Reducing Application of Nitrogen Fertilizer Increases Soil Bacterial Diversity and Drives Co-Occurrence Networks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description, Experiment Design and Sample Collection
2.2. Soil Physicochemical Properties
2.3. Soil DNA Extraction and 16S rRNA Gene Sequencing
2.4. Processing of Sequence Analysis
2.5. Statistical and Bioinformatic Analyses
3. Results
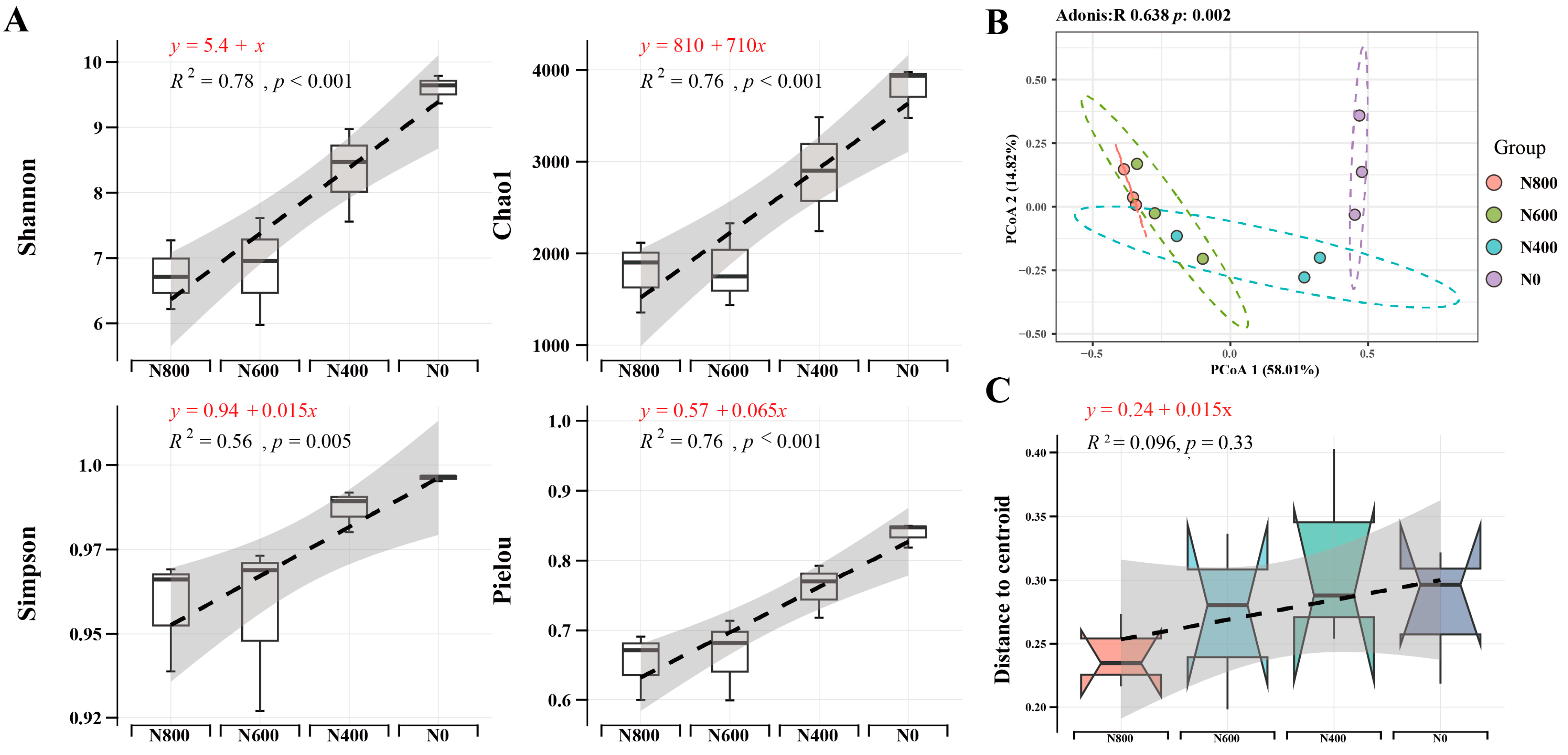
3.1. N Fertilizer Reduction Significantly Changed Soil Bacterial Community Structure
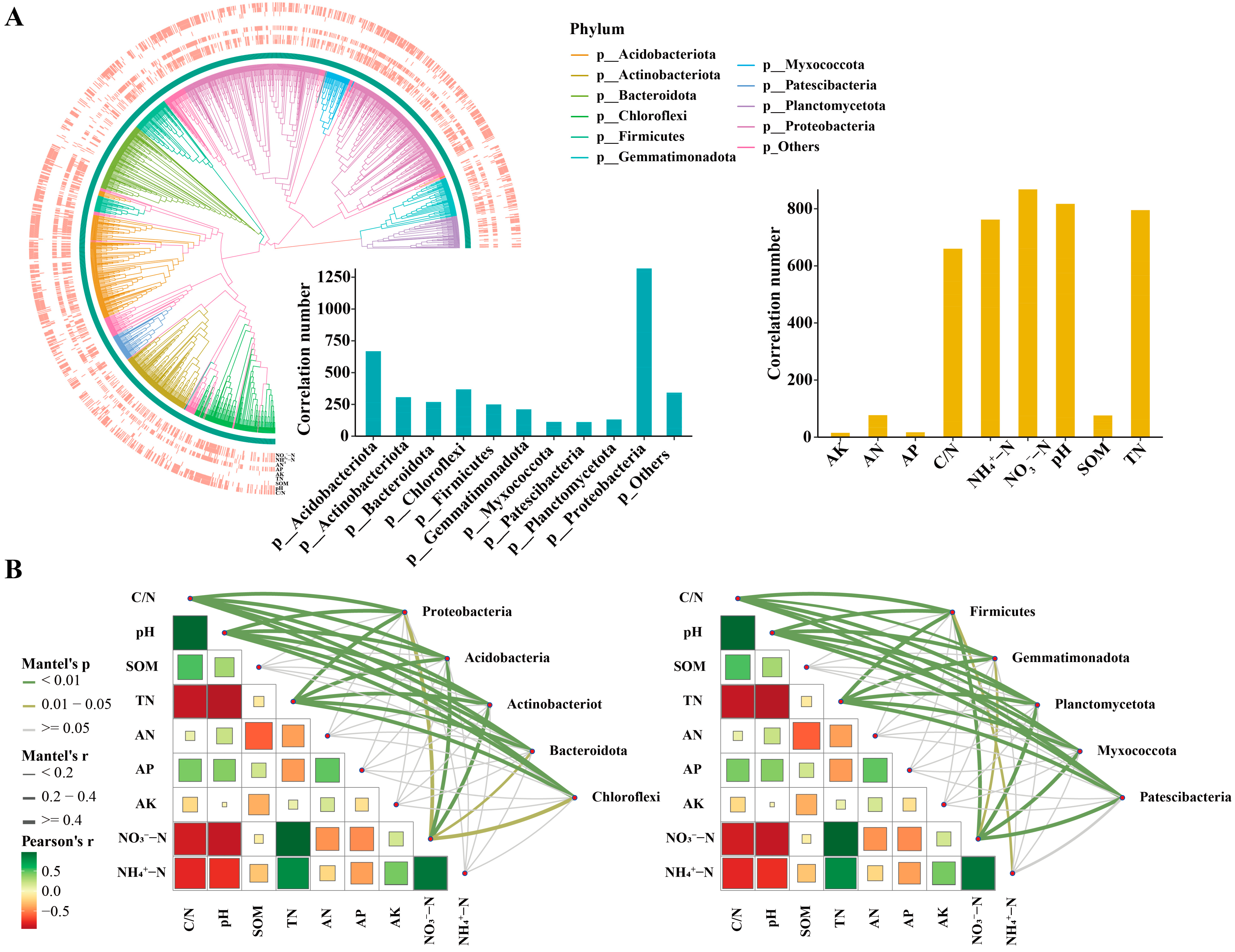
3.2. Correlations between Environmental Factors and Bacterial Community
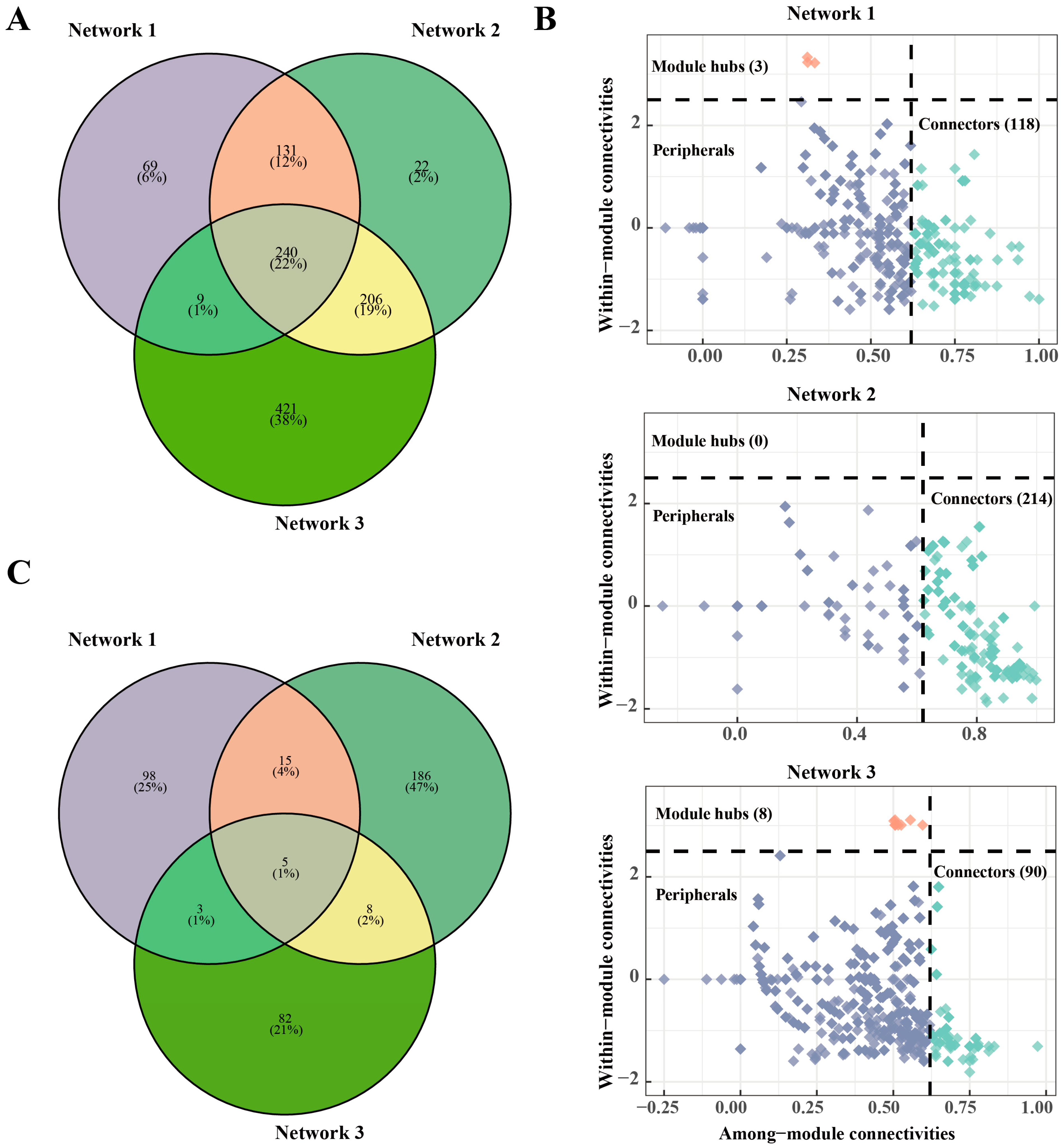
3.3. Bacterial Community Co-Occurrence Networks Are More Complex and Stable
3.4. Reduced Nitrogen Fertilizer Application Alters Soil Bacterial Community Network Composition
4. Discussion
4.1. Competition Release among Bacteria Facilitates Diversification of Soil Bacterial Communities
4.2. Competitive Release among Bacteria Promotes More Complex and Stable Bacterial Community Co-Occurrence Networks
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singh, J.S.; Gupta, V.K. Soil microbial biomass: A key soil driver in management of ecosystem functioning. Sci. Total Environ. 2018, 634, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Liu, J.; Jia, X.; Shangguan, Z.; Wang, R.; Yan, W. Microbial community assembly and metabolic function during wheat straw decomposition under different nitrogen fertilization treatments. Biol. Fertil. Soils 2020, 56, 697–710. [Google Scholar] [CrossRef]
