Abstract
Public health faces daily challenges due to increasing reports of pathogenic microorganisms with new antimicrobial resistance. Klebsiella michiganensis, an emerging pathogen, poses difficulty in its identification using conventional techniques. This study presents the first documented case of NDM-1-producing K. michiganensis in Brazil, identified as the new ST418. Initially, the isolate from a tracheal secretion was misidentified as K. oxytoca. However, accurate identification was achieved through ANI analyses. Whole-genome sequencing was conducted to characterize the genetic context of the resistance genes, to identify virulence factors, and to construct a phylogenetic tree. The blaNDM-1 gene was found to be harbored on an IncFIB plasmid approximately 112 kb in length, which was transferable in conjugation assays. The detection of carbapenem resistance genes in this species highlights the importance of public health vigilance, as it may serve as a reservoir and disseminator of significant resistance genes.
1. Introduction
Antimicrobial resistance presents a significant global threat, constraining treatment options for bacterial infections, thereby diminishing clinical effectiveness, escalating treatment expenses, and increasing mortality rates [1]. It is known that once resistance genes are successfully established in plasmids, they can spread resistance rapidly through different lineages, species, or even genera [2]. Klebsiella michiganensis, initially recovered from a toothbrush holder in 2013, is now recognized as an emerging critical human pathogen [3,4]. This species is part of the K. oxytoca complex, consisting of nine related species, including K. oxytoca, K. grimontii, K. huaxiensis, K. michiganensis, K. pasteurii, K. spallanzanii, and three unnamed taxa (taxons 1, 2, and 3), which are challenging to differentiate reliably based on their phenotypic characteristics [5]. It has been suggested that K. michiganensis may be more clinically relevant in human-associated infections than K. oxytoca, but differentiation between these two species is difficult, potentially leading to an underestimated real prevalence of K. michiganensis [6,7]. The complex can be divided into phylogroups based on variation in the sequence of the intrinsic gene blaOXY, encoding a β-lactamase that confers resistance to amino and carboxy-penicillin, of which K. michiganensis belongs to the phylogroup Ko1 (with Ko5 representing a sub-lineage) [5]. The presence of a wide range of accessory genes in the pan-genome of K. michiganensis suggests an open pan-genome, which indicates its ability to adapt to diverse environmental conditions and its potential genomic plasticity [8].
Moreover, the emergence of carbapenemase-producing K. michiganensis poses a new health threat, especially when bla-coding genes have the capability to horizontally spread through mobile genetic elements [3]. The resistance in these bacteria primarily involves genes such as blaKPC, blaNDM, and blaIMP, which are associated with plasmids. Strains carrying more than one gene have already been documented in recent years, all co-harboring blaNDM with another gene [9,10,11].
Unlike serine-β-lactamases, metallo-β-lactamases require an active site ion and are not inhibited by the so-called second generation of β-lactamase inhibitors, avibactam and vaborbactam, thus impacting antimicrobial therapy [12,13]. Currently, 61 variants of NDM have been identified (according to the BLDL database http://bldb.eu/, accessed on 20 June 2024) [14]. In Brazil, the first blaNDM gene was identified in a Providencia rettgeri in 2013 and the variant detected was the NDM-1 [15]. According to a recent review, between 2012 and 2021, NDM-producing isolates were identified in 14 bacterial species belonging to eight different genera in Brazil, with 80% belonging to Klebsiella spp. and NDM-1 being the predominant allele (54% of isolates) [16]. Another large study identified an increase in the prevalence of NDM-producing organisms in the southern region of Brazil [17].
The blaNDM gene is carried by various Inc-group plasmids, such as IncA/C, IncL/M, IncF, IncX, IncHI1A, IncHI1B, and IncN [18,19]. In terms of K. michiganensis, there have been few reported cases of strains carrying the blaNDM gene, with four from China [9,10,11,20], one from Japan [21], and one from South Africa [22], all of them located in the IncFIB or IncX3 plasmids. To the best of our knowledge, however, there are no reported cases of NDM-producing K. michiganensis on the American continents. In the present study, we characterized a clinical blaNDM-1-producing K. michiganensis strain that was isolated in Brazil and explored the phenotypic and genotypic characteristics of the isolate and the plasmid.
2. Materials and Methods
2.1. Bacterial Identification, Antimicrobial Susceptibility Testing, and PCR to blaNDM Detection
A strain of Klebsiella spp. was initially isolated from a tracheal secretion at the local hospital laboratory and sent to our reference laboratory (Bacteriology Center of the Instituto Adolfo Lutz, São Paulo, Brazil) in September 2021. The isolate was identified as ID_1060/21 and submitted for species identification via phenotypic (biochemical series) and molecular tests (MALDI-TOF MS, Bruker Daltonics, Bremen, Germany). The antimicrobial susceptibility test of isolate ID_1060/21 was evaluated by disk diffusion [23] against 21 antimicrobial agents: amikacin (30 µg), amoxicilin-clavulanic acid (20/10 µg), ampicilin-sulbactam (10/10 µg), aztreonam (30 µg), cefepime (30 µg), cefotaxime (30 µg), cefoxitin (30 µg), cefpodoxime (10 µg), ceftazidime (30 µg), ciprofloxacin (5 µg), chloramphenicol (30 µg), ertapenem (10 µg), gentamicin (10 µg), imipenem (10 µg), levofloxacin (5 µg), meropenem (10 µg), norfloxacin (10 µg), piperacillin-tazobactam (100/10 µg), trimethoprim-sulfamethoxazole (1.25/23.75 µg) tobramycin (10 µg), and tigecycline (15 µg). The results were interpreted according to the guidelines described in the Clinical Laboratory and Standards Institute document, M100 [24], and BrCAST 2023, when breakpoints were available. The in-house broth microdilution was performed with cation-adjusted Muller Hinton broth (Sigma-Aldrich, St. Louis, MO, USA) for polymyxin B, in plates ranging from 0.125 to 16 mg/L; MIC values >2 mg/L were considered resistant [25]. A multiplex PCR targeting the blaKPC, blaNDM, and blaOXA-48 genes was performed following established protocols [26]. The strain ID_1060/21 Klebsiella michiganensis has been deposited in the Department of Culture Collection of the Adolfo Lutz Institute, a public service collection, as IAL 10144.
