Temperature-Driven Activated Sludge Bacterial Community Assembly and Carbon Transformation Potential: A Case Study of Industrial Plants in the Yangtze River Delta
Abstract
:1. Introduction
2. Materials and Methods
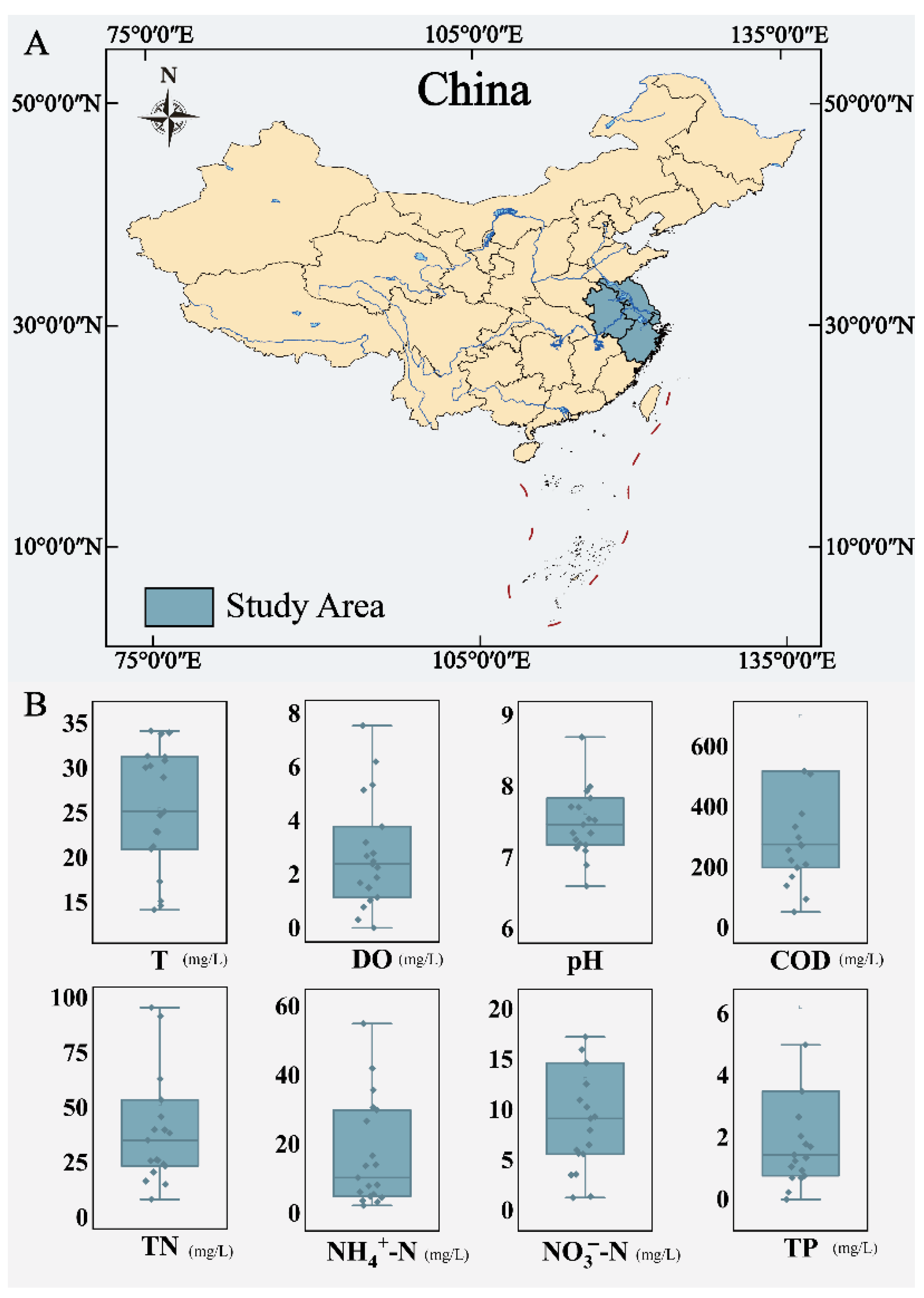
2.1. Sampling Points and Collection Methods