- Fierer, N.; Leff, J.W.; Adams, B.J.; Nielsen, U.N.; Bates, S.T.; Lauber, C.L.; Owens, S.; Gilbert, J.A.; Wall, D.H.; Caporaso, J.G. Cross-biome metagenomic analyses of soil microbial communities and their functional attributes. Proc. Natl. Acad. Sci. USA 2012, 109, 21390–21395. [Google Scholar] [CrossRef] [PubMed]
- Geisen, S.; Wall, D.H.; van der Putten, W.H. Challenges and opportunities for soil biodiversity in the anthropocene. Curr. Biol. 2019, 29, R1036–R1044. [Google Scholar] [CrossRef]
- Wang, C.; Liu, D.; Bai, E. Decreasing soil microbial diversity is associated with decreasing microbial biomass under nitrogen addition. Soil Biol. Biochem. 2018, 120, 126–133. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, L.; Yang, S.; Wang, Z.; Tian, R.; Peng, Z.; Chen, Y.; Zhang, X.; Kuang, J.; Ling, N. Critical transition of soil bacterial diversity and composition triggered by nitrogen enrichment. Ecology 2020, 101, e03053. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.-T.; Liu, Y.-N.; Zhong, H.-X.; Chen, X.-W.; Sui, X. Effects of simulated nitrogen deposition on the soil microbial community diversity of a Deyeuxia angustifolia wetland in the Sanjiang Plain, Northeastern China. Ann. Microbiol. 2022, 72, 11. [Google Scholar] [CrossRef]
