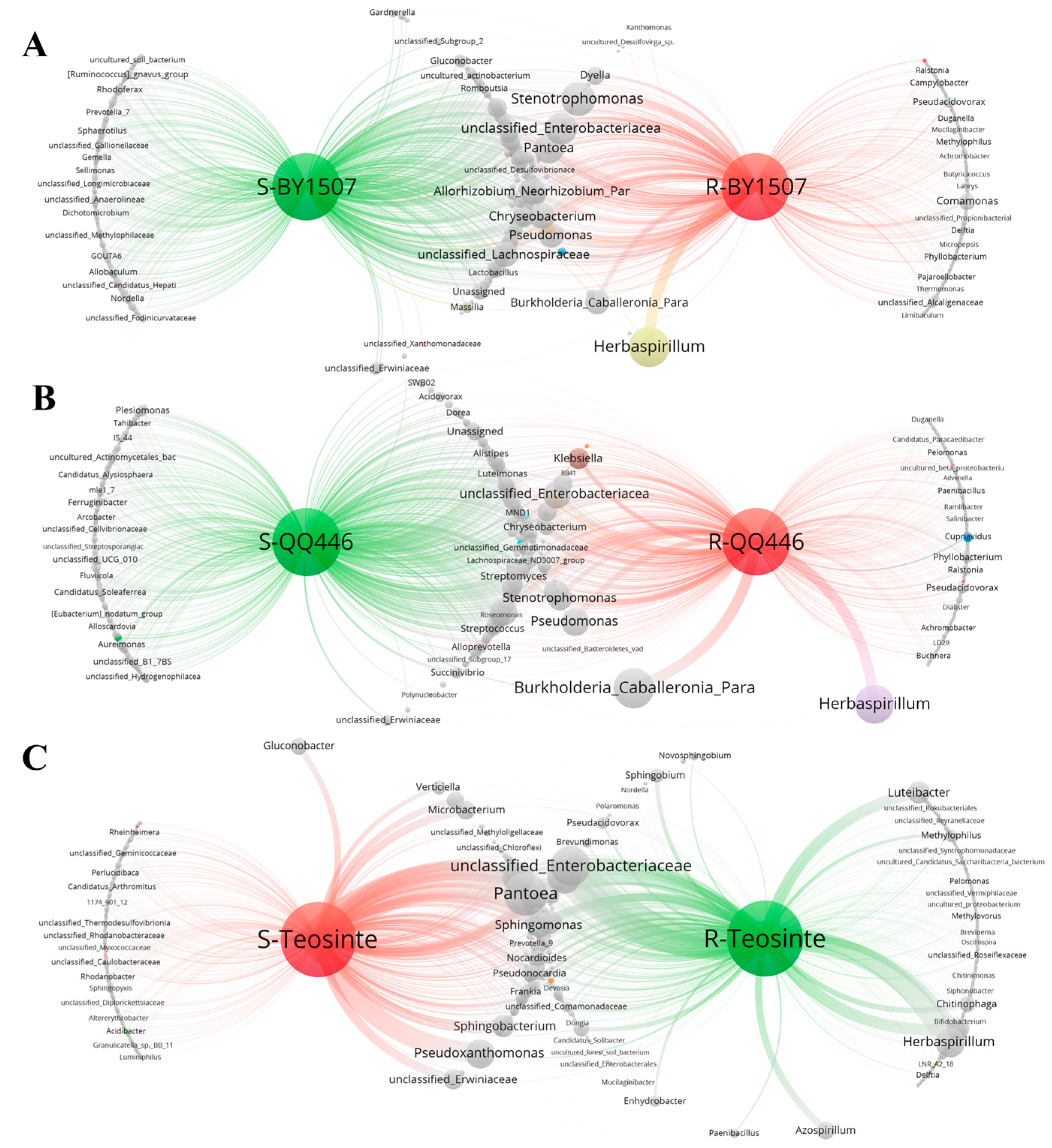
3.3. Co-Occurrence Network of Endophytic Bacteria in Seeds and Germinating Roots
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zou, Y.Y.; Liu, Y.; Wang, J.H.; Song, W. Advances in plant seed-associated microbial ecology. Acta. Ecol. Sin. 2011, 31, 2906–2914. [Google Scholar]
- Wang, X.; He, S.W.; Hou, J.W.; Wei, H.L.; Zhang, X.X. Advances in seed endophytic bacteriome. Acta. Microbiol. Sin. 2023, 63, 1365–1378. [Google Scholar]
- Locey, K.J.; Fisk, M.C.; Lennon, J.T. Microscale insight into microbial seed banks. Front. Microbiol. 2017, 7, 2040. [Google Scholar] [CrossRef] [PubMed]
- Barret, M.; Briand, M.; Bonneau, S.; Préveau, A.; Valière, S.; Bouchez, O.; Hunault, G.; Simoneau, P.; Jacques, M.A. Emergence shapes the structure of the seed microbiota. Appl. Environ. Microbiol. 2015, 81, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Klaedtke, S.; Jacques, M.A.; Raggi, L.; Préveaux, A.; Bonneau, S.; Negri, V.; Chable, V.; Barret, M. Terroir is a key driver of seed-associated microbial assemblages. Environ. Microbiol. 2016, 18, 1792–1804. [Google Scholar] [CrossRef] [PubMed]
- Adam, E.; Bernhart, M.; Müller, H.; Winkler, J.; Berg, G. The Cucurbita pepo seed microbiome: Genotype-specific composition and implications for breeding. Plant Soil 2018, 422, 35–49. [Google Scholar] [CrossRef]
- Kavamura, V.N.; Robinson, R.J.; Hayat, R.; Clark, I.M.; Hughes, D.; Rossmann, M.; Hirsch, P.R.; Mendes, R.; Mauchline, T.H. Land management and microbial seed load effect on rhizosphere and endosphere bacterial community assembly in wheat. Front. Microbiol. 2019, 10, 2625. [Google Scholar] [CrossRef] [PubMed]
- Girsowicz, R.; Moroenyane, I.; Steinberger, Y. Bacterial seed endophyte community of annual plants modulated by plant photosynthetic pathways. Microbiol. Res. 2019, 223, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.; Yeh, M.W.; Hsieh, Y.T.; Chung, W.C.; Lo, C.T.; Young, L.S. Diversity and functional characterization of bacterial endophytes dwelling in various rice (Oryza sativa L.) tissues, and their seed-borne dissemination into rhizosphere under gnotobiotic P-stress. Plant Soil 2015, 394, 177–197. [Google Scholar] [CrossRef]
- Denver, I.; Walitang, D.I.; Chan-Gi, K.C.G.; Sunyoung, J.S.; Yeongyeong, K.Y.; Sa, T. Conservation and transmission of seed bacterial endophytes across generations following crossbreeding and repeated inbreeding of rice at different geographic locations. MicrobiologyOpen. 2019, 8, e662. [Google Scholar]
- Hu, D.D.; Zhang, S.D.; Baskin, J.M.; Baskin, C.C.; Wang, Z.R.; Liu, R.; Du, J.; Yang, X.J.; Huang, Z.Y. Seed mucilage interacts with soil microbial community and physiochemical processes to affect seedling emergence on desert sand dunes. Plant Cell Environ. 2019, 42, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Danzberger, J.; Schöler, A.; Schröder, P.; Schloter, M.; Radl, V. Dominant groups of potentially active bacteria shared by barley seeds become less abundant in root associated microbiome. Front. Plant Sci. 2017, 8, 1005. [Google Scholar] [CrossRef] [PubMed]
