Intermediate Disturbances Enhance Microbial Enzyme Activities in Soil Ecosystems
Abstract
:1. Introduction
2. Materials and Methods
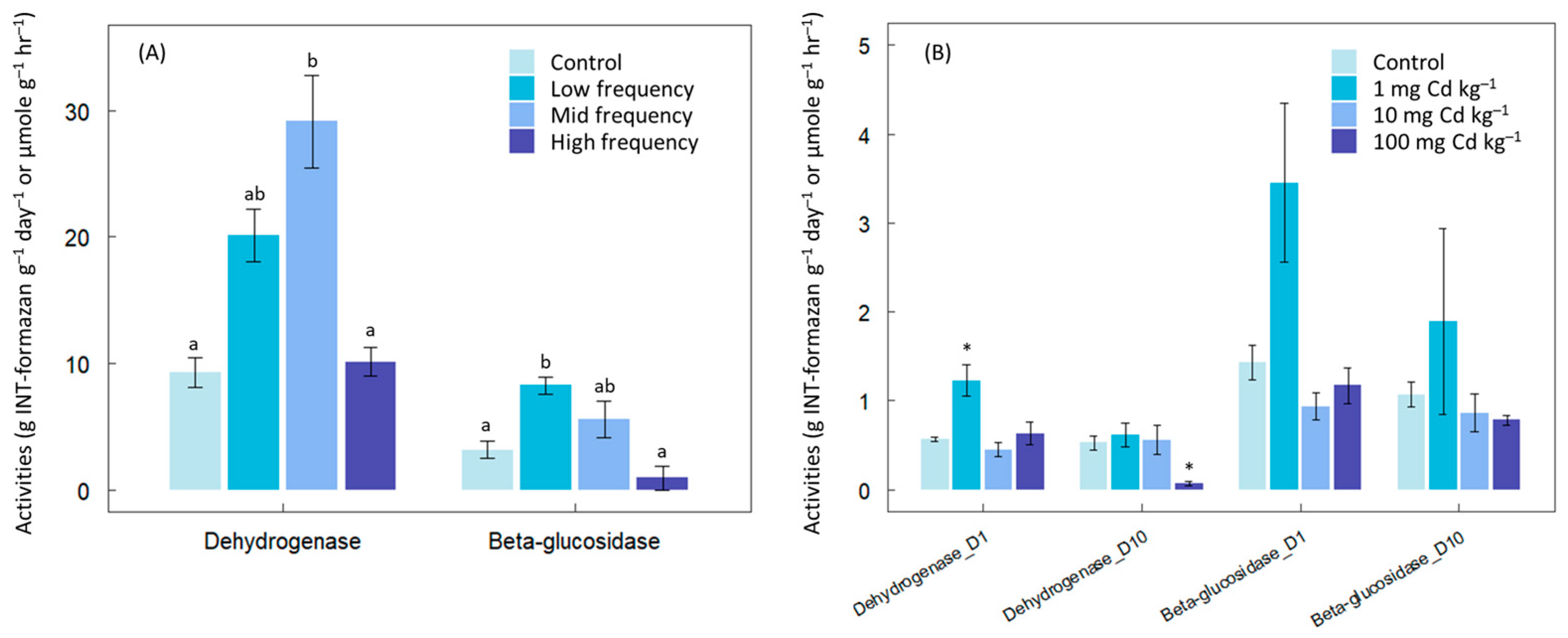
2.1. Chemical Contaminants
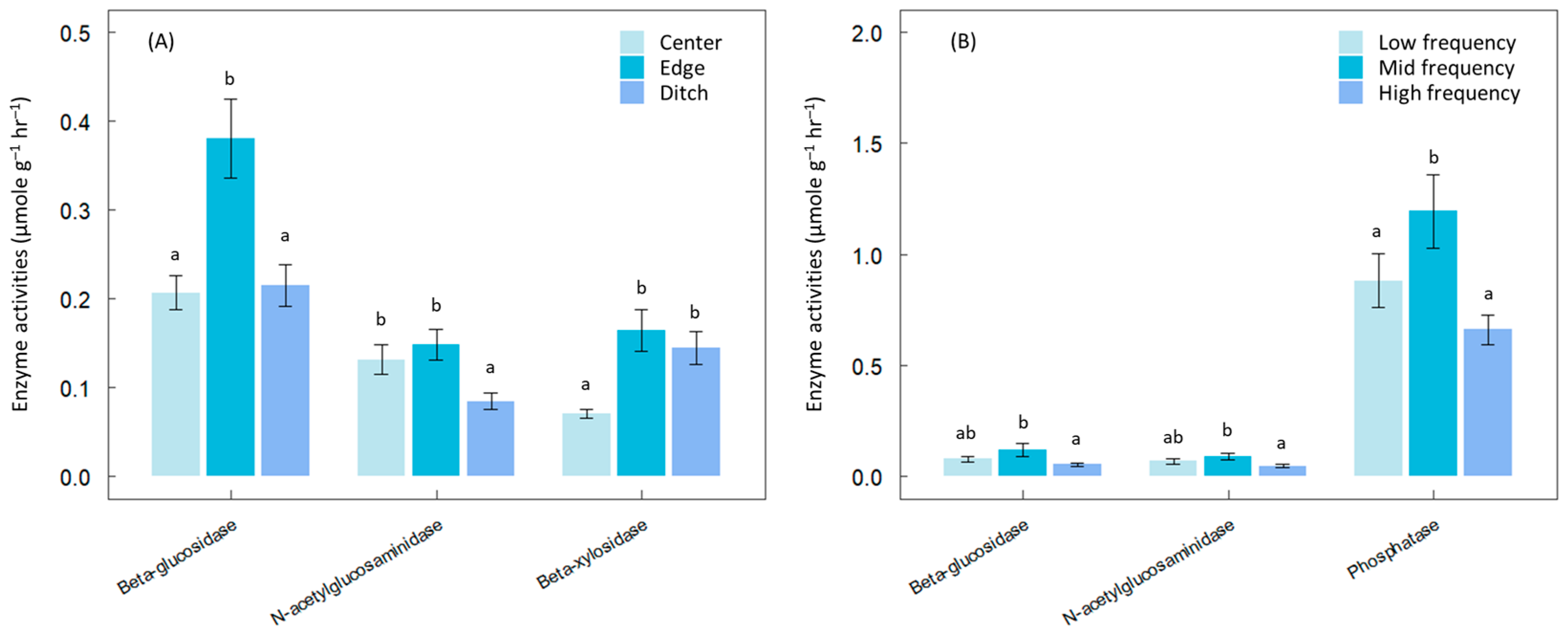
2.2. Hydrological Changes
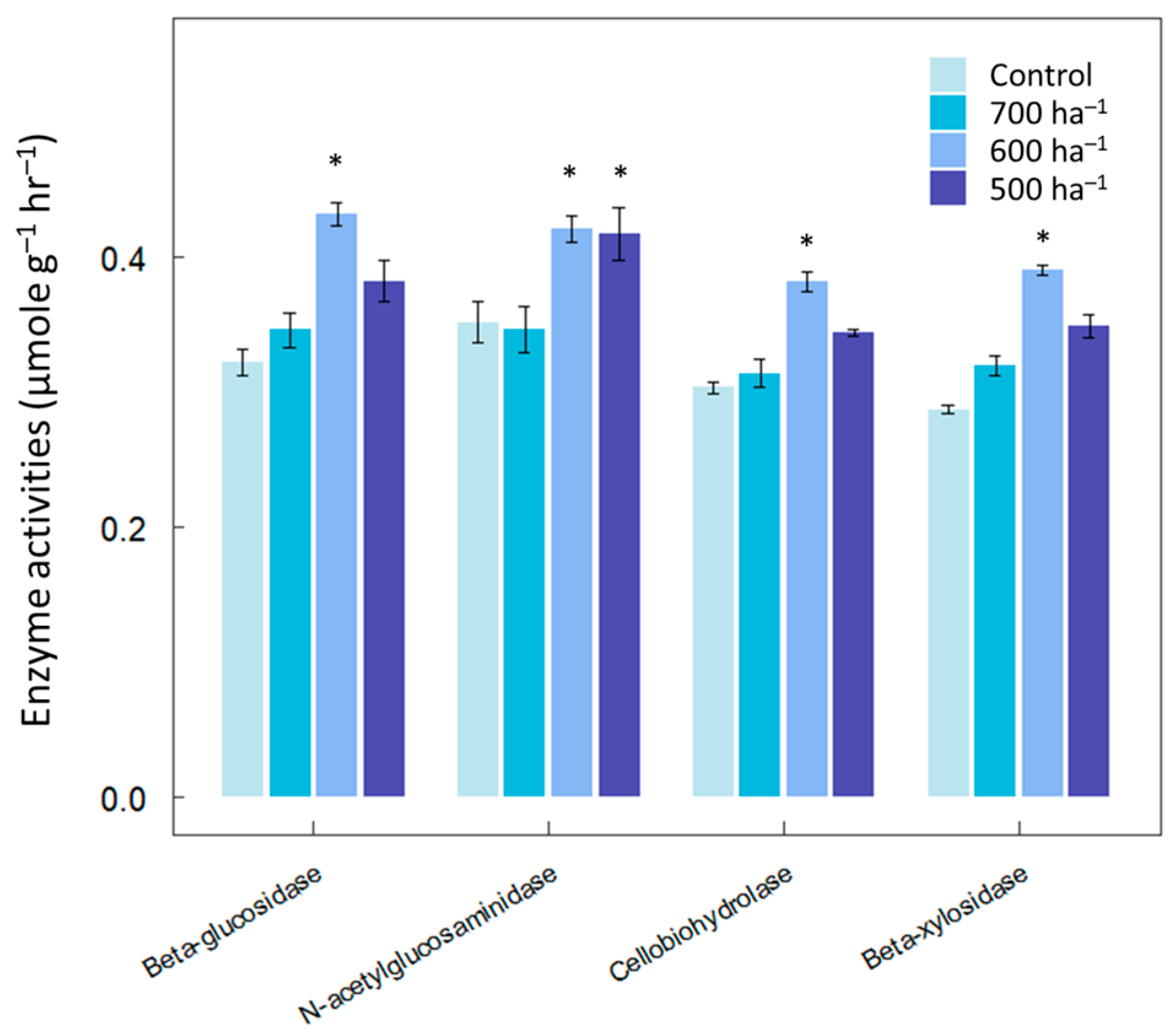
2.3. Thinning Management
2.4. Statistical Analysis
3. Results
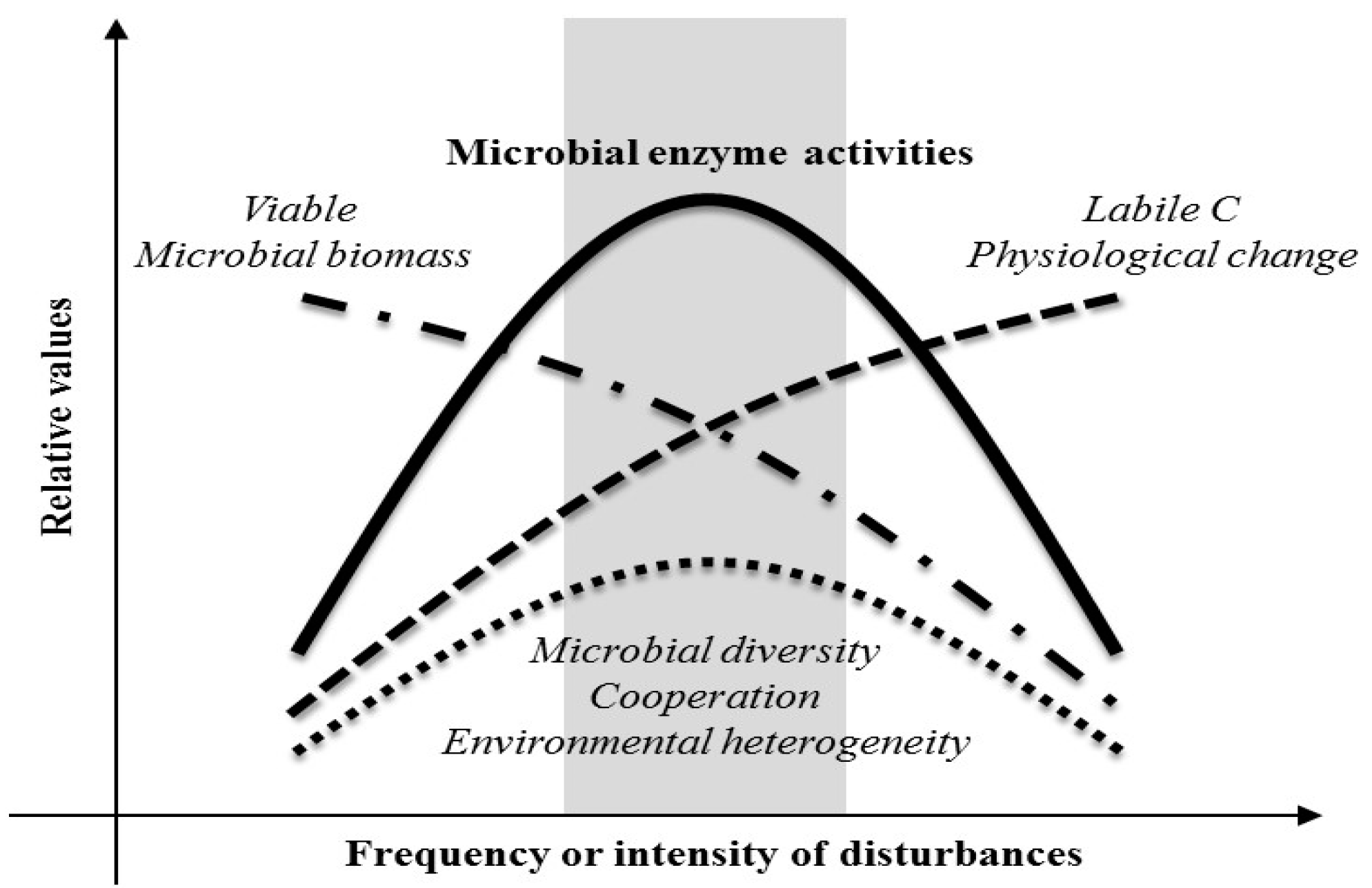
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fox, J.W. The intermediate disturbance hypothesis should be abandoned. Trends Ecol. Evol. 2013, 28, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Gardner, R.H.; Engelhardt, K.A. Spatial processes that maintain biodiversity in plant communities. Perspect. Plant Ecol. 2008, 9, 211–228. [Google Scholar] [CrossRef]
- Hall, A.R.; Miller, A.D.; Leggett, H.C.; Roxburgh, S.H.; Buckling, A.; Shea, K. Diversity-disturbance relationships: Frequency and intensity interact. Biol. Lett. 2012, 8, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Huston, M.A. Disturbance, productivity, and species diversity: Empiricism vs. logic in ecological theory. Ecology 2014, 95, 2382–2396. [Google Scholar] [CrossRef]
- Jiang, L.; Patel, S.N. Community assembly in the presence of disturbance: A microcosm experiment. Ecology 2008, 89, 1931–1940. [Google Scholar] [CrossRef]