2.2. Conjugation Assay and Plasmid Size Determination
A conjugation assay with Escherichia coli K12 strain J53 (sodium azide-resistant) was performed following Fernandes et al.’s (2017) protocol [27]. The isolates ID_1060/21 and E. coli J53 were reactivated on TSA and incubated at 35 °C for 24 h. One colony from each sample was inoculated into LB broth and incubated at 35 °C overnight. The optical density of the two samples was measured and equalized to 0.7 on the McFarland scale, and 100 µL of each was inoculated into the same tube with 3 mL of LB broth and placed at 35 °C overnight. Mueller Hinton plates were prepared with 2.0 µg/mL of imipenem and 100 µg/mL of sodium azide and, on the same day, 100 µL of growth was inoculated onto the plates and spread with a Drigalsk loop, and then incubated at 35 °C for 24 h. Colonies grown on the plates were reseeded onto TSA next to a ceftriaxone disk and isolated colonies grown close to the disk were identified, using MALDI-TOF, as E. coli. Of these, a new isolation was performed to obtain pure samples of the conjugate to proceed with the multiplex PCR, to confirm the transference of the resistance gene, and to perform the minimum inhibitory concentration with an E-test with beta lactams.
To determine the size of the plasmid, S1-nuclease pulsed-field gel electrophoresis was performed both in the parental (ID_1060/21) and in the transconjugant strain. The plugs were prepared from a suspension with a transmittance of 4–5%, measured in a spectrophotometer (Analyser, São Paulo, Brazil). Plugs were digested with 0.089 unit of S1-nuclease enzyme (Promega, Madison, WI, USA) for 45 min at 37 °C. For plasmid size determination, a molecular marker, Salmonella enterica serotype Braenderup H9812 [28] digested with 30 U XbaI at 37 °C/16 h, was employed. Electrophoresis was performed on the CHEF DR III (BioRad, Hercules, CA, USA) at 6 V/cm and 14 °C, with an initial time switch of 1.0 s and a final pulse of 40.0 s, for 17 h. The gel was stained with ethidium bromide (5 µg/mL) and visualized in a photo documenter (DNR, Bio Imaging Systems, Jerusalem, Israel). Plasmid size was determined using BioNumerics v.8.0 software (Applied Maths, Sint-Martens-Latem, Belgium).
2.3. Transconjugant Antimicrobial Susceptibility Testing (AST)
Minimal inhibitory concentrations (MICs) were determined with an E-test (ampicillin, cefepime, cefotaxime, ceftriaxone, cephalothin, imipenem and meropenem) for the ID_1060/21, the wild E. coli J53, and the transconjugant.
2.4. DNA Extraction and Whole-Genome Sequencing
Genomic DNA of the strain ID_1060/21 was extracted using the commercial Wizard® Genomic DNA Purification kit (Promega, Madison, WI, USA), starting from an initial volume of 1.4 mL of overnight bacterial growth at 35 °C in LB broth and following the manufacturer’s protocol. Purified DNA was initially assessed using a spectrophotometer from NanoDrop One (Thermo Fisher Scientific, Waltham, MA, USA) and then quantified in a Qubit 4 fluorometer (Thermo Fisher Scientific, Waltham, MA, USA). The DNA’s integrity was verified by E-gel (Invitrogen, Waltham, MA, USA) electrophoresis.
For short reads’ sequencing using Illumina technology (Illumina, San Diego, CA, USA), the genomic library was prepared using magnetic beads with the Illumina DNA Prep kit to cleave and tag the DNA. To form clusters, a PCR was performed with NexteraTM DNA CD Indexes. Sequencing was carried out at the Strategic Laboratory, Instituto Adolfo Lutz, Brazil, using Illumina MiSeq® equipment to generate 75 bp paired-end reads. For the long reads’ sequencing, libraries were prepared with the Rapid Barcoding Sequencing kit and sequenced using MinION equipment (Oxford Nanopore Technologies, Oxford, UK).
2.5. Genomic Analyzes
FastQC was used to verify the quality of the reads and GC content. The hybrid assembly of the genome’s short and long reads was performed using Unicycler and another assembly was performed, using Flye, for only the long reads [29]. To confirm the identity of ID_1060/21, an Average Nucleotide Identity (ANI) analysis was performed against a local database generated with representative genomes of all Klebsiella species available from the NCBI (Supplementary Table S1). From the assembled genomes, predicted resistance and gene locations were analyzed using ResFinder, PlasmidFinder, and MGEFinder (all using the Center for Genomic Epidemiology services available at http://www.genomicepidemiology.org/services/ accessed on 1 July 2024), and, to analyze the virulence genes, we employed the Virulence Factor Database [29], which compares the query genome with the references’ accession numbers: AAF37887, NP_752613, NP_286010, and NP_462662. The online tool PathogenWatch (https://pathogen.watch/ accessed on 1 July 2024) was used also for species identification and the determination of sequence length, sequence type (ST), antimicrobial resistance, and the virulence genes’ conference.
For the plasmids comparison, genome sequences were annotated with Rapid Annotation using Subsystem Technology (RAST) and the plasmids’ sequences were compared with BLAST Ring Image Generator (BRIG) software version 0.95. A partial alignment of the genetic environment of the resistance gene was visualized with Clinker (https://cagecat.bioinformatics.nl/tools/clinker accessed on 3 June 2024).
For phylogenetic analysis, 68 genomes (Supplementary Table S2) contained in PubMLST (website) were retrieved, together with their metadata, using the CSI phylogeny (http://www.genomicepidemiology.org/services/ accessed on 3 June 2024) tool and visualized in the Microreact platform (http://microreact.org accessed on 3 June 2024).
This Whole-Genome Shotgun project has been deposited in DDBJ/ENA/GenBank under the accession number JAOCNS000000000. The version described in this paper is version JAOCNS020000000.