- Cope-Selby, N.; Cookson, A.; Squance, M.; Donnison, T.; Flavell, R.; Farrar, K. Endophytic bacteria in Miscanthus seed: Implications for germination, vertical inheritance of endophytes, plant evolution and breeding. GCB Bioenergy 2017, 9, 57–77. [Google Scholar] [CrossRef]
- Majumdar, R.; Kandel, S.L.; Cary, J.W.; Rajasekaran, K. Changes in bacterial endophyte community following Aspergillus flavus infection in resistant and susceptible maize kernels. Int. J. Mol. Sci. 2021, 22, 3747. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhang, R.; Wu, X.; Xu, T.; Ahmad, S.; Zhang, X.; Zhao, J.; Liu, Y. An endophytic strain of the genus Bacillus isolated from the seeds of maize (Zea mays L.) has antagonistic activity against maize pathogenic strains. Microb. Pathog. 2020, 142, 104074. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.G. Maize seed endophytes. Mol. Plant Pathol. 2023, 24, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Prasanna, R.; Hossain, F.; Muthusamy, V.; Nain, L.; Das, S.; Shivay, Y.S.; Kumar, A. Priming maize seeds with cyanobacteria enhances seed vigour and plant growth in elite maize inbreds. 3 Biotech. 2020, 10, 154. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.B.; Li, T.; Shen, M.; Yang, Z.L.; Zhao, Z.W. Evidence for a dark septate endophyte (Exophiala pisciphila, H93) enhancing phosphorus absorption by maize seedlings. Plant Soil 2020, 452, 249–266. [Google Scholar] [CrossRef]
- David, C.G.; Bedmar, E.J.; Arone, G.J. Maize endophytic bacterial diversity as affected by soil cultivation history. Front Mirobiol. 2018, 9, 484. [Google Scholar]
- Cun, H.; Munir, S.; He, P.; Wu, Y.; He, P.; Ahmed, A.; Che, H.; Li, J.; He, Y. Diversity of root endophytic bacteria from maize seedling involved in biocontrol and plant growth promotion. Egypt. J. Biol Pest Co. 2022, 32, 1–9. [Google Scholar] [CrossRef]
- Johnston-Monje, D.; Lundberg, D.S.; Lazarovits, G.; Reis, V.M.; Raizada, M.N. Bacterial populations in juvenile maize rhizospheres originate from both seed and soil. Plant Soil 2016, 405, 337–355. [Google Scholar] [CrossRef]
- Anand, R.; Chanway, C.P. Detection of GFP-labeled Paenibacillus polymyxa in autofluorescing pine seedling tissues. Biol. Fert. Soils 2013, 214, 101–113. [Google Scholar] [CrossRef]
- Mashiane, R.A.; Ezeokoli, O.T.; Adeleke, R.A.; Bezuidenhout, C.C. Metagenomic analyses of bacterial endophytes associated with the phyllosphere of a Bt maize cultivar and its isogenic parental line from South Africa. World. J. Microb. Biot. 2017, 33, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.; Fernando, I.; Mónica, R.; Etcheverry, M.; Esperanza, M.R. Analysis of the bacterial diversity associated with the roots of maize (Zea mays L.) through culture-dependent and culture-independent methods. ISRN Ecol. 2011, 938546. [Google Scholar]
- Szilagyi-Zecchin, V.J.; Ikeda, A.C.; Hungria, M.; Adamoski, D.; Kava-Cordeiro, V.; Glienke, C.; Vitória Galli-Terasawa, L.V. Identification and characterization of endophytic bacteria from corn (Zea mays L.) roots with biotechnological potential in agriculture. AMB Express. 2014, 4, 26. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Fang, Z.J.; Xu, Y.R.; Lu, X.P.; Mu, C.H.; Shan, K.; Hao, L.J. Effects of starane on the community diversity of maize root endophytes analyzed using high-throughput sequencing technology. Crops 2018, 1, 160–165. [Google Scholar]