- Kershaw, H.M.; Mallik, A.U. Predicting plant diversity response to disturbance: Applicability of the intermediate disturbance hypothesis and mass ratio hypothesis. Crit. Rev. Plant Sci. 2013, 32, 383–395. [Google Scholar] [CrossRef]
- Salvarrey, A.V.B.; Kotzian, C.B.; Spies, M.R.; Braun, B. The influence of natural and anthropic environmental variables on the structure and spatial distribution along longitudinal gradient of macroinvertebrate communities in southern Brazilian streams. J. Insect Sci. 2014, 14, 13. [Google Scholar] [CrossRef]
- Telesh, I.; Schubert, H.; Skarlato, S. Life in the salinity gradient: Discovering mechanisms behind a new biodiversity pattern. Estuar. Coast. Shelf Sci. 2013, 135, 317–327. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.; Kang, H. Effects of elevated CO2 on communities of denitrifying bacteria and methanogens in a temperate marsh microcosm. Microb. Ecol. 2012, 64, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Prosser, J.I.; Bohannan, B.J.; Curtis, T.P.; Ellis, R.J.; Firestone, M.K.; Freckleton, R.P.; Green, J.L.; Green, L.E.; Killham, K.; Lennon, J.J. The role of ecological theory in microbial ecology. Nat. Rev. Microbiol. 2007, 5, 384–392. [Google Scholar] [CrossRef]
- Liu, Y.R.; Wang, J.J.; Zheng, Y.M.; Zhang, L.M.; He, J.Z. Patterns of bacterial diversity along a long-term mercury-contaminated gradient in the paddy soils. Microb. Ecol. 2014, 68, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Motegi, C.; Nagata, T.; Miki, T.; Weinbauer, M.G.; Legendre, L.; Rassoulzadegan, F. Interactive effects of viral and bacterial production on marine bacterial diversity. PLoS ONE 2013, 8, e76800. [Google Scholar] [CrossRef] [PubMed]
