Clostridial Myonecrosis: A Comprehensive Review of Toxin Pathophysiology and Management Strategies
Abstract
:1. Introduction
1.1. Background
1.2. Ecology
1.3. Pathogenicity
1.4. Toxin Classification
2. Types
2.1. Traumatic Gas Gangrene
2.2. Spontaneous or Hematogenous Gas Gangrene
3. Epidemiology
4. Clinical Presentation
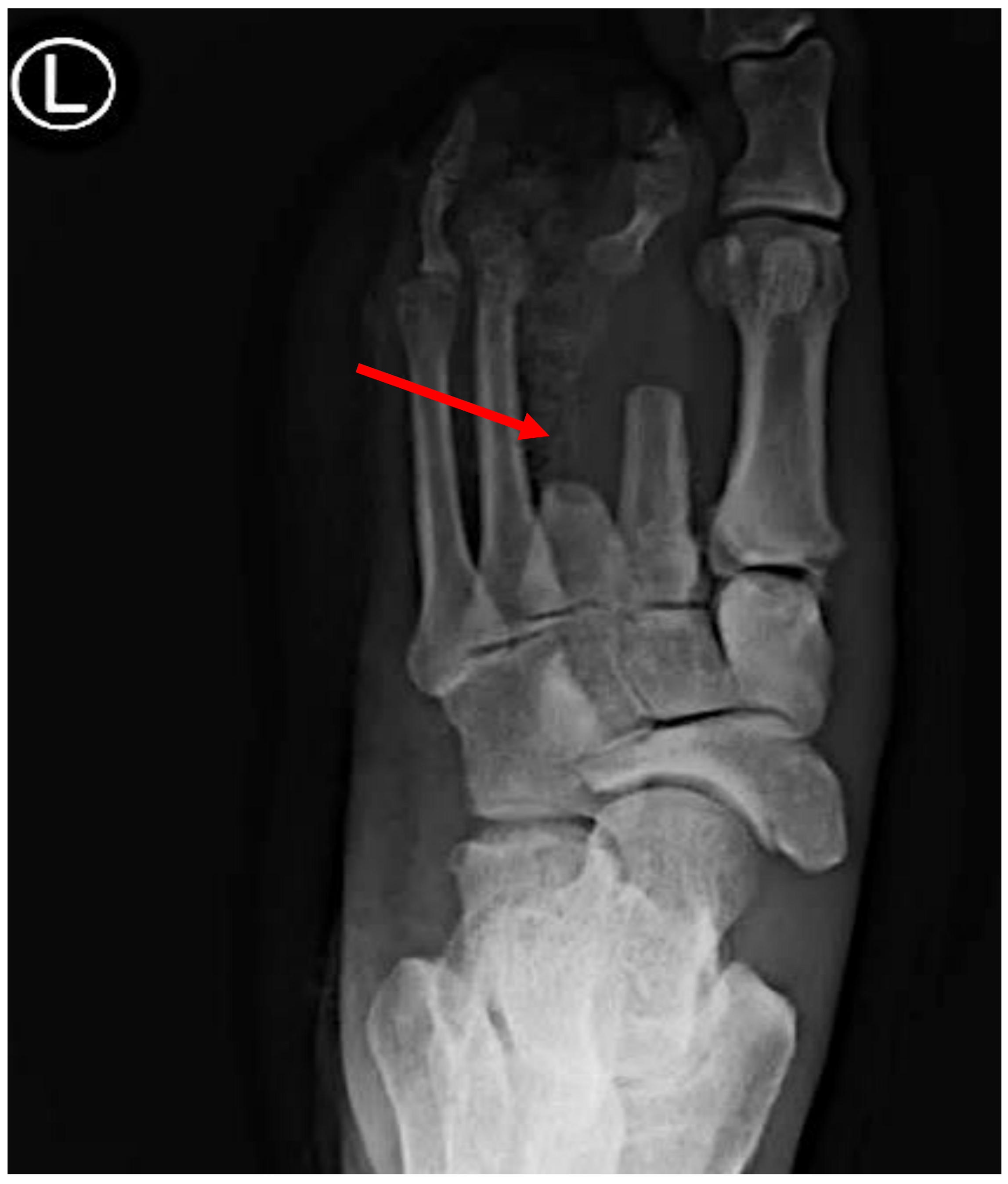
5. Diagnostic Steps
6. Etiology and Management
6.1. Etiology and Pathogenesis
6.2. Treatment
7. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, P.Y.; Annamaraju, P. Clostridium perfringens Infection; StatPearls [Internet]: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK559049/ (accessed on 29 June 2024).