3. Results
The isolate ID_1060/21 was initially misidentified as K. oxytoca by biochemical tests and MALDI-TOF MS. Nevertheless, ANI analyses identified ID_1060/21 as K. michiganensis, with values ≥ 0.9714 (Supplementary Table S1; Supplementary Figure S1). The presence of chromosomal blaOXY-5, according to ResFinder, confirms the species’ relationship to the variation in the sequence of this β-lactamase.
K. michiganensis ID_1060/21 was resistant to monobactams, penicillins, cephalosporins, tetracycline, and ertapenem, but presented susceptibility to almost all aminoglycosides, fluoroquinolones, phenicol, folate inhibitors, and polymyxin B (MIC 0.5 mg/L) (Table 1). With these phenotypic results, the isolate was classified as having multidrug resistance (MDR) [30].

Table 1.
Antimicrobial susceptibility testing, determined by disk diffusion, for ID_1060/21 and categorizations according to CLSI 2023.
The multiplex PCR assay was positive only for the blaNDM gene. The conjugative transfer of blaNDM was successfully achieved to an E. coli J53 recipient strain, confirmed by conventional PCR. The transconjugant strain showed more than a 60-fold increase in comparison to the wild E. coli J53 (Table 2). S1-PFGE revealed that isolate ID_1060/21 carries three plasmids sized 163.49 kb, 105.91 kb, and 50.52 kb, but only the 103.55 kb plasmid was transferred according to the S1-PFGE analysis (Supplementary Figure S2).

Table 2.
Results of comparative MICs between E. coli J53 receptor, transconjugant, and ID_1060/21.
Hybrid assembly successfully identified the presence of 10 contigs in a chromosome length of 6,071,905 bp. Three additional contigs were assembled, corresponding to the plasmids observed in S1-PFGE.
K. michiganensis ID_1060/21 was identified as the new sequence type ST418 (allelic profile: gapA 3; infB 50; mdh 15; pgi 22; phoE 18; rpoB 6; tonB 4). Sequencing analyses showed the presence of blaNDM-1, found in an IncFIB(K) plasmid, named pID_1060/21, ~112,325 bp in size, in accordance with the observed plasmids detected in the S1-PFGE of both the parental and transconjugant strain (Supplementary Figure S2). The beta-lactam resistance genes blaOXY-5 (chromosomal gene) and blaTEM-1 (IncN2 plasmid) were also detected. ID_1060/21 presented four virulence genes with >80% identity (accession numbers NP_752613, AAF37887, NP_286010, and NP_462662, respectively): entB (siderophores enterobactin), ompA (outer membrane protein), yagZ/ecpA (common pilus structural subunit), and mgtB (magnesium-transporting).
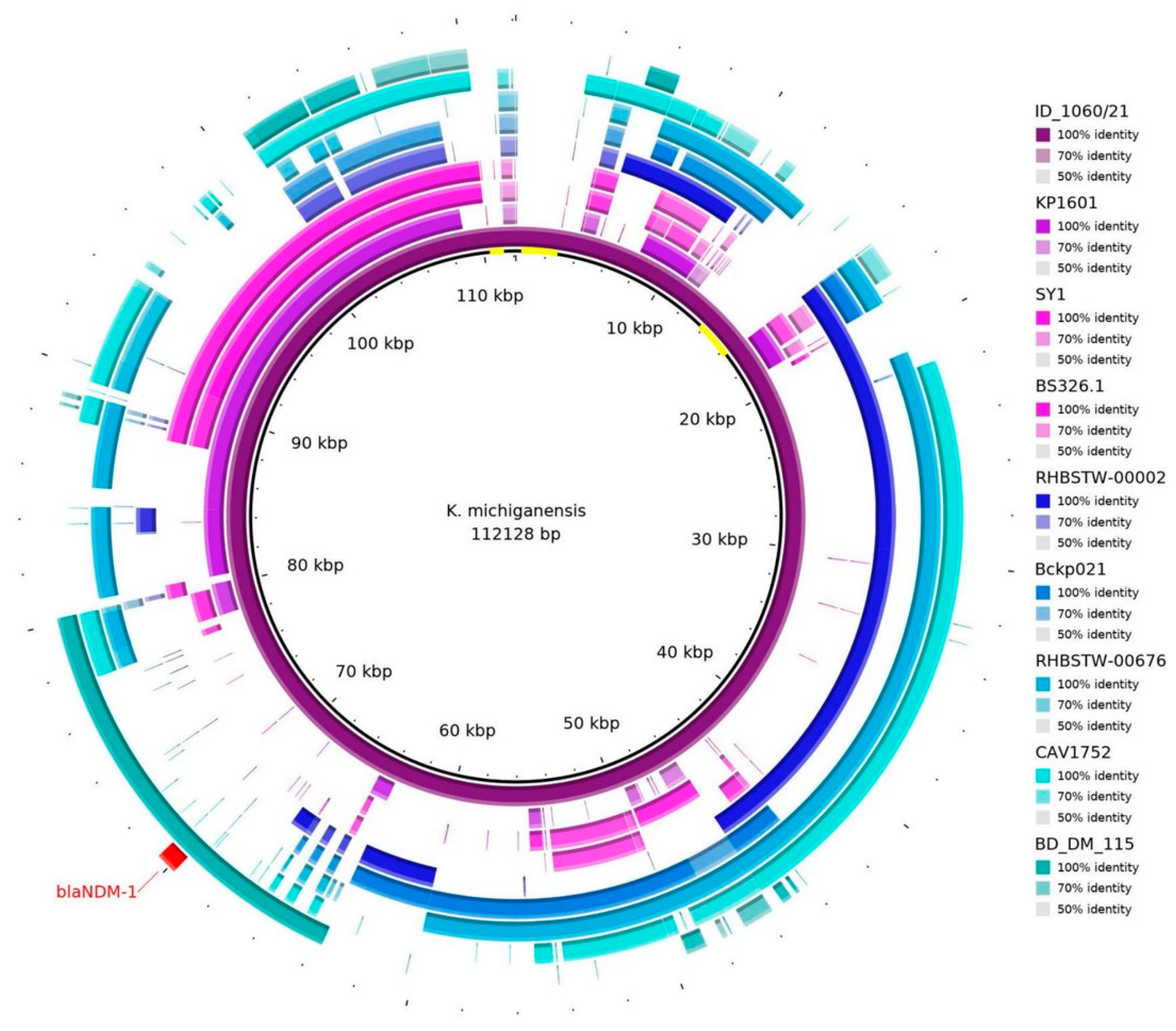
The sequence of the pID_1060/21 plasmid was blasted against the NCBI database, and eight different plasmid sequences (Supplementary Table S3) were necessary to almost cover the entire 112 kb sequence (Figure 1). Nevertheless, some regions were not identified in other plasmids (as highlighted in yellow in the center circle in Figure 1).