- Ding, L.L.; Shang, Y.S.; Zhang, W.; Zhang, Y.; Li, S.G.; Wei, X.; Chen, X.; Zhang, Y.J.; Song, X.L.; Chen, X.; et al. Disentangling the effects of driving forces on soil bacterial and fungal communities under shrub encroachment on the Guizhou Plateau of China. Sci. Total Environ. 2020, 709, 136207. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Huang, L.J.; Sun, X.F.; Zhao, L.L.; Wang, P.C. Transcriptomic and metabolomic analyses reveal key metabolites, pathways and candidate genes in Sophora davidii (Franch.) Skeels seedlings under drought stress. Front. Plant Sci. 2022, 13, 785702. [Google Scholar] [CrossRef] [PubMed]
- Pal, G.; Kumar, K.; Verma, A.; Kumar, V.S.K. Seed inhabiting bacterial endophytes of maize promote seedling establishment and provide protection against fungal disease. Microbiol. Res. 2022, 255, 126926. [Google Scholar] [CrossRef]
- Sheibani-Tezerji, R.; Naveed, M.; Jehl, M.A.; Sessitsch, A.; Rattei, T.; Mitter, B. The genomes of closely related Pantoeaananatis maize seed endophytes having different effects on the host plant differ in secretion system genes and mobile genetic elements. Front. Microbiol. 2015, 6, 440. [Google Scholar] [CrossRef]
- Mousa, W.K.; Shearer, C.R.; Limay-Rios, V.; Zhou, T.; Raizad, M.N. Bacterial endophytes from wild maize suppress Fusarium graminearum in modern maize and inhibit mycotoxin accumulation. Front. Plant Sci. 2015, 6, 805. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.X.; Bowatte, S.; Hou, F.J. Diversity of endophytic bacteria and fungi in seeds of Elymusnutans growing in four locations of Qinghai Tibet Plateau, China. Plant Soil 2021, 459, 49–63. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, R.; Ni, L.I.; Cao, Y.H.; Zhang, C.; Bai, F.R.; Zhang, X.; Yuan, L.P.; Wang, W.P.; Cheng, C. Diversity of endophytic bacterial communities in seeds of super hybrid rice (Oryza staiva L). Food Ferment. Ind. 2016, 42, 31–36. [Google Scholar]
- Parlapani, F.F. Microbial diversity of seafood. Curr. Opin. Food Sci. 2021, 37, 45–51. [Google Scholar] [CrossRef]
- Wang, P.C.; Ding, L.L.; Zou, C.; Zhang, Y.J.; Wang, M.Y. Rhizosphere element circling, multifunctionality, aboveground productivity and trade-offs are better predicted by rhizosphere rare taxa. Front. Plant Sci. 2022, 9, 985574. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.C.; Ding, L.L.; Zou, C.; Zhang, Y.J.; Wang, M.Y. Herbivore camping reshapes the taxonomy, function and network of pasture soil microbial communities. Peer J. 2022, 10, e14314. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Sun, X.F.; Zhao, L.L.; Wang, P.C.; Huang, L.J. Morphological, transcriptomic and metabolomic analyses Sophora davidii mutants for plant height. BMC Plant Biol. 2022, 22, 144. [Google Scholar] [CrossRef]
- Ding, L.L.; Tian, L.L.; Li, J.Y.; Zhang, Y.J.; Wang, M.Y.; Wang, P.C. Grazing lowers soil multifunctionality but boosts soil microbial network complexity and stability in a subtropical grassland of China. Front. Microbiol. 2023, 13, 1027097. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.Z.; Li, J.L.; Zhang, C.; Zeng, G.M.; Huang, D.L.; Tan, X.F.; Qin, D.Y.; Tan, H. Biochar in the 21st century: A data-driven visualization of collaboration, frontier identification, and future trend. Sci. Total Environ. 2022, 818, 151774. [Google Scholar] [CrossRef]
- Li, S.J.; Lei, Y.X.; Sun, M.G.; Liu, H.F.; Wang, X.M. Research progress in the diversity of endophytic bacteria in seeds and their interaction with plants. Biotechnol. Bull. 2023, 39, 166–175. [Google Scholar]
- Escalas, A.; Hale, L.; Voordeckers, J.W.; Yang, Y.F.; Firestone, M.K.; Alvarez, C.L.; Zhou, J.Z. Microbial functional diversity: From concepts to applications. Ecol. Evol. 2019, 9, 12000–12016. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, D.W.; Bai, E. Decreasing soil microbial diversity is associated with decreasing microbial biomass under nitrogen addition. Soil Biol. Biochem. 2018, 120, 126–133. [Google Scholar] [CrossRef]
- Ishida, A.; Furuya, T. Diversity and characteristics of culturable endophytic bacteria from Passiflora edulis seeds. MicrobiologyOpen 2021, 10, e1226. [Google Scholar] [CrossRef] [PubMed]
- Bodhankar, S.; Grover, M.; Hemanth, S.; Reddy, G.; Srinivasarao, C. Maize seed endophytic bacteria: Dominance of antagonistic, lytic enzyme-producing Bacillus spp. 3 Biotech. 2017, 7, 232. [Google Scholar] [CrossRef] [PubMed]
- David, J.M.; Raizada, M.N.; Gilbert, M.T.P. Conservation and diversity of seed associated endophytes in Zea across boundaries of evolution, ethnography and ecology. PLoS ONE 2011, 6, e20396. [Google Scholar]
- Liu, Y.; Zuo, S.; Zou, Y.; Wang, J.; Song, W. Investigation on diversity and population succession dynamics of endophytic bacteria from seeds of maize (Zea mays L., Nongda108) at different growth stages. Ann. Microbiol. 2013, 63, 71–79. [Google Scholar] [CrossRef]
- Truyens, S.; Weyens, N.; Cuypers, A.; Vangronsveld, J. Bacterial seed endophytes: Genera, vertical transmission and interaction with plants. Env. Microbiol. Rep. 2015, 7, 40–50. [Google Scholar] [CrossRef]
- Xie, H.L.; Wang, H.C.; Cai, L.T.; Zhou, H.; Liu, C.; Lu, N.; Shi, C.H.; Wang, X.P. Community structure and diversity of endophytic bacteria of tobacco seeds. Acta Microbiol. Sin. 2020, 60, 601–616. [Google Scholar]
- Hameeda, B.; Harini, G.; Rupela, O.P.; Wani, S.P.; Reddy, G. Growth promotion of maize by phosphate-solubilizing bacteria isolated from composts and macrofauna. Microbiol. Res. 2008, 163, 234–242. [Google Scholar] [CrossRef]
- Loiret, F.G.; Ortega, E.; Kleiner, D.; Ortega-Rodés, P.; Dong, Z. A putative new endophytic nitrogen-fixing bacterium pantoea sp. from sugarcane. J. Appl. Microbiol. 2004, 97, 504–511. [Google Scholar] [CrossRef]
- López-López, A.; Rogel, M.A.; Ormeno-Orrillo, E.; Julio, M.R.; Esperanz, M.R. Phaseolus vulgaris seed-borne endophytic community with novel bacterial species such as rhizobium endophyticum sp. nov. Syst. Appl. Microbiol. 2010, 33, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.J.; Shen, D.L.; Song, W. Rice endophyte Pantoea agglomerans YS19 promotes host plant growth and affects allocations of host photosynthates. J. Appl. Microbiol. 2006, 100, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Jiang, W. Application of high-throughput sequencing in understanding human oral microbiome related with health and disease. Front. Microbiol. 2014, 5, 00508. [Google Scholar] [CrossRef]