- Grenda, T.; Jarosz, A.; Sapała, M.; Grenda, A.; Patyra, E.; Kwiatek, K. Clostridium perfringens-Opportunistic Foodborne Pathogen, Its Diversity and Epidemiological Significance. Pathogens 2023, 12, 768. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, K.; Shimizu, T. Regulation of Toxin Production in Clostridium perfringens. Toxins 2016, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Brynestad, S.; Granum, P.E. Clostridium perfringens and foodborne infections. Int. J. Food Microbiol. 2002, 74, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Leiblein, M.; Wagner, N.; Adam, E.H.; Frank, J.; Marzi, I.; Nau, C. Clostridial Gas Gangrene—A Rare but Deadly Infection: Case series and Comparison to Other Necrotizing Soft Tissue Infections. Orthop. Surg. 2020, 12, 1733–1747. [Google Scholar] [CrossRef] [PubMed]
- Kiu, R.; Hall, L.J. An update on the human and animal enteric pathogen Clostridium perfringens. Emerg. Microbes Infect. 2018, 7, 141. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, K.G.; Fox, J.G. Chapter 16—Bacterial Diseases. In American College of Laboratory Animal Medicine. The Common Marmoset in Captivity and Biomedical Research; Academic Press: Cambridge, MA, USA, 2019; pp. 265–287. [Google Scholar]
- Aronoff, D.M.; Kazanjian, P.H. Historical and contemporary features of infections due to Clostridium novyi. Anaerobe 2018, 50, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Alenezi, T.; Sun, X. Clostridium perfringens-Induced Necrotic Diseases: An Overview. Immuno 2022, 2, 387–407. [Google Scholar] [CrossRef]
- Wells, C.L.; Wilkins, T.D. Clostridia: Sporeforming Anaerobic Bacilli. In Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; Chapter 18. Available online: https://www.ncbi.nlm.nih.gov/books/NBK8219/ (accessed on 29 June 2024).
- Milena, B.; Stefano, M.; Tiziana, S. Clostridium spp. Encyclopedia of Dairy Sciences, 3rd ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 431–438. [Google Scholar] [CrossRef]
- Nagahama, M.; Hayashi, S.; Morimitsu, S.; Sakurai, J. Biological activities and pore formation of Clostridium perfringens beta toxin in HL 60 cells. J. Biol. Chem. 2003, 278, 36934–36941. [Google Scholar] [CrossRef]
- Barbut, F.; Decré, D.; Lalande, V.; Burghoffer, B.; Noussair, L.; Gigandon, A.; Espinasse, F.; Raskine, L.; Robert, J.; Mangeol, A.; et al. Clinical features of Clostridium difficile-associated diarrhoea due to binary toxin (actin-specific ADP-ribosyltransferase)-producing strains. J. Med. Microbiol. 2005, 54, 181–185. [Google Scholar] [CrossRef]
- Li, J.; McClane, B.A. A novel small acid soluble protein variant is important for spore resistance of most Clostridium perfringens food poisoning isolates. PLoS Pathog. 2008, 4, e1000056. [Google Scholar] [CrossRef]
- Raju, D.; Setlow, P.; Sarker, M.R. Antisense-RNA-mediated decreased synthesis of small, acid-soluble spore proteins leads to decreased resistance of Clostridium perfringens spores to moist heat and UV radiation. Appl. Environ. Microbiol. 2007, 73, 2048–2053. [Google Scholar] [CrossRef]
- Harkness, J.M.; Li, J.; McClane, B.A. Identification of a lambda toxin-negative Clostridium perfringens strain that processes and activates epsilon prototoxin intracellularly. Anaerobe 2012, 18, 546–552. [Google Scholar] [CrossRef]
- Forti, K.; Ferroni, L.; Pellegrini, M.; Cruciani, D.; De Giuseppe, A.; Crotti, S.; Papa, P.; Maresca, C.; Severi, G.; Marenzoni, M.L.; et al. Molecular Characterization of Clostridium perfringens Strains Isolated in Italy. Toxins 2020, 12, 650. [Google Scholar] [CrossRef]
- Mehdizadeh Gohari, I.; A Navarro, M.; Li, J.; Shrestha, A.; Uzal, F.; A McClane, B. Pathogenicity and virulence of Clostridium perfringens. Virulence 2021, 12, 723–753. [Google Scholar] [CrossRef]
- Fourie, J.C.J.; Bezuidenhout, C.C.; Sanko, T.J.; Mienie, C.; Adeleke, R. Inside environmental Clostridium perfringens genomes: Antibiotic resistance genes, virulence factors and genomic features. J. Water Health 2020, 18, 477–493. [Google Scholar] [CrossRef] [PubMed]
- Watts, T.D.; Vidor, C.J.; Awad, M.M.; Lyras, D.; Rood, J.I.; Adams, V. pCP13, a representative of a new family of conjugative toxin plasmids in Clostridium perfringens. Plasmid 2019, 102, 37–45. [Google Scholar] [CrossRef]
