Disrupted Microbiota of Colon Results in Worse Immunity and Metabolism in Low-Birth-Weight Jinhua Newborn Piglets
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Piglets and Husbandry Practices
2.2. Experimental Design
2.3. Samples Collocation
2.4. Growth Performance and Organ Index Statistical Analysis
2.5. Histological Evaluation
2.6. Short Chain Fatty Acid (SCFA) Determination
2.7. RNA Extraction and Sequencing
2.8. RNA-Seq Quality Control, Read Mapping, and Filtering
2.9. RNA-Seq Differential Expression and Enrichment Analysis
2.10. 16S rRNA Extraction and Sequencing
2.11. Colonic Microbiome Data Processing
2.12. Colonic Microbiome Statistical Analysis
2.13. Correlation Analysis
2.14. Validation of DEGs Using qRT-PCR
3. Results
3.1. Low-Birth-Weight Piglets Had Lower Organ Indexes
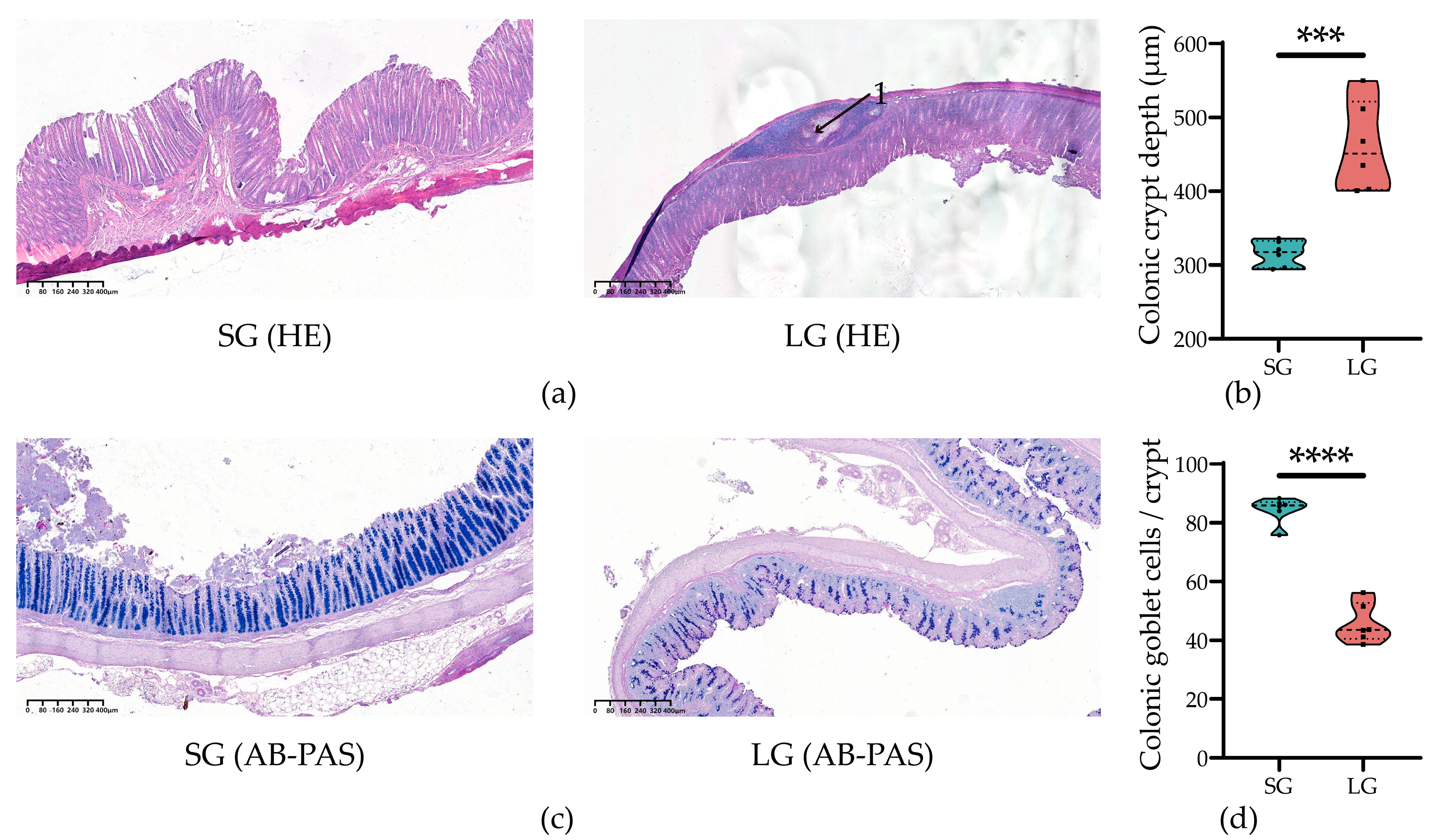
3.2. Low-Birth-Weight Piglets Had Worse Colonic Morphology and Secretory Function
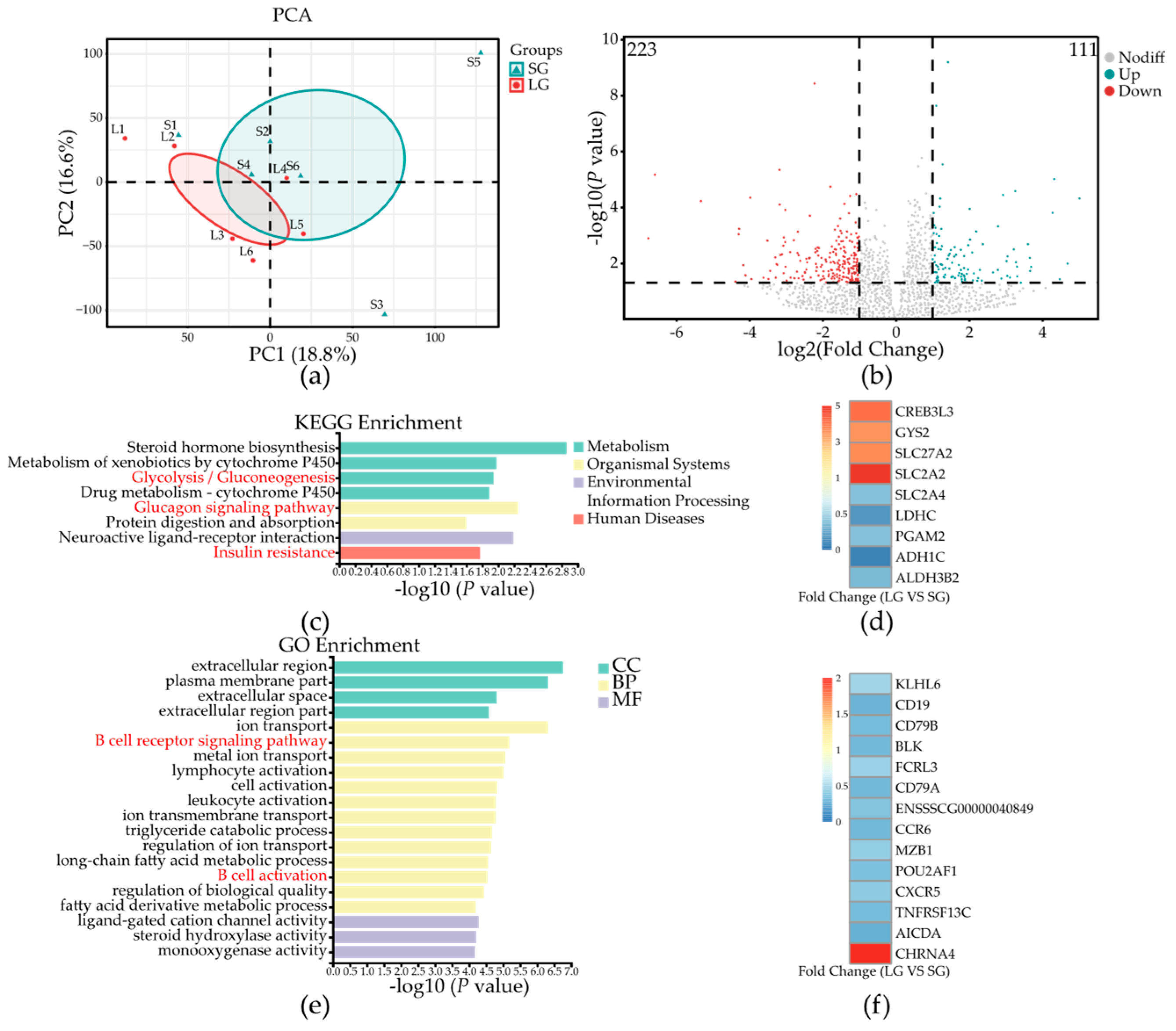
3.3. Low-Birth-Weight Piglets Have Differential Gene Expression in Colonic Mucosa
3.4. Low-Birth-Weight Piglets Had Lower Concentrations of Short Chain Fatty Acids in Colonic Digesta
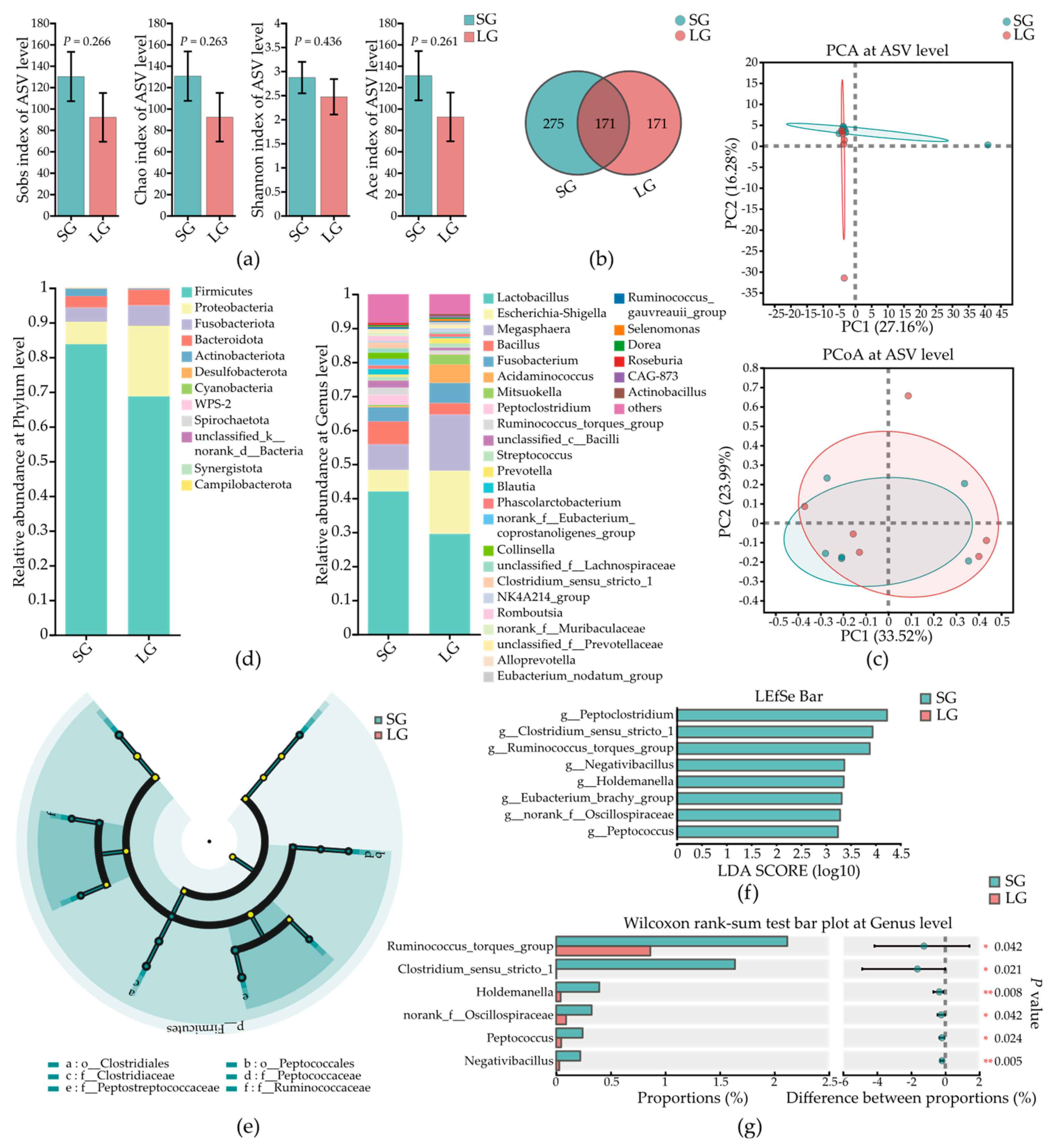
3.5. Low-Birth-Weight Piglets Had Differential Microbes in Colonic Mucosa
3.6. Low-Birth-Weight Piglets Had Differential Microbes in Colonic Contents
3.7. Interactions between Colonic Microbes and Glucose-Metabolism-Related Host Genes
3.8. Interactions between Colonic Microbes and B-Cell Immunity-Related Host Genes
3.9. Interactions between Colonic Microbes and Short Chain Fatty Acids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, N.-Y.; Zhang, S.-Q.; Peng, S.-H. Investigation on the distribution and their effects on reproduction traits of three major genes in Jinhua pigs. Yi Chuan Xue Bao = Acta Genet. Sin. 2003, 30, 1090–1096. [Google Scholar]
- Amdi, C.; Lynegaard, J.C.; Thymann, T.; Williams, A.R. Intrauterine growth restriction in piglets alters blood cell counts and impairs cytokine responses in peripheral mononuclear cells 24 days post-partum. Sci. Rep. 2020, 10, 4683. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; He, J.; Zhao, Y.; Shen, M.; Zhang, L.; Zhong, X.; Wang, C.; Wang, T. Effect of curcumin on growth performance, inflammation, insulin level, and lipid metabolism in weaned piglets with IUGR. Animals 2019, 9, 1098. [Google Scholar] [CrossRef] [PubMed]