- Bradford, M.A.; Wood, S.A.; Bardgett, R.D.; Black, H.I.; Bonkowski, M.; Eggers, T.; Grayston, S.J.; Kandeler, E.; Manning, P.; Setälä, H. Discontinuity in the responses of ecosystem processes and multifunctionality to altered soil community composition. Proc. Natl. Acad. Sci. USA 2014, 111, 14478–14483. [Google Scholar] [CrossRef]
- Yang, Z.; Dai, H.; Huang, Y.; Dong, B.; Fu, S.; Zhang, C.; Li, X.; Tan, Y.; Zhang, X.; Zhang, X. Driving mechanisms of soil bacterial α and β diversity under long-term nitrogen addition: Subtractive heterogenization based on the environment selection. Geoderma 2024, 445, 116886. [Google Scholar] [CrossRef]
- Cui, Y.; Fang, L.; Guo, X.; Wang, X.; Wang, Y.; Li, P.; Zhang, Y.; Zhang, X. Responses of soil microbial communities to nutrient limitation in the desert-grassland ecological transition zone. Sci. Total Environ. 2018, 642, 45–55. [Google Scholar] [CrossRef]
- Lloyd-Smith, J.O. Vacated niches, competitive release and the community ecology of pathogen eradication. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120150. [Google Scholar] [CrossRef] [PubMed]
- Ling, N.; Chen, D.; Guo, H.; Wei, J.; Bai, Y.; Shen, Q.; Hu, S. Differential responses of soil bacterial communities to long-term N and P inputs in a semi-arid steppe. Geoderma 2017, 292, 25–33. [Google Scholar] [CrossRef]
- Herbold, B.; Moyle, P.B. Introduced species and vacant niches. Am. Nat. 1986, 128, 751–760. [Google Scholar] [CrossRef]