- Pahle, J.; Kobelt, D.; Aumann, J.; Behrens, D.; Daberkow, O.; Mokritzkij, M.; Piontek, J.; Stein, U.; Walther, W. Effective Oncoleaking Treatment of Pancreatic Cancer by Claudin-Targeted Suicide Gene Therapy with Clostridium perfringens Enterotoxin (CPE). Cancers 2021, 13, 4393. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Wen, Q.; McClane, B.A. Multiplex PCR genotyping assay that distinguishes between isolates of Clostridium perfringens type A carrying a chromosomal enterotoxin gene (cpe) locus, a plasmid cpe locus with an IS1470-like sequence, or a plasmid cpe locus with an IS1151 sequence. J. Clin. Microbiol. 2004, 42, 1552–1558. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Wu, J.; Xiao, J.; Yu, J. Exploration of the binding mode of α/β-type small acid soluble proteins (SASPs) with DNA. J. Mol. Model. 2011, 17, 3183–3193. [Google Scholar] [CrossRef]
- Camargo, A.; Ramírez, J.D.; Kiu, R.; Hall, L.J.; Muñoz, M. Unveiling the pathogenic mechanisms of Clostridium perfringens toxins and virulence factors. Emerg. Microbes Infect. 2024, 13, 2341968. [Google Scholar] [CrossRef]
- Azimirad, M.; Gholami, F.; Yadegar, A.; Knight, D.R.; Shamloei, S.; Aghdaei, H.A.; Zali, M.R. Prevalence and characterization of Clostridium perfringens toxinotypes among patients with antibiotic-associated diarrhea in Iran. Sci. Rep. 2019, 9, 7792. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Okada, I.; Mizuno, J.; Nishida, S. Clostridium perfringens--specific lysin. Can. J. Microbiol. 1977, 23, 601–606. [Google Scholar] [CrossRef]
- Ohtani, K.; Shimizu, T. Regulation of toxin gene expression in Clostridium perfringens. Res. Microbiol. 2015, 166, 280–289. [Google Scholar] [CrossRef]
- Rood, J.I.; Adams, V.; Lacey, J.; Lyras, D.; McClane, B.A.; Melville, S.B.; Moore, R.J.; Popoff, M.R.; Sarker, M.R.; Songer, J.G.; et al. Expansion of the Clostridium perfringens toxin-based typing scheme. Anaerobe 2018, 53, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Freedman, J.C.; Theoret, J.R.; Wisniewski, J.A.; Uzal, F.A.; Rood, J.I.; McClane, B.A. Clostridium perfringens type A-E toxin plasmids. Res. Microbiol. 2015, 166, 264–279. [Google Scholar] [CrossRef]
- Titball, R.W. Clostridium perfringens vaccines. Vaccine 2009, 27 (Suppl. S4), D44–D47. [Google Scholar] [CrossRef]
- Lan, H.; Hosomi, K.; Kunisawa, J. Clostridium perfringens enterotoxin-based protein engineering for the vaccine design and delivery system. Vaccine 2019, 37, 6232–6239. [Google Scholar] [CrossRef]
- Miyamoto, K.; Li, J.; McClane, B.A. Enterotoxigenic Clostridium perfringens: Detection and identification. Microbes Environ. 2012, 27, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.S.; Kim, D.H.; Bae, D.; Kim, S.H.; Kim, H.; Moon, J.S.; Song, K.Y.; Chon, J.W.; Seo, K.H. Prevalence, toxin-typing, and antimicrobial susceptibility of Clostridium perfringens from retail meats in Seoul, Korea. Anaerobe 2020, 64, 102235. [Google Scholar] [CrossRef]
- Alimolaei, M.; Afzali, S. Prevalence of Clostridium perfringens toxinotypes in antibiotic-associated diarrheal (AAD) patients in Iranian hospitals; can toxinotype D serve as a possible zoonotic agent for humans? Acta Trop. 2023, 247, 107002. [Google Scholar] [CrossRef]
- Neumann, T.; Krüger, M.; Weisemann, J.; Mahrhold, S.; Stern, D.; Dorner, M.B.; Feraudet-Tarisse, C.; Pöhlmann, C.; Schulz, K.; Messelhäußer, U.; et al. Innovative and Highly Sensitive Detection of Clostridium perfringens Enterotoxin Based on Receptor Interaction and Monoclonal Antibodies. Toxins 2021, 13, 266. [Google Scholar] [CrossRef]
- Petit, L.; Gibert, M.; Popoff, M.R. Clostridium perfringens: Toxinotype and genotype. Trends Microbiol. 1999, 7, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, M.; Ochi, S.; Oda, M.; Miyamoto, K.; Takehara, M.; Kobayashi, K. Recent insights into Clostridium perfringens beta-toxin. Toxins 2015, 7, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.A.; Nakatani, M.; Nakajima, T.; Kohda, T.; Mukamoto, M. The cytotoxicity and molecular mechanisms of the Clostridium perfringens NetB toxin. J. Vet. Med. Sci. 2021, 83, 187–194. [Google Scholar] [CrossRef]
- Duracova, M.; Klimentova, J.; Myslivcova Fucikova, A.; Zidkova, L.; Sheshko, V.; Rehulkova, H.; Dresler, J.; Krocova, Z. Targeted Mass Spectrometry Analysis of Clostridium perfringens Toxins. Toxins 2019, 11, 177. [Google Scholar] [CrossRef] [PubMed]
- Alves, G.G.; Machado de Ávila, R.A.; Chávez-Olórtegui, C.D.; Lobato, F.C. Clostridium perfringens epsilon toxin: The third most potent bacterial toxin known. Anaerobe 2014, 30, 102–107. [Google Scholar] [CrossRef]