2.2. Analytical Methods
3. Results and Discussion
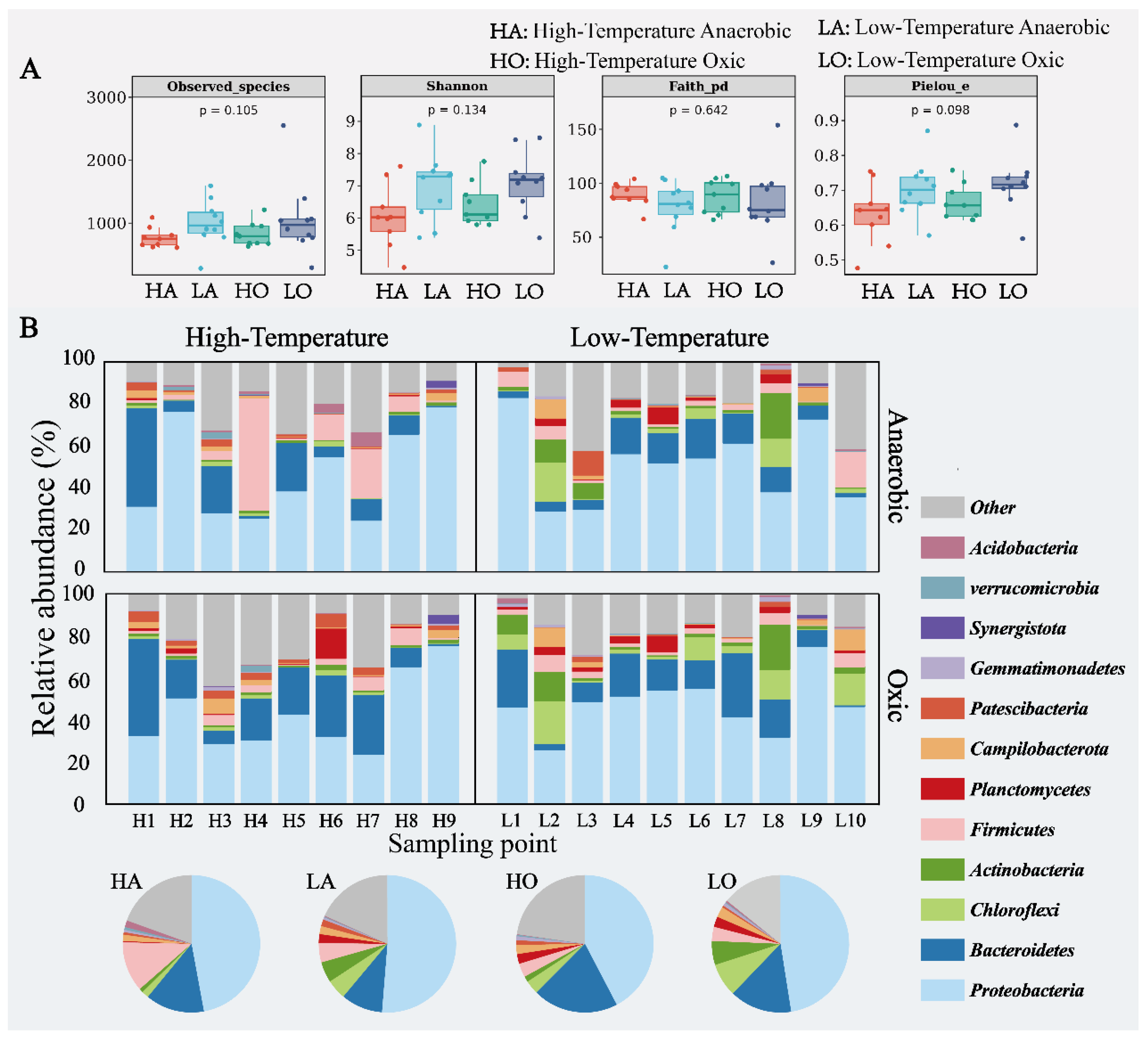
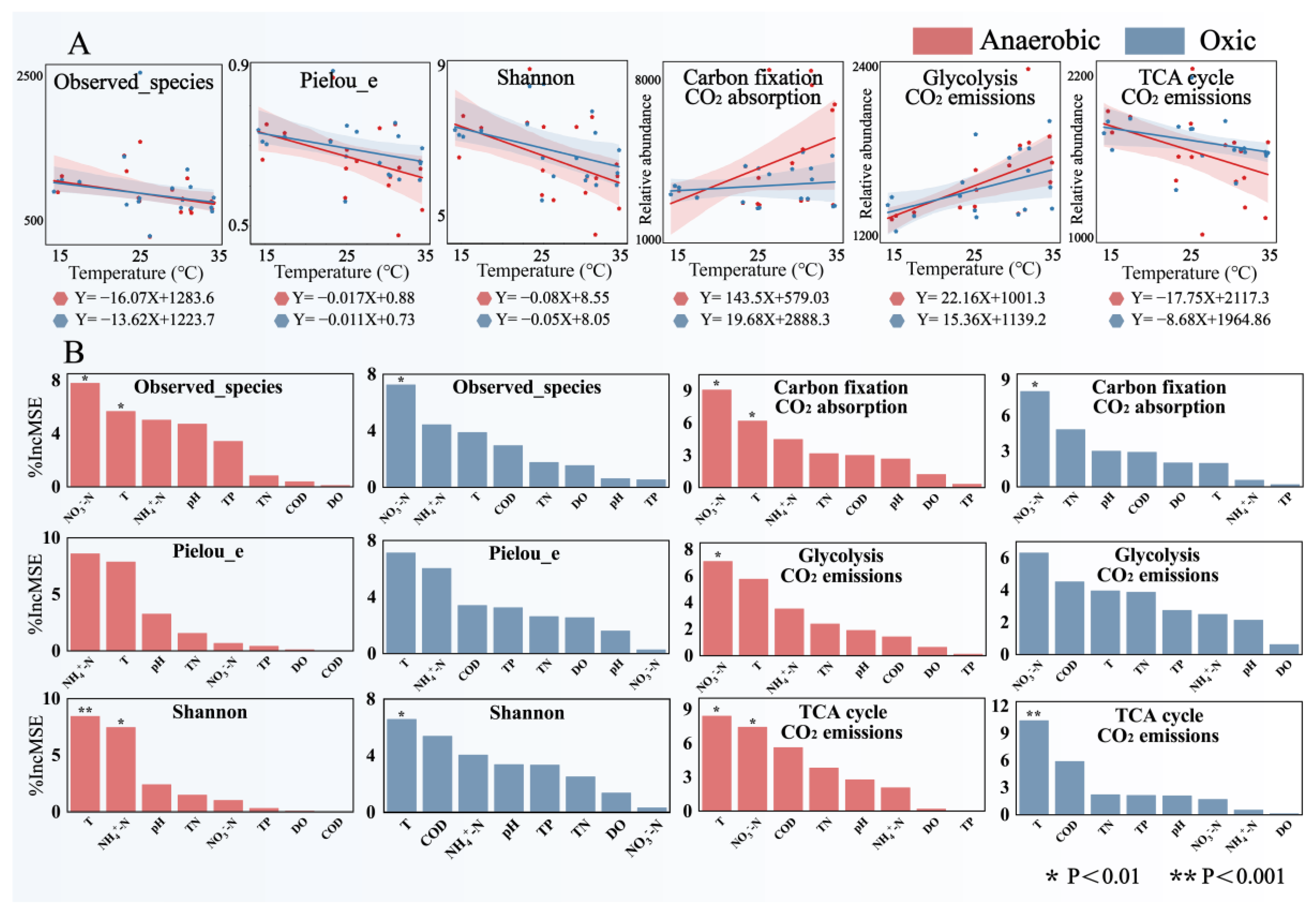
3.1. Microbial Community Evolution
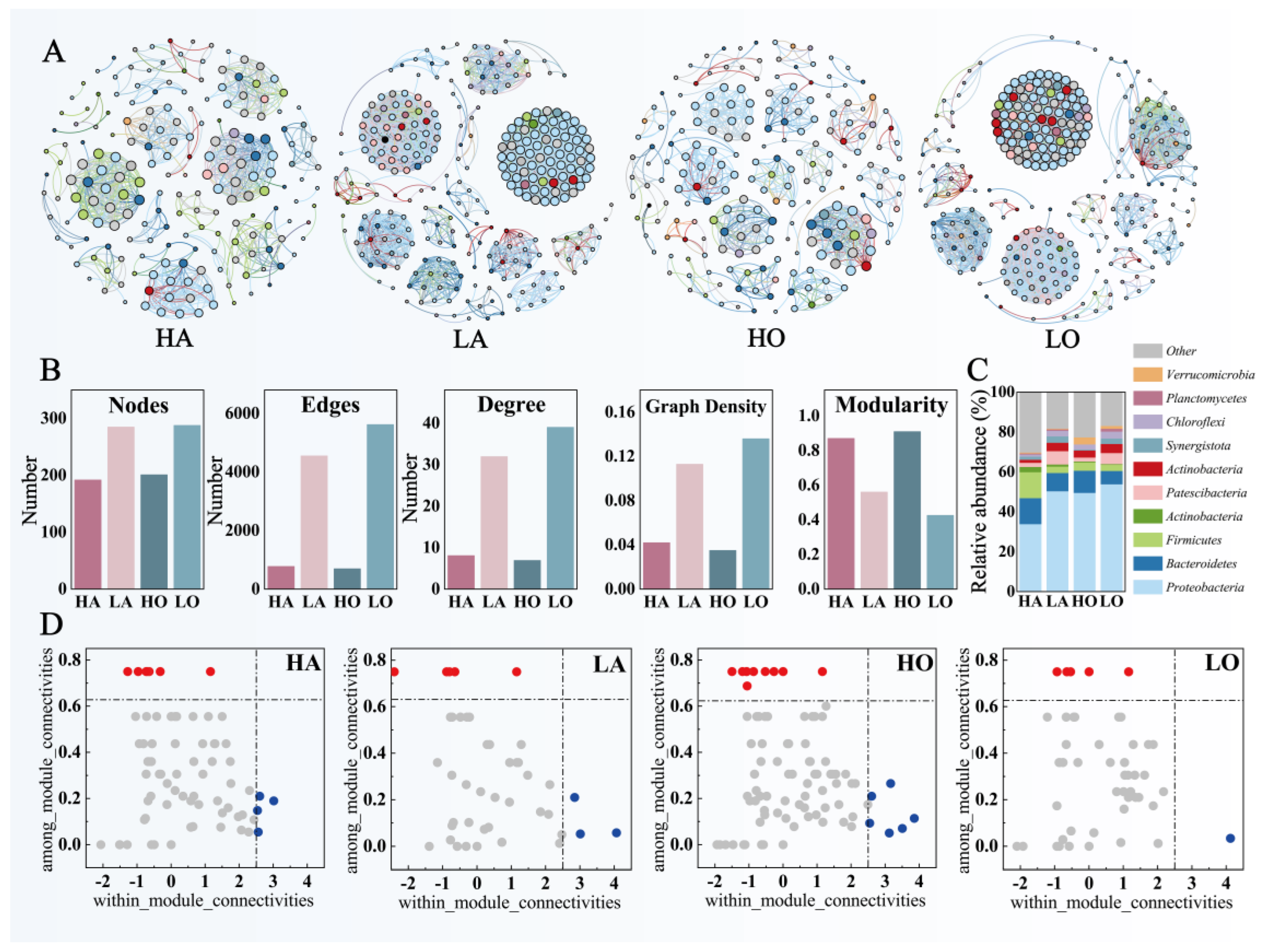
3.2. Microbial Community Functional Co-Occurrence
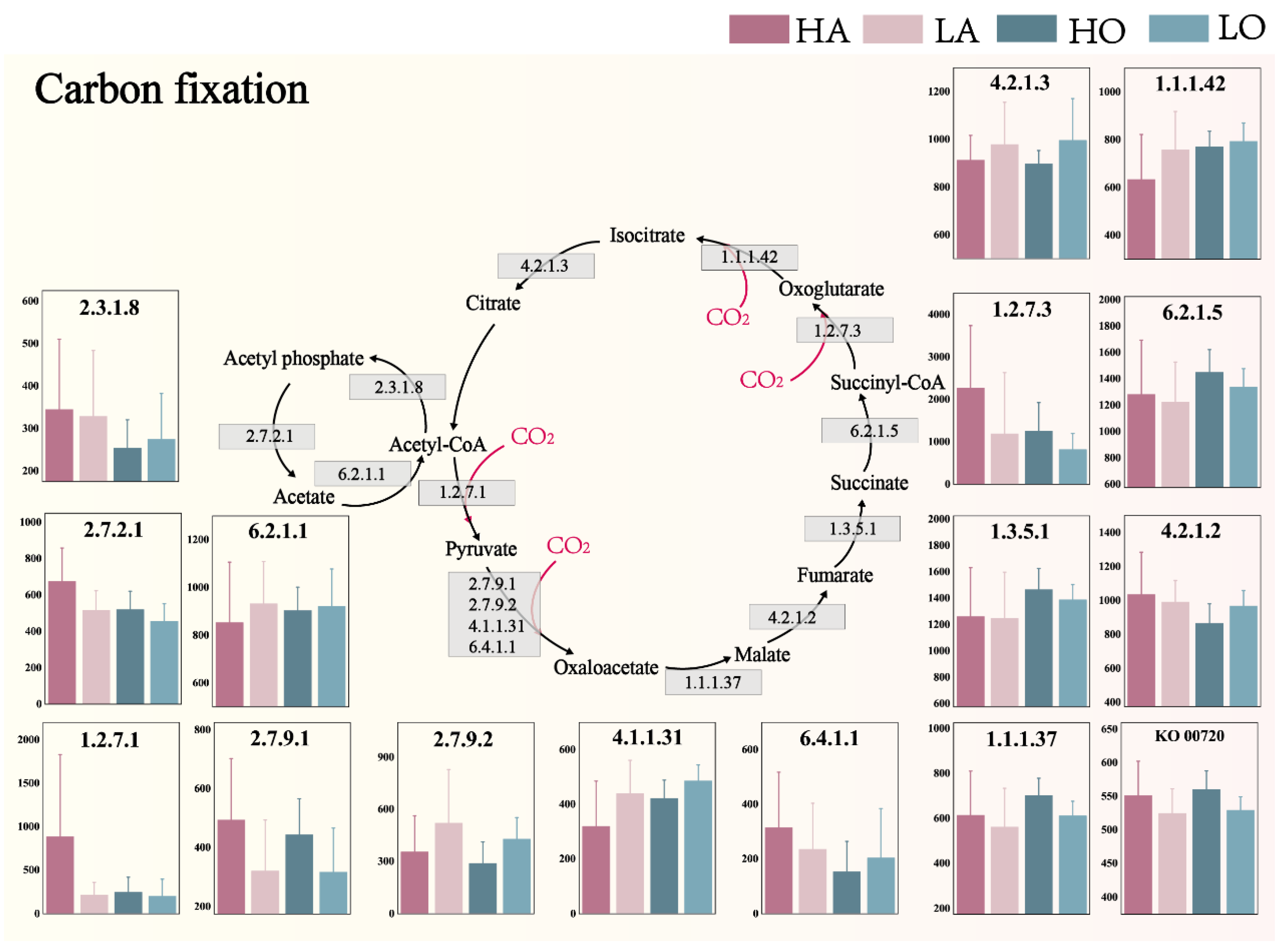
3.3. Microbial Community Carbon Fixation Potential
3.4. Microbial Community Glycolysis and TCA Cycle Potential
3.5. Microbial Community Contribution by Indicators
4. Conclusions
- As the operating temperature increases, the alpha diversity of A2O-activated sludge decreases, the community structure becomes simpler and more modular, and a few dominant species occupy more ecological niches. The relative abundance of Chloroflexi and Actinobacteria significantly decreased, while the relative abundance of Bacteroidetes and Firmicutes increased. Firmicutes exhibit high-temperature adaptability in anaerobic sludge communities.