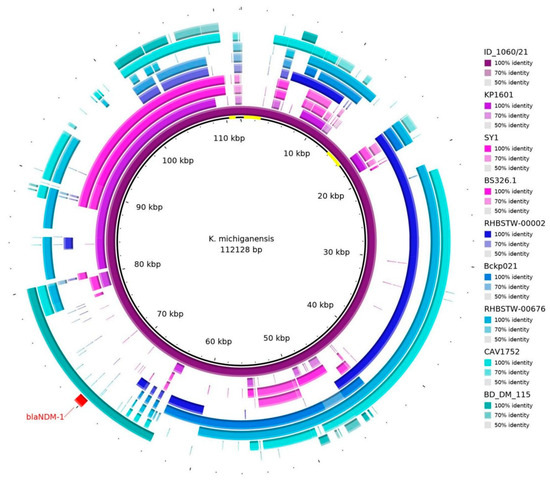
Figure 1.
Plasmid hits, according to blastn (https://blast.ncbi.nlm.nih.gov/Blast.cgi), against eight sequences in the database from the NCBI. It is possible to see that the blaNDM-1 gene (in red) is aligned with the BD_DM_115 sequence (plasmid p_kv_NDM1; accession: CP095680.1). Some regions were out of alignment with other sequences, as shown in yellow in the center of the representative circle.
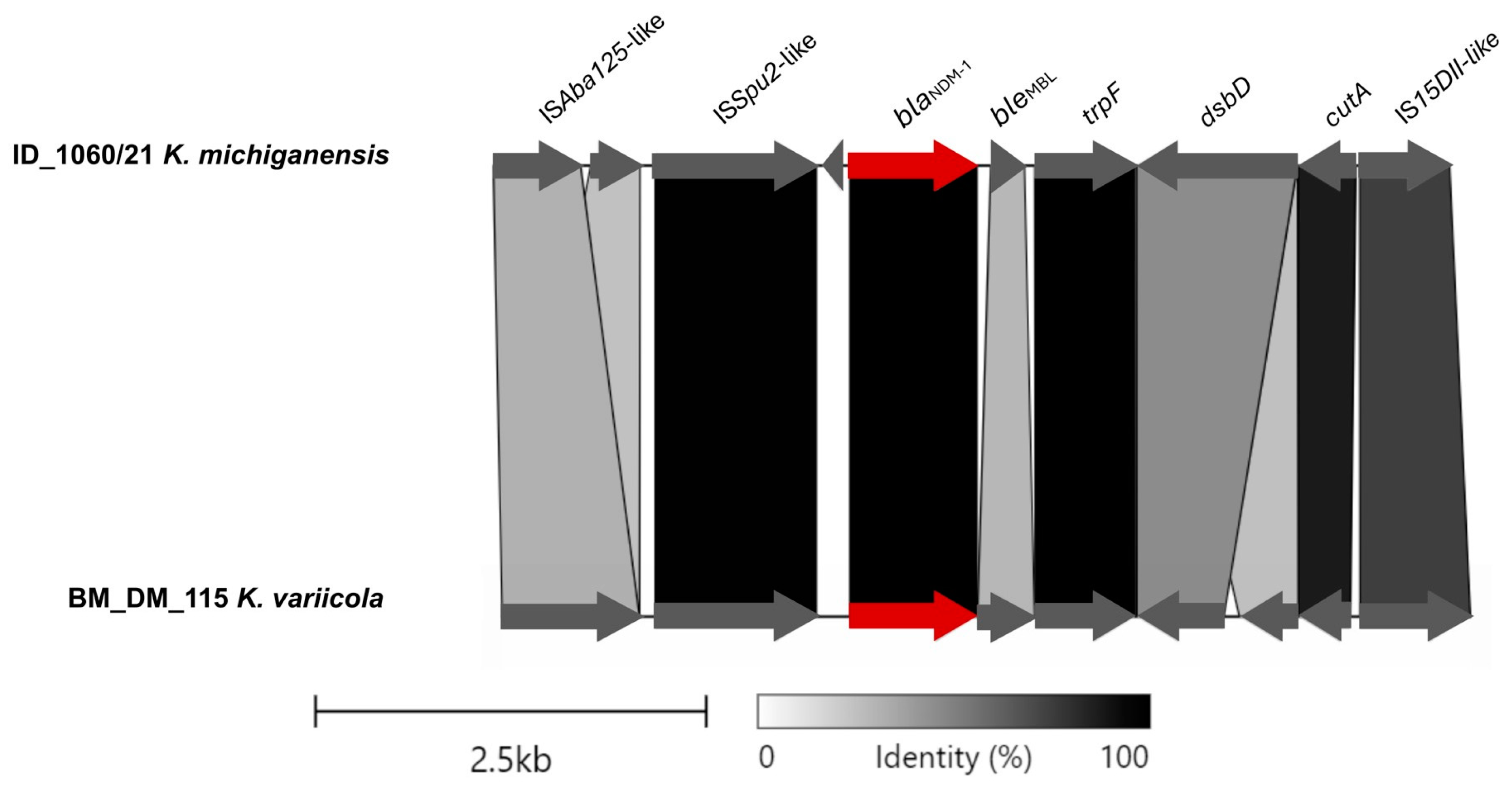
The region of pID_1060/21 containing the blaNDM-1 gene presented similarity with the plasmid p_kv_NDM1 (from K. variicola BM_DM_115, isolated from a blood sample in Bangladesh, 2016; accession CP095680.1), an IncFII(K)/IncFIB(pQil)-type plasmid (Figure 2). The ISAba125-like (IS30 family) truncated and ISSup2-like (IS630 family) element were identified as the mobile genetic elements located upstream of the gene blaNDM-1. Downstream, the blaNDM-1 gene, bleMBL, trpF, dsbD, and IS15DII-like (IS6 family) element were identified (Figure 2).
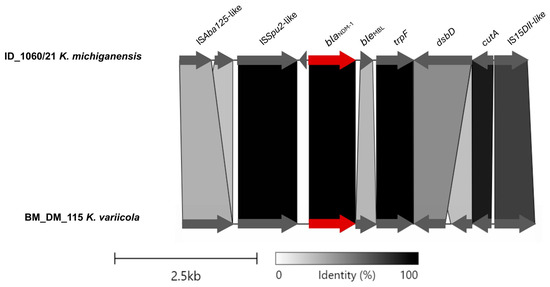
Figure 2.
Partial alignment of ID_1060/21 and BD_DM_115 plasmids and the linearized comparison of the blaNDM-1 genetic environment, with blaNDM-1 highlighted in red. This figure was created using the Clinker tool (available at https://cagecat.bioinformatics.nl/tools/clinker accessed on 3 June 2024).
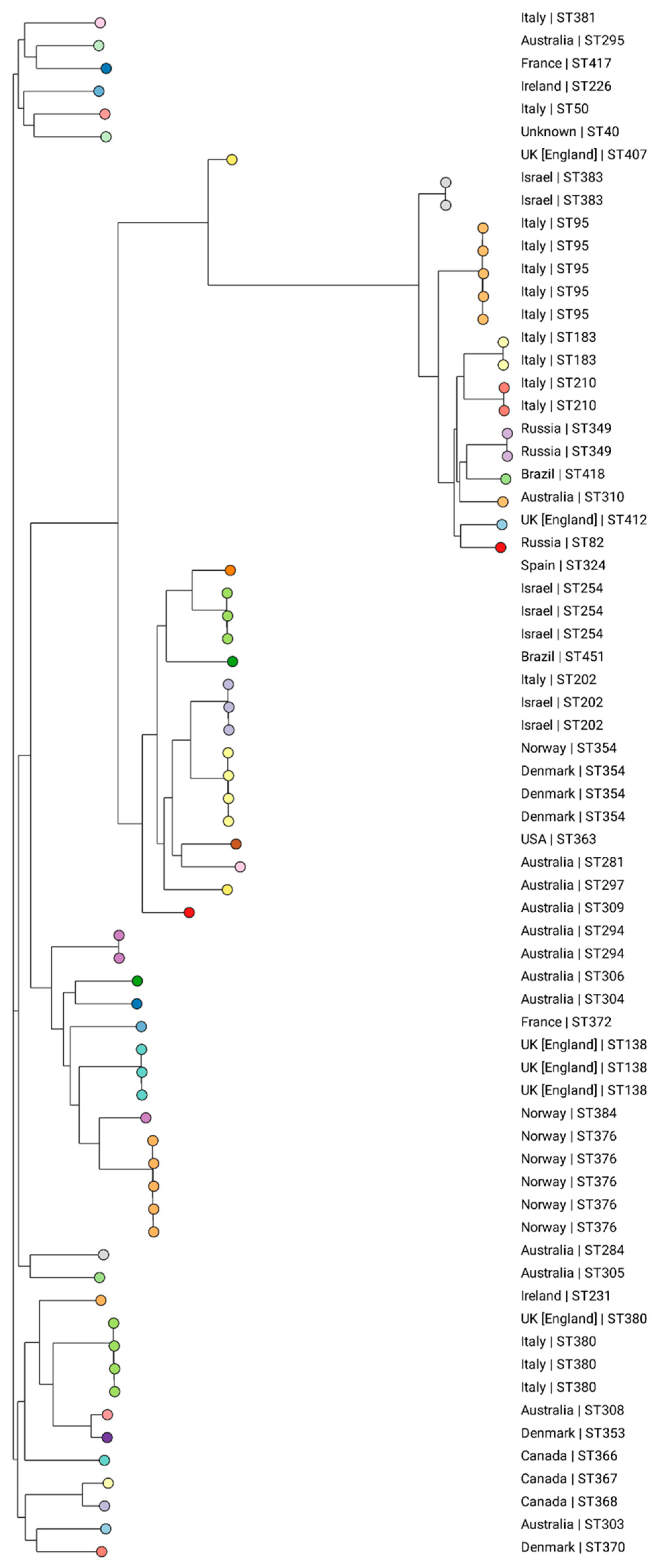
Based on the analysis of the K. michiganensis pubMLST collection, 43 different STs were identified among the 68 evaluated isolates retrieved from 14 countries: Australia (n = 13), Brazil (n = 2), Canada (n = 3), Denmark (n = 5), England (n = 6), France (n = 2), Ireland (n = 2), Israel (n = 7), Italy (n = 15), Norway (n = 7), Russia (n = 3), Spain (n = 1), the USA (n = 1), and unknown (n = 1) (Supplementary Table S2). This shows that there is a diversity to clones all over the world, with no ST predominating. An association of clones to certain regions was observed, such as ST354 in Denmark, ST376 in Norway, ST254 in Israel, and ST95 in Italy (Figure 3).
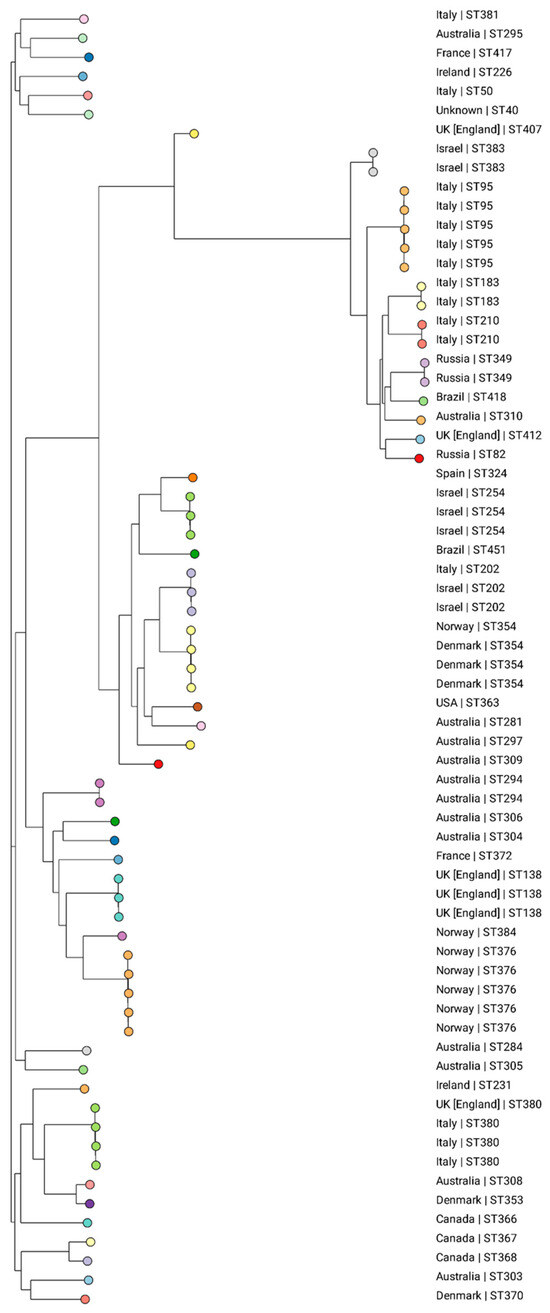
Figure 3.
Phylogenetic tree generated from the analysis of the SNPs of 68 global K. michiganensis isolates using CSIPhylogeny, visualized on the Microreact website. The colors represent the different STs and the country of isolation is presented for each strain. Isolate ID_1060/21 is clustered with two isolates from Russia (ST349).
4. Discussion
In this study, we report the first case in Brazil of K. michiganensis harboring the blaNDM-1 gene within a transferable IncFIB plasmid. The emergence of carbapenemase-producing K. michiganensis highlights its potential role as a reservoir and disseminator of clinically relevant resistance genes [9]. Noteworthy cases of K. michiganensis carrying NDM variants have been reported globally in recent years, spanning regions such as China [9,10,11,20], South Africa [22], and Japan [21].
Similar to our isolate, Zhang et al. (2022) identified an NDM-producing K. michiganensis in China presenting blaNDM-1 located in a 233,442 bp IncFIB(K)/IncFII(K) plasmid (Genbank accession: JAHNZR000000000.1) [9]. Conversely, Zheng et al. (2018) recovered a similar isolate from a stool sample carrying blaNDM-1 in a 106,140 bp IncFIB/IncFIIY plasmid (Genbank accession: NZ_CP022350.1) [11]. Notably, both studies detected multiple carbapenemase genes, including blaKPC-2, blaNDM-1, and blaIMP-4 [9] and blaKPC-2, blaNDM-1, and blaNDM-5 [11] in a single K. michiganensis strain, underscoring its potential as a reservoir for disseminating different resistance genes to other pathogens.
Diverging from our findings, previous reports of carbapenemase NDM in K. michiganensis have identified different Inc-type plasmids. NDM-1 was located in IncX3 plasmids in South Africa [22] and China [10], while NDM-5 was associated with IncX3 plasmids in Japan [21] and China [11]. The presence of resistance genes such as blaNDM and its variants in diverse plasmids may elucidate the broad dissemination of these enzymes [17], thus posing a significant public health challenge.
An in-depth analysis of the genetic context of the blaNDM-1 gene revealed a conserved triad of genes (bleMBL trpF, and dsbD) downstream of blaNDM-1, corroborating the existing literature [31].
According to the phylogenetic tree, ID_1061/21, identified as the new ST418, was closely associated with two isolates from Russia, both classified as ST349. Pan-genomic analyses conducted by Simoni et al. (2023) revealed that 35% of K. michiganensis genes are part of the core genome, emphasizing the existence of a diverse array of accessory genes within this species [8]. According to the authors, this suggests potential genomic plasticity linked to adaptation in novel environments, as well as the continuous microevolution of K. michiganensis. An escalating number of reports document K. michiganensis co-harboring genes implicated in carbapenem resistance. In a Chinese study encompassing 25 carbapenem-resistant K. oxytoca complex strains, K. michiganensis emerged as the predominant species in 16/25 (64% of isolates), with 5 harboring blaNDM resistance genes and an additional 10 strains containing blaKPC or blaIMP [32].
Since the initial identification of NDM in Brazil, numerous publications have spotlighted carbapenemase occurrences in Enterobacterales. This study represents the first report of NDM-producing K. michiganensis in the country, elucidating the plasmid and genetic context of the blaNDM-1 gene harbored by a transferable IncFIB plasmid. The increasing incidence of K. michiganensis carrying, accumulating, and disseminating resistance genes globally underscores the critical need for reinforced vigilance, aiming to identify and control the dissemination of carbapenemase-producing pathogens in healthcare settings.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/microorganisms12071408/s1, Figure S1: Heatmap visualization of Average Nucleotide Identity between the Klebsiella spp. genomes available in the NCBI database. A color key located in the upper left corner represents the percentage of identity between the analyzed genomes. The greater the percentage of identity between the genomes, the warmer the color. The clustering of ID_1060/21 with K. michiganensis genomes is observed, as is its distance from other species of the K. oxytoca complex. The database used to construct this figure is presented in Supplementary Table S1; Figure S2: S1-PFGE of ID_1060/21 (lane 1) and transconjugant E. coli J53 with a plasmid of ID_1060/21 (lane 2). It is observed that the wild type of the sample has three plasmids and one of them was transferred to the recipient E. coli J53. The plasmid sizes in the wild strain, from top to bottom, according to Bionumerics, are 163.49 kb, 105.91 kb, and 50.52 kb. (M—marker H9812; 1—wild sample ID_1060/21; 2—recipient E. coli J53); Supplementary Table S1: Representative genomes of Klebsiella species available in the NCBI database used to construct the heatmap visualization of Average Nucleotide Identity; Supplementary Table S2: Database of the 68 genomes contained in PubMLST used for the phylogenetic analysis; Supplementary Table S3: Eight different plasmid sequences aligned against pID_1060/21 plasmid from NCBI database.
Author Contributions
Conceptualization, C.H.C., D.B.d.A. and E.d.C.; methodology, A.R.d.S., C.H.C., A.Y.Y. and E.H.T.; software, C.H.C., A.Y.Y., E.d.C., K.R.C., C.T.S., M.P.V.C. and M.R.T.-C.; resources, C.H.C.; data curation, A.Y.Y., C.H.C. and C.T.S.; writing—original draft preparation, A.Y.Y.; writing—review and editing, C.H.C.; visualization, A.M.d.J.B. and M.R.T.-C.; supervision, C.H.C.; project administration, C.H.C.; funding acquisition, C.H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo, grant numbers 2024/10701-0; 2022/11794-7; 2020/06157-2; 2018/21192-9; 2017/50333-7); CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, grant number 302543/2021-0, 402158/2021-0; 444395/2023-7); and the Brazilian Ministry of Health (MoH).
Data Availability Statement
This Whole-Genome Shotgun project has been deposited in DDBJ/ENA/GenBank under the accession number JAOCNS000000000. The version described in this paper is version JAOCNS020000000. The strain ID_1060/21 was deposited in the Department of Culture Collection of the Adolfo Lutz Institute and is available as IAL 10144.
Acknowledgments
The authors are thankful to FESIMA (Fundo Especial de Saúde para Imunização em Massa e Controle de Doenças), FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo, CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), and the Brazilian Ministry of Health (MoH). C.H.C. and M.R.T.-C. have received Productivity Research Fellowships from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic Resistance: A Rundown of a Global Crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- von Wintersdorff, C.J.H.; Penders, J.; van Niekerk, J.M.; Mills, N.D.; Majumder, S.; van Alphen, L.B.; Savelkoul, P.H.M.; Wolffs, P.F.G. Dissemination of Antimicrobial Resistance in Microbial Ecosystems through Horizontal Gene Transfer. Front. Microbiol. 2016, 7, 173. [Google Scholar] [CrossRef]
- Campos-Madueno, E.I.; Sigrist, T.; Flückiger, U.M.; Risch, L.; Bodmer, T.; Endimiani, A. First Report of a BlaVIM-1 Metallo-β-Lactamase-Possessing Klebsiella michiganensis. J. Glob. Antimicrob. Resist. 2021, 25, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Saha, R.; Farrance, C.E.; Verghese, B.; Hong, S.; Donofrio, R.S. Klebsiella michiganensis sp. nov., a New Bacterium Isolated from a Tooth Brush Holder. Curr. Microbiol. 2013, 66, 72–78. [Google Scholar] [CrossRef]
- Yang, J.; Long, H.; Hu, Y.; Feng, Y.; McNally, A.; Zong, Z. Klebsiella oxytoca Complex: Update on Taxonomy, Antimicrobial Resistance, and Virulence. Clin. Microbiol. Rev. 2022, 35, e00006-21. [Google Scholar] [CrossRef]
- Gómez, M.; Valverde, A.; Del Campo, R.; Rodríguez, J.M.; Maldonado-Barragán, A. Phenotypic and Molecular Characterization of Commensal, Community-Acquired and Nosocomial Klebsiella spp. Microorganisms 2021, 9, 2344. [Google Scholar] [CrossRef]
- Shibu, P.; McCuaig, F.; McCartney, A.L.; Kujawska, M.; Hall, L.J.; Hoyles, L. Improved Molecular Characterization of the Klebsiella oxytoca Complex Reveals the Prevalence of the Kleboxymycin Biosynthetic Gene Cluster. Microb. Genom. 2021, 7, 000592. [Google Scholar] [CrossRef] [PubMed]
- Simoni, S.; Leoni, F.; Veschetti, L.; Malerba, G.; Carelli, M.; Lleò, M.M.; Brenciani, A.; Morroni, G.; Giovanetti, E.; Rocchegiani, E.; et al. The Emerging Nosocomial Pathogen Klebsiella michiganensis: Genetic Analysis of a KPC-3 Producing Strain Isolated from Venus Clam. Microbiol. Spectr. 2023, 11, e04235-22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, D.; Yang, X.; Wu, Y.; Liu, C.; Shen, Z.; Zhang, R. Emergence and Genomic Characterization of a KPC-2-, NDM-1-, and IMP-4-Producing Klebsiella michiganensis Isolate. Front. Microbiol. 2022, 12, 762509. [Google Scholar] [CrossRef]
- Li, X.; He, J.; Jiang, Y.; Peng, M.; Yu, Y.; Fu, Y. Genetic Characterization and Passage Instability of a Hybrid Co-Harboring blaIMP-4 and blaNDM-1 Reveal the Contribution of Insertion Sequences During Plasmid Formation and Evolution. Microbiol. Spectr. 2021, 9, e0157721. [Google Scholar] [CrossRef]
- Zheng, B.; Xu, H.; Yu, X.; Lv, T.; Jiang, X.; Cheng, H.; Zhang, J.; Chen, Y.; Huang, C.; Xiao, Y. Identification and Genomic Characterization of a KPC-2-, NDM-1- and NDM-5-Producing Klebsiella michiganensis Isolate. J. Antimicrob. Chemother. 2018, 73, 536–538. [Google Scholar] [CrossRef]
- Wu, W.; Feng, Y.; Tang, G.; Qiao, F.; Mcnally, A.; Zong, Z. NDM Metallo-Lactamases and Their Bacterial Producers in Health Care Settings. Clin. Microbiol. Rev. 2019, 32, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Lomovskaya, O.; Sun, D.; Rubio-Aparicio, D.; Nelson, K.; Tsivkovski, R.; Griffith, D.C.; Dudley, M.N. Vaborbactam: Spectrum of Beta-Lactamase Inhibition and Impact of Resistance Mechanisms on Activity in Enterobacteriaceae. Antimicrob. Agents Chemother. 2017, 61, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Naas, T.; Oueslati, S.; Bonnin, R.A.; Dabos, M.L.; Zavala, A.; Dortet, L.; Retailleau, P.; Iorga, B.I. Beta-Lactamase Database (BLDB)–Structure and Function. J. Enzyme Inhib. Med. Chem. 2017, 32, 917–919. [Google Scholar] [CrossRef]
- Carvalho-Assef, A.P.D.; Pereira, P.S.; Albano, R.M.; Berião, G.C.; Chagas, T.P.G.; Timm, L.N.; Da Silva, R.C.F.; Falci, D.R.; Asensi, M.D. Isolation of NDM-Producing Providencia rettgeri in Brazil. J. Antimicrob. Chemother. 2013, 68, 2956–2957. [Google Scholar] [CrossRef]
- Camargo, C.H. Current Status of NDM-Producing Enterobacterales in Brazil: A Narrative Review. Braz. J. Microbiol. 2022, 53, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Arend, L.N.V.S.; Bergamo, R.; Rocha, F.B.; Bail, L.; Ito, C.; Baura, V.A.; Balsanelli, E.; Pothier, J.F.; Rezzonico, F.; Pilonetto, M.; et al. Dissemination of NDM-Producing Bacteria in Southern Brazil. Diagn. Microbiol. Infect. Dis. 2023, 106, 115930. [Google Scholar] [CrossRef]
- Khong, W.X.; Xia, E.; Marimuthu, K.; Xu, W.; Teo, Y.Y.; Tan, E.L.; Neo, S.; Krishnan, P.U.; Ang, B.S.P.; Lye, D.C.B.; et al. Local Transmission and Global Dissemination of New Delhi Metallo-Beta-Lactamase (NDM): A Whole Genome Analysis. BMC Genom. 2016, 17, 452. [Google Scholar] [CrossRef]
- Marquez-Ortiz, R.A.; Haggerty, L.; Olarte, N.; Duarte, C.; Garza-Ramos, U.; Silva-Sanchez, J.; Castro, B.E.; Sim, E.M.; Beltran, M.; Moncada, M.V.; et al. Genomic Epidemiology of NDM-1-Encoding Plasmids in Latin American Clinical Isolates Reveals Insights into the Evolution of Multidrug Resistance. Genome Biol. Evol. 2017, 9, 1725–1741. [Google Scholar] [CrossRef]
- Jiang, T.; Li, G.; Huang, L.; Ding, D.; Ruan, Z.; Yan, J. Genomic and Phylogenetic Analysis of a Multidrug-Resistant Blandm-Carrying Klebsiella michiganensis in China. Infect. Drug Resist. 2023, 16, 3109–3116. [Google Scholar] [CrossRef]
- Prah, I.; Nukui, Y.; Yamaoka, S.; Saito, R. Emergence of a High-Risk Klebsiella michiganensis Clone Disseminating Carbapenemase Genes. Front. Microbiol. 2022, 13, 880248. [Google Scholar] [CrossRef] [PubMed]
- Founou, R.C.; Founou, L.L.; Allam, M.; Ismail, A.; Essack, S.Y. Genomic Characterisation of Klebsiella Co-Producing OXA-181 and NDM-1 Carbapenemases isolated from a Cancer Patient in Umgungundlovu, KwaZulu-Natal Province, South. Clin. Microbiol. Infect. 2014, 20, O1121–O1123. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic Susceptibility Testing by a Standardized Single Disk Method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards M100. Performance Standards for Antimicrobial Susceptibility Testing, 33rd ed.; Clinical and Laboratory Standards Institute: Berwyn, PA, USA, 2023. [Google Scholar]
- BrCAST Brazilian Committee on Antimicrobial Susceptibility Testing—BrCAST. Tabelas de Pontos de Corte Para Interpretação de CIMs e Diâmetros de Halo. 2023. Available online: https://brcast.org.br/documentos/ (accessed on 1 July 2024).
- Poirel, L.; Walsh, T.R.; Cuvillier, V.; Nordmann, P. Multiplex PCR for Detection of Acquired Carbapenemase Genes. Diagn. Microbiol. Infect. Dis. 2011, 70, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.A.; Camargo, C.H.; Francisco, G.R.; Bueno, M.F.C.; Garcia, D.O.; Doi, Y.; Casas, M.R.T. Prevalence of Extended-Spectrum β-Lactamases CTX-M-8 and CTX-M-2-Producing Salmonella Serotypes from Clinical and Nonhuman Isolates in Brazil. Microb. Drug Resist. 2017, 23, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Hunter, S.B.; Vauterin, P.; Lambert-Fair, M.A.; Van Duyne, M.S.; Kubota, K.; Graves, L.; Wrigley, D.; Barrett, T.; Ribot, E. Establishment of a Universal Size Standard Strain for Use with the Pulsenet Standardized Pulsed-Field Gel Electrophoresis Protocols: Converting the National Databases to the New Size Standard. J. Clin. Microbiol. 2005, 43, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Afgan, E.; Nekrutenko, A.; Grüning, B.A.; Blankenberg, D.; Goecks, J.; Schatz, M.C.; Ostrovsky, A.E.; Mahmoud, A.; Lonie, A.J.; Syme, A.; et al. The Galaxy Platform for Accessible, Reproducible and Collaborative Biomedical Analyses: 2022 Update. Nucleic Acids Res. 2022, 50, W345–W351. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Jia, X.; Liu, H.; Li, S.; Wu, X.; Huang, S. Emergence of NDM-5-Producing Escherichia coli in a Teaching Hospital in Chongqing, China: IncF-Type Plasmids May Contribute to the Prevalence of blaNDM–5. Front. Microbiol. 2020, 11, 334. [Google Scholar] [CrossRef]
- Wan, W.; Yang, X.; Yu, H.; Wang, M.; Jia, W.; Huang, B.; Qu, F.; Shan, B.; Tang, Y.W.; Chen, L.; et al. Genomic Characterization of Carbapenem-Resistant Klebsiella oxytoca Complex in China: A Multi-Center Study. Front. Microbiol. 2023, 14, 1153781. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).