- Li, J.; Adams, V.; Bannam, T.L.; Miyamoto, K.; Garcia, J.P.; Uzal, F.A.; Rood, J.I.; McClane, B.A. Toxin plasmids of Clostridium perfringens. Microbiol. Mol. Biol. Rev. 2013, 77, 208–233. [Google Scholar] [CrossRef]
- Tweten, R.K. Clostridium perfringens beta toxin and Clostridium septicum alpha toxin: Their mechanisms and possible role in pathogenesis. Vet. Microbiol. 2001, 82, 1–9. [Google Scholar] [CrossRef]
- Ohtani, K. Gene regulation by the VirS/VirR system in Clostridium perfringens. Anaerobe 2016, 41, 5–9. [Google Scholar] [CrossRef]
- Xu, C.; She, Y.; Lin, Y.; Xu, C. Molecular structure and function of the carboxy-terminus of the alpha-toxin from Clostridium perfringens type A. J. Anim. Physiol. Anim. Nutr. 2020, 104, 725–734. [Google Scholar] [CrossRef]
- Saadat, A.; Melville, S.B. Holin-Dependent Secretion of the Large Clostridial Toxin TpeL by Clostridium perfringens. J. Bacteriol. 2021, 203, e00580-20. [Google Scholar] [CrossRef] [PubMed]
- Ogbu, C.P.; Kapoor, S.; Vecchio, A.J. Structural Basis of Clostridium perfringens Enterotoxin Activation and Oligomerization by Trypsin. Toxins 2023, 15, 637. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, M.; Takehara, M.; Seike, S.; Sakaguchi, Y. Cellular Uptake and Cytotoxicity of Clostridium perfringens Iota-Toxin. Toxins 2023, 15, 695. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, H.D.; Schlesinger, M.S.; Streitenberger, N.; Henderson, E.; Asin, J.; Beingesser, J.; Uzal, F.A. Enterotoxemia produced by lambda toxin-positive Clostridium perfringens type D in 2 neonatal goat kids. J. Vet. Diagn. Invest. 2023, 35, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Awad, M.M.; Rood, J.I. Isolation of alpha-toxin, theta-toxin and kappa-toxin mutants of Clostridium perfringens by Tn916 mutagenesis. Microb. Pathog. 1997, 22, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Daube, G.; Simon, P.; Limbourg, B.; Manteca, C.; Mainil, J.; Kaeckenbeeck, A. Hybridization of 2,659 Clostridium perfringens isolates with gene probes for seven toxins (alpha, beta, epsilon, iota, theta, mu, and enterotoxin) and for sialidase. Am. J. Vet. Res. 1996, 57, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Kiu, R.; Sim, K.; Shaw, A.; Cornwell, E.; Pickard, D.; Kroll, J.S.; Hall, L.J. Genomic Analysis of Clostridium perfringens BEC/CPILE-Positive, Toxinotype D and E Strains Isolated from Healthy Children. Toxins 2019, 11, 543. [Google Scholar] [CrossRef] [PubMed]
- Gerding, D.N.; Johnson, S.; Rupnik, M.; Aktories, K. Clostridium difficile binary toxin CDT: Mechanism, epidemiology, and potential clinical importance. Gut Microbes 2014, 5, 15–27. [Google Scholar] [CrossRef]
- Popoff, M.R.; Bouvet, P. Clostridial toxins. Future Microbiol. 2009, 4, 1021–1064. [Google Scholar] [CrossRef]
- Oda, M.; Terao, Y.; Sakurai, J.; Nagahama, M. Membrane-Binding Mechanism of Clostridium perfringens Alpha-Toxin. Toxins 2015, 7, 5268–5275. [Google Scholar] [CrossRef]
- Navarro, M.A.; McClane, B.A.; Uzal, F.A. Mechanisms of action and cell death associated with Clostridium perfringens toxins. Toxins 2018, 10, 212. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.L.F.; Ferreira, M.R.A.; Donassolo, R.A.; Rodrigues, R.R.; Conceição, F.R. Clostridium septicum: A review in the light of alpha-toxin and development of vaccines. Vaccine 2021, 39, 4949–4956. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.; Ye, B.; Sun, M.; Qi, N.; Li, J.; Lv, M.; Lin, X.; Cai, H.; Hu, J.; Song, Y.; et al. Mechanisms of intestinal epithelial cell damage by Clostridium perfringens. Anaerobe 2024, 87, 102856. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, J.; Duncan, C.L. Some properties of the beta toxin produced by Clostridium perfringens type C. Infect. Immun. 1978, 21, 678–680. [Google Scholar] [CrossRef] [PubMed]
- Hunter, S.E.; Brown, J.E.; Oyston, P.C.; Sakurai, J.; Titball, R.W. Molecular genetic analysis of beta-toxin of Clostridium perfringens reveals sequence homology with alpha-toxin, gamma-toxin, and leukocidin of Staphylococcus aureus. Infect. Immun. 1993, 61, 3958–3965. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, M.; Seike, S.; Shirai, H.; Takagishi, T.; Kobayashi, K.; Takehara, M.; Sakurai, J. Role of P2X7 receptor in Clostridium perfringens beta-toxin-mediated cellular injury. Biochim. Biophys. Acta 2015, 1850, 2159–2167. [Google Scholar] [CrossRef]