- Vasileiadis, S.; Puglisi, E.; Arena, M.; Cappa, F.; van Veen, J.A.; Cocconcelli, P.S.; Trevisan, M. Soil microbial diversity patterns of a lowland spring environment. FEMS Microbiol. Ecol. 2013, 86, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Bao, T.; Gao, L.; Wang, S.; Yang, X.; Ren, W.; Zhao, Y. Moderate disturbance increases the PLFA diversity and biomass of microbial community in biocrusts in the Loess Pleteau region of China. Plant Soil 2020, 451, 499–513. [Google Scholar] [CrossRef]
- Santillan, E.; Seshan, H.; Constancias, F.; Drautz-Moses, D.J.; Wuertz, S. Frequency of disturbance alters diversity, function, and underlying assembly mechanisms of complex bacterial communities. NPJ Biofilms Microbiomes 2019, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, C.; Luo, Y. Meta-analysis of the impacts of global change factors on soil microbial diversity and functionality. Nat. Comm. 2020, 11, 3072. [Google Scholar] [CrossRef]
- Freeman, C.; Ostle, N.; Kang, H. An enzymic ‘latch’ on a global carbon store. Nature 2001, 409, 149. [Google Scholar] [CrossRef]
- Kwon, M.; Haraguchi, A.; Kang, H. Long-term water regime differentiates changes in decomposition and microbial properties in tropical peat soils exposed to the short-term drought. Soil Biol. Biochem. 2013, 60, 33–44. [Google Scholar] [CrossRef]
- Shimada, S.; Takahashi, H.; Haraguchi, A.; Kaneko, M. The carbon content characteristics of tropical peats in Central Kalimantan, Indonesia: Estimating their spatial variability in density. Biogeochemistry 2001, 53, 249–267. [Google Scholar] [CrossRef]
- Berga, M.; Székely, A.J.; Langenheder, S. Effects of disturbance intensity and frequency on bacterial community composition and function. PLoS ONE 2012, 7, e36959. [Google Scholar] [CrossRef]
- Rönnpagel, K.; Liß, W.; Ahlf, W. Microbial bioassays to assess the toxicity of solid-associated contaminants. Ecotoxicol. Environ. Saf. 1995, 31, 99–103. [Google Scholar] [CrossRef]
- Tandukar, M.; Oh, S.; Tezel, U.; Konstantinidis, T.K.; Pavlostathis, S.G. Long-term exposure to benzalkonium chloride disinfectants results in change of microbial community structure and increased antimicrobial resistance. Environ. Sci. Technol. 2013, 47, 9730–9738. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, D.S.; Powlson, D.S. The effects of biocidal treatments on metabolism in soil—V: A method for measuring soil biomass. Soil Biol. Biochem. 1976, 8, 209–213. [Google Scholar] [CrossRef]
