Epidemiological, Pathological, and Molecular Studies on Sheeppox Disease Outbreaks in Karnataka, India
Abstract
:1. Introduction
2. Materials and Methods
2.1. Passive Disease Outbreak Data Collection
2.2. Geographical Study Area and Sample Size
2.3. Clinical Examination and Collection of Samples
2.4. DNA Extraction, Amplification and Sequencing of P32 and RPO30 Gene
2.5. Phylogenetic Analysis and Multiple Sequence Alignment
3. Results
3.1. Retrospective Analysis of Sheeppox Outbreak and Seroprevalences
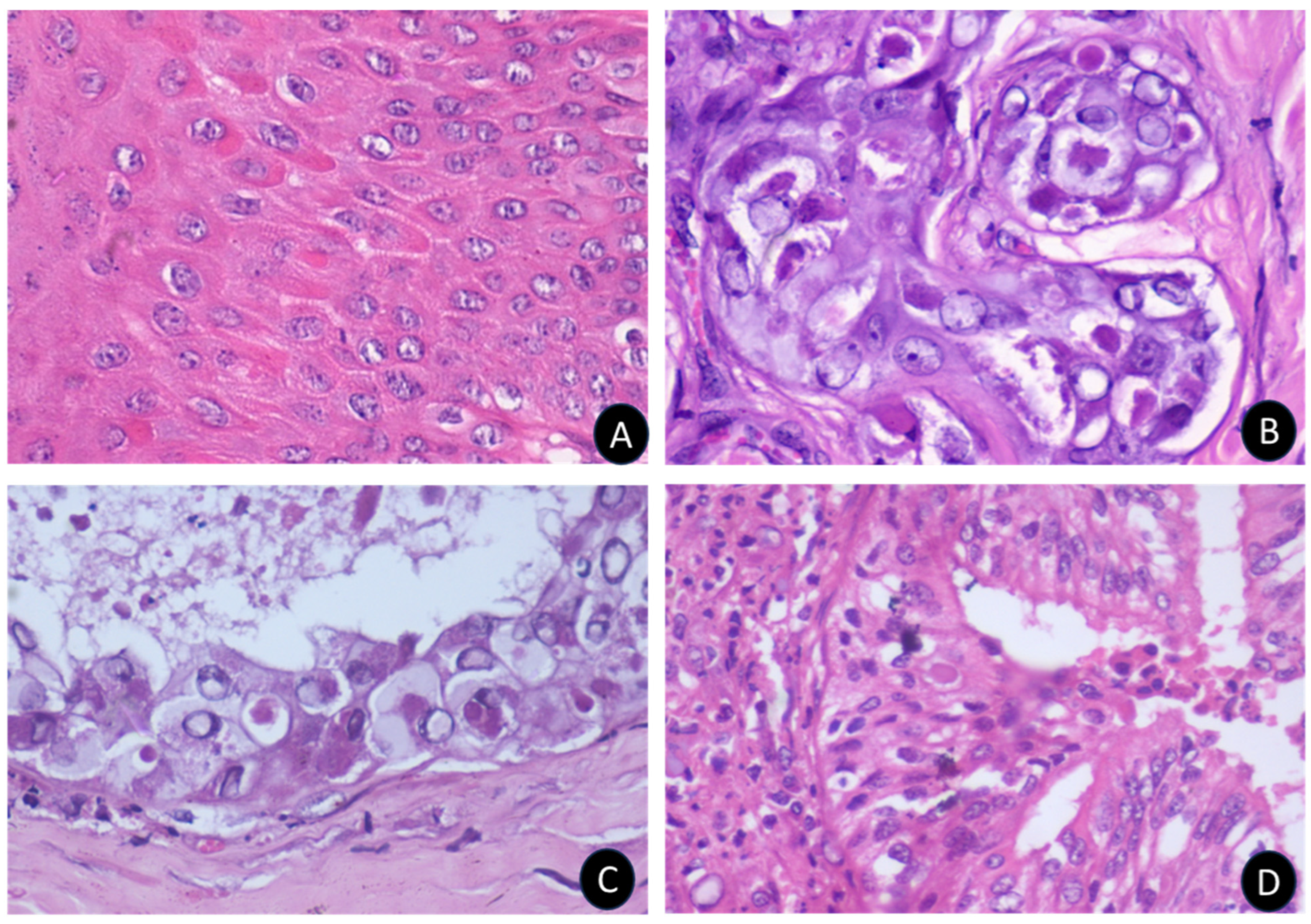
3.2. Epidemiological, Clinical, and Pathological Findings in Sheeppox Outbreaks
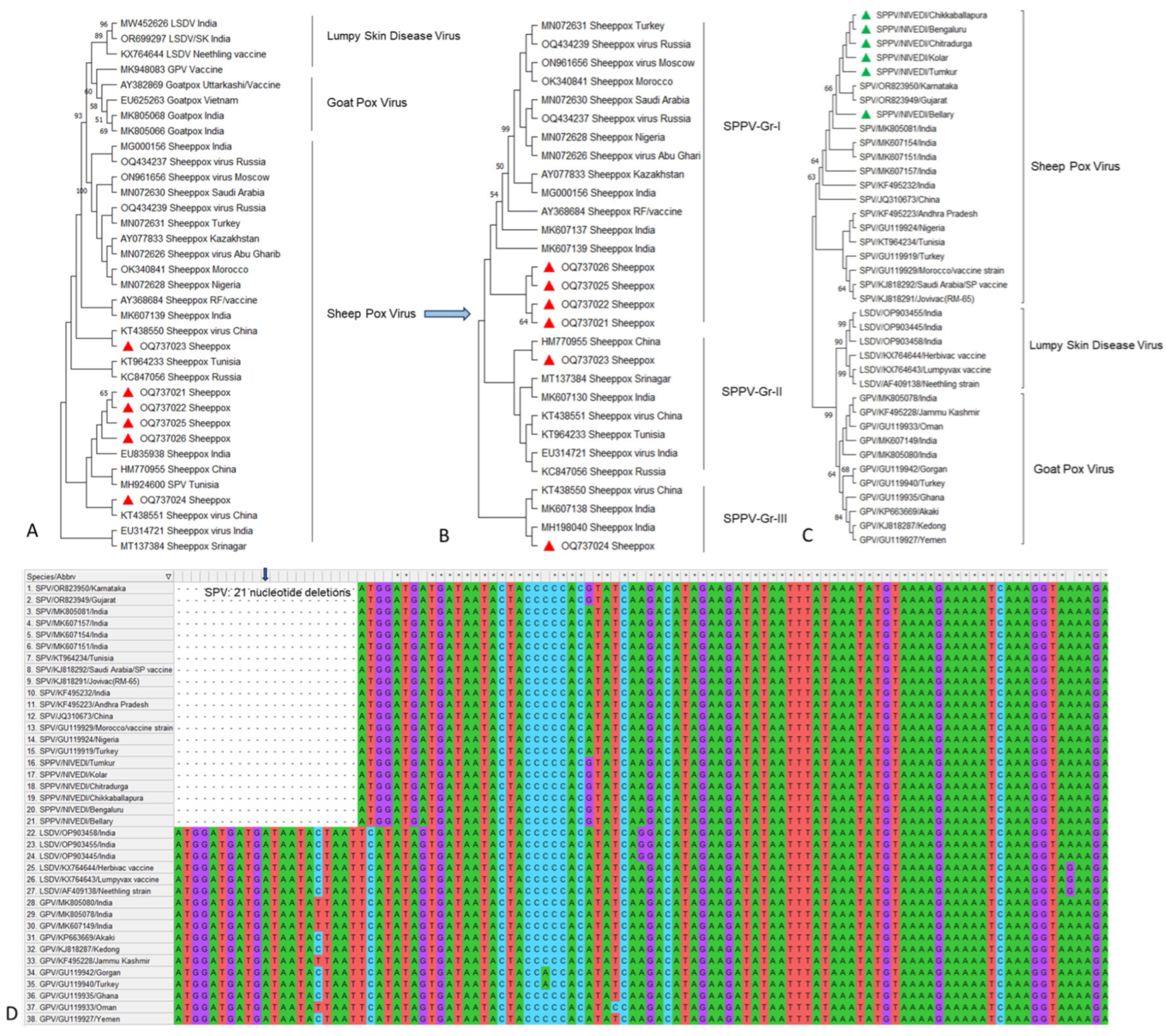
3.3. PCR and Sequencing Analysis of P32 and RPO30 Gene of Sheeppox Virus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhanuprakash, V.; Hosamani, M.; Singh, R.K. Prospects of control and eradication of capripox from the Indian subcontinent: A perspective. Antivir. Res. 2011, 91, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Rouby, S.R.; Bazid, A.H.; Wasfy, M.; El-Sayed, M. Capripoxviruses: Exploring the genetic relatedness between field and vaccine strains from Egypt. Vet. World 2019, 12, 1924–1930. [Google Scholar] [CrossRef] [PubMed]
- Babiuk, S.; Bowden, T.R.; Boyle, D.B.; Wallace, D.B.; Kitching, R.P. Capripoxviruses: An emerging worldwide threat to sheep, goats and cattle. Transbound. Emerg. Dis. 2008, 55, 263–272. [Google Scholar] [CrossRef] [PubMed]
- OIE Terrestrial Manual. 2012. Available online: https://www.oie.int/doc/ged/d12009.pdf (accessed on 12 December 2023).