- Bruggisser, J.; Tarek, B.; Wyder, M.; Müller, P.; von Ballmoos, C.; Witz, G.; Enzmann, G.; Deutsch, U.; Engelhardt, B.; Posthaus, H. CD31 (PECAM-1) Serves as the Endothelial Cell-Specific Receptor of Clostridium perfringens β-Toxin. Cell Host Microbe 2020, 28, 69–78.e6. [Google Scholar] [CrossRef] [PubMed]
- Hoonakker, M.; Zariri, A.; de Brouwer, L.; David, D.; Borgman, A.; Sloots, A. An in vitro assay for toxicity testing of Clostridium perfringens type C β-toxin. Front. Immunol. 2024, 15, 1373411. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, C.; Popescu, F.; Wyder, M.; Sutter, E.; Zeeh, F.; Frey, J.; von Schubert, C.; Posthaus, H. Rapid cytopathic effects of Clostridium perfringens beta-toxin on porcine endothelial cells. Infect. Immun. 2010, 78, 2966–2973. [Google Scholar] [CrossRef]
- Autheman, D.; Wyder, M.; Popoff, M.; D’Herde, K.; Christen, S.; Posthaus, H. Clostridium perfringens beta-toxin induces necrostatin-inhibitable, calpain-dependent necrosis in primary porcine endothelial cells. PLoS ONE 2013, 8, e64644. [Google Scholar] [CrossRef]
- Seike, S.; Takehara, M.; Kobayashi, K.; Nagahama, M. Role of pannexin 1 in Clostridium perfringens beta-toxin-caused cell death. Biochim. Biophys. Acta 2016, 1858, 3150–3156. [Google Scholar] [CrossRef] [PubMed]
- Theoret, J.R.; Uzal, F.A.; McClane, B.A. Identification and characterization of Clostridium perfringens beta toxin variants with differing trypsin sensitivity and in vitro cytotoxicity activity. Infect. Immun. 2015, 83, 1477–1486. [Google Scholar] [CrossRef]
- Uzal, F.A.; Saputo, J.; Sayeed, S.; Vidal, J.E.; Fisher, D.J.; Poon, R.; Adams, V.; Fernandez-Miyakawa, M.E.; Rood, J.I.; McClane, B.A. Development and application of new mouse models to study the pathogenesis of Clostridium perfringens type C Enterotoxemias. Infect. Immun. 2009, 77, 5291–5299. [Google Scholar] [CrossRef]
- Briggs, D.C.; Naylor, C.E.; Smedley, J.G.; Lukoyanova, N.; Robertson, S.; Moss, D.S.; McClane, B.A.; Basak, A.K. Structure of the food-poisoning Clostridium perfringens enterotoxin reveals similarity to the aerolysin-like pore-forming toxins. J. Mol. Biol. 2011, 413, 138–149. [Google Scholar] [CrossRef]
- Freedman, J.C.; Shrestha, A.; McClane, B.A. Clostridium perfringens enterotoxin: Action, genetics, and translational applications. Toxins 2016, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- Katahira, J.; Inoue, N.; Horiguchi, Y.; Matsuda, M.; Sugimoto, N. Molecular cloning and functional characterization of the receptor for Clostridium perfringens enterotoxin. J. Cell Biol. 1997, 136, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Uzal, F.A.; McClane, B.A. The interaction of Clostridium perfringens enterotoxin with receptor claudins. Anaerobe. 2016, 41, 18–26. [Google Scholar] [CrossRef]
- Saitoh, Y.; Suzuki, H.; Tani, K.; Nishikawa, K.; Irie, K.; Ogura, Y.; Tamura, A.; Tsukita, S.; Fujiyoshi, Y. Tight junctions. Structural insight into tight junction disassembly by Clostridium perfringens enterotoxin. Science 2015, 347, 775–778. [Google Scholar] [CrossRef]
- Yonogi, S.; Matsuda, S.; Kawai, T.; Yoda, T.; Harada, T.; Kumeda, Y.; Gotoh, K.; Hiyoshi, H.; Nakamura, S.; Kodama, T.; et al. BEC, a novel enterotoxin of Clostridium perfringens found in human clinical isolates from acute gastroenteritis outbreaks. Infect. Immun. 2014, 82, 2390–2399. [Google Scholar] [CrossRef]
- Miyata, S.; Matsushita, O.; Minami, J.; Katayama, S.; Shimamoto, S.; Okabe, A. Cleavage of a C-terminal peptide is essential for heptamerization of Clostridium perfringens epsilon-toxin in the synaptosomal membrane. J. Biol. Chem. 2001, 276, 13778–13783. [Google Scholar] [CrossRef]
- Minami, J.; Katayama, S.; Matsushita, O.; Matsushita, C.; Okabe, A. Lambda-toxin of Clostridium perfringens activates the precursor of epsilon-toxin by releasing its N- and C-terminal peptides. Microbiol. Immunol. 1997, 41, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, J.; Nagahama, M.; Oda, M.; Tsuge, H.; Kobayashi, K. Clostridium perfringens iota-toxin: Structure and function. Toxins 2009, 1, 208–228. [Google Scholar] [CrossRef] [PubMed]
- Gibert, M.; Petit, L.; Raffestin, S.; Okabe, A.; Popoff, M.R. Clostridium perfringens iota-toxin requires activation of both binding and enzymatic components for cytopathic activity. Infect. Immun. 2000, 68, 3848–3853. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, M.; Takekara, M.; Kobayashi, K. Interaction of Clostridium perfringens iota toxin and lipolysis-stimulated lipoprotein receptor (LSR). Toxins 2018, 10, 405. [Google Scholar] [CrossRef] [PubMed]
