A Clean and Health-Care-Focused Way to Reduce Indoor Airborne Bacteria in Calf House with Long-Wave Ultraviolet
Abstract
:1. Introduction
2. Materials and Methods
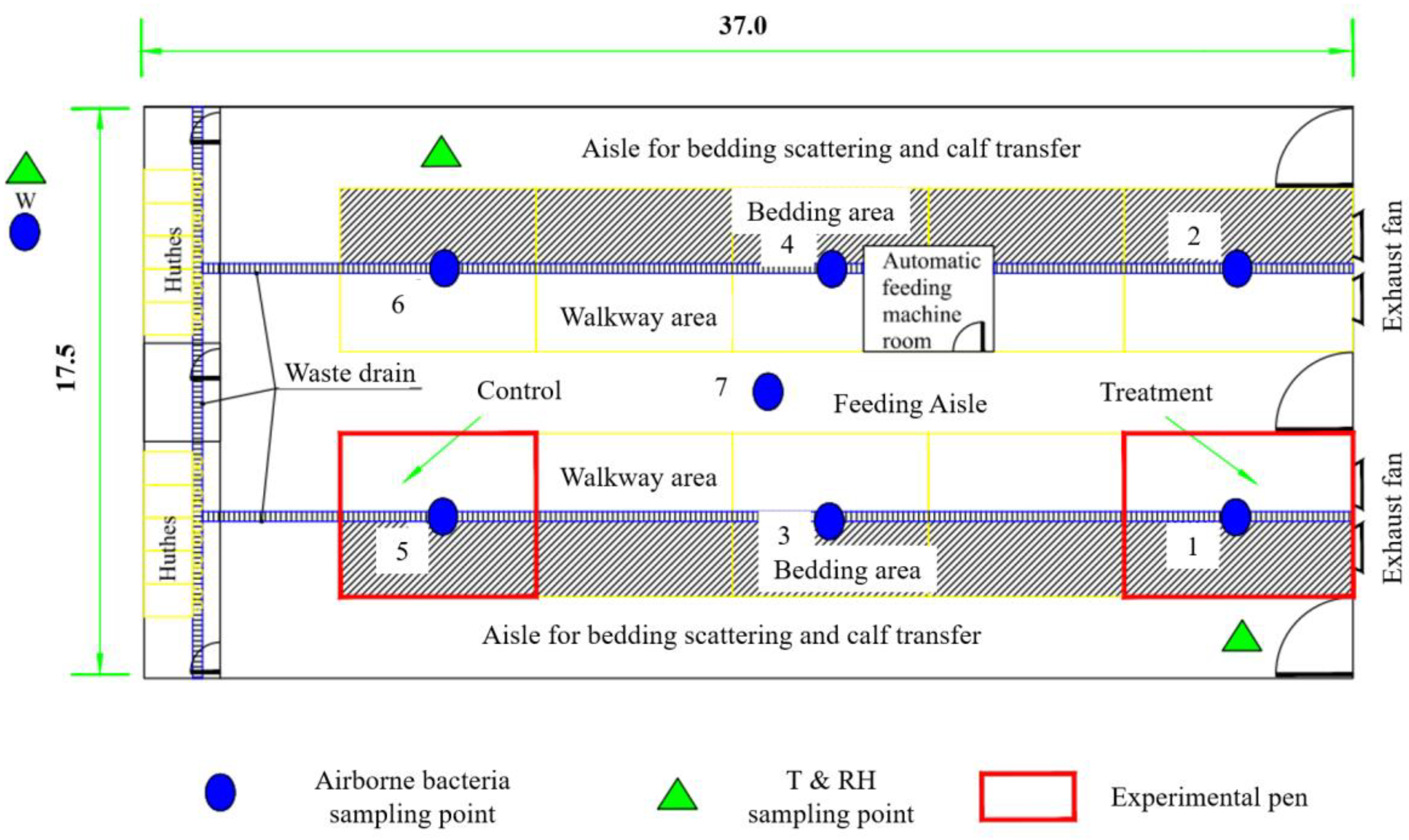
2.1. Experimental Calf House
2.2. Description of Tests and Sampling Strategies
2.2.1. Spatial Variation Test
2.2.2. Temporal Variation Test
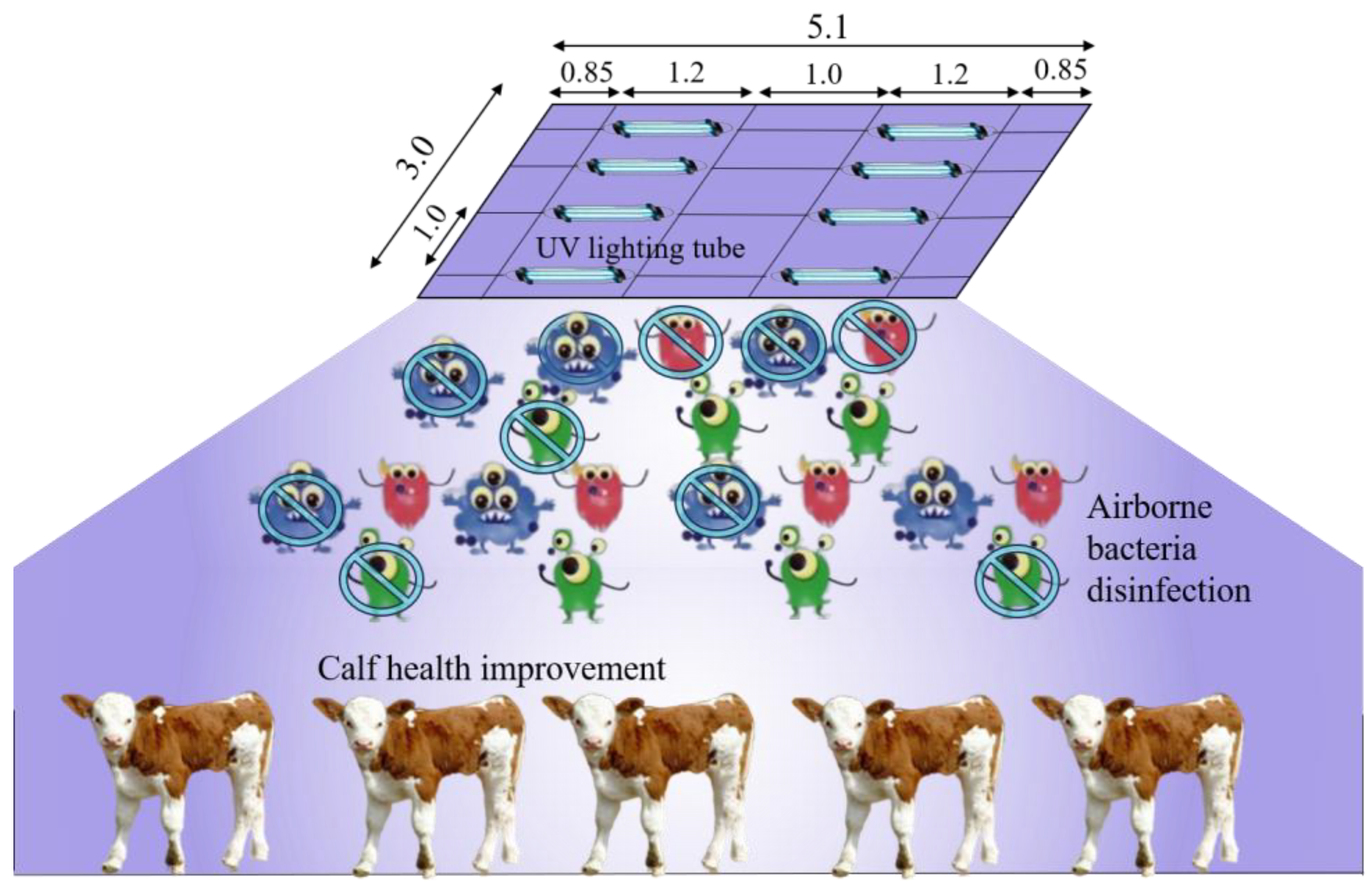
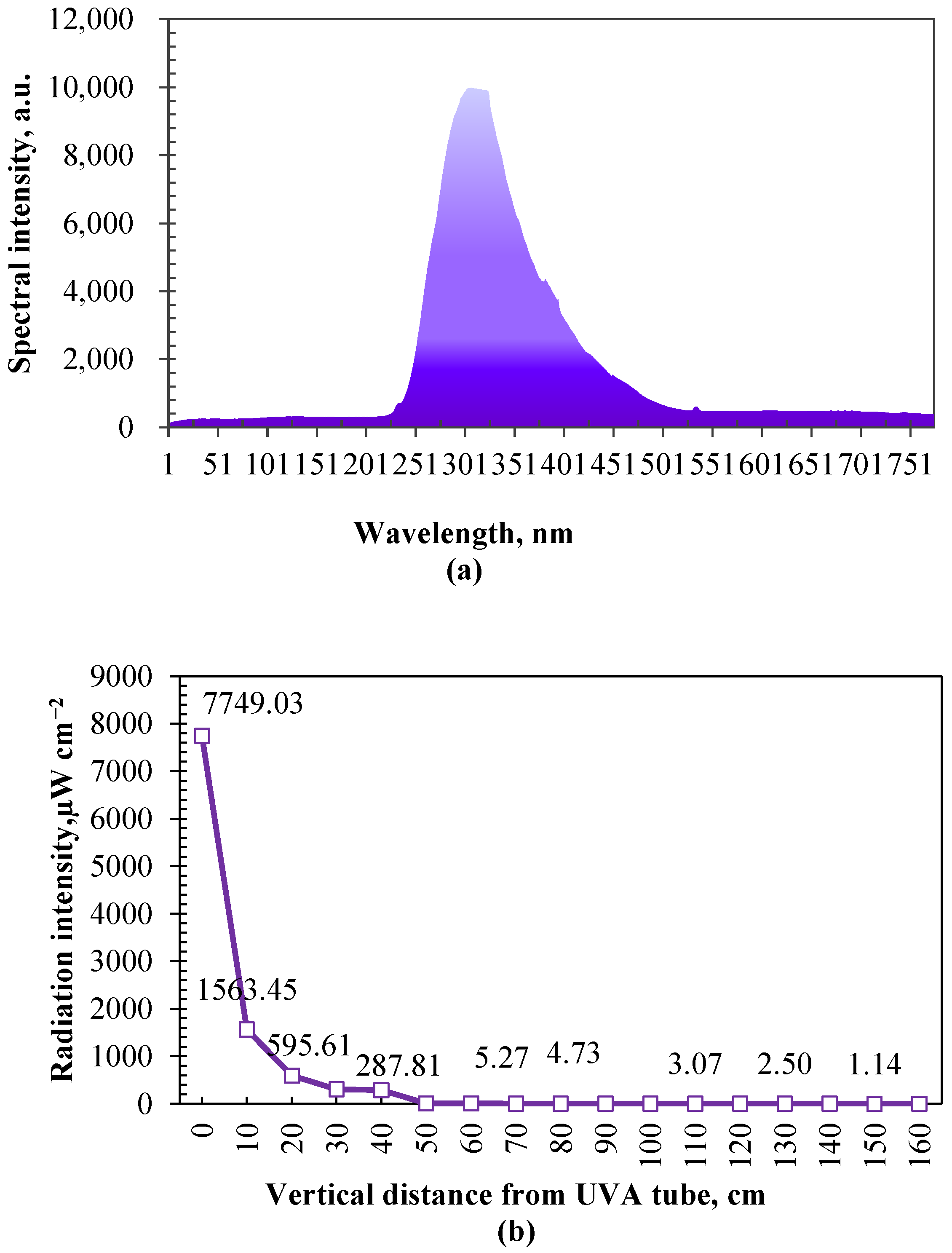
2.2.3. Bactericidal Effect Test of UVA
2.3. Data Processing and Analysis Methods
3. Results and Discussion
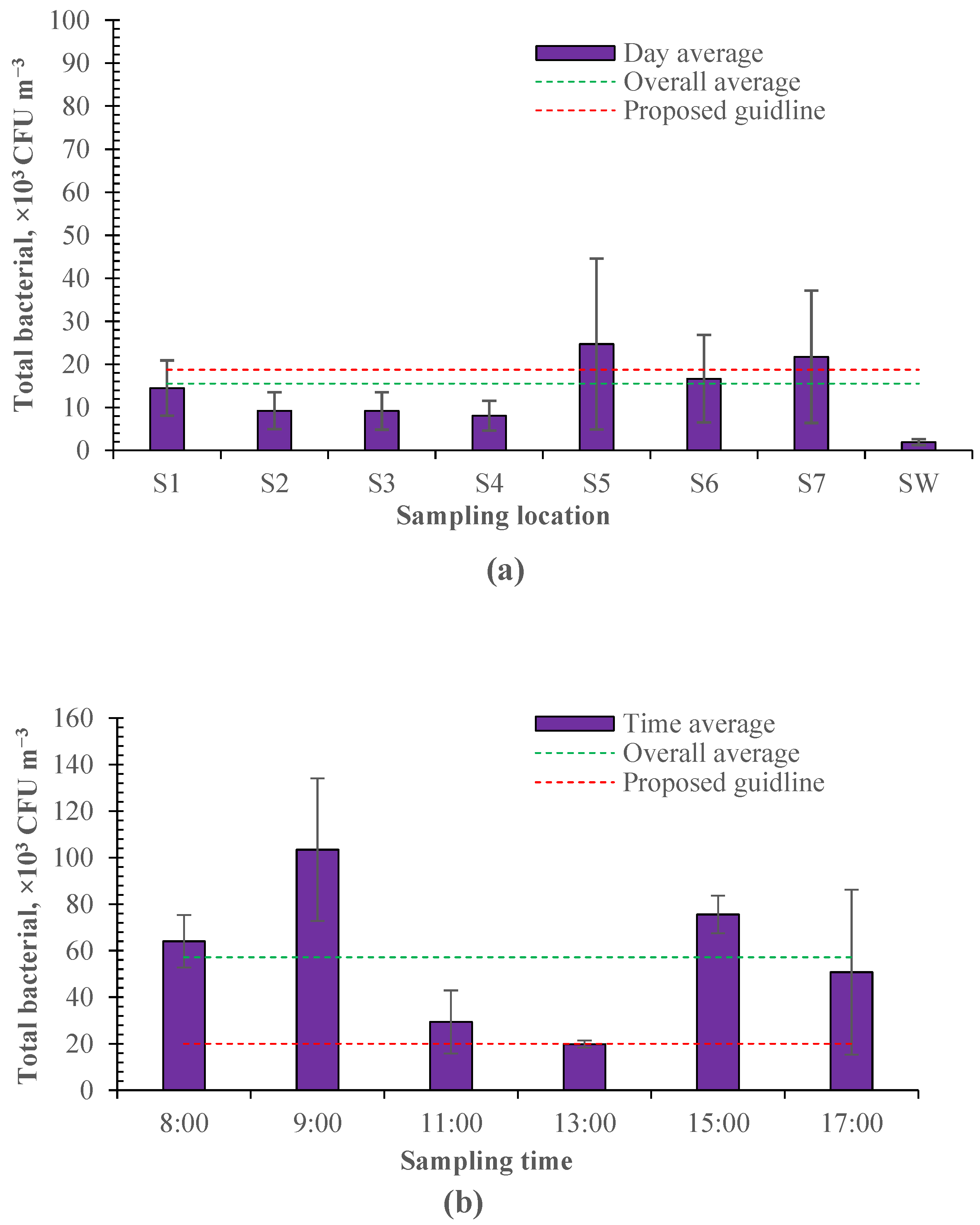
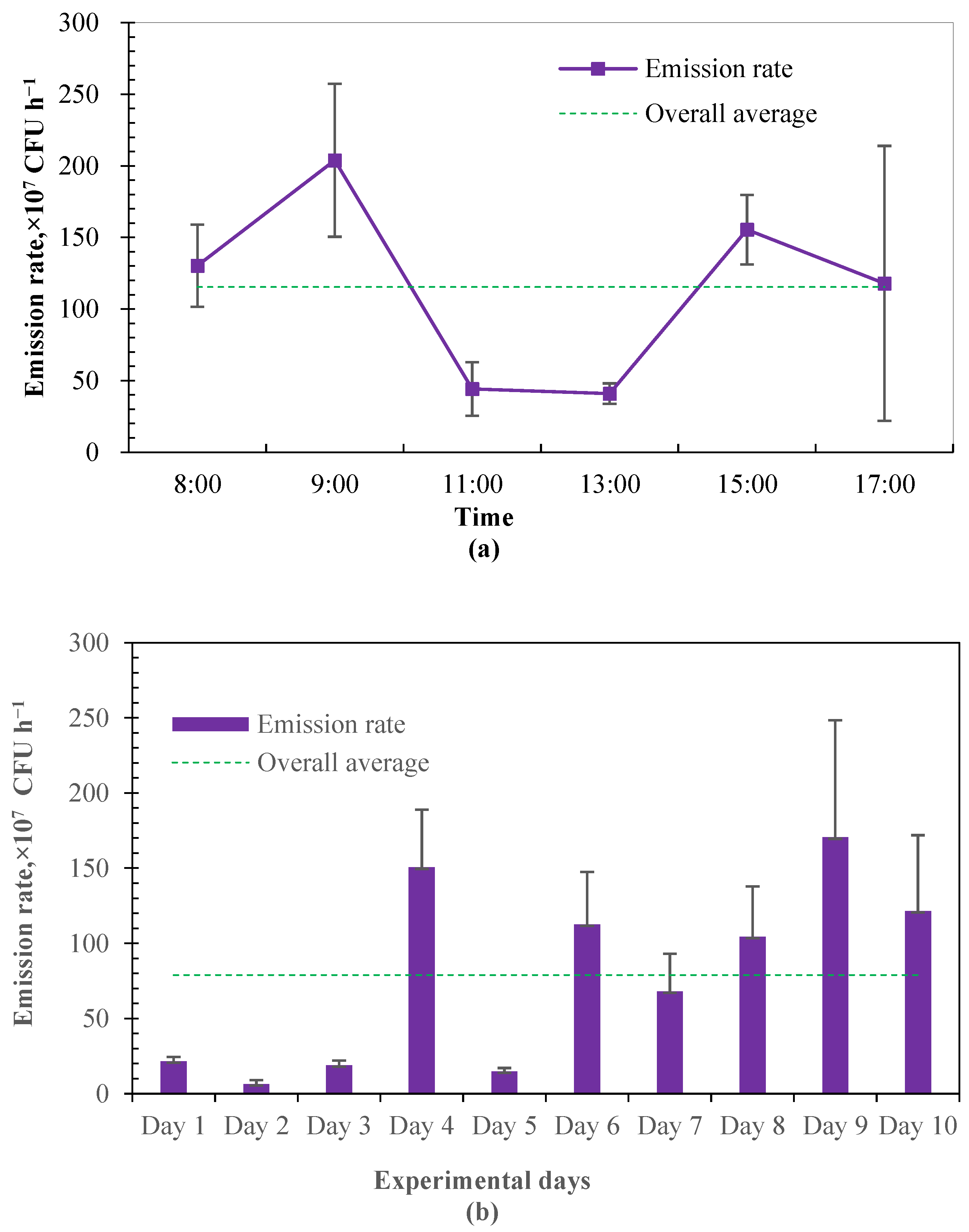
3.1. Spatial and Temporal Characteristics of Airborne Bacteria in Calf House
3.1.1. Concentration and Emission Rate
3.1.2. Size Distribution
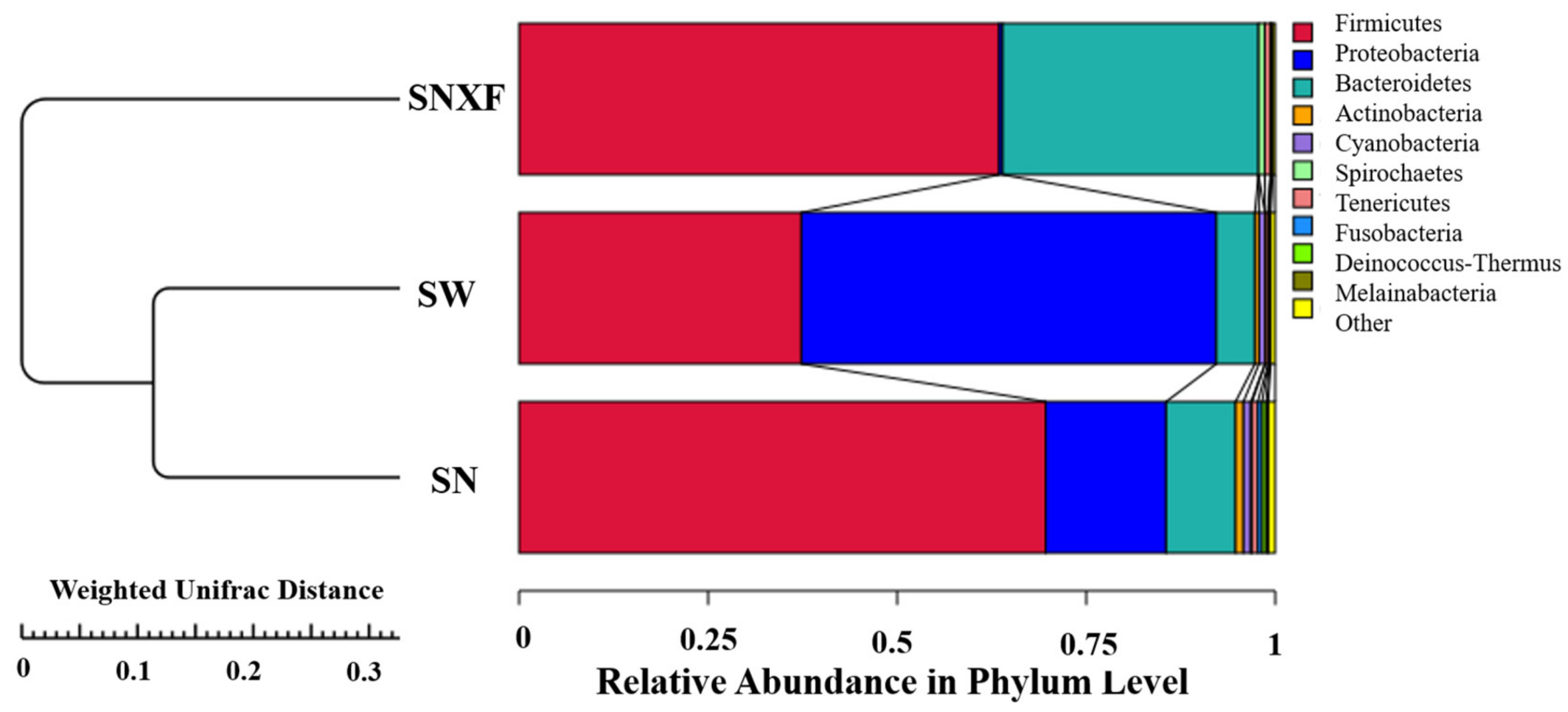
3.1.3. Microbial Composition and Diversity
3.2. Bactericidal Effect of UVA Irradiation
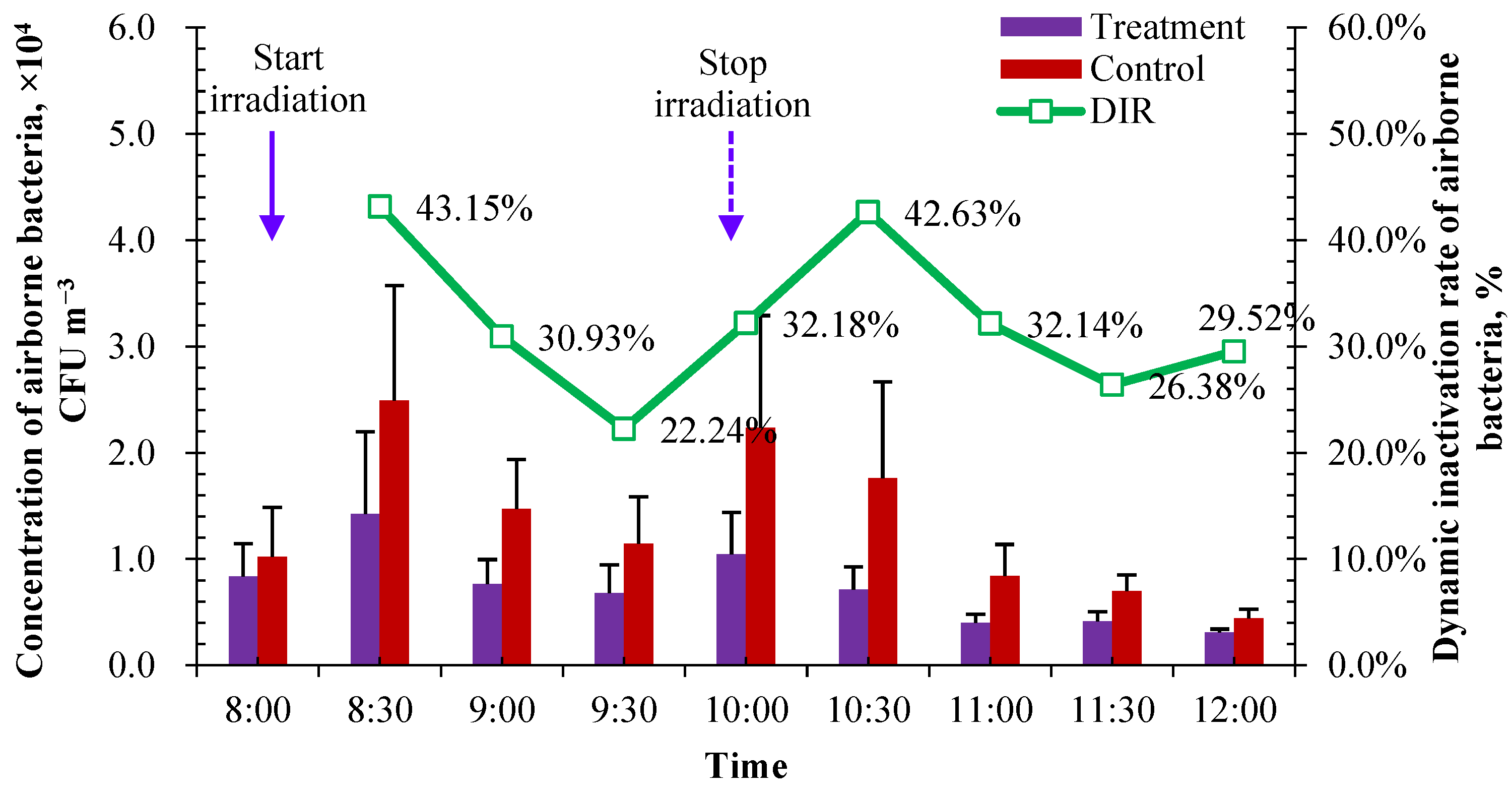
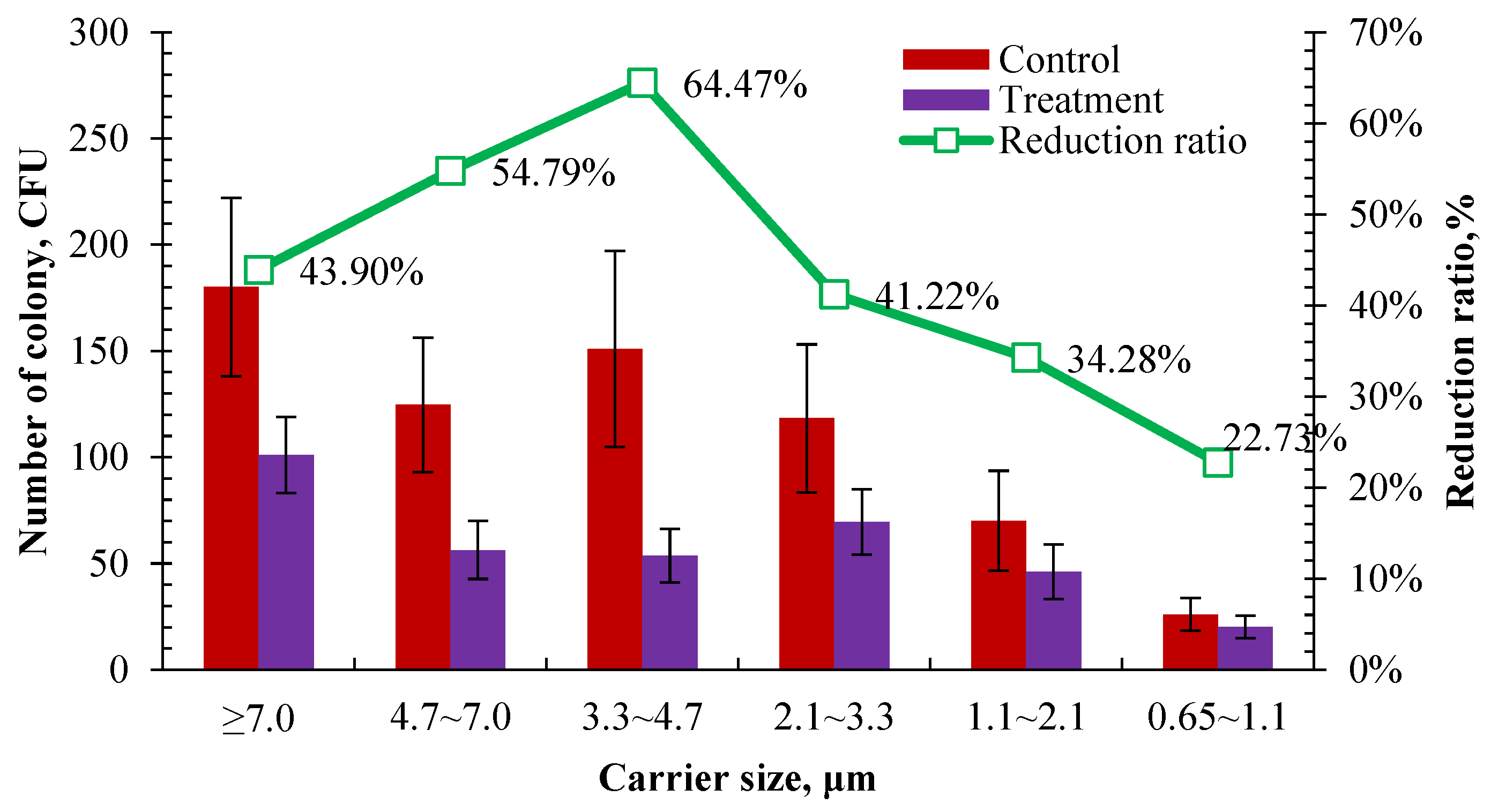
3.2.1. Reduction in Indoor Airborne Bacteria
3.2.2. Health Improvement for Enclose Housed Calves
4. Conclusions and Future Work
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cambra-López, M.; Aarnink, A.J.A.; Zhao, Y.; Calvet, S.; Torres, A.G. Airborne particulate matter from livestock production systems: A review of an air pollution problem. Environ. Pollut. 2010, 158, 1–17. [Google Scholar] [CrossRef]
- Douglas, P.; Robertson, S.; Gay, R.; Hansell, A.L.; Gant, T.W. A systematic review of the public health risks of bioaerosols from intensive farming. Int. J. Hyg. Environ. Health 2018, 221, 134–173. [Google Scholar] [CrossRef] [PubMed]