- Bello, A.; Han, Y.; Zhu, H.; Deng, L.; Yang, W.; Meng, Q.; Sun, Y.; Egbeagu, U.U.; Sheng, S.; Wu, X. Microbial community composition, co-occurrence network pattern and nitrogen transformation genera response to biochar addition in cattle manure-maize straw composting. Sci. Total Environ. 2020, 721, 137759. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Imamura, S.; Tatsumi, C.; Taniguchi, T.; Tateno, R. Microbial functions and soil nitrogen mineralisation processes in the soil of a cool temperate forest in northern Japan. Biogeochemistry 2021, 155, 359–379. [Google Scholar] [CrossRef]
- Yuan, M.M.; Guo, X.; Wu, L.; Zhang, Y.A.; Xiao, N.; Ning, D.; Shi, Z.; Zhou, X.; Wu, L.; Yang, Y.; et al. Climate warming enhances microbial network complexity and stability. Nat. Clim. Chang. 2021, 11, 343–348. [Google Scholar] [CrossRef]
- Mandakovic, D.; Rojas, C.; Maldonado, J.; Latorre, M.; Travisany, D.; Delage, E.; Bihouée, A.; Jean, G.; Díaz, F.P.; Fernández-Gómez, B.; et al. Structure and co-occurrence patterns in microbial communities under acute environmental stress reveal eco-logical factors fostering resilience. Sci. Rep. 2018, 8, 5875. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Li, J.; Wang, J.; Zhang, C.; Liu, G.; Dong, Q. Diversity and co-occurrence network modularization of bacterial communities determine soil fertility and crop yields in arid fertigation agroecosystems. Biol. Fertil. Soils 2021, 57, 809–824. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Qiu, T.; He, H.; Liu, J.; Duan, C.; Cui, Y.; Huang, M.; Wu, C.; Fang, L. High nitrogen fertilizer input enhanced the microbial network complexity in the paddy soil. Soil Ecol. Lett. 2024, 6, 230205. [Google Scholar] [CrossRef]
- CRGCST (Cooperative Research Group on Chinese Soil Taxonomy). Chinese Soil Taxonomy; Science Press: Beijing, China; New York, NY, USA, 2001. (In Chinese) [Google Scholar]
- Ma, B.; Wang, H.; Dsouza, M.; Lou, J.; He, Y.; Dai, Z.; Brookes, P.C.; Xu, J.; Gilbert, J.A. Geographic patterns of co-occurrence network topological features for soil microbiota at continental scale in eastern China. ISME J. 2016, 10, 1891–1901. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, H.; Liu, S.; Hussain, S.; Zhang, L.; Yu, X.; Cao, K.; Xin, X.; Cao, H.; Zhu, A. Response of Fungal Sub-Communities in a Maize-Wheat Rotation Field Subjected to Long-Term Conservation Tillage Management. Front. Microbiol. 2022, 13, 829152. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Yang, S.; Liebner, S.; Alawi, M.; Ebenhöh, O.; Wagner, D. Taxonomic database and cut-off value for processing mcrA gene 454 pyrosequencing data by MOTHUR. J. Microbiol. Methods 2014, 103, 3–5. [Google Scholar] [CrossRef]
- Jari Oksanen, F.; Blanchet, G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Wagner, H. vegan: Community Ecology Package, R Package Version 2.5-3. 2018. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 12 May 2024).