- Richard, J.F.; Mainguy, G.; Gibert, M.; Marvaud, J.C.; Stiles, B.G.; Popoff, M.R. Transcytosis of iota-toxin across polarized CaCo-2 cells. Mol. Microbiol. 2002, 43, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Hilger, H.; Pust, S.; von Figura, G.; Kaiser, E.; Stiles, B.G.; Popoff, M.R.; Barth, H. The long-lived nature of Clostridium perfringens iota toxin in mammalian cells induces delayed apoptosis. Infect. Immun. 2009, 77, 5593–5601. [Google Scholar] [CrossRef] [PubMed]
- Manich, M.; Knapp, O.; Gibert, M.; Maier, E.; Jolivet-Reynaud, C.; Geny, B.; Benz, R.; Popoff, M.R. Clostridium perfringens delta toxin is sequence related to beta toxin, NetB, and Staphylococcus pore-forming toxins, but shows functional differences. PLoS ONE 2008, 3, e3764. [Google Scholar] [CrossRef] [PubMed]
- Seike, S.; Takehara, M.; Kobayashi, K.; Nagahama, M. Clostridium perfringens Delta-Toxin Damages the Mouse Small Intestine. Toxins 2019, 11, 232. [Google Scholar] [CrossRef]
- Mehdizadeh Gohari, I.; Kropinski, A.M.; Weese, S.J.; Parreira, V.R.; Whitehead, A.E.; Boerlin, P.; Prescott, J.F. Plasmid Characterization and Chromosome Analysis of Two netF+ Clostridium perfringens Isolates Associated with Foal and Canine Necrotizing Enteritis. PLoS ONE 2016, 11, e0148344. [Google Scholar] [CrossRef]
- Burke, M.P.; Opeskin, K. Nontraumatic clostridial myonecrosis. Am. J. Forensic Med. Pathol. 1999, 2, 158–162. [Google Scholar] [CrossRef]
- Aronoff, D.M.; Marrazzo, J.M. Infections caused by Clostridium perfringens and Paeniclostridium sordellii after unsafe abortion. Lancet Infect. Dis. 2023, 23, e48–e55. [Google Scholar] [CrossRef] [PubMed]
- Suzaki, A.; Hayakawa, S. Clinical and Microbiological Features of Fulminant Haemolysis Caused by Clostridium perfringens Bacteraemia: Unknown Pathogenesis. Microorganisms 2023, 11, 824. [Google Scholar] [CrossRef]
- Chi, C.H.; Chen, K.W.; Huang, J.J.; Chuang, Y.C.; Wu, M.H. Gas composition in Clostridium septicum gas gangrene. J. Formos. Med. Assoc. 1995, 94, 757–759. [Google Scholar] [PubMed]
- Zhou, Y.; Li, D.; Li, D.; Chen, A.; He, L.; Luo, J.; Tao, L. LDLR, LRP1, and Megalin redundantly participate in the uptake of Clostridium novyi alpha-toxin. Commun. Biol. 2022, 5, 906. [Google Scholar] [CrossRef]
- Wang, C.; Schwaitzberg, S.; Berliner, E.; Zarin, D.A.; Lau, J. Hyperbaric oxygen for treating wounds: A systematic review of the literature. Arch. Surg. 2003, 138, 272–279. [Google Scholar] [CrossRef]
- Korhonen, K. Hyperbaric oxygen therapy in acute necrotizing infections. With a special reference to the effects on tissue gas tensions. Ann. Chir. Gynaecol. 2000, 89 (Suppl. S214), 7–36. [Google Scholar]
- Hirn, M. Hyperbaric oxygen in the treatment of gas gangrene and perineal necrotizing fasciitis. A clinical and experimental study. Eur. J. Surg. Suppl. 1993, 570, 1–36. [Google Scholar]
- Gibert, M.; Jolivet-Reynaud, C.; Popoff, M.R. Beta2 toxin, a novel toxin produced by Clostridium perfringens. Gene 1997, 203, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Jost, B.H.; Billington, S.J.; Trinh, H.T.; Bueschel, D.M.; Songer, J.G. Atypical cpb2 genes, encoding beta2-toxin in Clostridium perfringens isolates of nonporcine origin. Infect. Immun. 2005, 73, 652–656. [Google Scholar] [CrossRef]
- Kircanski, J.; Parreira, V.R.; Whiteside, S.; Pei, Y.; Prescott, J.F. The majority of atypical cpb2 genes in Clostridium perfringens isolates of different domestic animal origin are expressed. Vet. Microbiol. 2012, 159, 371–374. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Itoh, N.; Sugiyama, T.; Kurai, H. Clinical features of Clostridium bacteremia in cancer patients: A case series review. J. Infect. Chemother. 2020, 26, 92–94. [Google Scholar] [CrossRef] [PubMed]
- Sheff, B. Clostridium perfringens. Nursing 2004, 34, 31. [Google Scholar] [CrossRef] [PubMed]
- Redondo, L.M.; Carrasco, J.M.; Redondo, E.A.; Delgado, F.; Miyakawa, M.E. Clostridium perfringens type E virulence traits involved in gut colonization. PLoS ONE 2015, 10, e0121305. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Li, J.; McClane, B.A. Genotypic and phenotypic characterization of Clostridium perfringens isolates from Darmbrand cases in post-World War II Germany. Infect. Immun. 2012, 80, 4354–4363. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, B.; Hao, P.; Yan, M.N.; Dai, K.R. Comprehensive treatment for gas gangrene of the limbs in earthquakes. Chin. Med. J. 2013, 126, 3833–3839. [Google Scholar] [CrossRef] [PubMed]