- White, J.F.; Kingsley, K.I.; Kowalski, K.P.; Irizarry, I.; Micci, A.; Soares, M.A.; Bergen, M.S. Disease protection and allelopathic interactions of seed-transmitted endophytic pseudomonads of invasive reed grass (Phragmites australis). Plant Soil 2017, 422, 195–208. [Google Scholar] [CrossRef]
- Hardoim, P.R.; Hardoim, C.C.P.; Van, O.L.S.; Dirk, V.E.J.; Baker, S.E. Dynamics of seed-borne rice endophytes on early plant growth stages. PLoS ONE 2012, 7, e30438. [Google Scholar] [CrossRef] [PubMed]
- Mastretta, C.; Taghavi, S.; Daniel, V.D.L.; Mengoni, A.; Galardi, F.; Gonnelli, C.; Barac, T.; Boulet, J.; Weyens, N.; Vanggronsweld, J. Endophytic bacteria from seeds of Nicotiana tabacum can reduce cadmium phytotoxicity. Int. J. Phytoremediation 2009, 11, 251–267. [Google Scholar] [CrossRef]
- Mastretta, C.; Barac, T.; Vangronsveld, J.; Newman, L.; Taghavi, S.; Lelie, D.V.D. Endophytic bacteria and their potential application to improve the phytoremediation of contaminated environments. Biotechnol.Genet. Eng. 2006, 23, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Mounier, J.; Boncompain, G.; Senerovic, L.; Lagache, T.; Chrétien, F.; Perez, F.; Kolbe, M.; Olivo-Marin, J.C.; Sansonetti, P.J.; Sauvonnet, N. Shigella effector ipab-induced cholesterol relocation disrupts the golgi complex and recycling network to inhibit host cell secretion. Cell Host Microbe 2012, 12, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Marteyn, B.; Gazi, A.; Sansonetti, P. Shigella: A model of virulence regulation in vivo. Gut Microbes 2012, 3, 104–120. [Google Scholar] [CrossRef] [PubMed]
- Truyens, S.; Beckers, B.; Thijs, S.; Weyens, N.; Vangronsveld, J. The effects of the growth substrate on cultivable and total endophytic assemblages of Arabidopsis thaliana. Plant Soil 2016, 405, 325–336. [Google Scholar] [CrossRef]
- Wang, Z.S.; Li, N.; Wang, W.P.; Liu, Y. Research progress in endophytic bacteria in rice seeds. Biotechnol. Bull. 2022, 38, 236–246. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Zhang, Y.; Wang, P.; Zhao, L. Structure and Diversity of Endophytic Bacteria in Maize Seeds and Germinating Roots. Microorganisms 2024, 12, 1348. https://doi.org/10.3390/microorganisms12071348
Gao Y, Zhang Y, Wang P, Zhao L. Structure and Diversity of Endophytic Bacteria in Maize Seeds and Germinating Roots. Microorganisms. 2024; 12(7):1348. https://doi.org/10.3390/microorganisms12071348
Chicago/Turabian StyleGao, Yang, Yujun Zhang, Puchang Wang, and Lili Zhao. 2024. "Structure and Diversity of Endophytic Bacteria in Maize Seeds and Germinating Roots" Microorganisms 12, no. 7: 1348. https://doi.org/10.3390/microorganisms12071348
APA StyleGao, Y., Zhang, Y., Wang, P., & Zhao, L. (2024). Structure and Diversity of Endophytic Bacteria in Maize Seeds and Germinating Roots. Microorganisms, 12(7), 1348. https://doi.org/10.3390/microorganisms12071348