- Gladding, T.L.; Rolph, C.A.; Gwyther, C.L.; Kinnersley, R.; Walsh, K.; Tyrrel, S. Concentration and composition of bioaerosol emissions from intensive farms: Pig and poultry livestock. J. Environ. Manag. 2020, 272, 111052. [Google Scholar] [CrossRef]
- Douglas, P.; Hayes, E.T.; Williams, W.B.; Tyrrel, S.F.; Kinnersley, R.P.; Walsh, K.; Drew, G.H. Use of dispersion modelling for environmental impact assessment of biological air pollution from composting: Progress, problems and prospects. Waste Manag. 2017, 70, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Bruni, E.; Simonetti, G.; Bovone, B.; Casagrande, C.; Castellani, F.; Riccardi, C.; Uccelletti, D. Evaluation of bioaerosol bacterial components of a wastewater treatment plant through an integrate approach and in vivo assessment. Int. J. Environ. Res. Public Health 2020, 17, 273. [Google Scholar] [CrossRef] [PubMed]
- Dungan, R.S. Fate and transport of bioaerosols associated with livestock operations and manures. J. Anim. Sci. 2010, 88, 3693–3706. [Google Scholar] [CrossRef]
- Quintana, Á.R.; Seseña, S.; Garzón, A.; Arias, R. Factors affecting levels of airborne bacteria in dairy farms: A review. Animals 2020, 10, 526. [Google Scholar] [CrossRef]
- Lago, A.; McGuirk, S.M.; Bennett, T.B.; Cook, N.B.; Nordlund, K.V. Calf respiratory disease and pen microenvironments in naturally ventilated calf barns in winte. J. Dairy Sci. 2006, 89, 4014–4025. [Google Scholar] [CrossRef]
- Islam, M.A.; Ikeguchi, A.; Naide, T. Concentrations of Aerosol Numbers and Airborne Bacteria, and Temperature and Relative Humidity, and Their Interrelationships in a Tie-Stall Dairy Barn. Animals 2019, 9, 1023. [Google Scholar] [CrossRef]