- Vuguin, P. Animal models for assessing the consequences of intrauterine growth restriction on subsequent glucose metabolism of the offspring: A review. J. Matern.-Fetal Neonatal Med. 2002, 11, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Chen, Y.; Ma, Z.; Zhang, X.; Shi, D.; Khan, J.A.; Liu, H. Gut microbiota-derived short chain fatty acids are potential mediators in gut inflammation. Anim. Nutr. 2022, 8, 350–360. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, P.; Zheng, X.; Ma, Z.; Cui, C.; Wu, C.; Zeng, X.; Guan, W.; Chen, F. Altered Liver Metabolism, Mitochondrial Function, Oxidative Status, and Inflammatory Response in Intrauterine Growth Restriction Piglets with Different Growth Patterns before Weaning. Metabolites 2022, 12, 1053. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Tan, B.; Wang, J.; Liao, S.; Li, J.; Cui, Z.; Shao, Y.; Ji, P.; Yin, Y. Postnatal growth retardation is associated with deteriorated intestinal mucosal barrier function using a porcine model. J. Cell. Physiol. 2021, 236, 2631–2648. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, H.; Su, W.; Ying, Z.; Chen, Y.; Zhang, L.; Lu, Z.; Wang, T. Effects of dietary Bacillus amyloliquefaciens supplementation on growth performance, intestinal morphology, inflammatory response, and microbiota of intra-uterine growth retarded weanling piglets. J. Anim. Sci. Biotechnol. 2018, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, Y.; Feng, C.; Lin, G.; Wu, G.; Li, D.; Wang, J. Innate differences and colostrum-induced alterations of jejunal mucosal proteins in piglets with intra-uterine growth restriction. Br. J. Nutr. 2018, 119, 734–747. [Google Scholar] [CrossRef]
- Vaughan, A.T.; Roghanian, A.; Cragg, M.S. B cells—Masters of the immunoverse. Int. J. Biochem. Cell Biol. 2011, 43, 280–285. [Google Scholar] [CrossRef]
- Bouaziz, J.D.; Calbo, S.; Maho-Vaillant, M.; Saussine, A.; Bagot, M.; Bensussan, A.; Musette, P. IL-10 produced by activated human B cells regulates CD4+ T-cell activation in vitro. Eur. J. Immunol. 2010, 40, 2686–2691. [Google Scholar] [CrossRef] [PubMed]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the normal gut microbiota. World J. Gastroenterol. WJG 2015, 21, 8787. [Google Scholar] [CrossRef] [PubMed]
- Abraham, C.; Medzhitov, R. Interactions between the host innate immune system and microbes in inflammatory bowel disease. Gastroenterology 2011, 140, 1729–1737. [Google Scholar] [CrossRef] [PubMed]