- Hurisa, T.T.; Jing, Z.; Jia, H.; Chen, G.; He, X.B. A Review on Sheeppox and Goatpox: Insight of Epidemiology, Diagnosis, Treatment and Control Measures in Ethiopia. J. Infect. Dis. Epidemiol. 2018, 4, 057–065. [Google Scholar]
- Reddy, G.B.; Sumana, K.; Babu, S.; Yadav, J.; Balamuragan, V.; Hemadri, D.; Patil, S.S.; Suresh, K.P.; Gagendragud, M.R.; Rahman, H. Pathological and molecular characterization of Capripox virus outbreak in sheep and goats in Karnataka. Indian J. Vet. Pathol. 2015, 39, 11–14. [Google Scholar] [CrossRef]
- Manjunatha Reddy, G.B.; Sumana, K.; Apsana, R.; Yogisharadhya, R.; Prajapati, A.; Patil, S.S.; Balamuragan, V. Investigation of malignant form of sheep pox outbreak in fattening lambs in Mandya, Karnataka. Indian J. Vet. Pathol. 2017, 41, 184–188. [Google Scholar] [CrossRef]
- Lamien, C.E.; Lelenta, M.; Goger, W.; Silber, R.; Tuppurainen, E.; Matijevic, M.; Luckins, A.G.; Diallo, A. Real time PCR method for simultaneous detection, quantitation and differentiation of capripoxviruses. J. Virol. Methods 2011, 171, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Santhamani, R.; Yogisharadhya, R.; Venkatesan, G.; Shivachandra, S.B.; Pandey, A.B.; Ramakrishnan, M.A. Molecular characterization of Indian sheeppox and goatpox viruses based on RPO30 and GPCR genes. Virus Genes 2014, 49, 286–291. [Google Scholar] [CrossRef]
- Zeedan, G.S.G.; Mahmoud, A.H.; Abdalhamed, A.M.; Ghazy, A.A.; Abd EL-Razik, K.A. Rapid Detection and Differentiation between Sheep Pox and Goat Pox Viruses by Real-Time qPCR and Conventional PCR in Sheep and Goats in Egypt. World Vet. J. 2021, 10, 80–87. [Google Scholar] [CrossRef]
- Manjunatha Reddy, G.B.; Sumana, K.; Yogisharadhya, R.; Mohan, H.V.; Lavanya, V.K.; Chethankumar, B.H.; Shivasharanappa, N.; Saminathan, M.; Basavaraj, S.; Dhama, K.; et al. Structural and sequence analysis of the RPO30 gene of sheeppox and goatpox viruses from India. Vet. Q. 2024, 44, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Bhanuprakash, V.; Moorthy, A.R.; Krishnappa, G.; Srinivasa Gowda, R.N.; Indrani, B.K. An epidemiological study of sheep pox infection in Karnataka State, India. Rev. Sci. Tech. 2005, 24, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Rao, T.V.S.; Bandyopadhyay, S.K. A comprehensive review of goat pox and sheep pox and their diagnosis. Anim. Health Res. Rev. 2000, 1, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Chahota, R.; Sharma, P.; Kumar, R.; Gupta, T.; Sharma, M. Investigation of an outbreak of sheeppox among native sheep breeds in the Western Himalayas of India. Vet. Res. Commun. 2022, 46, 101–107. [Google Scholar] [CrossRef]