- As the operating temperature increases, the carbon conversion potential of A2O-activated sludge increases overall. The absorption potential of CO2 is enhanced, especially in anaerobic sludge. During glycolysis and TCA cycle, the potential for CO2 release increases and decreases, respectively. Upcoming studies should focus on analyzing transport and primary metabolism-related proteins that can directly illuminate links between carbon uptake and biomass synthesis. This will not only empirically validate the study’s conclusions but also enhance our understanding of temperature-driven impacts on activated sludge microbial community metabolism.
- Temperature is an important factor in explaining the characteristics of microbial communities and the differences in CO2 absorption and emission potential. In terms of the absorption and emission potential of CO2, the influence of temperature on anaerobic sludge is more significant.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Reynolds, L.J.; Sala-Comorera, L.; Khan, M.F.; Martin, N.A.; Whitty, M.; Stephens, J.H.; Nolan, T.M.; Joyce, E.; Fletcher, N.F.; Murphy, C.D.; et al. Coprostanol as a Population Biomarker for SARS-CoV-2 Wastewater Surveillance Studies. Water 2022, 14, 225. [Google Scholar] [CrossRef]
- El Hattab, N.A.; El-Seddik, M.M.; Abdel-Halim, H.S.; Matta, M.E. Simulation of a full-scale wastewater treatment plant performance at various temperatures using Extended Activated Sludge Model No.1. Desalination Water Treat. 2021, 213, 190–201. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, J.; Li, X.; Wang, S.; Hu, B.; Ding, X. The Denitrification Characteristics and Microbial Community in the Cathode of an MFC with Aerobic Denitrification at High Temperatures. Front. Microbiol. 2017, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, E.; D’Onghia, G.; Lopopolo, L.; Gikas, P.; Ranieri, F.; Gika, E.; Spagnolo, V.; Herrera, J.A.; Ranieri, A.C. Influence of climate change on wastewater treatment plants performances and energy costs in Apulia, south Italy. Chemosphere 2024, 350, 141087. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Murphy, C.D. Bacterial degradation of the anti-depressant drug fluoxetine produces trifluoroacetic acid and fluoride ion. Appl. Microbiol. Biotechnol. 2021, 105, 9359–9369. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Hof, C.; Niemcova, P.; Murphy, C.D. Chapter Eleven—Biotransformation of fluorinated drugs and xenobiotics by the model fungus Cunninghamella elegans. In Methods in Enzymology; Stockbridge, R.B., Ed.; Academic Press: Cambridge, MA, USA, 2024; pp. 251–285. [Google Scholar]
- Lood, R.; Ertürk, G.; Mattiasson, B. Revisiting Antibiotic Resistance Spreading in Wastewater Treatment Plants—Bacteriophages as a Much Neglected Potential Transmission Vehicle. Front. Microbiol. 2017, 8, 2298. [Google Scholar] [CrossRef] [PubMed]
- Dubey, M.; Rajpal, A.; Vellanki, B.P.; Kazmi, A.A. Occurrence, removal, and mass balance of contaminants of emerging concern in biological nutrient removal-based sewage treatment plants: Role of redox conditions in biotransformation and sorption. Sci. Total. Environ. 2022, 808, 152131. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Zheng, J.; Han, Y.; Xu, X.; Li, M.; Schwarz, C.; Zhu, L. Comprehensive insights into core microbial assemblages in activated sludge exposed to textile-dyeing wastewater stress. Sci. Total Environ. 2021, 791, 148145. [Google Scholar] [CrossRef]
- Guo, H.; Ji, M.; Du, T.; Xu, W.; Liu, J.; Bai, R.; Teng, Z.; Li, T. Salt stress altered anaerobic microbial community and carbon metabolism characteristics: The trade-off between methanogenesis and chain elongation. J. Environ. Manag. 2023, 341, 118111. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Altamirano, M.; Maza-Márquez, P.; Pérez, S.; Rodelas, B.; Pozo, C.; Osorio, F. Fate of pharmaceutically active compounds in a pilot-scale A2O integrated fixed-film activated sludge (IFAS) process treating municipal wastewater. J. Environ. Chem. Eng. 2021, 9, 105398. [Google Scholar] [CrossRef]
- Khan, M.F.; Hof, C.; Niemcová, P.; Murphy, C.D. Recent advances in fungal xenobiotic metabolism: Enzymes and applications. World J. Microbiol. Biotechnol. 2023, 39, 296. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Zheng, X.; Li, X.; Liu, T.; Wang, Y.; Ma, Y.; Ji, Y.; Qiu, X.; Wan, Y.; Pan, B. Variation of effluent organic matter (EfOM) during anaerobic/anoxic/oxic (A2O) wastewater treatment processes. Water Res. 2020, 178, 115830. [Google Scholar] [CrossRef] [PubMed]