- Nicholas, A.B.; Thissen, J.B.; Fofanov, V.Y.; Allen, E.E.; Rojas, M.; Golovko, G.; Fofanov, Y.; Koshinsky, H.; Jaing, C.J. Metagenomic analysis of the airborne environment in urban spaces. Microb. Ecol. 2015, 69, 346–355. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Z.; Hou, J.; Mao, D.; Lin, H.; Xue, Y.; Luo, Y. Monitoring Antibiotic Resistomes and Bacterial Microbiomes in the Aerosols from Fine, Hazy, and Dusty Weather in Tianjin, China Using a Developed High-volume Tandem Liquid Impinging Sampler. Sci. Total Environ. 2020, 731, 139242. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liang, Z.; Liao, W.; Ye, Z.; Li, G.; An, T. Contributions of Meat Waste Decomposition to the Abundance and Diversity of Pathogens and Antibiotic-resistance Genes in the Atmosphere. Sci. Total Environ. 2021, 784, 147128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lu, B.; Chen, G.; Wang, L.; Lin, B.; Peng, Z.; Chen, J. Culturable and inhalable airborne bacteria in a semiunderground municipal wastewater treatment plant: Distribution, transmission, and health risk assessment. J. Hazard. Mater. 2023, 459, 132234. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.A.; Siddiqua, S.A.; Faruk, M.O.; Md. Towfiqul Islam, A.R.; Salam, M.A. Systematic review and meta-analysis of the potential threats to respiratory health from microbial Bioaerosol exposures. Environ. Pollut. 2024, 341, 122972. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.A.; Miyaoka, Y.; Kabir, M.H.; Kadota, C.; Hakim, H.; Shoham, D.; Murakami, H.; Takehara, K. Evaluation of Virucidal Quantitative Carrier Test towards Bovine Viruses for Surface Disinfectants While Simulating Practical Usage on Livestock Farms. Microorganisms 2022, 10, 1320. [Google Scholar] [CrossRef] [PubMed]