- Hota, A.; Biswal, S.; Sahoo, N.; Venkatesan, G.; Arya, S.; Kumar, A.; Ramakrishnan, M.A.; Pandey, A.B.; Rout, M. Seroprevalence of Capripoxvirus infection in sheep and goats among different agro-climatic zones of Odisha, India. Vet. World 2018, 11, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Masoud, F.; Mahmood, M.; Hussain, I. Seroepidemiology of goat pox disease in district Layyah, Punjab, Pakistan. J. Vet. Med. Res. 2016, 3, 1043. [Google Scholar]
- Bhanuprakash, V.; Indrani, B.K.; Hosamani, M.; Singh, R.K. The current status of sheep pox disease. Comp Immunol Microbiol. Infect. Dis. 2006, 29, 27–60. [Google Scholar] [CrossRef] [PubMed]
- Yune, N.; Abdela, N. Epidemiology and economic importance of sheep and goat pox: A review on past and current aspects. J. Vet. Sci. Technol. 2017, 8, 2. [Google Scholar] [CrossRef]
- Yeruham, I.; Yadin, H.; Van Ham, M.; Bumbarov, V.; Soham, A.; Perl, S. Economic and epidemiological aspects of an outbreak of sheeppox in a dairy sheep flock. Vet. Rec. 2007, 160, 236–237. [Google Scholar] [CrossRef] [PubMed]
- Manimaran, K.; Mahaprabhu, R.; Jaisree, S.; Hemalatha, S.; Ravimurugan, T.; Pazhanivel, N.; Roy, P. An outbreak of sheep pox in an organized farm of Tamil Nadu, India. Indian J. Anim. Res. 2017, 51, 162–164. [Google Scholar]
- Beard, P.M.; Sugar, S.; Bazarragchaa, E.; Gerelmaa, U.; Tserendorj, S.H.; Tuppurainen, E.; Sodnomdarjaa, R. A description of two outbreaks of capripoxvirus disease in Mongolia. Vet. Microbiol. 2010, 142, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.Z.E.; Khslsgslls, A.I.; Abdellatif, M.M. An epidemiological study of sheep and goat pox outbreaks in the Sudan. Food Biol. 2016, 5, 1–5. [Google Scholar]
- Zangana, I.K.; Abdullah, M.A. Epidemiological, clinical and histopathological studies of lamb and kid pox in Duhok, Iraq. Bangladesh J. Vet. Med. 2013, 16, 133–138. [Google Scholar]
- Bhanuprakash, V.; Venkatesan, G.; Balamurugan, V.; Hosamani, M.; Yogisharadhya, R.; Chauhan, R.S.; Pande, A.; Mondal, B.; Singh, R.K. Pox outbreaks in sheep and goats at Makhdoom (Uttar Pradesh), India: Evidence of sheeppox virus infection in goats. Transbound. Emerg. Dis. 2010, 57, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Mangana, O.; Kottaridi, C.; Nomikou, K. The