- D’Inca, R.; Kloareg, M.; Gras-Le Guen, C.; Le Huërou-Luron, I. Intrauterine growth restriction modifies the developmental pattern of intestinal structure, transcriptomic profile, and bacterial colonization in neonatal pigs. J. Nutr. 2010, 140, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ma, C.; Xie, P.; Zhu, Q.; Wang, X.; Yin, Y.; Kong, X. Gut microbiota of newborn piglets with intrauterine growth restriction have lower diversity and different taxonomic abundances. J. Appl. Microbiol. 2019, 127, 354–369. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zhang, W.; Azad, M.A.K.; Ma, C.; Zhu, Q.; Kong, X. Metabolome, microbiome, and gene expression alterations in the colon of newborn piglets with intrauterine growth restriction. Front. Microbiol. 2022, 13, 989060. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Xia, B.; Zhong, R.; Shen, D.; Li, J.; Chen, L.; Zhang, H. Early-Life Nutrition Interventions Improved Growth Performance and Intestinal Health via the Gut Microbiota in Piglets. Front. Nutr. 2021, 8, 783688. [Google Scholar] [CrossRef] [PubMed]
- Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and immune function, supplementation and clinical translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, L.; Lu, C.; Dou, M.; Jiao, Y.; Bao, Y.; Shi, W. Effects of compound small peptides of Chinese medicine on intestinal immunity and cecal intestinal flora in CTX immunosuppressed mice. Front. Microbiol. 2022, 13, 959726. [Google Scholar] [CrossRef]
- Teixeira, L.G.; Leonel, A.J.; Aguilar, E.C.; Batista, N.V.; Alves, A.C.; Coimbra, C.C.; Ferreira, A.V.; de Faria, A.M.C.; Cara, D.C.; Alvarez Leite, J.I. The combination of high-fat diet-induced obesity and chronic ulcerative colitis reciprocally exacerbates adipose tissue and colon inflammation. Lipids Health Dis. 2011, 10, 204. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Zhong, R.; Wu, W.; Luo, C.; Meng, Q.; Gao, Q.; Zhao, Y.; Chen, L.; Zhang, S.; Zhao, X. Mucin O-glycan-microbiota axis orchestrates gut homeostasis in a diarrheal pig model. Microbiome 2022, 10, 139. [Google Scholar] [CrossRef]
- Houghteling, P.D.; Walker, W.A. Why is initial bacterial colonization of the intestine important to infants’ and children’s health? J. Pediatr. Gastroenterol. Nutr. 2015, 60, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Shimano, H. CREBH regulates systemic glucose and lipid metabolism. Int. J. Mol. Sci. 2018, 19, 1396. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Satoh, A.; Yabe, S.; Furusawa, M.; Tokushige, N.; Tezuka, H.; Mikami, M.; Iwata, W.; Shingyouchi, A.; Matsuzaka, T. Hepatic CREB3L3 controls whole-body energy homeostasis and improves obesity and diabetes. Endocrinology 2014, 155, 4706–4719. [Google Scholar] [CrossRef] [PubMed]