- Anderson, M.J. Permutational multivariate analysis of variance (PERMANOVA). In Wiley StatsRef: Statistics Reference Online; Wiley: Hoboken, NJ, USA, 2014; pp. 1–15. [Google Scholar]
- Breslow, N. A generalized Kruskal-Wallis test for comparing K samples subject to unequal patterns of censorship. Biometrika 1970, 57, 579–594. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Oliverio, A.M.; Bradford, M.A.; Fierer, N. Identifying the microbial taxa that consistently respond to soil warming across time and space. Glob. Chang. Biol. 2017, 23, 2117–2129. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification and regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- Hou, E.; Chen, C.; Luo, Y.; Zhou, G.; Kuang, Y.; Zhang, Y.; Heenan, M.; Lu, X.; Wen, D. Effects of climate on soil phosphorus cycle and availability in natural terrestrial ecosystems. Glob. Chang. Biol. 2018, 24, 3344–3356. [Google Scholar] [CrossRef]
- Jiao, S.; Lu, Y. Abundant fungi adapt to broader environmental gradients than rare fungi in agricultural fields. Glob. Chang. Biol. 2020, 26, 4506–4520. [Google Scholar] [CrossRef]
- Zhang, C.; Xiang, X.; Yang, T.; Liu, X.; Ma, Y.; Zhang, K.; Liu, X.; Chu, H. Nitrogen fertilization reduces plant diversity by changing the diversity and stability of arbuscular mycorrhizal fungal community in a temperate steppe community. Sci. Total Environ. 2024, 170775. [Google Scholar] [CrossRef]
- Guimera, R.; Nunes Amaral, L.A. Functional cartography of complex metabolic networks. Nature 2005, 433, 895–900. [Google Scholar] [CrossRef]
- Grandy, A.S.; Daly, A.B.; Bowles, T.M.; Gaudin, A.C.; Jilling, A.; Leptin, A.; McDaniel, M.D.; Wade, J.; Waterhouse, H. The nitrogen gap in soil health concepts and fertility measurements. Soil Biol. Biochem. 2022, 175, 108856. [Google Scholar] [CrossRef]
- Sabir, M.S.; Shahzadi, F.; Ali, F.; Shakeela, Q.; Niaz, Z.; Ahmed, S. Comparative effect of fertilization practices on soil microbial diversity and activity: An overview. Curr. Microbiol. 2021, 78, 3644–3655. [Google Scholar] [CrossRef]
- Wang, X.; Feng, J.; Ao, G.; Qin, W.; Han, M.; Shen, Y.; Liu, M.; Chen, Y.; Zhu, B. Globally nitrogen addition alters soil microbial community structure, but has minor effects on soil microbial diversity and richness. Soil Biol. Biochem. 2023, 179, 108982. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, X.; Song, L.; Lin, X.; Zhang, H.; Shen, C.; Chu, H. Nitrogen fertilization directly affects soil bacterial diversity and indirectly affects bacterial community composition. Soil Biol. Biochem. 2016, 92, 41–49. [Google Scholar] [CrossRef]
- Yang, R.; Yang, Z.; Yang, S.; Chen, L.-l.; Xin, J.; Xu, L.; Zhang, X.; Zhai, B.; Wang, Z.; Zheng, W. Nitrogen inhibitors improve soil ecosystem multifunctionality by enhancing soil quality and alleviating microbial nitrogen limitation. Sci. Total Environ. 2023, 880, 163238. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Q.; Wang, Z.; Zheng, H.; Chen, Y.; Chen, X.; Wang, L.; Li, H.; Zhang, J. Nitrogen addition alleviates microbial nitrogen limitations and promotes soil respiration in a subalpine coniferous forest. Forests 2019, 10, 1038. [Google Scholar] [CrossRef]