epidemiology of sheep pox in Greece from 1987 to 2007. Rev. Sci. Tech. 2008, 27, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Purushothaman, V.; Sreekumar, C.; Tamizharasan, S.; Chandramohan, A. Sheep pox disease outbreaks in Madras Red and Mechery breeds of indigenous sheep in Tamilnadu, India. Res. Vet. Sci. 2008, 85, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Verma, L.K.; Gupta, V.K.; Katoch, V.C.; Dogra, V.; Pal, B.; Sharma, M. Emerging Capripoxvirus disease outbreaks in Himachal Pradesh, a northern state of India. Transbound. Emerg. Dis. 2011, 58, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Al- Shabebi, A.A.; El-Sabagh, I.M.; Abu-Elzein, E.M.; Zaghawa, A.A.; Al-Naeem, A.A.; Housawi, F.M. Molecular detection and phylogenetic analysis of Sheep pox virus in Al—Hassa of Eastern Province of Saudi Arabia. Adv. Anim. Vet. Sci. 2014, 2, 31–34. [Google Scholar] [CrossRef]
- Sumana, K.; Revanaiah, Y.; Apsana, R.; Roy, P.; Manjunatha Reddy, G.B. Molecular characterization of sheeppox virus from outbreaks in Karnataka, India. Vet. World 2020, 13, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Nashiruddullah, N.; AHMED, J.A. Pathology of Spontaneous Pox Virus Infection of Sheep and Goat in Jammu Region. Int. J. Curr. Microbiol. App. Sci. 2020, 9, 1204–1226. [Google Scholar] [CrossRef]
- Beytut, E. Sheep pox virus induces proliferation of type II pneumocytes in the lungs. J. Comp. Pathol. 2010, 143, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Lafar, S.; Zro, K.; Haegeman, A.; Khayli, M.; De Clercq, K.; Lancelot, R.; Ennaji, M. Clinical and epidemiological evolution of sheep pox in Morocco. J. Agric. Sci. Technol. 2019, 9, 103–113. [Google Scholar] [CrossRef]
- Tulman, E.R.; Afonso, C.L.; Lu, Z.; Zsak, L.; Sur, J.H.; Sandybaev, N.T.; Kerembekova, U.Z.; Zaitsev, V.L.; Kutish, G.F.; Rock, D.L. The genomes of sheeppox and goatpox viruses. J. Virol. 2002, 76, 6054–6061. [Google Scholar] [CrossRef] [PubMed]
- Stein, R.A.; Staros, J.V. Evolutionary analysis of the ErbB receptor and ligand families. J. Mol. Evol. 2000, 50, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Ozmen, O.; Kale, M.; Haligur, M.; Yavru, S. Pathological, serological, and virological findings in sheep infected simultaneously with Bluetongue, Peste-des-petits ruminants, and Sheeppox viruses. Trop. Anim. Health Prod. 2009, 41, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Haegeman, A.; Zro, K.; Vandenbussche, F.; Demeestere, L.; Van Campe, W.; Ennaji, M.M.; De Clercq, K. Development and validation of three Capripoxvirus real-time PCRs for parallel testing. J. Virol. Methods. 