- Min, A.-K.; Jeong, J.; Go, Y.; Choi, Y.-K.; Kim, Y.-D.; Lee, I.-K.; Park, K.-G. cAMP response element binding protein H mediates fenofibrate-induced suppression of hepatic lipogenesis. Diabetologia 2013, 56, 412–422. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ruppert, P.M.; Park, J.-G.; Xu, X.; Hur, K.Y.; Lee, A.-H.; Kersten, S. Transcriptional profiling of PPARα−/− and CREB3L3−/− livers reveals disparate regulation of hepatoproliferative and metabolic functions of PPARα. BMC Genom. 2019, 20, 199. [Google Scholar] [CrossRef] [PubMed]
- Sampieri, L.; Di Giusto, P.; Alvarez, C. CREB3 transcription factors: ER-golgi stress transducers as hubs for cellular homeostasis. Front. Cell Dev. Biol. 2019, 7, 123. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, S.; Jia, X.; Lai, S. Association of a synonymous mutation of the PGAM2 gene and growth traits in rabbits. Czech J. Anim. Sci 2015, 60, 139–144. [Google Scholar] [CrossRef]
- Xu, Y.; Li, F.; Lv, L.; Li, T.; Zhou, X.; Deng, C.-X.; Guan, K.-L.; Lei, Q.-Y.; Xiong, Y. Oxidative stress activates SIRT2 to deacetylate and stimulate phosphoglycerate mutase. Cancer Res. 2014, 74, 3630–3642. [Google Scholar] [CrossRef]
- Naini, A.; Toscano, A.; Musumeci, O.; Vissing, J.; Akman, H.O.; DiMauro, S. Muscle phosphoglycerate mutase deficiency revisited. Arch. Neurol. 2009, 66, 394–398. [Google Scholar] [CrossRef]
- Zhou, H.; Brekman, A.; Zuo, W.-L.; Ou, X.; Shaykhiev, R.; Agosto-Perez, F.J.; Wang, R.; Walters, M.S.; Salit, J.; Strulovici-Barel, Y. POU2AF1 functions in the human airway epithelium to regulate expression of host defense genes. J. Immunol. 2016, 196, 3159–3167. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, L.; Emslie, D.; Kratina, T.; Shi, W.; Hirsch, S.; Taubenheim, N.; Chevrier, S. Oct2 and Obf1 as facilitators of B: T cell collaboration during a humoral immune response. Front. Immunol. 2014, 5, 108. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Reth, M. Oligomeric organization of the B-cell antigen receptor on resting cells. Nature 2010, 467, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Pelanda, R.; Braun, U.; Hobeika, E.; Nussenzweig, M.C.; Reth, M. B cell progenitors are arrested in maturation but have intact VDJ recombination in the absence of Ig-α and Ig-β. J. Immunol. 