- de Santana, C.O.; Spealman, P.; Azulai, D.; Reid, M.; Dueker, M.E.; Perron, G.G. Bacteria communities and water quality parameters in riverine water and sediments near wastewater discharges. Sci. Data 2022, 9, 578. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, Z.; Chu, X.; Deng, Y.; Qing, S.; Sun, C.; Wang, Q.; Zhou, H.; Cheng, H.; Zhan, W.; et al. Temperature mediated the balance between stochastic and deterministic processes and reoccurrence of microbial community during treating aniline wastewater. Water Res. 2022, 221, 118741. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Xie, J.; Wang, G.; Li, Z.; Zhang, K.; Li, H.; Xia, Y.; Tian, J.; Yu, E.; Xie, W.; et al. Community assembly patterns and processes of bacteria in a field-scale aquaculture wastewater treatment system. Sci. Total Environ. 2024, 907, 167913. [Google Scholar] [CrossRef] [PubMed]
- Seth, N.; Vats, S.; Lakhanpaul, S.; Arafat, Y.; Mazumdar-Leighton, S.; Bansal, M.; Babu, C.R. Microbial community diversity of an integrated constructed wetland used for treatment of sewage. Front. Microbiol. 2024, 15, 1355718. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yue, Z.; Ma, D.; Xu, W.; Li, Z.; Wang, J. Mechanisms and responses of nitrogen transformations mediated by microbial communities in constructed tailwater wetlands to salinity stress. J. Water Process. Eng. 2023, 56, 104491. [Google Scholar] [CrossRef]
- Ma, K.-L.; Li, X.-K.; Wang, K.; Ren, Y.-H.; Chu, Z.-R.; Zhang, J. Role of temperature on microbial community profiles in an anaerobic bioreactor for treating PTA wastewater. Chem. Eng. J. 2017, 308, 256–263. [Google Scholar] [CrossRef]
- Huang, X.; Dong, W.; Wang, H.; Jiang, S. Biological nutrient removal and molecular biological characteristics in an anaerobic-multistage anaerobic/oxic (A-MAO) process to treat municipal wastewater. Bioresour. Technol. 2017, 241, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Ross, B.N.; Lancellotti, B.V.; Brannon, E.Q.; Loomis, G.W.; Amador, J.A. Greenhouse gas emissions from advanced nitrogen-removal onsite wastewater treatment systems. Sci. Total Environ. 2020, 737, 140399. [Google Scholar] [CrossRef] [PubMed]
- Safitri, A.S.; Kaster, K.M.; Kommedal, R. Effect of low temperature and municipal wastewater organic loading on anaerobic granule reactor performance. Bioresour. Technol. 2022, 360, 127616. [Google Scholar] [CrossRef]
- Li, T.; Bo, L.; Yang, F.; Zhang, S.; Wu, Y.; Yang, L. Comparison of the removal of COD by a hybrid bioreactor at low and room temperature and the associated microbial characteristics. Bioresour. Technol. 2012, 108, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Lougheed, V.L.; Tweedie, C.E.; Andresen, C.G.; Armendariz, A.M.; Escarzaga, S.M.; Tarin, G. Patterns and Drivers of Carbon Dioxide Concentrations in Aquatic Ecosystems of the Arctic Coastal Tundra. Glob. Biogeochem. Cycles 2020, 34, e2020GB006552. [Google Scholar] [CrossRef]
- Minick, K.J.; Mitra, B.; Li, X.; Fischer, M.; Aguilos, M.; Prajapati, P.; Noormets, A.; King, J.S. Wetland microtopography alters response of potential net CO2 and CH4 production to temperature and moisture: Evidence from a laboratory experiment. Geoderma 2021, 402, 115367. [Google Scholar] [CrossRef]
- Koschorreck, M.; Knorr, K.H.; Teichert, L. Temporal patterns and drivers of CO2 emission from dry sediments in agroyne field of a large river. Biogeosciences 2022, 19, 5221–5236. [Google Scholar] [CrossRef]
- Zhang, S.; Li, H.; Zhang, Q.; Tian, X.; Shi, F. Uncovering the impacts of industrial transformation on low-carbon development in the Yangtze River Delta. Resour. Conserv. Recycl. 2019, 150, 104442. [Google Scholar] [CrossRef]
- Chen, W.; Wei, J.; Su, Z.; Wu, L.; Liu, M.; Huang, X.; Yao, P.; Wen, D. Deterministic mechanisms drive bacterial communities assembly in industrial wastewater treatment system. Environ. Int. 2022, 168, 107486. [Google Scholar] [CrossRef]
- Gao, P.; Xu, W.; Sontag, P.; Li, X.; Xue, G.; Liu, T.; Sun, W. Correlating microbial community compositions with environmental factors in activated sludge from four full-scale municipal wastewater treatment plants in Shanghai, China. Appl. Microbiol. Biotechnol. 2016, 100, 4663–4673. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shen, Z.; Fang, W.; Gao, G. Composition of bacterial communities in municipal wastewater treatment plant. Sci. Total Environ. 2019, 689, 1181–1191. [Google Scholar] [CrossRef]