- Schwaiger, K.; Harms, K.S.; Bischoff, M.; Preikschat, P.; Molle, G.; Bauer-Unkauf, I.; Lindorfer, S.; Thalhammer, S.; Bauer, J.; Hölzel, C.S. Insusceptibility to disinfectants in bacteria from animals, food and humans-is there a link to antimicrobial resistance? Front. Microbiol. 2014, 5, 88. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.Y.; Hu, H.; Chen, G.; Li, Z.Y.; Li, A.F.; Zhang, J.Y. Chlorine disinfectants promote microbial resistance in Pseudomonas sp. Environ. Res. 2021, 199, 111296. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Hu, A.; Ren, G.; Chen, M.; Zhou, S.; He, Z. Biophotoelectrochemistry for renewable energy and environmental applications. iScience 2021, 24, 102828. [Google Scholar] [CrossRef]
- Matsumura, Y.; Ananthaswamy, H.N. Toxic effects of ultraviolet radiation on the skin. Toxicol. Appl. Pharm. 2004, 195, 298–308. [Google Scholar] [CrossRef]
- Nunayon, S.S.; Mui, K.-W.; Wong, L.-T. Mapping the knowledge pattern of ultraviolet germicidal irradiation for cleaner indoor air through the lens of bibliometrics. J. Clean. Prod. 2023, 391, 135974. [Google Scholar] [CrossRef]
- Zhao, N.; Lv, L.-P.; Ma, P.; Zhang, Y.-Y.; Deng, J.; Zhang, Y.-Y. A novel exposure mode based on UVA-LEDs for bacterial inactivation. J. Photochem. Photobiol. B 2023, 239, 112641. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, J.; Imafuku, S.; Mori, T.; Sato, C. Narrowband ultraviolet B irradiation increases the serum level of vitamin D3 in patients with neurofibromatosis 1. J. Dermatol. 2013, 40, 829–831. [Google Scholar] [CrossRef]