- Kirchweger, P.; Wundsam, H.; Bosse, F.; Fritz, A.; Kratzer, T.; Kalteis, M.; Kupferthaler, A.; Böhm, G.; Kern, D.; Forstner, M.; et al. Systematic literature review and meta-analysis of Clostridium septicum aortitis. J. Vasc. Surg. 2022, 76, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Prah, I.; Mahazu, S.; Ablordey, A.; Saito, R. Types A and F Clostridium perfringens in healthcare wastewater from Ghana. Appl. Environ. Microbiol. 2023, 89, e0161923. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.L.; Cho, S.Y.; Lee, D.G.; Ko, Y.; Hyun, J.I.; Kim, B.K.; Seo, J.H.; Lee, J.W.; Lee, S. A Fatal Spontaneous Gas Gangrene due to Clostridium perfringens during Neutropenia of Allogeneic Stem Cell Transplantation: Case Report and Literature Review. Infect. Chemother. 2014, 46, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Lanting, B.; Athwal, G.S.; Naudie, D.D. Spontaneous Clostridium perfringens myonecrosis of the shoulder: A case report. Clin. Orthop. Relat. Res. 2007, 461, 20–24. [Google Scholar] [CrossRef]
- Robertson, S.; Smedley, J.G.; McClane, B.A. Identification of a claudin-4 residue important for mediating the host cell binding and action of Clostridium perfringens enterotoxin. Infect. Immun. 2010, 78, 505–517. [Google Scholar] [CrossRef]
- Mehdizadeh Gohari, I.; Li, J.; Navarro, M.; Uzal, F.; McClane, B. Effects of Claudin-1 on the Action of Clostridium perfringens Enterotoxin in Caco-2 Cells. Toxins 2019, 11, 582. [Google Scholar] [CrossRef]
- Chen, J.; Theoret, J.R.; Shrestha, A.; Smedley, J.G.; McClane, B.A. Cysteine-scanning mutagenesis supports the importance of Clostridium perfringens enterotoxin amino acids 80 to 106 for membrane insertion and pore formation. Infect. Immun. 2012, 80, 4078–4088. [Google Scholar] [CrossRef]
- Benz, R.; Popoff, M.R. Clostridium perfringens enterotoxin. The toxin forms highly cation-selective channels in lipid bilayers. Toxins 2018, 10, 341. [Google Scholar] [CrossRef] [PubMed]
- Chakarbarti, G.; Zhou, X.; McClane, B.A. Death pathways activated in CaCo-2 cells by Clostridium perfringens enterotoxin. Infect. Immun. 2003, 71, 4260–4270. [Google Scholar] [CrossRef]
- Mehdizadeh Gohari, I.; Parreira, V.R.; Nowell, V.J.; Nicholson, V.M.; Oliphant, K.; Prescott, J.F. A novel pore-forming toxin in type A Clostridium perfringens is associated with both fatal canine hemorrhagic gastroenteritis and fatal foal necrotizing enterocolitis. PLoS ONE 2015, 10, e0122684. [Google Scholar]
- Gurjar, A.; Li, J.; McClane, B.A. Characterization of toxin plasmids in Clostridium perfringens type C isolates. Infect. Immun. 2010, 78, 4860–4869. [Google Scholar] [CrossRef] [PubMed]
- Adams, V.; Watts, T.D.; Bulach, D.M.; Lyras, D.; Rood, J.I. Plasmid partitioning systems of conjugative plasmids from Clostridium perfringens. Plasmid 2015, 80, 90–96. [Google Scholar] [CrossRef]
- Bueschel, D.M.; Jost, B.H.; Billington, S.J.; Trinh, H.T.; Songer, J.G. Prevalence of cpb2, encoding beta2 toxin, in Clostridium perfringens field isolates: Correlation of genotype with phenotype. Vet. Microbiol. 2003, 94, 121–129. [Google Scholar] [CrossRef]
- Serroni, A.; Colabella, C.; Cruciani, D.; Ciullo, M.; Crotti, S.; Papa, P.; Di Paolo, A.; Gobbi, M.; Forti, K.; Pellegrini, M.; et al. Identification and Characterization of Clostridium perfringens Atypical CPB2 Toxin in Cell Cultures and Field Samples Using Monoclonal Antibodies. Toxins 2022, 14, 796. [Google Scholar] [CrossRef]
- Srivastava, I.; Aldape, M.J.; Bryant, A.E.; Stevens, D.L. Spontaneous C. septicum gas gangrene: A literature review. Anaerobe 2017, 48, 165–171. [Google Scholar] [CrossRef]
- Hoy, S.M. Collagenase Clostridium Histolyticum: A Review in Peyronie’s Disease. Clin. Drug Investig. 2020, 40, 83–92. [Google Scholar] [CrossRef]
- Van Asbroeck, E.; Vasileiadou, O.; De Laere, S.; Van Hedent, E.; Devue, K. Clostridium myonecrosis-a rare and underdiagnosed condition in the elderly: A case with severe skipping lesions and an overview of treatment guidelines. Int. J. Emerg. Med. 2022, 15, 56. [Google Scholar] [CrossRef] [PubMed]
- Aldape, M.J.; Bayer, C.R.; Rice, S.N.; Bryant, A.E.; Stevens, D.L. Comparative efficacy of antibiotics in treating experimental Clostridium septicum infection. Int. J. Antimicrob. Agents 2018, 52, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Dennis, L.; Stevens Alan, L.; Bisno Henry, F.; Chambers, E.; Patchen Dellinger Ellie, J.C.; Goldstein Sherwood, L.; Gorbach Jan, V.; Hirschmann Sheldon, L.; Kaplan Jose, G.; Montoya James, C. Wade Practice Guidelines for the Diagnosis and Management of Skin and Soft Tissue Infections: 2014 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2014, 59, e10–e52. [Google Scholar]
- Alves, G.G.; Gonçalves, L.A.; Assis, R.A.; Oliveira Júnior, C.A.; Silva, R.O.S.; Heneine, L.G.D.; Lobato, F.C.F. Production and purification of Clostridium perfringens type D epsilon toxin and IgY antitoxin. Anaerobe 2021, 69, 102354. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Hu, J.; Qu, Y.; Sun, F.; Leng, X.; Li, H.; Zhan, S. Interventions for treating gas gangrene. Cochrane Database Syst. Rev. 2015, 3, CD010577. [Google Scholar] [CrossRef] [PubMed]
- Stiehl, J.B. Early wound bed preparation: Irrigation and debridement. J. Wound Care 2021, 30, S8–S16. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.X.; Zheng, H.R.; Wang, Y.Y.; Bai, L.L.; Du, X.L.; Wu, Y.; Lu, J.X. Molecular characteristics and phylogenetic analysis of Clostridium perfringens from different regions in China, from 2013 to 2021. Front. Microbiol. 2023, 14, 1195083. [Google Scholar] [CrossRef]
- García-Vela, S.; Martínez-Sancho, A.; Said, L.B.; Torres, C.; Fliss, I. Pathogenicity and Antibiotic Resistance Diversity in Clostridium perfringens Isolates from Poultry Affected by Necrotic Enteritis in Canada. Pathogens 2023, 12, 905. [Google Scholar] [CrossRef]
- Forti, K.; Cagiola, M.; Pellegrini, M.; Anzalone, L.; Di Paolo, A.; Corneli, S.; Severi, G.; De Giuseppe, A. Generation of recombinant baculovirus expressing atoxic C-terminal CPA toxin of Clostridium perfringens and production of specific antibodies. BMC Biotechnol. 2020, 20, 7. [Google Scholar] [CrossRef]
- Ha, E.; Son, B.; Ryu, S. Clostridium perfringens Virulent Bacteriophage CPS2 and Its Thermostable Endolysin LysCPS2. Viruses 2018, 10, 251. [Google Scholar] [CrossRef] [PubMed]
- Hamid, S.; Gadré, A.; Fornander, L.; Sjöwall, J.; Muhrbeck, M. Clostridium septicum myonecrosis following gardening: A case report. Int. J. Surg. Case Rep. 2023, 105, 108000. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, B.; Cerulli, P.; Lucilla, L.; Fusco, A.; Di Pasquali, C.; Bocchini, I.; Orlandi, F.; Agovino, A.; Cervelli, V. Spontaneous clostridial myonecrosis after pregnancy-emergency treatment to the limb salvage and functional recovery: A case report. Int. Wound J. 2014, 11, 93–97. [Google Scholar] [CrossRef]
- Gray, K.M.; Padilla, P.L.; Sparks, B.; Dziewulski, P. Distant myonecrosis by atraumatic Clostridium septicum infection in a patient with metastatic breast cancer. IDCases 2020, 20, e00784. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, H.; Fadel, A.; Garcia, E.; Hernandez, R.J.; Saadoon, Z.F.; Naseer, L.; Casmartino, E.; Hamad, M.; Schnepp, T.; Sarfraz, R.; et al. Clostridial Myonecrosis: A Comprehensive Review of Toxin Pathophysiology and Management Strategies. Microorganisms 2024, 12, 1464. https://doi.org/10.3390/microorganisms12071464
Hussain H, Fadel A, Garcia E, Hernandez RJ, Saadoon ZF, Naseer L, Casmartino E, Hamad M, Schnepp T, Sarfraz R, et al. Clostridial Myonecrosis: A Comprehensive Review of Toxin Pathophysiology and Management Strategies. Microorganisms. 2024; 12(7):1464. https://doi.org/10.3390/microorganisms12071464
Chicago/Turabian StyleHussain, Hussain, Aya Fadel, Efrain Garcia, Robert J. Hernandez, Zahraa F. Saadoon, Lamia Naseer, Ekaterina Casmartino, Mohammad Hamad, Taylor Schnepp, Rehan Sarfraz, and et al. 2024. "Clostridial Myonecrosis: A Comprehensive Review of Toxin Pathophysiology and Management Strategies" Microorganisms 12, no. 7: 1464. https://doi.org/10.3390/microorganisms12071464
APA StyleHussain, H., Fadel, A., Garcia, E., Hernandez, R. J., Saadoon, Z. F., Naseer, L., Casmartino, E., Hamad, M., Schnepp, T., Sarfraz, R., Angly, S., & Jayakumar, A. R. (2024). Clostridial Myonecrosis: A Comprehensive Review of Toxin Pathophysiology and Management Strategies. Microorganisms, 12(7), 1464. https://doi.org/10.3390/microorganisms12071464