2002, 169, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Zhou, N.; Busino, L. KLHL6 is a tumor suppressor gene in diffuse large B-cell lymphoma. Cell Cycle 2019, 18, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Pizzorno, J.E.; Murray, M.T.; Joiner-Bey, H. The Clinician’s Handbook of Natural Medicine E-Book; Elsevier Health Sciences: Maryland Heights, MO, USA, 2016. [Google Scholar]
- Pan, H.; Liu, Z. Roles of dietary fiber and gut microbial metabolites short-chain fatty acids in regulating mitochondrial function in central nervous system. In Molecular Nutrition and Mitochondria; Elsevier: Amsterdam, The Netherlands, 2023; pp. 243–251. [Google Scholar]
- Thakur, B.K.; Dasgupta, N.; Ta, A.; Das, S. Physiological TLR5 expression in the intestine is regulated by differential DNA binding of Sp1/Sp3 through simultaneous Sp1 dephosphorylation and Sp3 phosphorylation by two different PKC isoforms. Nucleic Acids Res. 2016, 44, 5658–5672. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, J.; Liu, Z.; Jiang, A.; Li, S.; Wu, D.; Zhang, Y.; Zhu, X.; Zhou, E.; Wei, Z. Sodium butyrate alleviates lipopolysaccharide-induced inflammatory responses by down-regulation of NF-κB, NLRP3 signaling pathway, and activating histone acetylation in bovine macrophages. Front. Vet. Sci. 2020, 7, 579674. [Google Scholar] [CrossRef]
- Lee, C.; Kim, B.G.; Kim, J.H.; Chun, J.; Im, J.P.; Kim, J.S. Sodium butyrate inhibits the NF-kappa B signaling pathway and histone deacetylation, and attenuates experimental colitis in an IL-10 independent manner. Int. Immunopharmacol. 2017, 51, 47–56. [Google Scholar] [CrossRef]
- Heimann, E.; Nyman, M.; Pålbrink, A.-K.; Lindkvist-Petersson, K.; Degerman, E. Branched short-chain fatty acids modulate glucose and lipid metabolism in primary adipocytes. Adipocyte 2016, 5, 359–368. [Google Scholar] [CrossRef]
- Schroeder, B.O. Fight them or feed them: How the intestinal mucus layer manages the gut microbiota. Gastroenterol. Rep. 2019, 7, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Azad, M.A.K.; Zhu, Q.; Yu, Z.; Kong, X. Dietary bile acid supplementation alters plasma biochemical and hormone indicators, intestinal digestive capacity, and microbiota of piglets with normal birth weight and intrauterine growth retardation. Front. Microbiol. 2022, 13, 1053128. [Google Scholar] [CrossRef]