2013, 193, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Noyce, R.S.; Babiuk, L.A.; Lung, O.; Bulach, D.M.; Bowden, T.R.; Boyle, D.B.; Babiuk, S.; Evans, D.H. Extended sequencing of vaccine and wild-type capripoxvirus isolates provides insights into genes modulating virulence and host range. Transbound. Emerg. Dis. 2020, 67, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Lachheb, J.; Turki, A.; Mastour, H.; Nsiri, J.; El Behi, I.; Larbi, I.; Ghram, A. Phylogenetic analyses of P32, RPO30, GPCR, ORF117, and Kelch-like genes of Tunisian sheeppox virus isolates. Turkish J. Vet. Anim. Sci. 2019, 43, 603–614. [Google Scholar] [CrossRef]
- Zhou, T.; Jia, H.; Chen, G.; He, X.; Fang, Y.; Wang, X.; Guan, Q.; Zeng, S.; Cui, Q.; Jing, Z. Phylogenetic analysis of Chinese sheeppox and goatpox virus isolates. Virol. J. 2012, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha Reddy, G.B.; Pabbineedi, S.M.; Nagaraj, S.; Bijalwan, S.; Tadakod, S.; Bhutia, Z.; Palmu, D.; Rai, S.; Bhutia, P.D.; Bhutia, P.T.; et al. Lumpy Skin Disease (LSD) in Yak (Bos grunniens): An Evidence of Species Spillover from Cattle in India. Microorganisms 2023, 11, 2823. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Godara, B.; Chander, Y.; Kachhawa, J.P.; Dedar, R.K.; Verma, A.; Riyesh, T.; Pal, Y.; Barua, S.; Tripathi, B.N.; et al. Evidence of lumpy skin disease virus infection in camels. Acta Trop. 2023, 242, 106922. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.B.M.; Mounica, P.S.; Sudeep, N.; Vikram, R.; Garam, G.B.; Lalzampuia, H.; Ragulraj, S.; Pal, S.; Khate, K.; Bijalwan, S.; et al. First evidence of lumpy skin disease in mithun (Bos frontalis) in India. Arch. Virol. 2024, 169, 65. [Google Scholar] [CrossRef] [PubMed]
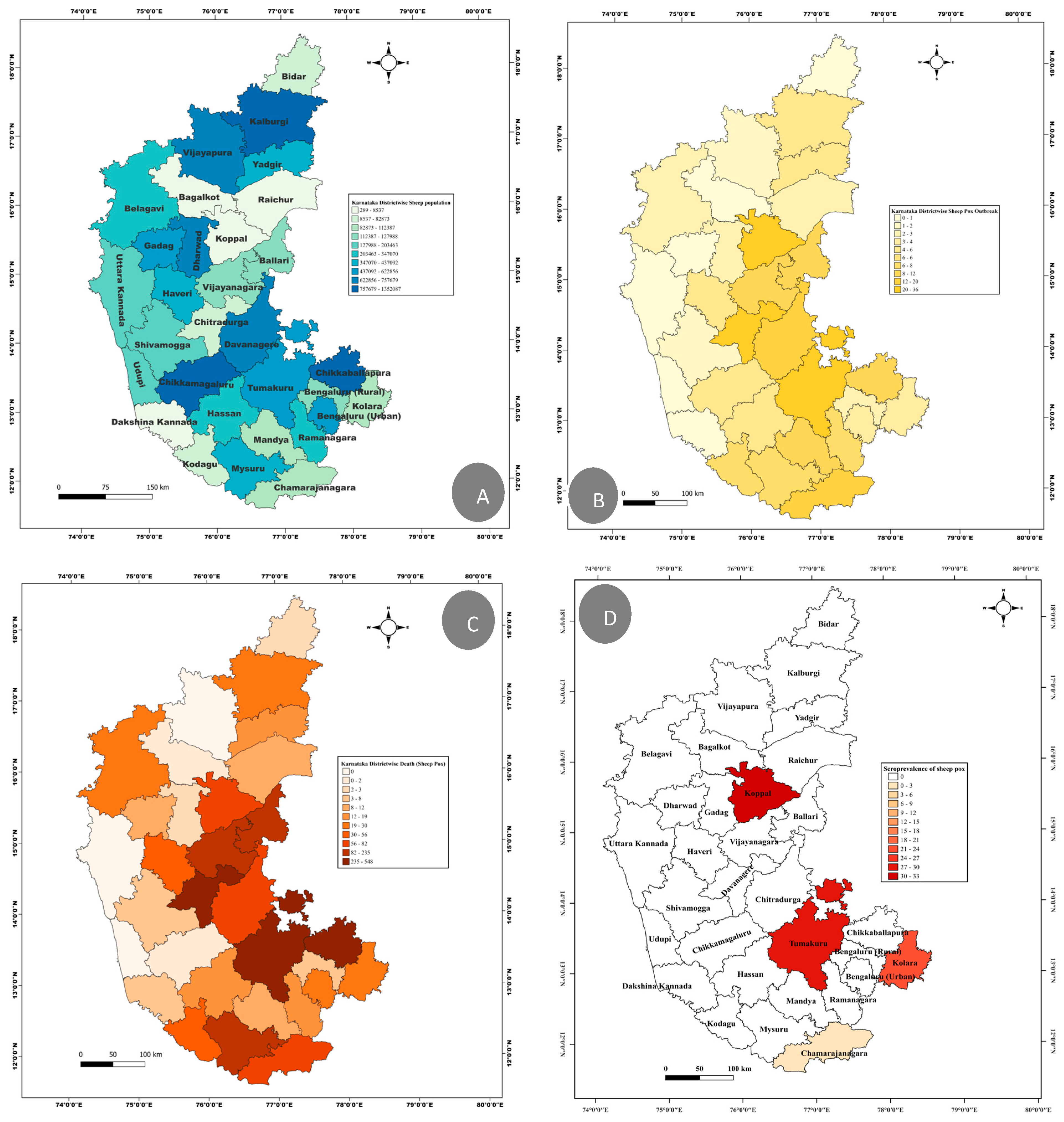



| District | Blocks Affected | Rearing System | Total Number (Susceptible) | Clinically Affected | SPPV-PCR | Morbidity (%) | Mortality (%) | Case Fatality Rate (CFR%) |
|---|---|---|---|---|---|---|---|---|
| Chikkaballapura | Gouribidanur | Organized | 52 | 08 | 08 | 15.38 | 03.85 | 25.00 |
| Backyard | 27 | 12 | 10 | 37.04 | 14.81 | 40.00 | ||
| Shiddlagatta | Backyard | 40 | 04 | 03 | 07.50 | 07.50 | 100.0 | |
| Erbachenahalli | Organized | 60 | 08 | 07 | 11.67 | 10.00 | 85.71 | |