- Zhou, J.; Guan, D.; Zhou, B.; Zhao, B.; Ma, M.; Qin, J.; Jiang, X.; Chen, S.; Cao, F.; Shen, D. Influence of 34-years of fertilization on bacterial communities in an intensively cultivated black soil in northeast China. Soil Biol. Biochem. 2015, 90, 42–51. [Google Scholar] [CrossRef]
- Ramirez, K.S.; Craine, J.M.; Fierer, N. Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Glob. Chang. Biol. 2012, 18, 1918–1927. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Dai, Z.; Su, W.; Chen, H.; Barberán, A.; Zhao, H.; Yu, M.; Yu, L.; Brookes, P.C.; Schadt, C.W.; Chang, S.X. Long-term nitrogen fertilization decreases bacterial diversity and favors the growth of Actinobacteria and Proteobacteria in agro-ecosystems across the globe. Glob. Chang. Biol. 2018, 24, 3452–3461. [Google Scholar] [CrossRef]
- Ramirez, K.S.; Lauber, C.L.; Knight, R.; Bradford, M.A.; Fierer, N. Consistent effects of nitrogen fertilization on soil bacterial communities in contrasting systems. Ecology 2010, 91, 3463–3470. [Google Scholar] [CrossRef]
- Machado, D.; Maistrenko, O.M.; Andrejev, S.; Kim, Y.; Bork, P.; Patil, K.R.; Patil, K.R. Polarization of microbial communities between competitive and cooperative metabolism. Nat. Ecol. Evol. 2021, 5, 195–203. [Google Scholar] [CrossRef]
- Wagg, C.; Bender, S.F.; Widmer, F.; Van Der Heijden, M.G. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Natl. Acad. Sci. USA 2014, 111, 5266–5270. [Google Scholar] [CrossRef]
- Banerjee, S.; Schlaeppi, K.; van der Heijden, M.G. Keystone taxa as drivers of microbiome structure and functioning. Nat. Rev. Microbiol. 2018, 16, 567–576. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, Y.; Liang, C.; Luo, Y.; Xu, Q.; Han, C.; Zhao, Q.; Sun, B. Competitive interaction with keystone taxa induced negative priming under biochar amendments. Microbiome 2019, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Morrison-Whittle, P.; Goddard, M.R. Quantifying the relative roles of selective and neutral processes in defining eukaryotic microbial communities. ISME J. 2015, 9, 2003–2011. [Google Scholar] [CrossRef] [PubMed]
- Peay, K.G.; Kennedy, P.G.; Talbot, J.M. Dimensions of biodiversity in the Earth mycobiome. Nat. Rev. Microbiol. 2016, 14, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Van Der Heijden, M.G.; Bardgett, R.D.; Van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef]
- Jousset, A.; Bienhold, C.; Chatzinotas, A.; Gallien, L.; Gobet, A.; Kurm, V.; Küsel, K.; Rillig, M.C.; Rivett, D.W.; Salles, J.F. Where less may be more: How the rare biosphere pulls ecosystems strings. ISME J. 2017, 11, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Liu, H.; Yao, H.; Zhang, B.; Li, Y.; Jin, S.; Cao, H. Reducing Application of Nitrogen Fertilizer Increases Soil Bacterial Diversity and Drives Co-Occurrence Networks. Microorganisms 2024, 12, 1434. https://doi.org/10.3390/microorganisms12071434
Wang F, Liu H, Yao H, Zhang B, Li Y, Jin S, Cao H. Reducing Application of Nitrogen Fertilizer Increases Soil Bacterial Diversity and Drives Co-Occurrence Networks. Microorganisms. 2024; 12(7):1434. https://doi.org/10.3390/microorganisms12071434
Chicago/Turabian StyleWang, Feng, Hao Liu, Hongyan Yao, Bo Zhang, Yue Li, Shuquan Jin, and Hui Cao. 2024. "Reducing Application of Nitrogen Fertilizer Increases Soil Bacterial Diversity and Drives Co-Occurrence Networks" Microorganisms 12, no. 7: 1434. https://doi.org/10.3390/microorganisms12071434