- Brockhurst, M.A.; Buckling, A.; Gardner, A. Cooperation peaks at intermediate disturbance. Curr. Biol. 2007, 17, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Wakelin, S.A.; Chu, G.; Lardner, R.; Liang, Y.; McLaughlin, M. A single application of Cu to field soil has long-term effects on bacterial community structure, diversity, and soil processes. Pedobiologia 2010, 53, 149–158. [Google Scholar] [CrossRef]
- Kang, H.; Kwon, M.; Kim, S.; Lee, S.; Jones, T.G.; Johncock, A.C.; Haraguchi, A.; Freeman, C. Biologically driven DOC release from peatlands during recovery from acidification. Nat. Commun. 2018, 9, 3807. [Google Scholar] [CrossRef]
- Zhong, Y.; Jiang, M.; Middleton, B.A. Effects of water level alteration on carbon cycling in peatlands. Ecosyst. Health Sust. 2020, 6, 1806113. [Google Scholar] [CrossRef]
- Zeide, B. Thinning and growth: A full turnaround. J. For. 2001, 99, 20–25. [Google Scholar] [CrossRef]
- Wu, R.; Cheng, X.; Han, H. The effect of forest thinning on soil microbial community structure and function. Forests 2019, 10, 352. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, C.; Zhou, Z. Impacts of forest thinning on soil microbial community structure and extracellular enzyme activities: A global meta-analysis. Soil Biol. Biochem. 2020, 149, 107915. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Huenneke, L.F. Disturbance, diversity, and invasion—Implications for conservations. Conserv. Biol. 1992, 6, 324–337. [Google Scholar] [CrossRef]
| Disturbance | Target Enzymes | Sample Sources | |
|---|---|---|---|
| Chemical contaminations | Bacteriocide | Dehydrogenase β-glucosidase | Forest soil |
| Cd addition | Dehydrogenase, β-glucosidase | Marsh sediment | |
| Hydrological stress | Drought | β-glucosidase N-acetylglucosaminidase β-xylosidase | Tropical peatland |
| Tidal flow | β-glucosidase N-acetylglucosaminidase β-xylosidase | Mud-flat sediment | |
| Management | Thinning | β-glucosidase Cellobiohydrolase N-acetylglucosaminidase β-xylosidase | Forest soils |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, H.; Kim, S.; Song, K.; Kwon, M.-J.; Lee, J. Intermediate Disturbances Enhance Microbial Enzyme Activities in Soil Ecosystems. Microorganisms 2024, 12, 1401. https://doi.org/10.3390/microorganisms12071401
Kang H, Kim S, Song K, Kwon M-J, Lee J. Intermediate Disturbances Enhance Microbial Enzyme Activities in Soil Ecosystems. Microorganisms. 2024; 12(7):1401. https://doi.org/10.3390/microorganisms12071401
Chicago/Turabian StyleKang, Hojeong, Sunghyun Kim, Keunyea Song, Min-Jung Kwon, and Jaehyun Lee. 2024. "Intermediate Disturbances Enhance Microbial Enzyme Activities in Soil Ecosystems" Microorganisms 12, no. 7: 1401. https://doi.org/10.3390/microorganisms12071401
APA StyleKang, H., Kim, S., Song, K., Kwon, M.-J., & Lee, J. (2024). Intermediate Disturbances Enhance Microbial Enzyme Activities in Soil Ecosystems. Microorganisms, 12(7), 1401. https://doi.org/10.3390/microorganisms12071401