- Jiang, L.; Feng, C.; Tao, S.; Li, N.; Zuo, B.; Han, D.; Wang, J. Maternal imprinting of the neonatal microbiota colonization in intrauterine growth restricted piglets: A review. J. Anim. Sci. Biotechnol. 2019, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Garg, N.; Hasan, S.; Shirodkar, S. Prevotella: An insight into its characteristics and associated virulence factors. Microb. Pathog. 2022, 169, 105673. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E. Prevotella in the gut: Choose carefully. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 69–70. [Google Scholar] [CrossRef]
- Tong, L.-T.; Xiao, T.; Wang, L.; Lu, C.; Liu, L.; Zhou, X.; Wang, A.; Qin, W.; Wang, F. Plant protein reduces serum cholesterol levels in hypercholesterolemia hamsters by modulating the compositions of gut microbiota and metabolites. Iscience 2021, 24, 103435. [Google Scholar] [CrossRef]
- Ormerod, K.L.; Wood, D.L.; Lachner, N.; Gellatly, S.L.; Daly, J.N.; Parsons, J.D.; Dal’Molin, C.G.; Palfreyman, R.W.; Nielsen, L.K.; Cooper, M.A. Genomic characterization of the uncultured Bacteroidales family S24-7 inhabiting the guts of homeothermic animals. Microbiome 2016, 4, 36. [Google Scholar] [CrossRef]
- Lopetuso, L.R.; Scaldaferri, F.; Petito, V.; Gasbarrini, A. Commensal Clostridia: Leading players in the maintenance of gut homeostasis. Gut Pathog. 2013, 5, 23. [Google Scholar] [CrossRef]
- Li, Z.; Liu, S.; Zhao, Y.; Wang, J.; Ma, X. Compound organic acid could improve the growth performance, immunity and antioxidant properties, and intestinal health by altering the microbiota profile of weaned piglets. J. Anim. Sci. 2023, 101, skad196. [Google Scholar] [CrossRef]
- Accetto, T.; Avguštin, G. The diverse and extensive plant polysaccharide degradative apparatuses of the rumen and hindgut Prevotella species: A factor in their ubiquity? Syst. Appl. Microbiol. 2019, 42, 107–116. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Ge, F.; Yao, X.; Guo, X.; Bao, P.; Ma, X.; Wu, X.; Chu, M.; Yan, P.; Liang, C. Microbiome and metabolomics reveal the effects of different feeding systems on the growth and ruminal development of yaks. Front. Microbiol. 2021, 12, 682989. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Luo, Y.; Su, R.; Yao, D.; Hou, Y.; Liu, C.; Du, R.; Jin, Y. Impact of feeding regimens on the composition of gut microbiota and metabolite profiles of plasma and feces from Mongolian sheep. J. Microbiol. 