Gut Microbiota Composition Is Causally Linked to Multiple Sclerosis: A Mendelian Randomization Analysis
Abstract
:1. Introduction
2. Materials and Methods
- (1)
- The most comprehensive study in this domain, by the MiBioGen consortium, in which 16S rRNA sequencing profiles were collected from 24 cohorts, for a total of 18,340 individuals [14];
- (2)
- The work by Qin and colleagues, in which metagenomic sequencing was performed in a single population-based cohort of 5959 subjects [15];
- (3)
- The work by Lopera-Maya and colleagues, which assessed SNP-to-taxon and SNP-to-microbial function associations from stool shotgun metagenomic studies of 7738 participants in the Dutch Microbiome Project [16].
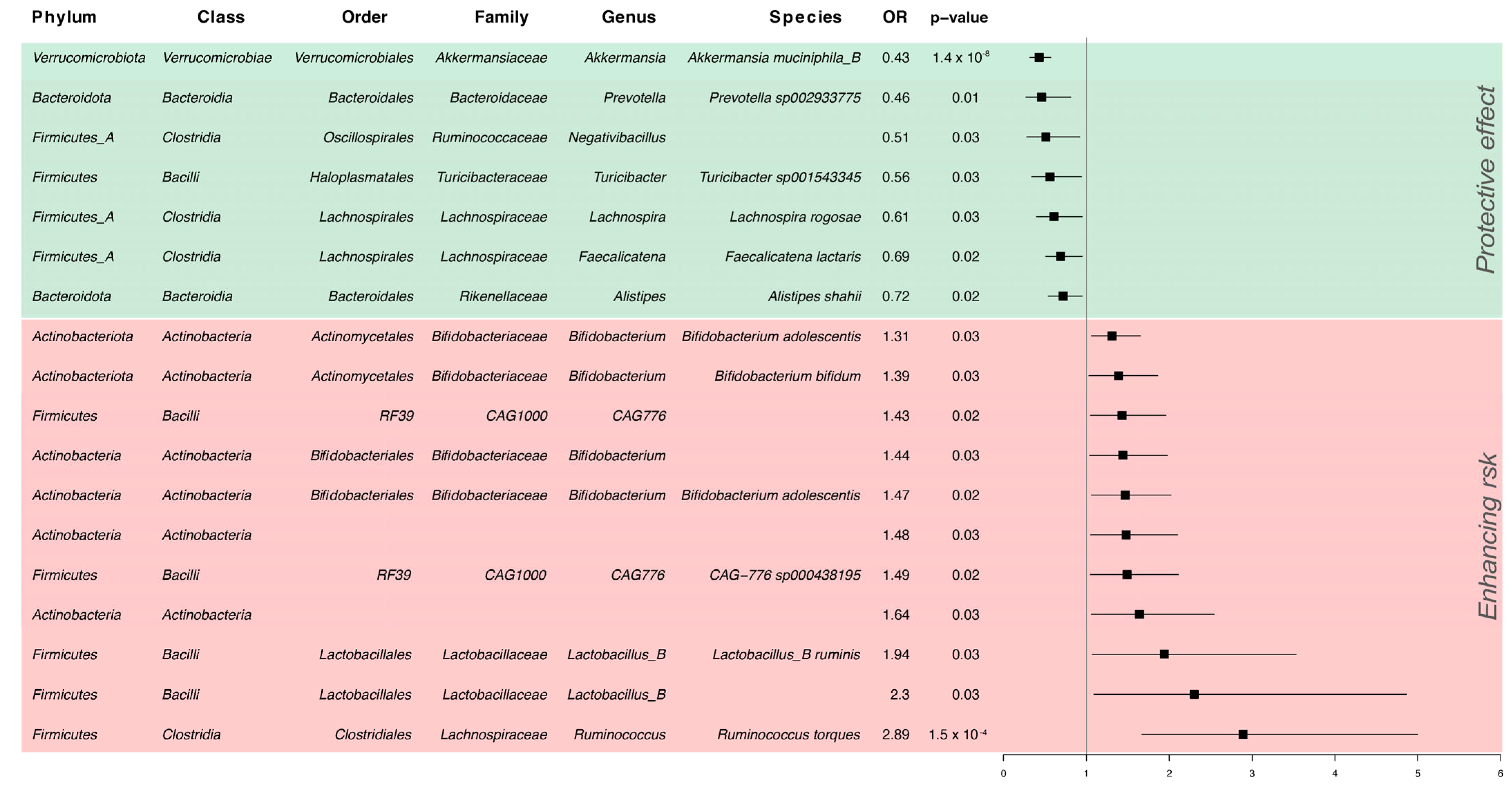
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- McGinley, M.P.; Goldschmidt, C.H.; Rae-Grant, A.D. Diagnosis and Treatment of Multiple Sclerosis: A Review. JAMA—J. Am. Med. Assoc. 2021, 325, 765–779. [Google Scholar] [CrossRef] [PubMed]
- Aloisi, F.; Giovannoni, G.; Salvetti, M. Review Epstein-Barr Virus as a Cause of Multiple Sclerosis: Opportunities for Prevention and Therapy. Lancet Neurol. 2023, 22, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liang, Z.; Wang, S.; Ma, D.; Zhu, M.; Feng, J. Role of Gut Microbiota in Multiple Sclerosis and Potential Therapeutic Implications. Curr. Neuropharmacol. 2022, 20, 1413–1426. [Google Scholar] [CrossRef] [PubMed]
- Correale, J.; Hohlfeld, R.; Baranzini, S.E. The Role of the Gut Microbiota in Multiple Sclerosis. Nat. Rev. Neurol. 2022, 18, 544–558. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Baumann, R.; Gao, X.; Mendoza, M.; Singh, S.; Katz Sand, I.; Xia, Z.; Cox, L.M.; Chitnis, T.; Yoon, H.; et al. Gut Microbiome of Multiple Sclerosis Patients and Paired Household Healthy Controls Reveal Associations with Disease Risk and Course. Cell 2022, 185, 3467–3486.e16. [Google Scholar] [CrossRef] [PubMed]