- Liang, J.; Mai, W.; Tang, J.; Wei, Y. Highly effective treatment of petrochemical wastewater by a super-sized industrial scale plant with expanded granular sludge bed bioreactor and aerobic activated sludge. Chem. Eng. J. 2019, 360, 15–23. [Google Scholar] [CrossRef]
- Jiang, Y.; Ma, D.; Wang, J.; Xu, Q.; Fang, J.; Yue, Z. Regulatory of salinity on assembly of activated sludge microbial communities and nitrogen transformation potential in industrial plants of the lower Yangtze River basin. Environ. Res. 2024, 251, 118769. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Najar, I.N.; Sharma, P.; Tamang, S.; Mondal, K.; Das, S.; Sherpa, M.T.; Thakur, N. Temperature—A critical abiotic paradigm that governs bacterial heterogeneity in natural ecological system. Environ. Res. 2023, 234, 116547. [Google Scholar] [CrossRef] [PubMed]
- García, F.C.; Bestion, E.; Warfield, R.; Yvon-Durocher, G. Changes in temperature alter the relationship between biodiversity and ecosystem functioning. Proc. Natl. Acad. Sci. USA 2018, 115, 10989–10994. [Google Scholar] [CrossRef]
- Louca, S.; Polz, M.F.; Mazel, F.; Albright, M.B.; Huber, J.A.; O’Connor, M.I.; Ackermann, M.; Hahn, A.S.; Srivastava, D.S.; Crowe, S.A.; et al. Function and functional redundancy in microbial systems. Nat. Ecol. Evol. 2018, 2, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.C.; Murugapiran, S.K.; Hamilton, T.L. Temperature impacts community structure and function of phototrophic Chloroflexi and Cyanobacteria in two alkaline hot springs in Yellowstone National Park. Environ. Microbiol. Rep. 2020, 12, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Haihan, Z.; Manli, M.; Tinglin, H.; Yutian, M.; Haiyun, L.; Kaiwen, L.; Wanqiu, Y.; Ben, M. Spatial and temporal dynamics of actinobacteria in drinking water reservoirs: Novel insights into abundance, community structure, and co-existence model. Sci. Total Environ. 2022, 814, 152804. [Google Scholar]
- Li, J.; Zhang, W.; Li, X.; Ye, T.; Gan, Y.; Zhang, A.; Chen, H.; Xue, G.; Liu, Y. Production of lactic acid from thermal pretreated food waste through the fermentation of waste activated sludge: Effects of substrate and thermal pretreatment temperature. Bioresour. Technol. 2018, 247, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, W.; Sun, X.; Shen, J.; Wang, L. A novel acetogenic bacteria isolated from waste activated sludge and its potential application for enhancing anaerobic digestion performance. J. Environ. Manag. 2020, 255, 109842. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Rene, E.R.; Wang, J.; Ma, W. Biodegradation of polyaromatic hydrocarbons and the influence of environmental factors during the co-composting of sewage sludge and green forest waste. Bioresour. Technol. 2020, 297, 122434. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chang, S. Impact of temperatures on microbial community structures of sewage sludge biological hydrolysis. Bioresour. Technol. 2017, 245, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yuan, L.J.; Lv, J.H.; Nie, K. Influence of temperature on sludge settleability and bacterial community structure in enhanced biological phosphorus removal systems. Desalination Water Treat. 2016, 57, 9900–9913. [Google Scholar] [CrossRef]
- Ferrer, I.; Passos, F.; Romero, E.; Vázquez, F.; Font, X. Optimising sewage sludge anaerobic digestion for resource recovery in wastewater treatment plants. Renew. Energy 2024, 224, 120123. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, M.; Yang, S.; Gong, H.; Ma, J.; Li, C.; Wang, K. Performance and microbial community evaluation of full-scale two-phase anaerobic digestion of waste activated sludge. Sci. Total Environ. 2022, 814, 152525. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mao, Q.; Su, Y.-A.; Zhang, H.; Liu, H.; Fu, B.; Su, Z.; Wen, D. Thermophilic rather than mesophilic sludge anaerobic digesters possess lower antibiotic resistant genes abundance. Bioresour. Technol. 2021, 329, 124924. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, J.; Tian, J.; Pan, Z.; Chen, Y.; Ming, F.; Wang, R.; Wang, L.; Zhou, H.; Li, J.; et al. Co-cultivation of microalgae-activated sludge for municipal wastewater treatment: Exploring the performance, microbial co-occurrence patterns, microbiota dynamics and function during the startup stage. Bioresour. Technol. 2023, 374, 128733. [Google Scholar] [CrossRef] [PubMed]