2020, 58, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Meng, L.; Sun, Z.; Sun, S.; Huang, X.; Lin, N.; Zhang, J.; Lu, W.; Yang, Q.; Chi, J. Yellow wine polyphenolic compound protects against doxorubicin-induced cardiotoxicity by modulating the composition and metabolic function of the gut microbiota. Circ. Heart Fail. 2021, 14, e008220. [Google Scholar] [CrossRef] [PubMed]
- Lundell, A.-C.; Björnsson, V.; Ljung, A.; Ceder, M.; Johansen, S.; Lindhagen, G.; Törnhage, C.-J.; Adlerberth, I.; Wold, A.E.; Rudin, A. Infant B cell memory differentiation and early gut bacterial colonization. J. Immunol. 2012, 188, 4315–4322. [Google Scholar] [CrossRef]
- Huang, Y.; Tang, J.; Cai, Z.; Zhou, K.; Chang, L.; Bai, Y.; Ma, Y. Prevotella induces the production of Th17 cells in the colon of mice. J. Immunol. Res. 2020, 2020, 9607328. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Hirota, K.; Sakaguchi, S. Impaired T cell receptor signaling and development of T cell–mediated autoimmune arthritis. Immunol. Rev. 2020, 294, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 2017, 151, 363–374. [Google Scholar] [CrossRef]
- Hua, Z.; Hou, B. TLR signaling in B-cell development and activation. Cell. Mol. Immunol. 2013, 10, 103–106. [Google Scholar] [CrossRef]
- Bunker, J.J.; Flynn, T.M.; Koval, J.C.; Shaw, D.G.; Meisel, M.; McDonald, B.D.; Ishizuka, I.E.; Dent, A.L.; Wilson, P.C.; Jabri, B. Innate and adaptive humoral responses coat distinct commensal bacteria with immunoglobulin A. Immunity 2015, 43, 541–553. [Google Scholar] [CrossRef]
- Babbar, A.; Hitch, T.C.; Pabst, O.; Clavel, T.; Hübel, J.; Eswaran, S.; Wagner, N.; Schippers, A. The compromised mucosal immune system of β7 integrin-deficient mice has only minor effects on the fecal microbiota in homeostasis. Front. Microbiol. 2019, 10, 2284. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Sun, C.; Li, J.; Shao, X.; Wu, J.; Zhang, M.; Yao, H.; Wu, X. Characterization of Purified Mulberry Leaf Glycoprotein and Its Immunoregulatory Effect on Cyclophosphamide-Treated Mice. Foods 2022, 11, 2034. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Xie, S.; Lv, D.; Wang, P.; He, H.; Zhang, T.; Zhou, Y.; Lin, Q.; Zhou, H.; Jiang, J. Alteration of the gut microbiota in Chinese population with chronic kidney disease. Sci. Rep. 2017, 7, 2870. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Zhang, M.; Wang, X.; Bai, X.; Yue, T.; Gao, Z. Cloudy apple juice fermented by Lactobacillus prevents obesity via modulating gut microbiota and protecting intestinal tract health. Nutrients 2021, 13, 971. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Sun, G.; Duan, J.; Luo, C.; Yangji, C.; Zhong, R.; Chen, L.; Zhu, Y.; Wangdui, B.; Zhang, H. Alterations in gut microbiota improve SCFA production and fiber utilization in Tibetan pigs fed alfalfa diet. Front. Microbiol. 2022, 13, 969524. [Google Scholar] [CrossRef]
- Wang, L.; Ren, B.; Hui, Y.; Chu, C.; Zhao, Z.; Zhang, Y.; Zhao, B.; Shi, R.; Ren, J.; Dai, X. Methionine restriction regulates cognitive function in high-fat diet-fed mice: Roles of diurnal rhythms of SCFAs producing-and inflammation-related microbes. Mol. Nutr. Food Res. 2020, 64, 2000190. [Google Scholar] [CrossRef]



| Parameters | SG | LG | Pooled SD | p Value |
|---|---|---|---|---|
| Heart index (mg/g) | 4.80 ± 1.56 | 3.18 ± 0.81 | 1.24 | 0.048 |
| Spleen index (mg/g) | 2.05 ± 0.79 | 1.35 ± 0.33 | 0.60 | 0.071 |
| Liver index (mg/g) | 28.53 ± 7.50 | 16.53 ± 0.70 | 5.33 | 0.011 |
| Kidney index (mg/g) | 4.10 ± 1.12 | 2.56 ± 0.26 | 0.81 | 0.020 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wei, Z.; Lou, F.; Zhang, X.; Duan, J.; Luo, C.; Hu, X.; Tu, P.; Liu, L.; Zhong, R.; et al. Disrupted Microbiota of Colon Results in Worse Immunity and Metabolism in Low-Birth-Weight Jinhua Newborn Piglets. Microorganisms 2024, 12, 1371. https://doi.org/10.3390/microorganisms12071371
Li J, Wei Z, Lou F, Zhang X, Duan J, Luo C, Hu X, Tu P, Liu L, Zhong R, et al. Disrupted Microbiota of Colon Results in Worse Immunity and Metabolism in Low-Birth-Weight Jinhua Newborn Piglets. Microorganisms. 2024; 12(7):1371. https://doi.org/10.3390/microorganisms12071371
Chicago/Turabian StyleLi, Jiaheng, Zeou Wei, Fangfang Lou, Xiaojun Zhang, Jiujun Duan, Chengzeng Luo, Xujin Hu, Pingguang Tu, Lei Liu, Ruqing Zhong, and et al. 2024. "Disrupted Microbiota of Colon Results in Worse Immunity and Metabolism in Low-Birth-Weight Jinhua Newborn Piglets" Microorganisms 12, no. 7: 1371. https://doi.org/10.3390/microorganisms12071371
APA StyleLi, J., Wei, Z., Lou, F., Zhang, X., Duan, J., Luo, C., Hu, X., Tu, P., Liu, L., Zhong, R., Chen, L., Du, X., & Zhang, H. (2024). Disrupted Microbiota of Colon Results in Worse Immunity and Metabolism in Low-Birth-Weight Jinhua Newborn Piglets. Microorganisms, 12(7), 1371. https://doi.org/10.3390/microorganisms12071371