- Khashayar, E.; Meagan-Helen Henderson, B.; Hanieh, Z.; Robin, B.; Pehr, K.; Margaret, R.; Wilson, H.M. Narrowband ultraviolet B therapy for refractory immune-related lichenoid dermatitis on PD-1 therapy: A case report. J. Immunother. Cancer 2021, 9, e001831. [Google Scholar] [CrossRef]
- Wang, X.; Fang, W.; Zhao, Z. Design of UVA ultraviolet disinfection system for nutrient solution residual liquid and development of microbial online monitoring system. Sustainability 2023, 15, 173. [Google Scholar] [CrossRef]
- Qi, W.; Zhu, S.; Shitu, A.; Ye, Z.; Liu, D. Low concentration peroxymonosulfate and UVA-LED combination for E. coli inactivation and wastewater disinfection from recirculating aquaculture systems. J. Water Process. Eng. 2020, 36, 101362. [Google Scholar] [CrossRef]
- Mazar, Y.; Cytryn, E.; Erel, Y.; Rudich, Y. Effect of dust storms on the atmospheric microbiome in the Eastern Mediterranean. Environ. Sci. Technol. 2016, 50, 4194–4202. [Google Scholar] [CrossRef]
- Šantl-Temkiv, T.; Gosewinkel, U.; Starnawski, P.; Lever, M.; Finster, K. Aeolian dispersal of bacteria in southwest Greenland: Their sources, abundance, diversity and physiological states. FEMS Microbiol. Ecol. 2018, 94, 29481623. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, C.; Di Filippo, D.; Pomata, D.; Simonetti, G.; Castellani, F.; Uccelletti, D.; Buiarelli, F. Comparison of analytical approaches for identifying airborne microorganisms in a livestock facility. Sci. Total Environ. 2021, 783, 147044. [Google Scholar] [CrossRef]
- Ribeiro, E.; Mira, A.R.; Ponte, T.; Oliveira, K. Airborne transmission of bacteria bioburden. In Viruses, Bacteria & Fungi in the Built Environment; Woodhead Publishing: Sawston, UK, 2022; p. 245995162. [Google Scholar] [CrossRef]
- Jahne, M.A.; Rogers, S.W.; Holsen, T.M.; Grimberg, S.J.; Ramler, I.P. Emission and dispersion of bioaerosols from dairy manure application sites: Human health risk assessment. Environ. Sci. Technol. 2015, 49, 9842–9849. [Google Scholar] [CrossRef]
- Van Leuken, J.P.G.; Swart, A.N.; Havelaar, A.H.; Van Pul, A.V.; Van der Hoek, W.; Heederik, D. Atmospheric dispersion modelling of bioaerosols that are pathogenic to humans and livestock—A review to inform risk assessment studies. Microb. Risk Anal. 2016, 1, 19–39. [Google Scholar] [CrossRef]
- Šantl-Temkiv, T.; Sikoparija, B.; Maki, T.; Carotenuto, F.; Amato, P.; Yao, M.; Huffman, J.A. Bioaerosol field measurements: Challenges and perspectives in outdoor studies. Aerosp. Sci. Technol. 2020, 54, 520–546. [Google Scholar] [CrossRef]
- Nouri, H.; Ravelet, F.; Bakir, F.; Sarraf, C.; Rey, R. Design and experimental validation of a ducted counter-rotating axial-flow fans system. J. Fluid. Eng. 2012, 134, 104504. [Google Scholar] [CrossRef]
- Downey, B.C.; Jensen, M.B.; Tucker, C.B. Hay provision affects 24-h performance of normal and abnormal oral behaviors in individually housed dairy calves. J. Dairy Sci. 2022, 105, 4434–4448. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Xu, X.; Zhang, N.; Tu, Y.; Diao, Q. Effects of different feeding methods and space allowance on the growth performance, individual and social behaviors of Holstein calves. J. Integr. Agric. 2017, 16, 1375–1382. [Google Scholar] [CrossRef]
- NY/T 388; Environmental Quality Standard for the Livestock and Poultry Farm. China Standards Press: Beijing, China, 1999.
- Matković, K.; Vučemilo, M.; Vinković, B.; Šeol, B.; Pavičic, Ž.; Matković, S. Qualitative structure of airborne bacteria and fungi in dairy barn and nearby environment. Czech. J. Anim. Sci. 2007, 52, 249–253. [Google Scholar] [CrossRef]
- Xiang, R.; Zhang, A.; Lei, C.; Kong, L.; Ye, X.; Zhang, X.; Wang, H.-N. Spatial variability and evaluation of airborne bacteria concentration in manure belt poultry houses. Poult. Sci. 2019, 98, 1202–1210. [Google Scholar] [CrossRef]
- Zhao, Y.; Aarnink, A.J.A.; De Jong, M.C.M.; Groot Koerkamp, P.W.G. Airborne microorganisms from livestock production systems and their relation to dust. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1071–1128. [Google Scholar] [CrossRef] [PubMed]
- Scheuch, G.; Stahlhofen, W. Deposition and dispersion of aerosols in the airways of the human respiratory tract: The effect of particle size. Exp. Lung Res. 1992, 18, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Salma, I.; Balásházy, I.; Winkler-Heil, R.; Hofmann, W.; Záray, G. Effect of particle mass size distribution on the deposition of aerosols in the human respiratory system. J. Aerosol. Sci. 2002, 33, 119–132. [Google Scholar] [CrossRef]
- Hu, D.; Wang-Li, L.; Simmons, O.D.; Classen, J.J.; Osborne, J.A. Size distributions of bioaerosols in an egg production facility and its vicinity. Environ. Eng. Sci. 2016, 33, 215–223. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, W.; Wang, Y.; Li, B.; Wang, Y. Spatiotemporal variations in the association between particulate matter and airborne bacteria based on the size-resolved respiratory tract deposition in concentrated layer feeding operations. Environ. Int. 2021, 150, 106413. [Google Scholar] [CrossRef]
- Ru, L.; Ding, L.; Deng, S.; Li, Q.; Zhao, W.; Wang, R.; Yao, C. Distribution characteristics and factors influencing culturable bacterial bioaerosols on a dairy farm in Northern China. Agriculture 2023, 13, 1752. [Google Scholar] [CrossRef]
- Zhong, Z.; Wang, N. The size distrition and health risk assessment of microbe bacterial aerosol in livestock and poultry house. China Anim. Health Insp. 2014, 31, 101–105, (In Chinese with English Abstract). [Google Scholar]
- Popescu, S.; Borda, C.; Hegedus, I.C.; Stefan, R.; Diugan, E.A.; Köfer, J.; Schobesberger, H. Airborne bacteria in free-stall dairy barns. In Proceedings of the XVth International Congress of the International Society for Animal Hygiene, Vienna, Austria, 3–7 July 2011; Volume 3. [Google Scholar]
- Hamamoto, A.; Mori, M.; Takahashi, A.; Nakano, M.; Wakikawa, N.; Akutagawa, M.; Kinouchi, Y. New water disinfection system using UVA light-emitting diodes. J. Appl. Microbiol. 2007, 103, 2291–2298. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, L.; Wang, Y.; Zheng, T.; Liu, J. Characterization, factors, and UV reduction of airborne bacteria in a rural wastewater treatment station. Sci. Total Environ. 2021, 751, 141811. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G.; Ishida, K. Biological mechanisms underlying the ultraviolet radiation-induced formation of skin wrinkling and sagging I: Reduced skin elasticity, highly associated with enhanced dermal elastase activity, triggers wrinkling and sagging. Int. J. Mol. Sci. 2015, 16, 7753–7775. [Google Scholar] [CrossRef] [PubMed]
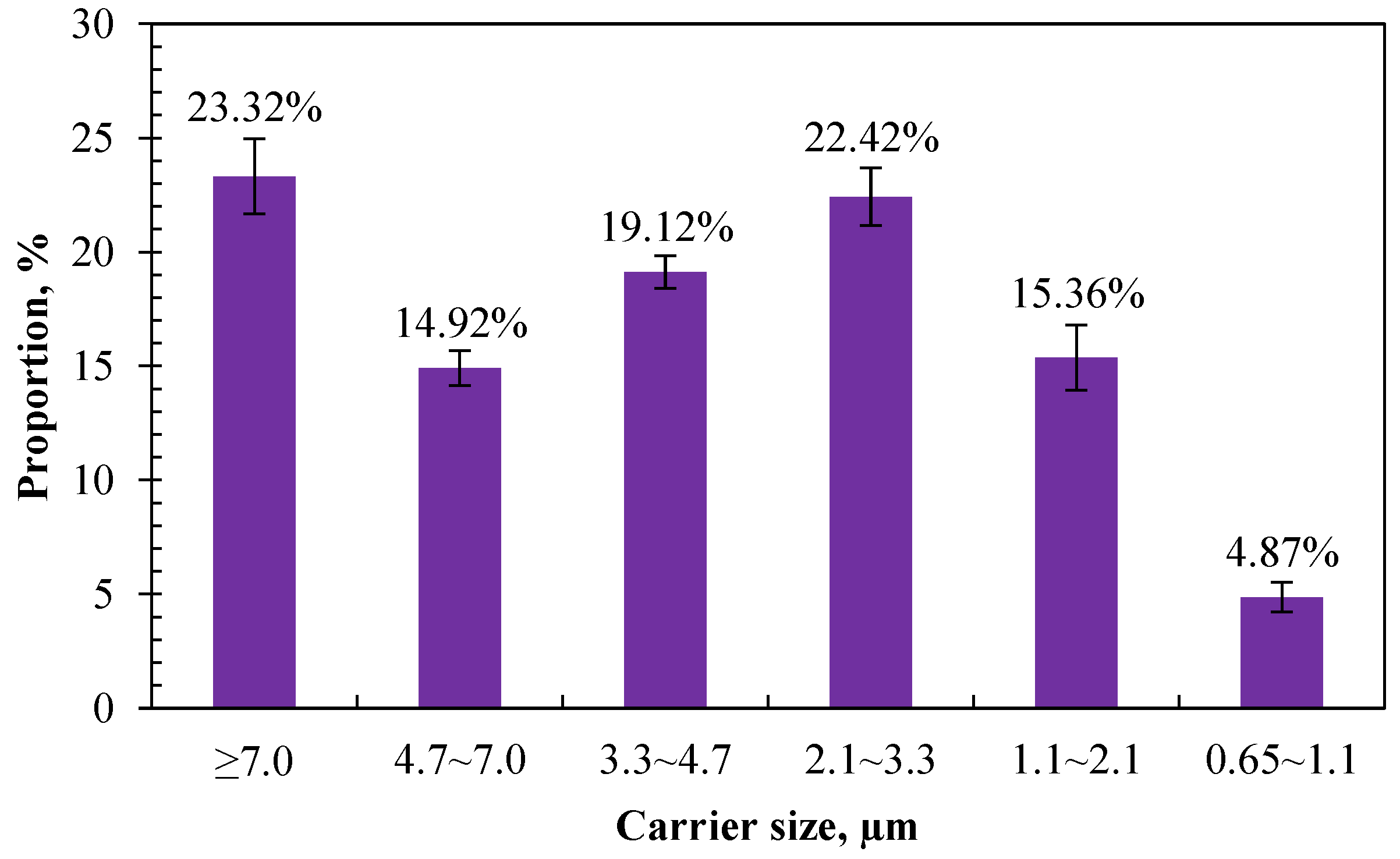

| No. | Behavior | Description | Reference |
|---|---|---|---|
| 1 | Grooming | Touching hair with the tongue or mouth on calf’s own body or a neighboring animal. | Adapted from [34] |
| 2 | Tongue rolling | Tongue is held in a full or partial circular position or moves in a full or partial circular motion; this can occur when the tongue is held within the border of the lips inside the mouth or extended outside the border of the lips. This cannot occur while any other behaviors are being performed (the tongue is not touching any feed or non-nutritive items), and does not need to be repeated. | [34] |
| 3 | Non-nutritive oral manipulation (NNOM) | Licking, chewing, or sucking directed toward a non-nutritive item (includes bars, hutch, bedding, empty bottle), excluding the calf’s own body or that of a neighboring animal. Tongue or lips must be touching a non-nutritive item, or such item must be held inside the mouth. | [34] |
| 4 | Itching | The calf rhythmically rubs a part of its body repeatedly against an object. | |
| 5 | Cough | A reflex action of throat that produces a sound accompanied by vocal cord vibration. | |
| 6 | Cross-sucking (mouth, ear, neck, under belly or body) | The calf is sucking on the mouth, ear, neck, under belly or body of another calf; the sucking movements are performed with the body part in the mouth. | [35] |
| 7 | Headbang | The head quickly turns from one direction to another without the body moving, and repeats more than two consecutive times. |
| Phyla | Indoor Air | Outdoor Air | Calf Manure |
|---|---|---|---|
| Firmicutes | 69.65% | 37.34% | 63.49% |
| Proteobacteria | 15.91% | 54.87% | 0.47% |
| Bacteroidetes | 9.19% | 5.11% | 33.83% |
| Actinobacteria | 1.04% | 0.54% | 0.00% |
| Cyanobacteria | 0.99% | 0.77% | 0.00% |
| Tenericutes | 0.75% | 0.38% | 0.74% |
| Fusobacteria | 0.57% | 0.08% | 0.05% |
| Deinococcus-Thermus | 0.54% | 0.11% | 0.00% |
| Melainabacteria | 0.27% | 0.18% | 0.27% |
| Spirochaetes | 0.15% | 0.05% | 0.83% |
| Others | 0.92% | 0.59% | 0.33% |
| NO. | Behavior | Before | After | ||
|---|---|---|---|---|---|
| Treatment (%) | Control (%) | Treatment (%) | Control (%) | ||
| 1 | Grooming | 11.82 ± 10.28 ns | 12.24 ± 9.44 | 6.12 ± 5.20 ns | 8.16 ± 6.46 |
| 2 | Tongue rolling | 22.45 ± 7.64 * | 4.08 ± 6.97 | 26.53 ± 5.00 * | 4.08 ± 6.45 |
| 3 | NNOM | 24.49 ± 10.80 ns | 30.61 ± 17.36 | 18.37 ± 14.72 ns | 22.45 ± 16.83 |
| 4 | Headbang | 6.12 ± 7.64 * | 0 | 0 * | 4.08 ± 10.00 |
| 5 | Cough | 34.69 ± 17.18 ns | 32.65 ± 24.35 | 24.49 ± 10.00 ns* | 38.78 ± 16.59 |
| 6 | Itching | 18.37 ± 6.97 ns | 20.41 ± 13.94 | 24.49 ± 16.58 ns* | 10.20 ± 10.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, L.; Zhang, Q.; Wang, C.; Yao, C.; Shan, F.; Li, Q. A Clean and Health-Care-Focused Way to Reduce Indoor Airborne Bacteria in Calf House with Long-Wave Ultraviolet. Microorganisms 2024, 12, 1472. https://doi.org/10.3390/microorganisms12071472
Ding L, Zhang Q, Wang C, Yao C, Shan F, Li Q. A Clean and Health-Care-Focused Way to Reduce Indoor Airborne Bacteria in Calf House with Long-Wave Ultraviolet. Microorganisms. 2024; 12(7):1472. https://doi.org/10.3390/microorganisms12071472
Chicago/Turabian StyleDing, Luyu, Qing Zhang, Chaoyuan Wang, Chunxia Yao, Feifei Shan, and Qifeng Li. 2024. "A Clean and Health-Care-Focused Way to Reduce Indoor Airborne Bacteria in Calf House with Long-Wave Ultraviolet" Microorganisms 12, no. 7: 1472. https://doi.org/10.3390/microorganisms12071472