- Bahadur, A.; Zhang, L.; Guo, W.; Sajjad, W.; Ilahi, N.; Banerjee, A.; Faisal, S.; Usman, M.; Chen, T.; Zhang, W. Temperature-dependent transformation of microbial community: A systematic approach to analyzing functional microbes and biogas production. Environ. Res. 2024, 249, 118351. [Google Scholar] [CrossRef] [PubMed]
- Barton, S.; Jenkins, J.; Buckling, A.; Schaum, C.-E.; Smirnoff, N.; Raven, J.A.; Yvon-Durocher, G. Evolutionary temperature compensation of carbon fixation in marine phytoplankton. Ecol. Lett. 2020, 23, 722–733. [Google Scholar] [CrossRef] [PubMed]
- Olzhausen, J.; Grigat, M.; Seifert, L.; Ulbricht, T.; Schüller, H.J. Increased biosynthesis of acetyl-CoA in the yeast Saccharomyces cerevisiae by overexpression of a deregulated pantothenate kinase gene and engineering of the coenzyme A biosynthetic pathway. Appl. Microbiol. Biotechnol. 2021, 105, 7321–7337. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Du, H.X.; Xie, H.Y.; Zhu, J.M.; Zeng, S.F.; Igarashi, Y.; Luo, F. Dual-chamber differs from single-chamber microbial electrosynthesis in biogas production performance under low temperature (15celcius). Bioresour. Technol. 2021, 337, 125377. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zeng, S.; Luo, L.; Xu, Y.; Yasuo, I.; Luo, F. Metatranscriptome revealed how carbon brush addition affected the fermentation of food wastewater in the low-temperature environment. Environ. Res. 2023, 239, 117382. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-H.; Yang, J.-W.; Zhang, H. Increase metabolic heat to compensate for low temperature in activated sludge systems. Water Res. 2024, 250, 121068. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Wu, X.; Zhao, M.; Zhang, J. Lactic acid accumulation under heat stress related to accelerated glycolysis and mitochondrial dysfunction inhibits the mycelial growth of Pleurotus ostreatus. Appl. Microbiol. Biotechnol. 2020, 104, 6767–6777. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Pan, Y.; Chen, Y.; Zhang, X.; Huang, Y.; Song, Z. Effects of seasonal temperature variations on phosphorus removal, recovery, and key metabolic pathways in the suspended biofilm. Biochem. Eng. J. 2021, 176, 108187. [Google Scholar] [CrossRef]
- Hasunuma, T.; Matsuda, M.; Kato, Y.; Vavricka, C.J.; Kondo, A. Temperature enhanced succinate production concurrent with increased central metabolism turnover in the cyanobacterium Synechocystis sp. PCC 6803. Metab. Eng. 2018, 48, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.C.; Tamadonfar, K.O.; Seymour, C.O.; Lai, D.; Dodsworth, J.A.; Murugapiran, S.K.; Eloe-Fadrosh, E.A.; Dijkstra, P.; Hedlund, B.P. Position-Specific Metabolic Probing and Metagenomics of Microbial Communities Reveal Conserved Central Carbon Metabolic Network Activities at High Temperatures. Front. Microbiol. 2019, 10, 1427. [Google Scholar] [CrossRef] [PubMed]
- Bagra, K.; Kneis, D.; Padfield, D.; Szekeres, E.; Teban-Man, A.; Coman, C.; Singh, G.; Berendonk, T.U.; Klümper, U. Contrary effects of increasing temperatures on the spread of antimicrobial resistance in river biofilms. mSphere 2024, 9, e0057323. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Jia, L.; Li, Y.; Wu, W. Strengthening in microbiota dynamics and C, N, S transformation induced by novel synthesized pyrite/PHBV composites for advanced nitrogen and phosphate removal: Overlooked sulfate reduction process. Chem. Eng. J. 2023, 463, 142315. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Q.; Jiang, Y.; Wang, J.; Deng, R.; Yue, Z. Temperature-Driven Activated Sludge Bacterial Community Assembly and Carbon Transformation Potential: A Case Study of Industrial Plants in the Yangtze River Delta. Microorganisms 2024, 12, 1454. https://doi.org/10.3390/microorganisms12071454
Xu Q, Jiang Y, Wang J, Deng R, Yue Z. Temperature-Driven Activated Sludge Bacterial Community Assembly and Carbon Transformation Potential: A Case Study of Industrial Plants in the Yangtze River Delta. Microorganisms. 2024; 12(7):1454. https://doi.org/10.3390/microorganisms12071454
Chicago/Turabian StyleXu, Qingsheng, Yifan Jiang, Jin Wang, Rui Deng, and Zhengbo Yue. 2024. "Temperature-Driven Activated Sludge Bacterial Community Assembly and Carbon Transformation Potential: A Case Study of Industrial Plants in the Yangtze River Delta" Microorganisms 12, no. 7: 1454. https://doi.org/10.3390/microorganisms12071454




