Adsorption of Cd2+ by Lactobacillus plantarum Immobilized on Distiller’s Grains Biochar: Mechanism and Action
Abstract
1. Introduction
2. Materials and Methods
2.1. Biochar Preparation
2.2. Adsorption Experiment Design
2.3. Characterization of Biochar
2.4. Data Analysis
2.4.1. Calculation of Adsorption Capacity and Adsorption Efficiency
2.4.2. Isothermal Adsorption Model
2.4.3. Kinetic Model
3. Results
3.1. Analysis of Physical and Chemical Properties of Biochar
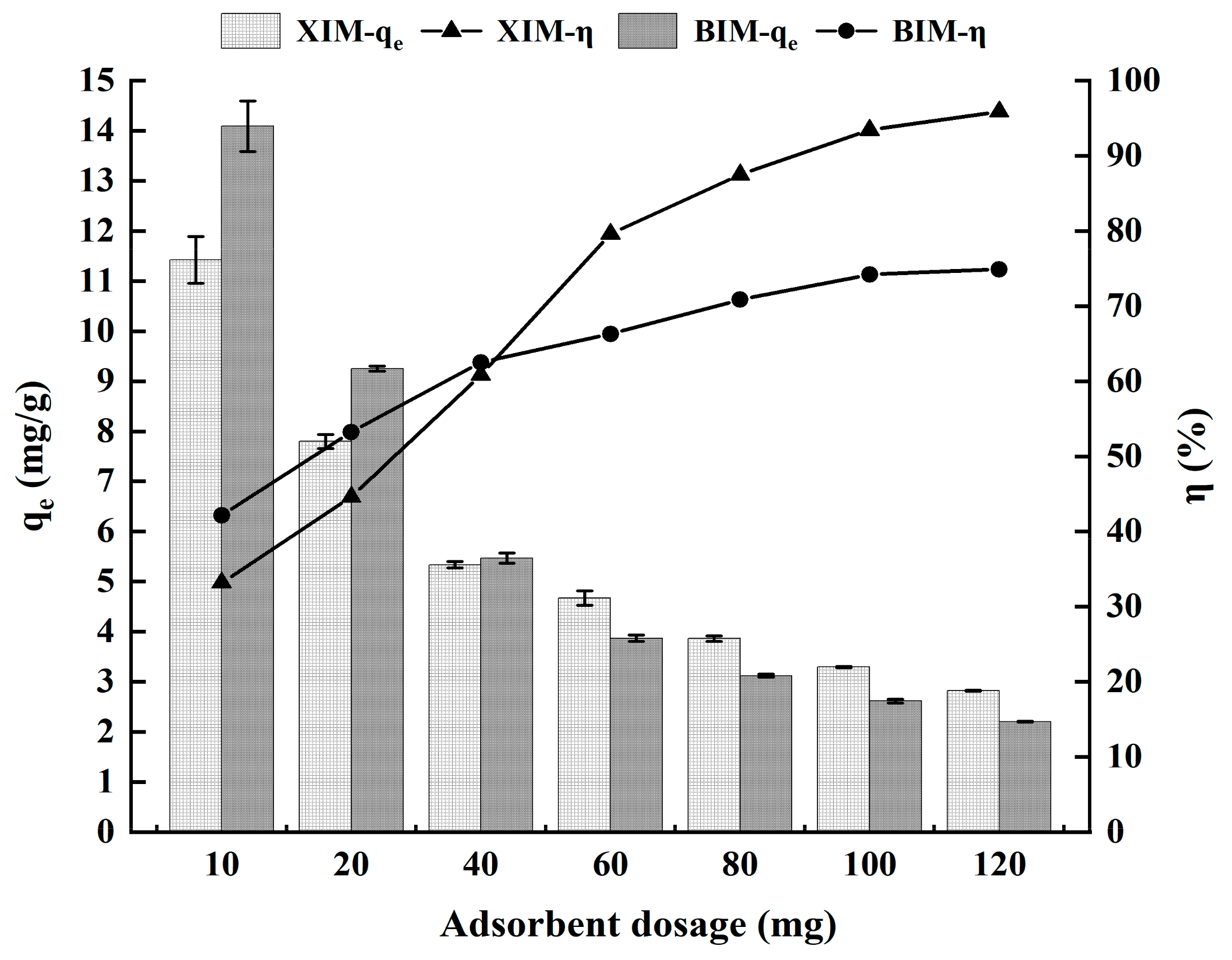
3.2. Effect of Dosage on the Adsorption Performance of Cd2+
3.3. Effect of pH on the Adsorption Properties of Cd2+
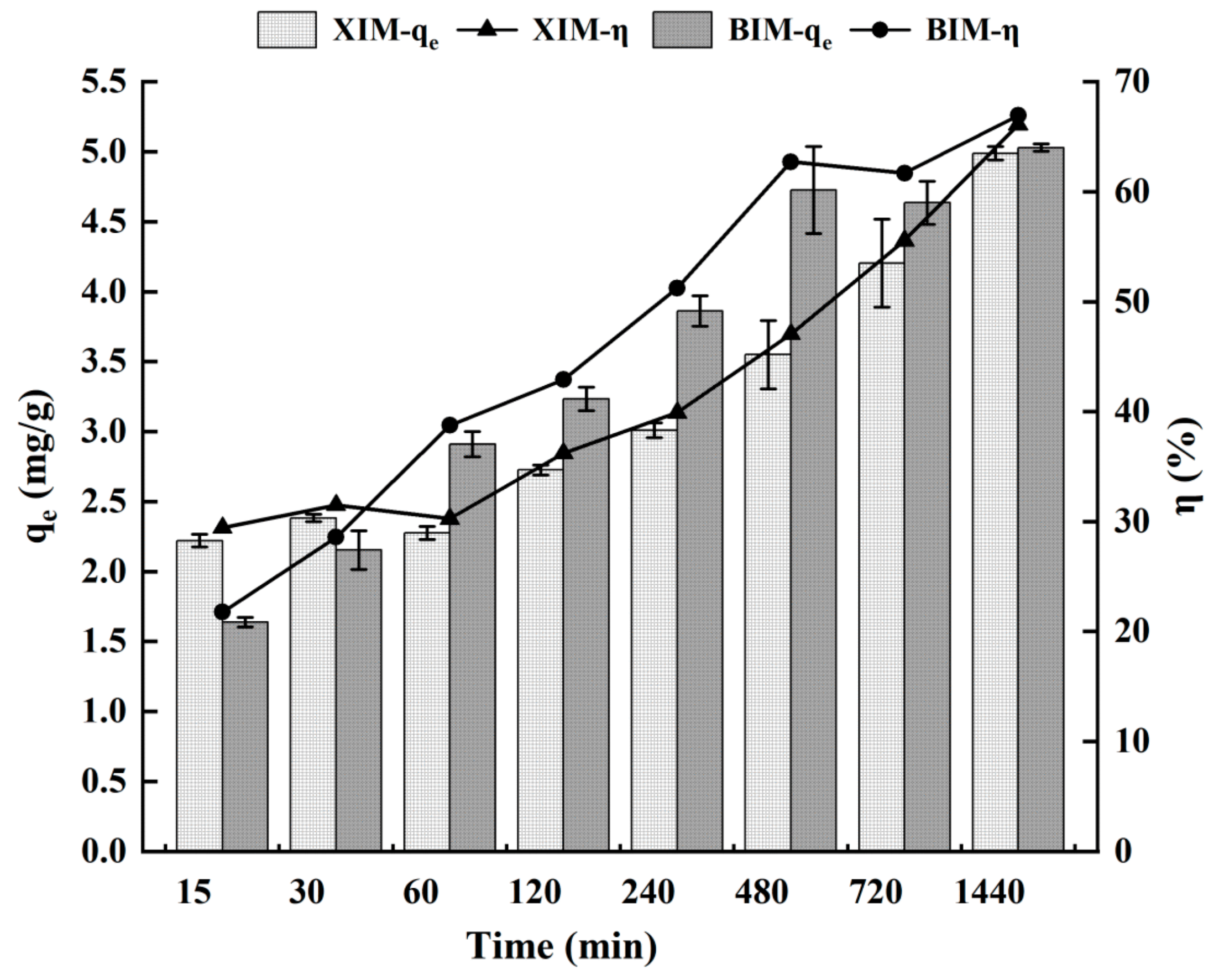
3.4. Effect of Adsorption Time on the Adsorption Properties of Cd2+
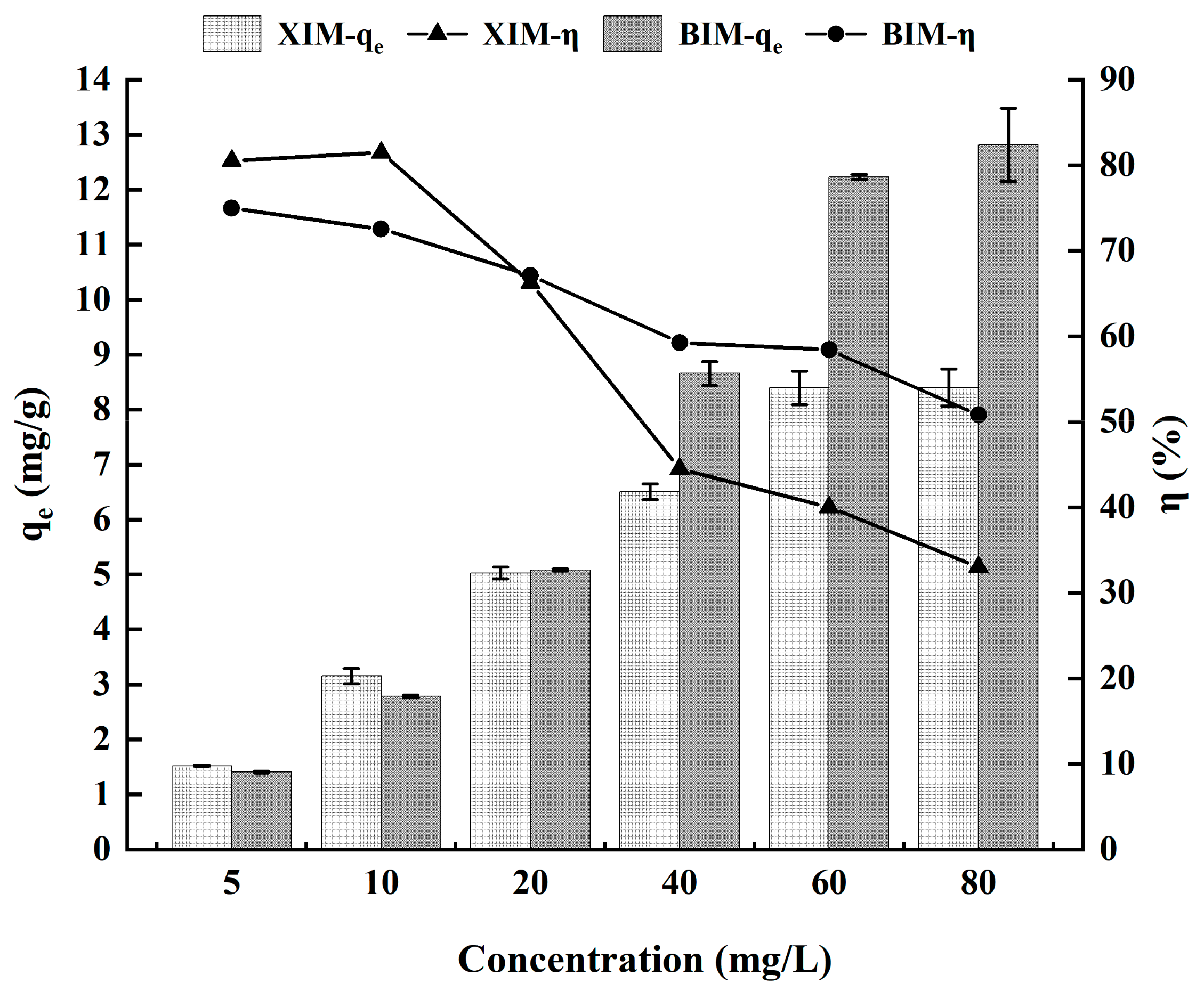
3.5. Effect of Cd2+ Concentration on Adsorption Performance
3.6. Adsorption Isotherm Analysis
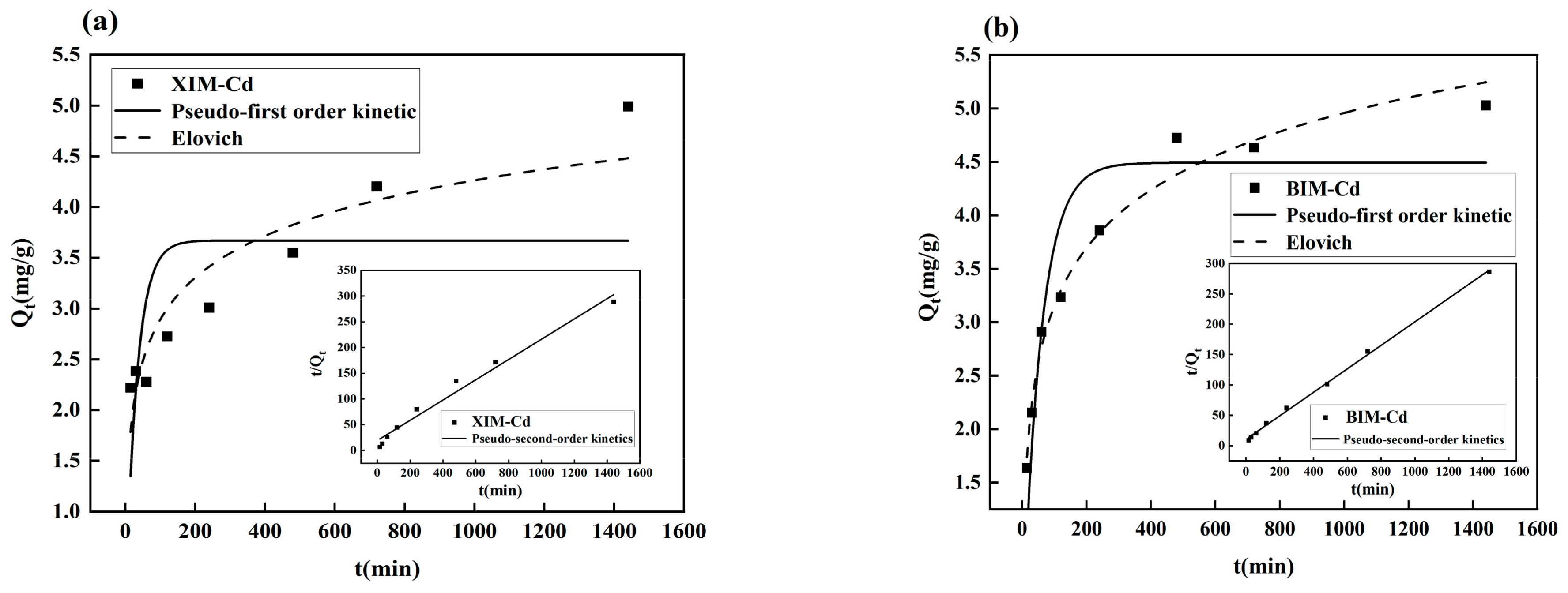
3.7. Adsorption Kinetic Analysis
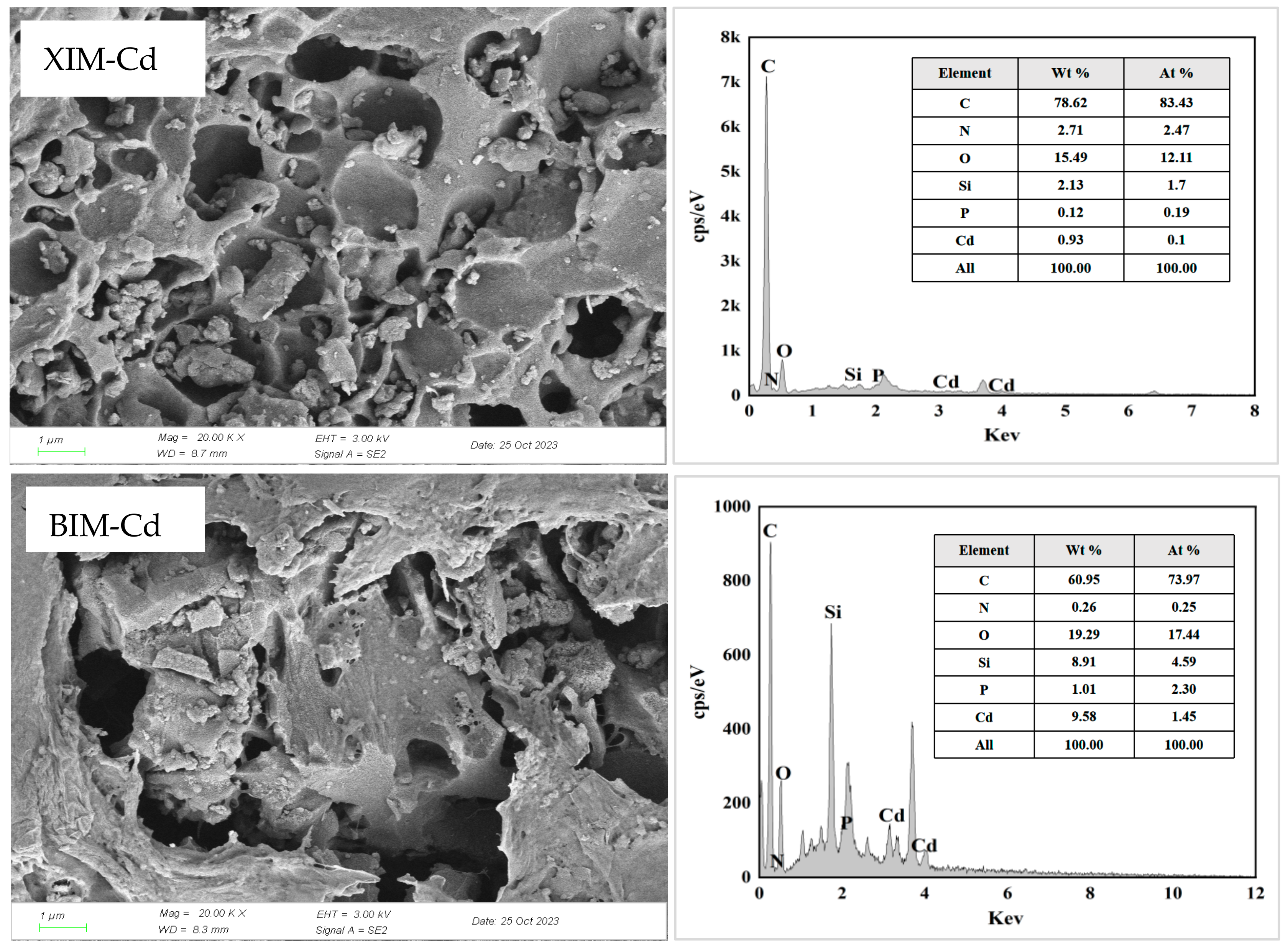
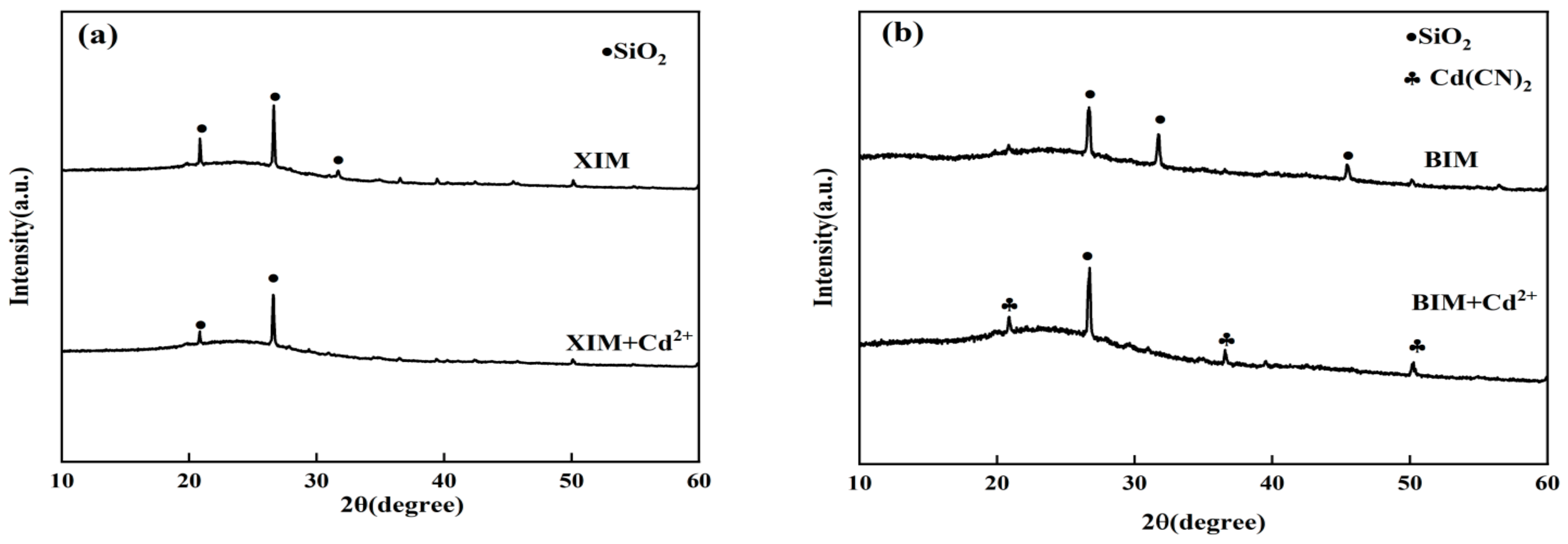
3.8. Post-Adsorption Characterization Results and Analysis
4. Discussion
5. Conclusions
- (1)
- With increasing dosages of XIM and BIM, the unit adsorption amount of Cd2+ gradually decreases, whereas the adsorption efficiency shows an upward trend. The adsorption of Cd2+ increases with higher solution pH, reaching maximum levels of adsorption amount and efficiency at a pH of 6.0.
- (2)
- The adsorption of Cd by XIM and BIM exhibited an increase with the concentration of Cd2+. XIM and BIM reached equilibrium at a Cd2+ concentration of 60 mg/L. BIM consistently demonstrated significantly higher Cd2+ adsorption than XIM across high Cd2+ concentrations.
- (3)
- The adsorption of Cd2+ by both XIM and BIM increased gradually with the extension of time. Cd2+ adsorption by XIM primarily involved surface and porous adsorption mechanisms. In contrast, the adsorption process of Cd2+ by BIM encompassed surface adsorption and intra-particle diffusion.
- (4)
- The adsorption of Cd2+ by XIM predominantly entailed physical adsorption and void retention. Conversely, the adsorption mechanism of Cd2+ by BIM predominantly involved the formation of Cd(CN)2 with Cd2+. Furthermore, the specific surface area, pore size, and functional group content inherent to XIM and BIM materials collectively affected their Cd2+ d adsorption processes.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, M.; Chen, Z.F.; Song, W.; Hong, D.Z.; Huang, L.; Li, Y.H. A review on cadmium exposure in the population and intervention strategies against cadmium toxicity. Bull. Environ. Contam. Toxicol. 2021, 106, 65–74. [Google Scholar] [CrossRef]
- Šrut, M.; Menke, S.; Höckner, M.; Sommer, S. Earthworms and cadmium–heavy metal resistant gut bacteria as indicators for heavy metal pollution in soils? Ecotoxicol. Environ. Saf. 2019, 171, 843–853. [Google Scholar] [CrossRef]
- Deng, Y.Y.; Huang, S.; Laird, D.A.; Wang, X.G.; Meng, Z.W. Adsorption behaviour and mechanisms of cadmium and nickel on rice straw biochars in single and binary-metal systems. Chemosphere 2019, 218, 308–318. [Google Scholar] [CrossRef]
- Pastorelli, A.A.; Angeletti, R.; Binato, G.; Mariani, M.B.; Cibin, V.; Morelli, S.; Ciardullo, S.; Stacchini, P. Exposure to cadmium through Italian rice (Oryza sativa L.): Consumption and implications for human health. J. Food Compos. Anal. 2018, 69, 115–121. [Google Scholar] [CrossRef]
- Qu, F.; Zheng, W.W. Cadmium Exposure: Mechanisms and Pathways of Toxicity and Implications for Human Health. Toxics 2024, 12, 388. [Google Scholar] [CrossRef]
- Schaefer, H.R.; Flannery, B.M.; Crosby, L.; Jones-Dominic, O.E.; Punzalan, C.; Middleton, K. A systematic review of adverse health effects associated with oral cadmium exposure. Regul. Toxicol. Pharmacol. 2022, 134, 105243. [Google Scholar] [CrossRef] [PubMed]
- Ayangbenro, A.S.; Babalola, O.O. A new strategy for heavy metal polluted environments: A review of microbial biosorbents. Int. J. Environ. Res. Public Health 2017, 14, 94. [Google Scholar] [CrossRef]
- Verma, S.; Kuila, A. Bioremediation of heavy metals by microbial process. Environ. Technol. Innov. 2019, 14, 100369. [Google Scholar] [CrossRef]
- Zhai, Q.; Wang, G.; Zhao, J.; Narbad, A.; Chen, Y.Q.; Zhang, H.; Tian, F.; Chen, W. Protective effects of Lactobacillus plantarum CCFM8610 against chronic cadmium toxicity in mice indicate routes of protection besides intestinal sequestration. Appl. Environ. Microbiol. 2014, 80, 4063–4071. [Google Scholar] [CrossRef]
- Ameen, F.A.; Hamdan, A.M.; El-Naggar, M.Y. Assessment of the heavy metal bioremediation efficiency of the novel marine lactic acid bacterium, Lactobacillus plantarum MF042018. Sci. Rep. 2020, 10, 314. [Google Scholar] [CrossRef]
- Pham, V.H.T.; Kim, J.; Chang, S.; Chung, W. Bacterial biosorbents, an efficient heavy metals green clean-up strategy: Prospects, challenges, and opportunities. Microorganisms 2022, 10, 610. [Google Scholar] [CrossRef]
- Rahman, Z.; Singh, V.P. Bioremediation of toxic heavy metals (THMs) contaminated sites: Concepts, applications and challenges. Env. Sci. Pollut. Res. 2020, 27, 27563–27581. [Google Scholar] [CrossRef] [PubMed]
- Bai, N.; Wang, S.; Sun, P.; Abuduaini, R.; Zhu, X.; Zhao, Y. Degradation of nonylphenol polyethoxylates by functionalized Fe3O4 nanoparticle-immobilized Sphingomonas sp. Y2. Sci. Total Environ. 2018, 615, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yang, F.; Dai, M.; Ali, I.; Shen, X.; Hou, X.; Naz, I. Application of microbial immobilization technology for remediation of Cr (VI) contamination: A review. Chemosphere 2022, 286, 131721. [Google Scholar] [CrossRef]
- Ji, X.; Wan, J.; Wang, X.; Peng, C.; Wang, G.; Liang, W.; Zhang, W. Mixed bacteria-loaded biochar for the immobilization of arsenic, lead, and cadmium in a polluted soil system: Effects and mechanisms. Sci. Total Environ. 2022, 811, 152112. [Google Scholar] [CrossRef] [PubMed]
- Velkova, Z.; Kirova, G.; Stoytcheva, M.; Kostadinova, S.; Todorova, K.; Gochev, V. Immobilized microbial biosorbents for heavy metals removal. Eng. Life Sci. 2018, 18, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Schommer, V.A.; Vanin, A.P.; Nazari, M.T.; Ferrari, V.; Dettmer, A.; Colla, L.M.; Piccin, J.S. Biochar-immobilized Bacillus spp. for heavy metals bioremediation: A review on immobilization techniques, bioremediation mechanisms and effects on soil. Sci. Total Environ. 2023, 881, 163385. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Gou, J.L.; Chen, X.M.; Xiao, S.Q.; Ali, I.; Shang, R.; Wang, D.; Wu, Y.W.; Han, M.W.; Luo, X.G. Application of mixed bacteria-loaded biochar to enhance uranium and cadmium immobilization in a co-contaminated soil. J. Hazard. Mater. 2021, 401, 123823. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Li, K.; Wu, R.R.; Yan, Y.J.; Xiao, R.B. Insight into the Cd2+ biosorption by viable Bacillus cereus RC-1 immobilized on different biochars: Roles of bacterial cell and biochar matrix. J. Clean. Prod. 2020, 272, 122743. [Google Scholar] [CrossRef]
- O’Connor, D.; Peng, T.; Zhang, J.; Tsang, D.C.; Alessi, D.S.; Shen, Z.; Bolan, N.S.; Hou, D. Biochar application for the remediation of heavy metal polluted land: A review of in situ field trials. Sci. Total Environ. 2018, 619, 815–826. [Google Scholar] [CrossRef]
- Gholizadeh, M.; Hu, X. Removal of heavy metals from soil with biochar composite: A critical review of the mechanism. J. Environ. Chem. Eng. 2021, 9, 105830. [Google Scholar] [CrossRef]
- Liu, M.S.; Almatrafi, E.; Zhang, Y.; Xu, P.; Song, B.; Zhou, C.Y.; Zeng, G.M.; Zhu, Y. A critical review of biochar-based materials for the remediation of heavy metal contaminated environment: Applications and practical evaluations. Sci. Total Environ. 2022, 806, 150531. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.J.; Khan, A.; Zhang, K.; Sun, P.; Akther, S.; Zhang, Y. Biochar remediation of soil: Linking biochar production with function in heavy metal contaminated soils. Plant Soil Environ. 2021, 67, 183–201. [Google Scholar]
- Li, X. Resourcezation of distillers grains of Jiangxiang Baijiu. Liquor-Mak. Sci. Technol. 2023, 8, 128–131. [Google Scholar]
- Bouabidi, Z.B.; El-Naas, M.H.; Zhang, Z. Immobilization of microbial cells for the biotreatment of wastewater: A review. Environ. Chem. Lett. 2019, 17, 241–257. [Google Scholar] [CrossRef]
- An, Q.; Ran, B.B.; Deng, S.M.; Jin, N.J.; Zhao, B.; Song, J.L.; Fu, S.Y. Peanut shell biochar immobilized pseudomonas hibiscicola strain L1 to remove electroplating mixed-wastewater. J. Environ. Chem. Eng. 2023, 11, 109411. [Google Scholar] [CrossRef]
- Valdivia-Rivera, S.; Ayora-Talavera, T.; Lizardi-Jiménez, M.A.; García-Cruz, U.; Cuevas-Bernardino, J.C.; Pacheco, N. Encapsulation of microorganisms for bioremediation: Techniques and carriers. Rev. Environ. Sci. Bio/Technol. 2021, 20, 815–838. [Google Scholar] [CrossRef]
- Ashjari, M.; Garmroodi, M.; Ahrari, F.; Yousefi, M.; Mohammadi, M. Soluble enzyme cross-linking via multi-component reactions: A new generation of cross-linked enzymes. Chem. Commun. 2020, 56, 9683–9686. [Google Scholar] [CrossRef] [PubMed]
- Altinkaynak, C.; Tavlasoglu, S.; Ocsoy, I. A new generation approach in enzyme immobilization: Organic-inorganic hybrid nanoflowers with enhanced catalytic activity and stability. Enzym. Microb. Technol. 2016, 9, 105–112. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Wnętrzak, R.; Leahy, J.J.; Hayes, M.H.B.; Kwapiński, W.; Hubicki, Z.J.C.E.J. Kinetic and adsorptive characterization of biochar in metal ions removal. Chem. Eng. J. 2012, 197, 295–305. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Dong, Z.M.; Dai, Y.; Xiao, S.; Cao, X.H.; Liu, Y.H.; Guo, W.H.; Luo, M.B.; Le, Z.G. Amidoxime-functionalized hydrothermal carbon materials for uranium removal from aqueous solution. RSC Adv. 2016, 6, 102462–102471. [Google Scholar] [CrossRef]
- Singh, S.; Bajwa, B.S.; Kaur, I. (Zn/Co)-zeolitic imidazolate frameworks: Room temperature synthesis and application as promising U(VI) scavengers –A comparative study. J. Ind. Eng. Chem. 2021, 93, 351–360. [Google Scholar] [CrossRef]
- Pütün, A.E.; Özbay, N.; Önal, E.P.; Pütün, E. Fixed-bed pyrolysis of cotton stalk for liquid and solid products. Fuel Process. Technol. 2005, 86, 1207–1219. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Xu, M.; Wu, J. Adsorption of Cd2+ by potassium ferrate/potassium permanganate-modified vinasse biochar. J. Agro-Environ. Sci. 2021, 40, 876–883. [Google Scholar]
- Tiwari, D.; Laldanwngliana, C.; Choi, C.H.; Lee, S.M. Manganese-modified natural sand in the remediation of aquatic environment contaminated with heavy metal toxic ions. Chem. Eng. J. 2011, 171, 958–966. [Google Scholar] [CrossRef]
- Chen, X.; Chen, G.; Chen, L.; Chen, Y.; Lehmann, J.; McBride, M.B.; Hay, A.G. Adsorption of copper and zinc by biochars produced from pyrolysis of hardwood and corn straw in aqueous solution. Bioresour. Technol. 2011, 10, 8877–8884. [Google Scholar] [CrossRef]
- Teng, Z.; Shao, W.; Zhang, K.; Yu, F.; Huo, Y.; Li, M. Enhanced passivation of lead with immobilized phosphate solubilizing bacteria beads loaded with biochar/nanoscale zero valent iron composite. J. Hazard. Mater. 2020, 384, 121505. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Li, Q.; Li, X. Removal of heavy metals from wastewater using biochars: Adsorption and mechanisms. Environ. Pollut. Bioavailab. 2022, 34, 385–394. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Niazi, N.K.; Hassan, N.E.E.; Bibi, I.; Wang, H.L.; Tsang, D.C.W.; Ok, Y.S.; Bolan, N.; Rinklebe, J. Wood-based biochar for the removal of potentially toxic elements in water and wastewater: A critical review. Int. Mater. Rev. 2019, 64, 216–247. [Google Scholar] [CrossRef]
- Li, A.Y.; Deng, H.; Jiang, Y.H.; Ye, C.H.; Yu, B.G.; Zhou, X.L.; Ma, A.Y. Superefficient removal of heavy metals from wastewater by Mg-loaded biochars: Adsorption characteristics and removal mechanisms. Langmuir 2020, 36, 9160–9174. [Google Scholar] [CrossRef]
- Vassileva, P.S.; Radoykova, T.H.; Detcheva, A.K.; Avramova, I.A.; Aleksieva, K.I.; Nenkova, S.K.; Valchev, I.V.; Mehandjiev, D.R. Adsorption of Ag+ ions on hydrolyzed lignocellulosic materials based on willow, paulownia, wheat straw and maize stalks. Int. J. Environ. Sci. Technol. 2016, 13, 1319–1328. [Google Scholar] [CrossRef]
- Regmi, P.; Moscoso, J.L.G.; Kumar, S.; Cao, X.; Mao, J.; Schafran, G. Removal of copper and cadmium from aqueous solution using switchgrass biochar produced via hydrothermal carbonization process. J. Environ. Manag. 2012, 109, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhou, D.; Zhu, L. Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures. Environ. Sci. Technol. 2008, 42, 5137–5143. [Google Scholar] [CrossRef]
- Manikandan, S.K.; Pallavi, P.; Shetty, K.; Bhattacharjee, D.; Giannakoudakis, D.A.; Katsoyiannis, I.A.; Nair, V. Effective usage of biochar and microorganisms for the removal of heavy metal ions and pesticides. Molecules 2023, 28, 719. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.A. Isotherm, kinetic, and thermodynamic studies on Hg(II) adsorption from aqueous solution by silica- multiwall carbon nanotubes. Environ. Sci. Pollut. Res. 2015, 22, 16721–16731. [Google Scholar] [CrossRef]
- Liu, Y.L.; Zhu, H.C.; Peng, O.; Li, D.Y.; Yang, R.X.; Peng, J.; Tie, B.Q. Adsorption of cadmium and arsenic by corn stalk biochar solidified microorganism. Environ. Sci. 2020, 41, 4322–4332. [Google Scholar]
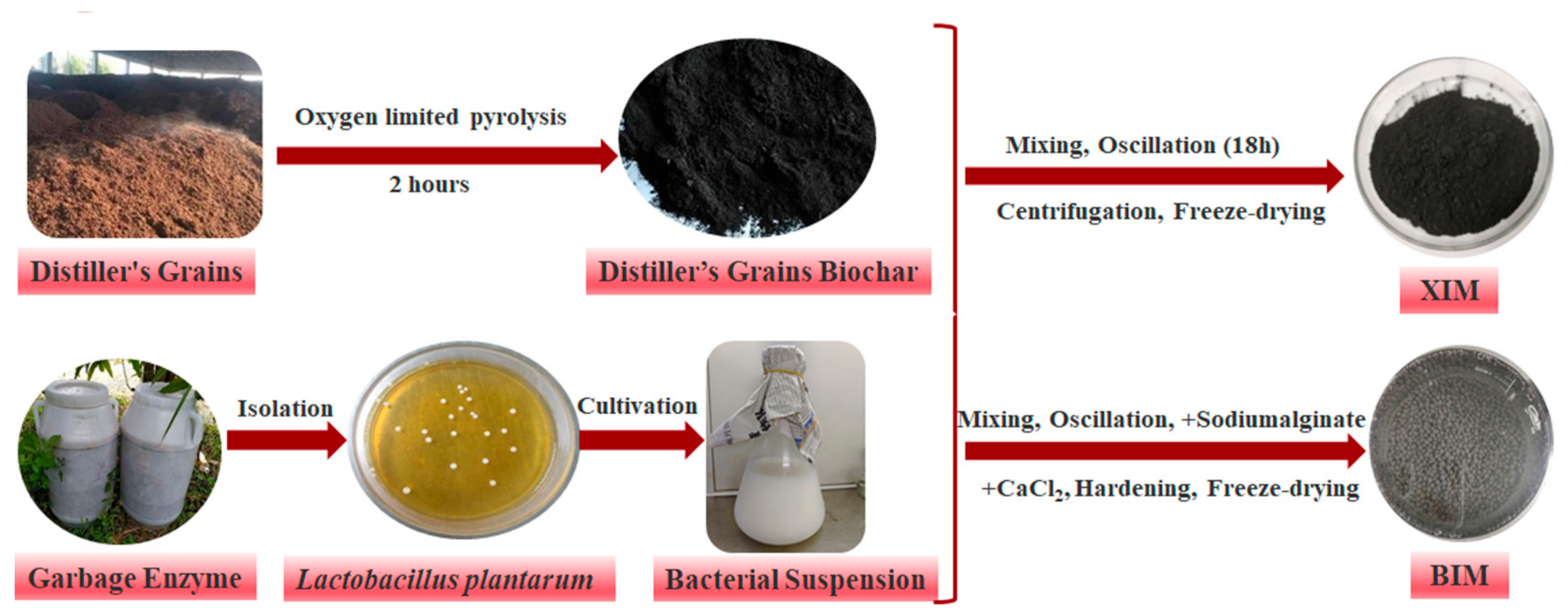



| Absorbent Material | Experimental Projects | Variable Settings | Experimental Condition |
|---|---|---|---|
| XIM BIM | One-way experiment | Dosage: 10, 20, 40, 60, 80, 100, 120 mg | Cd2+ Concentration: 20 mg/L, pH = 6.0, Reaction time: 1440 min (24 h) |
| pH: 3, 4, 5, 6, 7 | Cd2+ Concentration: 20 mg/L, XIM/BIM dosage: 50 mg, Reaction time: 1440 min (24 h) | ||
| Kinetic experiment | Reaction time: 15, 30, 60, 120, 240, 480, 720, 1440 | Cd2+ Concentration: 20 mg/L, pH = 6.0, XIM/BIM dosage: 50 mg | |
| Isothermal adsorption experiment | Cd2+ solution: 5, 10, 20, 40, 60, 80 mg/L | pH = 6.0, XIM/BIM dosage: 50 mg, Reaction time: 1440 min (24 h) |
| Biochar | pH | Specific Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|---|
| BC | 9.94 | 5.3207 | 0.0210 | 15.8109 |
| XIM | 7.23 | 5.8500 | 0.0195 | 13.3140 |
| BIM | 7.78 | 7.0270 | 0.0146 | 8.3068 |
| Experimental Projects | Variable | Variable Settings | Adsorption Amount (mg/g) | Adsorption Efficiency (%) | ||
|---|---|---|---|---|---|---|
| XIM | BIM | XIM | BIM | |||
| One-way experiment | Dosage | 10 mg | 11.42 ± 0.93 | 14.09 ± 1.01 | 33.18% ± 1.93% | 42.17% ± 2.66% |
| 20 mg | 7.80 ± 0.28 | 9.25 ± 0.11 | 44.60% ± 1.18% | 53.24% ± 0.52% | ||
| 40 mg | 5.34 ± 0.13 | 5.47 ± 0.20 | 60.84% ± 1.28% | 62.50% ± 2.15% | ||
| 60 mg | 4.67 ± 0.30 | 3.87 ± 0.13 | 79.61% ± 5.23% | 66.31% ± 2.17% | ||
| 80 mg | 3.86 ± 0.11 | 3.12 ± 0.06 | 87.51% ± 2.30% | 70.85% ± 1.19% | ||
| 100 mg | 3.29 ± 0.03 | 2.61 ± 0.08 | 93.44% ± 1.02% | 74.20% ± 2.14% | ||
| 120 mg | 2.82 ± 0.02 | 2.20 ± 0.02 | 95.91% ± 0.64% | 74.91% ± 0.59% | ||
| pH | 3 | 1.85 ± 0.26 | 4.09 ± 0.17 | 23.42% ± 3.30% | 51.72% ± 2.03% | |
| 4 | 4.42 ± 0.05 | 4.90 ± 0.08 | 58.38% ± 0.72% | 64.56% ± 0.86% | ||
| 5 | 4.71 ± 0.17 | 4.97 ± 0.13 | 23.42% ± 1.96% | 51.72% ± 1.39% | ||
| 6 | 4.73 ± 0.10 | 4.93 ± 0.18 | 58.38% ± 1.45% | 64.56% ± 2.12% | ||
| 7 | 4.53 ± 0.05 | 4.80 ± 0.16 | 63.01% ± 0.50% | 66.91% ± 1.83% | ||
| Kinetic experiment | Time | 15 min | 2.22 ± 0.09 | 1.64 ± 0.07 | 29.44% ± 1.30% | 21.78% ± 0.86% |
| 30 min | 2.38 ± 0.05 | 2.15 ± 0.28 | 31.51% ± 0.75% | 28.57% ± 3.63% | ||
| 60 min | 2.28 ± 0.09 | 2.91 ± 0.18 | 30.26% ± 1.25% | 38.76% ± 2.25% | ||
| 120 min | 2.73 ± 0.07 | 3.23 ± 0.17 | 36.21% ± 0.98% | 42.91% ± 2.12% | ||
| 240 min | 3.01 ± 0.11 | 3.86 ± 0.22 | 39.91% ± 1.49% | 51.25% ± 2.68% | ||
| 480 min | 3.55 ± 0.49 | 4.73 ± 0.62 | 47.06% ± 6.43% | 62.71% ± 8.20% | ||
| 720 min | 4.20 ± 0.63 | 4.64 ± 0.31 | 55.55% ± 8.30% | 61.69% ± 4.14% | ||
| 1440 min | 4.99 ± 0.10 | 5.03 ± 0.05 | 66.10% ± 1.40% | 66.93% ± 0.67% | ||
| Isothermal adsorption experiment | Cd2+ solution | 5 mg/L | 1.52 ± 0.01 | 1.41 ± 0.03 | 80.52% ± 0.80% | 74.98% ± 1.51% |
| 10 mg/L | 3.15 ± 0.14 | 2.78 ± 0.05 | 81.47% ± 3.50% | 72.55% ± 1.15% | ||
| 20 mg/L | 5.03 ± 0.11 | 5.08 ± 0.04 | 66.27% ± 1.39% | 67.09% ± 0.66% | ||
| 40 mg/L | 6.51 ± 0.14 | 8.66 ± 0.44 | 44.51% ± 0.96% | 59.25% ± 3.13% | ||
| 60 mg/L | 8.40 ± 0.30 | 12.23 ± 0.10 | 40.03% ± 1.42% | 58.42% ± 0.55% | ||
| 80 mg/L | 8.41 ± 0.34 | 12.82 ± 1.33 | 33.03% ± 1.34% | 50.79% ± 5.33% | ||
| Absorbent Material | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qm | KL | R2 | KF | 1/n | R2 | |
| XIM | 11.64 | 0.0357 | 0.9879 | 1.0136 | 0.4991 | 0.9607 |
| BIM | 27.43 | 0.0118 | 0.9909 | 0.6075 | 0.7110 | 0.9798 |
| Absorbent Material | Pseudo-First Order Kinetic | Pseudo-Second Order Kinetics | Elovich | ||||||
|---|---|---|---|---|---|---|---|---|---|
| K1 | qe | R2 | K2 | qe | R2 | α | β | R2 | |
| XIM | 0.0305 | 3.67 | 0.3434 | 0.0020 | 5.08 | 0.9797 | 0.7484 | 1.6735 | 0.8701 |
| BIM | 0.0175 | 4.49 | 0.8499 | 0.0035 | 5.17 | 0.9983 | 0.4224 | 1.2674 | 0.9805 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, G.; Wang, X.; Du, R.; Wen, S.; Du, L.; Tu, Q. Adsorption of Cd2+ by Lactobacillus plantarum Immobilized on Distiller’s Grains Biochar: Mechanism and Action. Microorganisms 2024, 12, 1406. https://doi.org/10.3390/microorganisms12071406
Zhu G, Wang X, Du R, Wen S, Du L, Tu Q. Adsorption of Cd2+ by Lactobacillus plantarum Immobilized on Distiller’s Grains Biochar: Mechanism and Action. Microorganisms. 2024; 12(7):1406. https://doi.org/10.3390/microorganisms12071406
Chicago/Turabian StyleZhu, Guangxu, Xingfeng Wang, Ronghui Du, Shuangxi Wen, Lifen Du, and Qiang Tu. 2024. "Adsorption of Cd2+ by Lactobacillus plantarum Immobilized on Distiller’s Grains Biochar: Mechanism and Action" Microorganisms 12, no. 7: 1406. https://doi.org/10.3390/microorganisms12071406
APA StyleZhu, G., Wang, X., Du, R., Wen, S., Du, L., & Tu, Q. (2024). Adsorption of Cd2+ by Lactobacillus plantarum Immobilized on Distiller’s Grains Biochar: Mechanism and Action. Microorganisms, 12(7), 1406. https://doi.org/10.3390/microorganisms12071406