- Berer, K.; Gerdes, L.A.; Cekanaviciute, E.; Jia, X.; Xiao, L.; Xia, Z.; Liu, C.; Klotz, L.; Stauffer, U.; Baranzini, S.E.; et al. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc. Natl. Acad. Sci. USA 2017, 114, 10719–10724. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Farshbafnadi, M.; Agah, E.; Rezaei, N. The Second Brain: The Connection between Gut Microbiota Composition and Multiple Sclerosis. J. Neuroimmunol. 2021, 360, 577700. [Google Scholar] [CrossRef] [PubMed]
- Mirza, A.; Mao-Draayer, Y. The gut microbiome and microbial translocation in multiple sclerosis. Clin. Immunol. 2017, 183, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Pröbstel, A.K.; Baranzini, S.E. The Role of the Gut Microbiome in Multiple Sclerosis Risk and Progression: Towards Characterization of the “MS Microbiome”. Neurotherapeutics 2018, 15, 126–134. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cekanaviciute, E.; Yoo, B.B.; Runia, T.F.; Debelius, J.W.; Singh, S.; Nelson, C.A.; Kanner, R.; Bencosme, Y.; Lee, Y.K.; Hauser, S.L.; et al. Gut Bacteria from Multiple Sclerosis Patients Modulate Human T Cells and Exacerbate Symptoms in Mouse Models. Proc. Natl. Acad. Sci. USA 2017, 114, 10713–10718. [Google Scholar] [CrossRef]
- Allman, P.H.; Aban, I.B.; Tiwari, H.K.; Cutter, G.R. An Introduction to Mendelian Randomization with Applications in Neurology. Mult. Scler. Relat. Disord. 2018, 24, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Ni, J.J.; Han, B.X.; Yan, S.S.; Wei, X.T.; Feng, G.J.; Zhang, H.; Zhang, L.; Li, B.; Pei, Y.F. Causal Relationship Between Gut Microbiota and Autoimmune Diseases: A Two-Sample Mendelian Randomization Study. Front. Immunol. 2022, 12, 746998. [Google Scholar] [CrossRef] [PubMed]
- Xiang, K.; Wang, P.; Xu, Z.; Hu, Y.Q.; He, Y.S.; Chen, Y.; Feng, Y.T.; Yin, K.J.; Huang, J.X.; Wang, J.; et al. Causal Effects of Gut Microbiome on Systemic Lupus Erythematosus: A Two-Sample Mendelian Randomization Study. Front. Immunol. 2021, 12, 667097. [Google Scholar] [CrossRef] [PubMed]
- Kurilshikov, A.; Medina-Gomez, C.; Bacigalupe, R.; Radjabzadeh, D.; Wang, J.; Demirkan, A.; Le Roy, C.I.; Raygoza Garay, J.A.; Finnicum, C.T.; Liu, X.; et al. Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat. Genet. 2021, 53, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Havulinna, A.S.; Liu, Y.; Jousilahti, P.; Ritchie, S.C.; Tokolyi, A.; