Isolation and Characterization of Potassium-Solubilizing Rhizobacteria (KSR) Promoting Cotton Growth in Saline–Sodic Regions
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Soil Sampling
2.3. Soil Properties Analysis
2.4. Mineral Samples and Medium
2.5. Isolation of KSR from Cotton Rhizosphere
2.6. Potassium Solubilizing Ability of KSR
2.7. Assessment of Strain Viability Under Different pH Conditions
2.8. DNA Sequencing and Phylogenic Analysis of KSR
2.9. Determination of Organic Acids and Enzyme Types and Contents
2.10. Pot Experiment
2.11. Data Analysis
3. Results
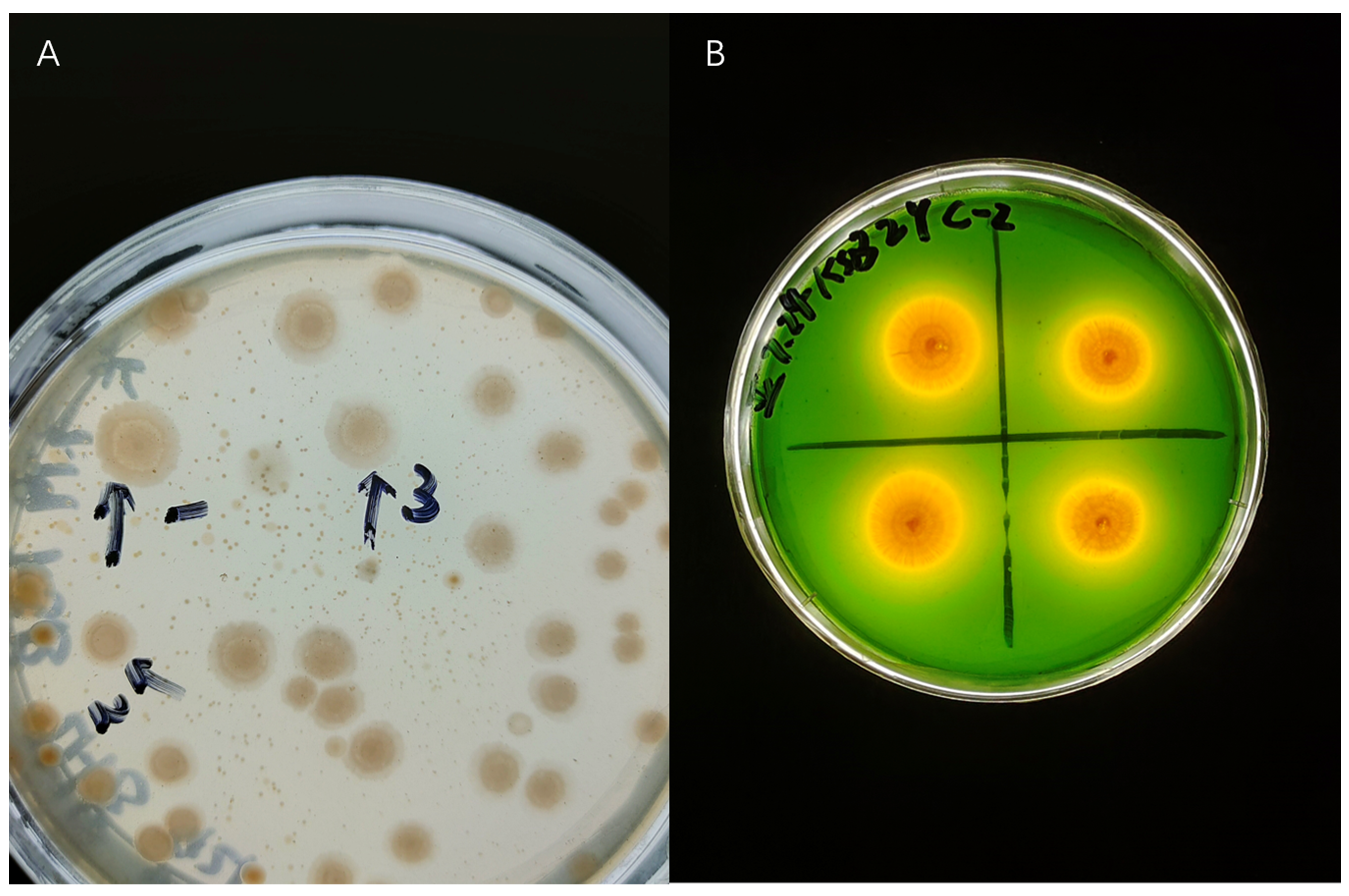
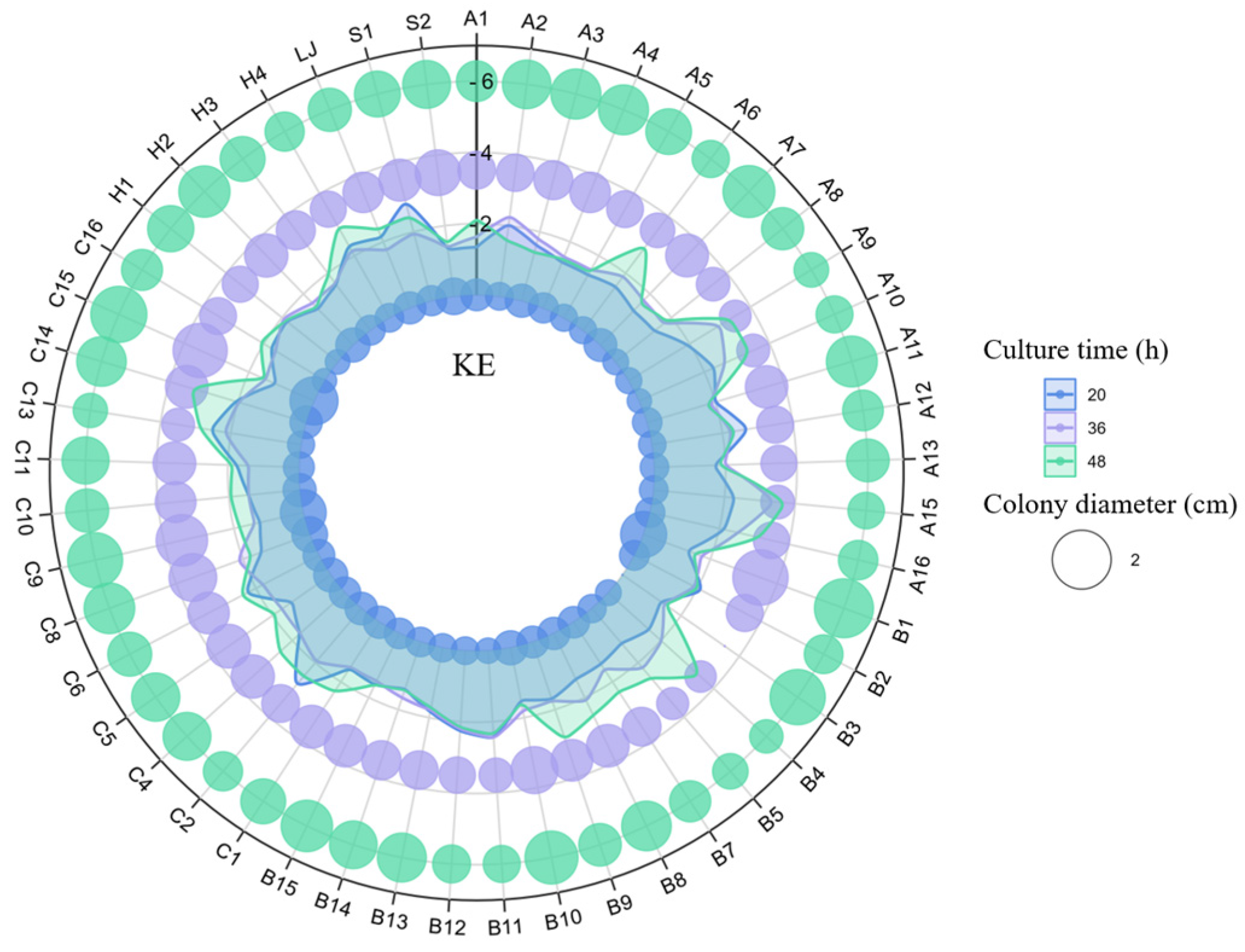
3.1. Isolation and Potassium Solubilizing Ativity of Cotton Rhizosphere KSR
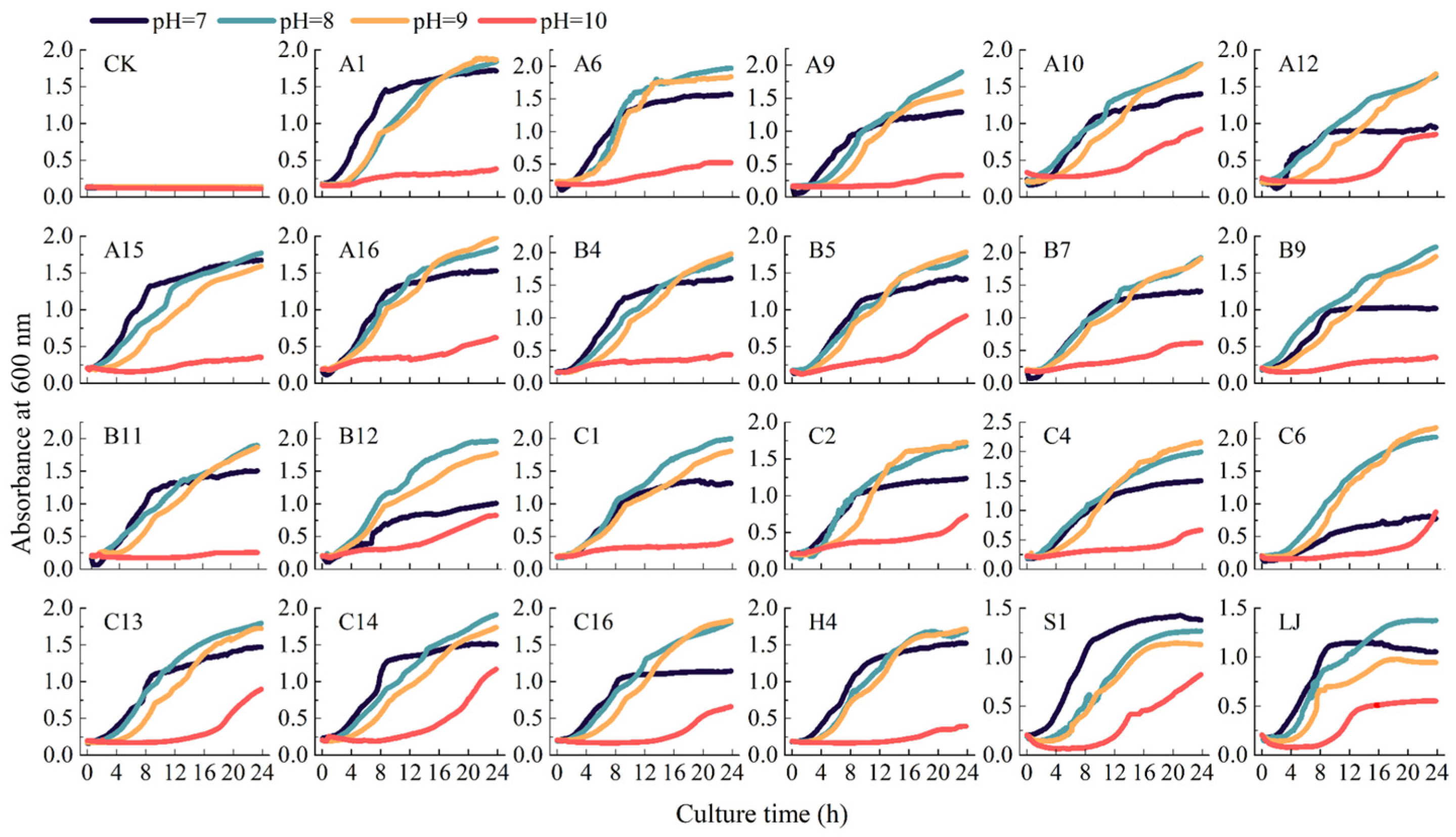
3.2. Effect of Alkaline Stress on KSR
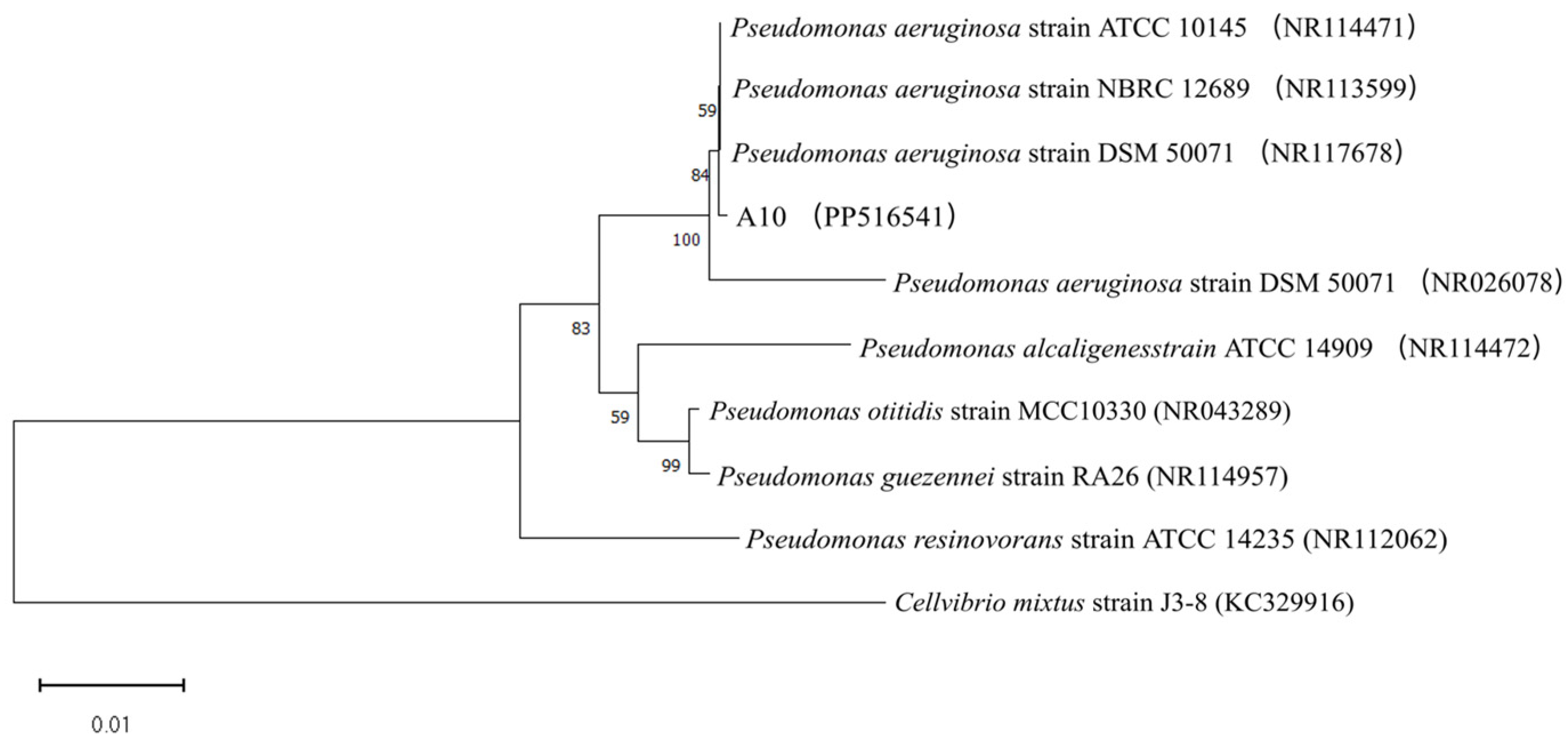
3.3. Molecular and Biochemical Identification of Efficient KSR Strains
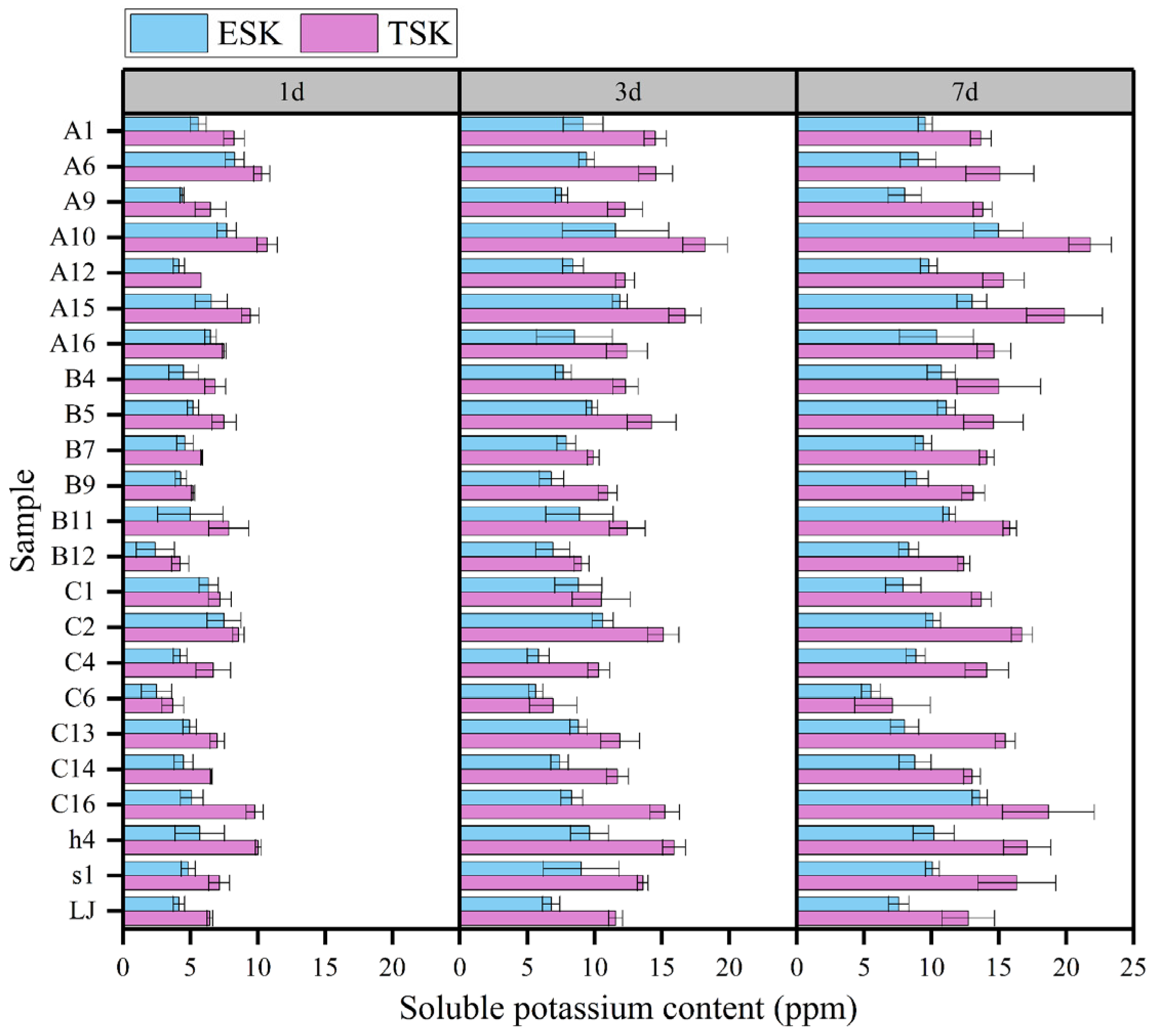
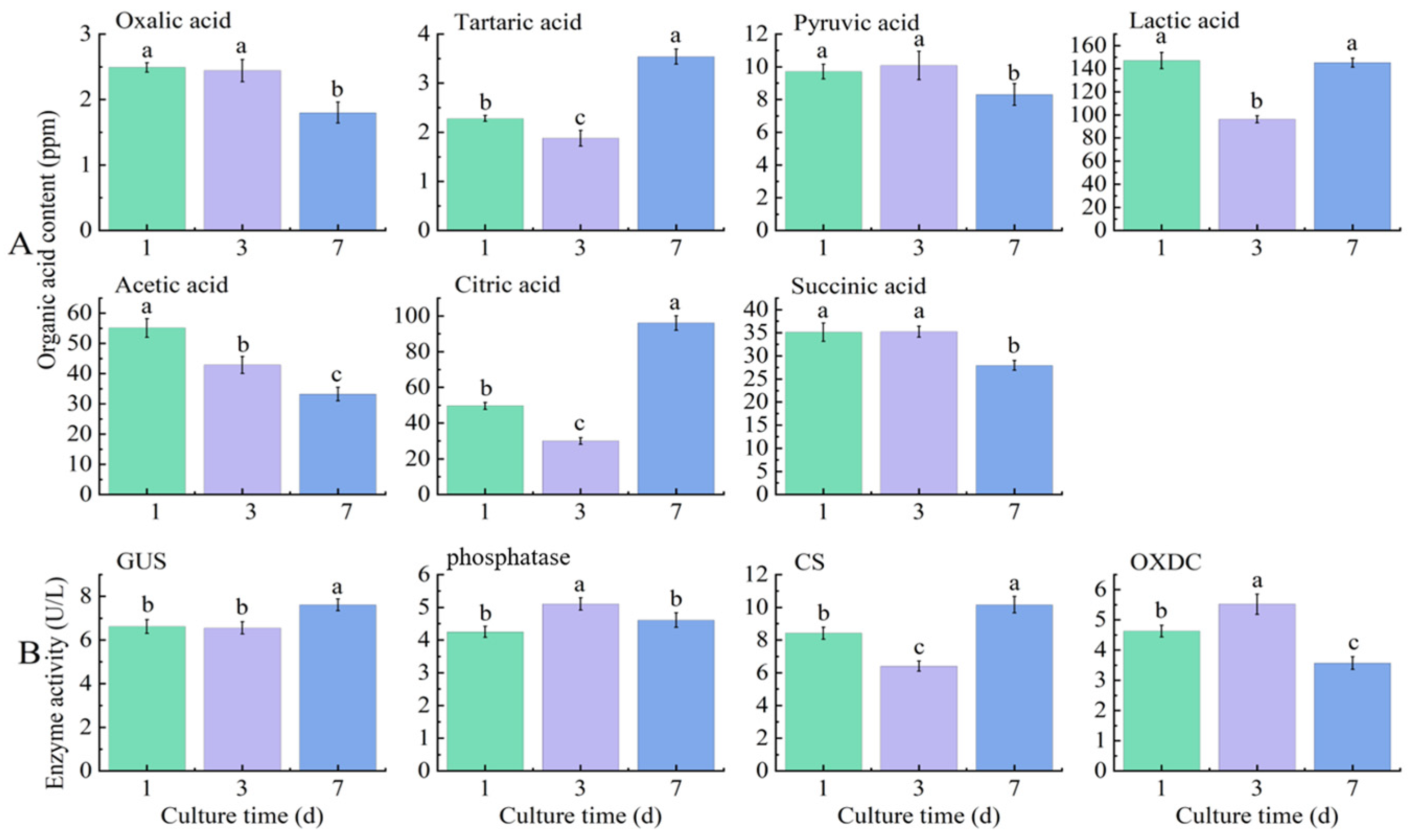
3.4. Quantification of Organic Acids and Enzymes
3.5. Promotional Effects of KSR on Cotton Growth
4. Discussion
4.1. Isolation, Identification, and Evaluation of Potassium Solubilization Capacity of KSR
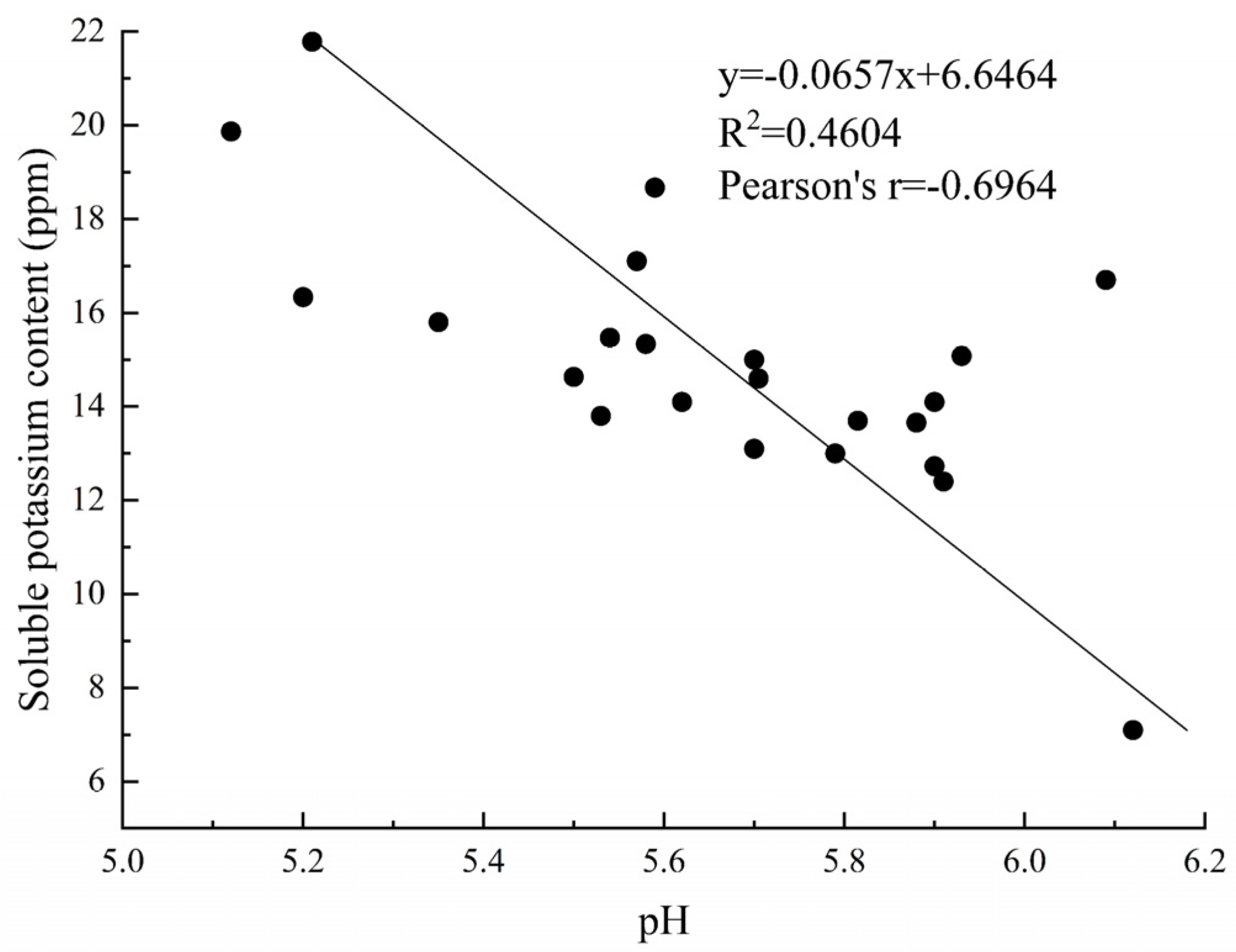
4.2. The pH Dynamics and Mechanisms of Potassium Solution
4.3. Survival Capability of KSR Under Alkaline Stress
4.4. Plant Growth of Cotton in the Presence of KSR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahanger, M.A.; Tittal, M.; Mir, R.A.; Agarwal, R. Alleviation of water and osmotic stress-induced changes in nitrogen metabolizing enzymes in Triticum aestivum L. cultivars by potassium. Protoplasma 2017, 254, 1953–1963. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Li, F.; Yi, F.; Eneji, A.E.; Tian, X.; Li, Z. Transcriptome analysis unravels key factors involved in response to potassium deficiency and feedback regulation of K+ uptake in cotton roots. Int. J. Mol. Sci. 2021, 22, 3133. [Google Scholar] [CrossRef] [PubMed]
- Fontana, J.E.; Wang, G.; Sun, R.; Xue, H.; Li, Q.; Liu, J.; Davis, K.E.; Thornburg, T.E.; Zhang, B.; Zhang, Z. Impact of potassium deficiency on cotton growth, development and potential microRNA-mediated mechanism. Plant Physiol. Biochem. 2020, 153, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Cassman, K.G.; Bryant, D.C.; Higashi, S.L.; Roberts, B.A.; Kerby, T.A. Soil potassium balance and cumulative cotton response to annual potassium additions on a vermiculitic soil. Soil Sci. Soc. Am. J. 1989, 53, 805–812. [Google Scholar] [CrossRef]
- Ali, S.; Hafeez, A.; Ma, X.; Tung, S.A.; Liu, A.; Shah, A.N.; Chattha, M.S.; Zhang, Z.; Yang, G. Potassium relative ratio to nitrogen considerably favors carbon metabolism in late-planted cotton at high planting density. Field Crop. Res. 2018, 223, 48–56. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, S.; Huang, J.; Zhang, Y.; Feng, L.; Zhao, W.; Qi, H.; Zhou, G.; Deng, N. Prospects for cotton self-sufficiency in China by closing yield gaps. Eur. J. Agron. 2022, 133, 126437. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X. Loss and conservation of soil organic carbon and nutrients in arid and semiarid China during aeolian dust emissions. Soil Tillage Res. 2024, 235, 105910. [Google Scholar] [CrossRef]
- Ma, W.; Chen, Y.; Wang, F.; Wei, C. Analysis of nutrient supply of nitrogen, phosphorus and potassium in arable land in Xinjiang. Xinjiang Agric. Sci. 2022, 59, 1401–1408. (in Chinese). [Google Scholar]
- Huang, C.; Zeng, F.; Lei, J. Impact of cultivation practices in oasis agriculture on soil fertility dynamics and the relationship with cotton nitrogen-use efficiency in the southern rim of the Tarim Basin, Xinjiang, China. Commun. Soil Sci. Plant Anal. 2014, 45, 2621–2635. [Google Scholar] [CrossRef]
- Song, X.; Geng, Y.; Zhang, Y.; Zhang, X.; Gao, Z.; Li, M. Dynamic potassium flows analysis in China for 2010–2019. Resour. Policy 2022, 78, 102803. [Google Scholar] [CrossRef]
- Meena, V.S.; Maurya, B.R.; Verma, J.P.; Aeron, A.; Kumar, A.; Kim, K.; Bajpai, V.K. Potassium solubilizing rhizobacteria (KSR): Isolation, identification, and K-release dynamics from waste mica. Ecol. Eng. 2015, 81, 340–347. [Google Scholar] [CrossRef]
- Shi, X.; Hao, X.; Li, N.; Li, J.; Shi, F.; Han, H.; Tian, Y.; Chen, Y.; Wang, J.; Luo, H. Organic liquid fertilizer coupled with single application of chemical fertilization improves growth, biomass, and yield components of cotton under mulch drip irrigation. Front. Plant Sci. 2022, 12, 763525. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, D.; Riaz, M.; Zhang, L.; Jiang, C. Investigating the effect of biochar on the potential of increasing cotton yield, potassium efficiency and soil environment. Ecotox. Environ. Safe. 2019, 182, 109451.1–109451.7. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhang, J.; Zhang, Z.; Abdelrahman, A.M.; Gao, Q. Effect of different fertilization managements on nitrate accumulation in a mollisol of northeast China. Chem. Biol. Technol. Agric. 2016, 3, 16. [Google Scholar] [CrossRef]
- Tan, M.; Li, W.; Zong, R.; Li, X.; Han, Y.; Luo, P.; Dhital, Y.P.; Lin, H.; Li, H.; Wang, Z. Long-term mulched drip irrigation enhances the stability of soil aggregates by increasing organic carbon stock and reducing salinity. Soil Tillage Res. 2024, 240, 106069. [Google Scholar] [CrossRef]
- Zong, R.; Wang, Z.; Li, W.; Li, H.; Ayantobo, O.O. Effects of practicing long-term mulched drip irrigation on soil quality in northwest China. Sci. Total Environ. 2023, 878, 163247. [Google Scholar] [CrossRef]
- Sattar, A.; Naveed, M.; Ali, M.; Zahir, Z.A.; Nadeem, S.M.; Yaseen, M.; Meena, V.S.; Farooq, M.; Singh, R.; Rahman, M. Perspectives of potassium solubilizing microbes in sustainable food production system: A review. Appl. Soil Ecol. 2019, 133, 146–159. [Google Scholar] [CrossRef]
- Shen, H.; He, X.; Liu, Y.; Chen, Y.; Tang, J.; Guo, T. A complex inoculant of N2-fixing, P- and K-solubilizing bacteria from a purple soil improves the growth of kiwifruit (Actinidia chinensis) plantlets. Front. Microbiol. 2016, 7, 841. [Google Scholar] [CrossRef]
- Meena, V.S.; Maurya, B.R.; Verma, J.P. Does a rhizospheric microorganism enhance K+ availability in agricultural soils? Microbiol. Res. 2014, 169, 337–347. [Google Scholar] [CrossRef]
- Han, D.; Wang, L.; Luo, Y. Isolation, identification, and the growth promoting effects of two antagonistic actinomycete strains from the rhizosphere of Mikania micrantha Kunth. Microbiol. Res. 2018, 208, 1–11. [Google Scholar] [CrossRef]
- Sun, F.; Ou, Q.; Wang, N.; Guo, Z.; Ou, Y.; Li, N.; Peng, C. Isolation and identification of potassium-solubilizing bacteria from mikania micrantha rhizospheric soil and their effect on M. micrantha plants. Glob. Ecol. Conserv. 2020, 23, e01141. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, H.; Shen, Z.; Ye, J. Whole-genome sequencing and potassium-solubilizing mechanism of Bacillus aryabhattai SK1-7. Front. Microbiol. 2022, 12, 722379. [Google Scholar] [CrossRef]
- Yaghoubi Khanghahi, M.; Pirdashti, H.; Rahimian, H.; Nematzadeh, G.; Ghajar Sepanlou, M. Potassium solubilising bacteria (KSB) isolated from rice paddy soil: From isolation, identification to K use efficiency. Symbiosis 2018, 76, 13–23. [Google Scholar] [CrossRef]
- Sarikhani, M.R.; Oustan, S.; Ebrahimi, M.; Aliasgharzad, N. Isolation and identification of potassium-releasing bacteria in soil and assessment of their ability to release potassium for plants. Eur. J. Soil Sci. 2018, 69, 1078–1086. [Google Scholar] [CrossRef]
- Nawaz, A.; Qamar, Z.U.; Marghoob, M.U.; Imtiaz, M.; Imran, A.; Mubeen, F. Contribution of potassium solubilizing bacteria in improved potassium assimilation and cytosolic K+/Na+ Ratio in Rice (Oryza sativa L.) under saline-sodic conditions. Front. Microbiol. 2023, 14, 1196024. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, M.; Yuan, M.; Wang, E.; Bai, Y.; Crowther, T.W.; Zhou, J.; Ma, Z.; Zhang, L.; Wang, Y.; et al. Root microbiota confers rice resistance to aluminum toxicity and phosphorus deficiency in acidic soils. Nat. Food 2023, 4, 912–924. [Google Scholar] [CrossRef]
- Han, M.; Zhu, X.; Ruan, C.; Wu, H.; Chen, G.; Zhu, K.; Liu, Y.; Wang, G. Micro-biophysical interactions at bacterium-mineral interfaces determine potassium dissolution. Environ. Technol. Innov. 2024, 33, 103524. [Google Scholar] [CrossRef]
- Lorenz, N.; Verdell, K.; Ramsier, C.; Dick, R.P. A Rapid assay to estimate soil microbial biomass potassium in agricultural soils. Soil Sci. Soc. Am. J. 2010, 74, 512–516. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, C.; Ji, C.; Liu, Z.; Song, X.; Liu, Y.; Li, C.; Yan, D.; Li, H.; Qin, Y.; et al. Isolation and characterization of endophytic bacteria for controlling root rot disease of Chinese jujube. J. Appl. Microbiol. 2021, 130, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fan, B.; Guo, L. Soil salinization after long-term mulched drip irrigation poses a potential risk to agricultural sustainability. Eur. J. Soil Sci. 2019, 70, 20–24. [Google Scholar] [CrossRef]
- Zong, R.; Tan, M.; Han, Y.; Zou, R.; Wang, Z. Changes in soil microbial community following the conversion of wasteland to cotton land in saline-sodic region of northwest China. Appl. Soil Ecol. 2022, 174, 104424. [Google Scholar] [CrossRef]
- Sarikhani, M.R.; Khoshru, B.; Oustan, S. Efficiency of some bacterial strains in potassium release from mica and phosphate solubilization under in vitro conditions. Geomicrobiol. J. 2016, 33, 832–838. [Google Scholar] [CrossRef]
- Costa, R.; Salles, J.F.; Berg, G.; Smalla, K. Cultivation-independent analysis of Pseudomonas species in soil and in the rhizosphere of field-grown Verticillium dahliae host plants. Environ. Microbiol. 2006, 8, 2136–2149. [Google Scholar] [CrossRef]
- Yamashita, K.; Honjo, H.; Nishida, M.; Kimura, M.; Asakawa, S. Estimation of microbial biomass potassium in paddy field soil. Soil Sci. Plant Nutr. 2014, 60, 512–519. [Google Scholar] [CrossRef]
- Wu, Y.; Cai, P.; Jing, X.; Niu, X.; Ji, D.; Ashry, N.M.; Gao, C.; Huang, Q. Soil biofilm formation enhances microbial community diversity and metabolic activity. Environ. Int. 2019, 132, 105116. [Google Scholar] [CrossRef]
- Rasamiravaka, T.; Labtani, Q.; Duez, P.; El Jaziri, M. The formation of biofilms by pseudomonas aeruginosa: A review of the natural and synthetic compounds interfering with control mechanisms. Biomed Res. Int. 2015, 2015, 759348. [Google Scholar] [CrossRef]
- Berg, H.C.; Turner, L. Chemotaxis of bacteria in glass capillary arrays. escherichia coli, motility, microchannel plate, and light scattering. Biophys. J. 1990, 58, 919–930. [Google Scholar] [CrossRef]
- Basak, B.B.; Sarkar, B.; Biswas, D.R.; Sarkar, S.; Sanderson, P.; Naidu, R. Bio-intervention of naturally occurring silicate minerals for alternative source of potassium. Adv. Agron. 2017, 141, 115–145. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Safari Sinegani, A.A.; Sarikhani, M.R.; Aliasgharzad, N. Assessment of soluble and biomass k in culture medium is a reliable tool for estimation of k releasing efficiency of bacteria. Geomicrobiol. J. 2019, 36, 873–880. [Google Scholar] [CrossRef]
- Keshavarz Zarjani, J.; Aliasgharzad, N.; Oustan, S.; Emadi, M.; Ahmadi, A. Isolation and characterization of potassium solubilizing bacteria in some iranian soils. Arch. Agron. Soil Sci. 2013, 59, 1713–1723. [Google Scholar] [CrossRef]
- Sheng, X.F.; He, L.Y. Solubilization of potassium-bearing minerals by a wild-type strain of Bacillus edaphicus and its mutants and increased potassium uptake by wheat. Can. J. Microbiol. 2006, 52, 66–72. [Google Scholar] [CrossRef]
- Liu, W.; Xu, X.; Wu, X.; Yang, Q.; Luo, Y.; Christie, P. Decomposition of silicate minerals by Bacillus mucilaginosus in liquid culture. Environ. Geochem. Health 2006, 28, 133–140. [Google Scholar] [CrossRef]
- Alekseev, K.V.; Dubina, M.V.; Komov, V.P. Molecular-genetic and biochemical characteristics of citrate synthase from the citric-acid producing fungus Aspergillus niger. Appl. Biochem. Microbiol. 2016, 52, 810–817. [Google Scholar] [CrossRef]
- Mäkelä, M.R.; Hildén, K.; Lundell, T.K. Oxalate decarboxylase: Biotechnological update and prevalence of the enzyme in filamentous fungi. Appl. Microbiol. Biotechnol. 2010, 87, 801–814. [Google Scholar] [CrossRef]
- Yu, L.; Huang, H.; Wang, X.; Li, S.; Feng, N.; Zhao, H.; Huang, X.; Li, Y.; Li, H.; Cai, Q. Novel phosphate-solubilising bacteria isolated from sewage sludge and the mechanism of phosphate solubilisation. Sci. Total Environ. 2019, 658, 474–484. [Google Scholar] [CrossRef]
- Nordgaard, M.; Blake, C.; Maróti, G.; Hu, G.; Wang, Y.; Strube, M.L.; Kovács, Á.T. Experimental evolution of Bacillus subtilis on Arabidopsis thaliana roots reveals fast adaptation and improved root colonization. iScience 2022, 25, 104406. [Google Scholar] [CrossRef]
- Wang, R.; Wang, C.; Feng, Q.; Liou, R.-M.; Lin, Y. Biological inoculant of salt-tolerant bacteria for plant growth stimulation under different saline soil conditions. J. Microbiol. Biotechnol. 2021, 31, 398–407. [Google Scholar] [CrossRef]
- Timofeeva, A.; Galyamova, M.; Sedykh, S. Prospects for using phosphate-solubilizing microorganisms as natural fertilizers in agriculture. Plants 2022, 11, 2119. [Google Scholar] [CrossRef]
- Scervino, J.M.; Papinutti, V.L.; Godoy, M.S.; Rodriguez, M.A.; Della Monica, I.; Recchi, M.; Pettinari, M.J.; Godeas, A.M. Medium pH, carbon and nitrogen concentrations modulate the phosphate solubilization efficiency of Penicillium purpurogenum through organic acid production. J. Appl. Microbiol. 2011, 110, 1215–1223. [Google Scholar] [CrossRef]
- Jung, J.; Shin, R.; Schachtman, D.P. Ethylene mediates response and tolerance to potassium deprivation in arabidopsis. Plant Cell 2009, 21, 607–621. [Google Scholar] [CrossRef]
- Zhu, R.; Jin, L.; Sang, Y.; Hu, S.; Wang, B.; Jin, F. Characterization of potassium-solubilizing fungi, Mortierella spp., isolated from a poplar plantation rhizosphere soil. Arch. Microbiol. 2024, 206, 157. [Google Scholar] [CrossRef]
- Zhang, C.; Kong, F. Isolation and identification of potassium-solubilizing bacteria from tobacco rhizospheric soil and their effect on tobacco plants. Appl. Soil Ecol. 2014, 82, 18–25. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, J.; Wen, Y.; Zhang, J.; Yin, F.; Guo, L.; Li, W.; He, J.; Ma, J.; Liang, Y. Optimizing cotton yield through appropriate irrigation water salinity: Coordinating above- and below-ground growth and enhancing photosynthetic capacity. Eur. J. Agron. 2024, 154, 127095. [Google Scholar] [CrossRef]
- Ortiz-Castro, R.; Pelagio-Flores, R.; Méndez-Bravo, A.; Ruiz-Herrera, L.F.; Campos-García, J.; López-Bucio, J. Pyocyanin, a virulence factor produced by Pseudomonas aeruginosa, alters root development through reactive oxygen species and ethylene signaling in Arabidopsis. Mol. Plant Microbe Interact. 2014, 27, 364–378. [Google Scholar] [CrossRef]
- Wu, D.; Ye, J.; Ou, H.; Wei, X.; Huang, X.; He, Y.; Xu, Y. Genomic analysis and temperature-dependent transcriptome profiles of the rhizosphere originating strain Pseudomonas aeruginosa M18. BMC Genom. 2011, 12, 438. [Google Scholar] [CrossRef]

| Total Potassium (g·kg−1) | Available Potassium (mg·kg−1) | Total Phosphorus (g·kg−1) | Total Nitrogen (mg·kg−1) | Soil Moisture (g·g−1) | pH |
|---|---|---|---|---|---|
| 15.61 ± 1.21 | 148.11 ± 7.13 | 1.19 ± 0.07 | 0.59 ± 0.13 | 0.13 ± 0.10 | 8.28 ± 0.11 |
| Strain | pH = 7 | pH = 8 | pH = 9 | pH = 10 |
|---|---|---|---|---|
| A1 | 8.25 ± 0.94 aCDE | 7.49 ± 0.50 abCD | 7.07 ± 0.42 bC | 3.11 ± 0.06 cB |
| A6 | 10.30 ± 0.74 aA | 9.49 ± 1.24 abAB | 8.71 ± 0.68 bB | 3.76 ± 0.07 cA |
| A9 | 6.50 ± 1.40 aFGH | 6.12 ± 0.55 aEF | 5.13 ± 0.11 aF | 2.35 ± 0.09 bEFG |
| A10 | 10.70 ± 0.91 aA | 10.41 ± 0.98 aA | 9.63 ± 0.18 aA | 4.24 ± 0.12 bA |
| A12 | 5.80 ± 0.56 aGH | 4.67 ± 0.40 bG | 4.02 ± 0.13 bGH | 2.75 ± 0.14 cBCDE |
| A15 | 9.45 ± 0.78 aABC | 9.19 ± 0.90 aB | 8.56 ± 0.35 aB | 4.09 ± 0.08 bA |
| A16 | 7.50 ± 0.17 aDEFG | 7.13 ± 0.31 aCDE | 5.40 ± 0.35 bF | 2.94 ± 0.10 cBCD |
| B4 | 6.85 ± 0.96 aEFGH | 6.82 ± 0.39 aDE | 6.08 ± 0.22 aE | 2.21 ± 0.11 bFGH |
| B5 | 7.50 ± 1.10 aDEFG | 6.37 ± 0.50 aEF | 4.21 ± 0.12 bGH | 2.61 ± 0.09 cCDEF |
| B7 | 5.85 ± 0.09 aGH | 4.94 ± 0.14 bG | 4.25 ± 0.07 cGH | 2.13 ± 0.06 dFGHI |
| B9 | 5.20 ± 0.18 aI | 5.03 ± 0.24 aG | 4.31 ± 0.17 bGH | 1.71 ± 0.10 cIJ |
| B11 | 7.85 ± 1.82 aDEF | 8.10 ± 0.45 aC | 7.24 ± 0.33 aC | 2.36 ± 0.18 bEFG |
| B12 | 4.25 ± 0.78 aI | 4.57 ± 0.29 aG | 4.41 ± 0.17 aG | 1.36 ± 0.13 bJ |
| C1 | 7.20 ± 1.04 aDEFG | 5.37 ± 0.38 bFG | 5.05 ± 0.18 bF | 3.23 ± 0.11 cB |
| C2 | 8.57 ± 0.52 aBCD | 7.72 ± 0.67 bCD | 6.54 ± 0.18 cD | 1.99 ± 0.11 dGHI |
| C4 | 6.70 ± 1.57 abEFGH | 6.87 ± 0.54 aDE | 5.22 ± 0.13 bF | 2.55 ± 0.07 cDEF |
| C6 | 3.70 ± 0.99 aI | 4.52 ± 0.16 aG | 3.89 ± 0.09 aH | 1.76 ± 0.05 bHIJ |
| C13 | 7.00 ± 0.66 aDEFG | 7.15 ± 0.62 aCDE | 7.02 ± 0.17 aC | 3.09 ± 0.52 bBC |
| C14 | 6.55 ± 0.09 aEFGH | 6.68 ± 0.47 aDE | 6.57 ± 0.38 aD | 2.30 ± 0.34 bEFG |
| C16 | 9.78 ± 0.78 aABC | 9.25 ± 0.33 abB | 8.47 ± 0.26 bF | 3.21 ± 0.56 cB |
| h4 | 10.05 ± 0.26 aABC | 10.09 ± 0.85 aAB | 9.74 ± 0.14 aA | 4.01 ± 0.79 bA |
| s1 | 7.15 ± 0.95 aDEFG | 6.33 ± 0.30 abEF | 5.40 ± 0.07 bF | 3.82 ± 0.32 cA |
| LJ | 6.45 ± 0.26 aFGH | 6.81 ± 0.57 aDE | 5.14 ± 0.10 bF | 2.77 ± 0.23 cBCDE |
| Organic Acid | Oxalic Acid (μg/mL) | Tartaric Acid (μg/mL) | Pyruvic Acid (μg/mL) | Lactic Acid (μg/mL) | Acetic Acid (μg/mL) | Citric Acid (μg/mL) | Succinic Acid (μg/mL) |
|---|---|---|---|---|---|---|---|
| pH = 7 | 2.49 ± 0.23 a | 2.28 ± 0.12 b | 9.71 ± 0.68 a | 147.09 ± 10.32 a | 55.12 ± 3.44 b | 49.65 ± 1.68 d | 35.12 ± 4.33 b |
| pH = 8 | 2.23 ± 0.19 a | 2.86 ± 0.20 a | 8.15 ± 0.83 b | 92.15 ± 5.56 b | 21.73 ± 1.80 c | 107.36 ± 5.57 a | 57.00 ± 5.49 a |
| pH = 9 | 2.56 ± 0.13 a | 2.23 ± 0.20 b | 6.91 ± 0.60 bc | 87.65 ± 7.27 b | 64.33 ± 5.29 b | 79.26 ± 1.65 b | 20.91 ± 2.28 c |
| pH = 10 | 2.50 ± 0.26 a | 0.75 ± 0.12 c | 6.62 ± 0.67 c | 35.05 ± 3.47 c | 109.22 ± 7.94 a | 67.29 ± 4.21 c | 15.57 ± 1.51 c |
| Treatment | Root | Aboveground | Root–Shoot Ratio | ||
|---|---|---|---|---|---|
| Total Length (cm) | Dry Weight (g) | Plant Height (cm) | Dry Weight (g) | ||
| Control | 377.04 ± 42.51 d | 0.60 ± 0.05 c | 10.56 ± 0.71 d | 1.15 ± 0.40 d | 0.52 |
| K | 437.26 ± 48.50 c | 0.64 ± 0.04 b | 11.73 ± 0.50 c | 1.25 ± 0.12 c | 0.51 |
| A10 | 473.80 ± 18.07 b | 0.67 ± 0.04 b | 12.95 ± 0.71 b | 1.35 ± 0.09 b | 0.50 |
| K + A10 | 533.05 ± 29.21 a | 0.74 ± 0.03 a | 14.22 ± 0.57 a | 1.68 ± 0.08 a | 0.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Liang, H.; Zhang, J.; Chen, Y.; Dhital, Y.P.; Zhao, T.; Wang, Z. Isolation and Characterization of Potassium-Solubilizing Rhizobacteria (KSR) Promoting Cotton Growth in Saline–Sodic Regions. Microorganisms 2024, 12, 1474. https://doi.org/10.3390/microorganisms12071474
Zhao Y, Liang H, Zhang J, Chen Y, Dhital YP, Zhao T, Wang Z. Isolation and Characterization of Potassium-Solubilizing Rhizobacteria (KSR) Promoting Cotton Growth in Saline–Sodic Regions. Microorganisms. 2024; 12(7):1474. https://doi.org/10.3390/microorganisms12071474
Chicago/Turabian StyleZhao, Yue, Hongbang Liang, Jihong Zhang, Yu Chen, Yam Prasad Dhital, Tao Zhao, and Zhenhua Wang. 2024. "Isolation and Characterization of Potassium-Solubilizing Rhizobacteria (KSR) Promoting Cotton Growth in Saline–Sodic Regions" Microorganisms 12, no. 7: 1474. https://doi.org/10.3390/microorganisms12071474
APA StyleZhao, Y., Liang, H., Zhang, J., Chen, Y., Dhital, Y. P., Zhao, T., & Wang, Z. (2024). Isolation and Characterization of Potassium-Solubilizing Rhizobacteria (KSR) Promoting Cotton Growth in Saline–Sodic Regions. Microorganisms, 12(7), 1474. https://doi.org/10.3390/microorganisms12071474