| Bengaluru urban | DG Halli | Backyard | 17 | 10 | 10 | 58.82 | 29.41 | 50.00 |
| Bengaluru rural | Hesaragatta | Backyard | 35 | 03 | 03 | 08.57 | 02.86 | 33.33 |
| Kolar | Shrinivaspur | Organized | 176 | 07 | 06 | 03.41 | 01.14 | 33.33 |
| Bellari | Harappanahalli | Organized | 160 | 08 | 08 | 05.00 | 01.88 | 37.50 |
| Chigateri | Organized | 260 | 15 | 14 | 05.38 | 01.92 | 35.71 | |
| Tumkur | Sira | Backyard | 16 | 13 | 11 | 68.75 | 37.50 | 54.55 |
| Chitradurga | Hosadurga | Backyard | 46 | 17 | 15 | 32.61 | 10.87 | 33.33 |
| Total | 889 | 105 | 95 | 10.69 | 04.72 | 45.26 | ||
| Hematology Parameters | Control Sheep (n = 6) | Sheeppox Positive (n = 71) |
|---|---|---|
| TLC (×103 /µL) | 6.335 ± 0.2871 | 10.88 ± 0.3191 *** |
| TEC (×106 /µL) | 11.49 ± 0.5396 | 9.579 ± 0.2493 * |
| Hb (g/dL) | 9.355 ± 0.3703 | 7.453 ± 0.2242 * |
| HCT (%) | 30.98 ± 0.5605 | 23.36 ± 0.6026 *** |
| PLT (105 /µL) | 2.475 ± 0.1817 | 2.964 ± 0.2142 |
| Biochemical Parameters | Control Sheep (n = 6) | Sheeppox Positive (n = 71) |
|---|---|---|
| AST (U/I) | 157.8 ± 15.29 | 234.8 ± 18.48 |
| ALP (U/I) | 175.5 ± 15.53 | 296.0 ± 26.97 |
| Total Protein (g/dL) | 6.578 ± 0.2140 | 6.967 ± 0.3505 |
| Albumin g/dL | 3.967 ± 0.1687 | 4.293 ± 0.2336 |
| GGT (U/I) | 57.27 ± 3.560 | 65.06 ± 2.057 |
| ALT (U/I) | 29.64 ± 1.053 | 29.68 ± 3.380 |
| Creatinine (mg/dL) | 1.005 ± 0.1132 | 1.002 ± 0.09801 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manjunatha Reddy, G.B.; Krishnappa, V.K.; Siddalingaiah, C.D.; Rao, S.; Nayakvadi, S.; Harlipura Basavarajappa, C.K.; Gualti, B.R. Epidemiological, Pathological, and Molecular Studies on Sheeppox Disease Outbreaks in Karnataka, India. Microorganisms 2024, 12, 1373. https://doi.org/10.3390/microorganisms12071373
Manjunatha Reddy GB, Krishnappa VK, Siddalingaiah CD, Rao S, Nayakvadi S, Harlipura Basavarajappa CK, Gualti BR. Epidemiological, Pathological, and Molecular Studies on Sheeppox Disease Outbreaks in Karnataka, India. Microorganisms. 2024; 12(7):1373. https://doi.org/10.3390/microorganisms12071373
Chicago/Turabian StyleManjunatha Reddy, Gundallahalli Bayyappa, Varun Kumar Krishnappa, Chandan Dypasandra Siddalingaiah, Suguna Rao, Shivasharanappa Nayakvadi, Chethan Kumar Harlipura Basavarajappa, and Baldev Raj Gualti. 2024. "Epidemiological, Pathological, and Molecular Studies on Sheeppox Disease Outbreaks in Karnataka, India" Microorganisms 12, no. 7: 1373. https://doi.org/10.3390/microorganisms12071373





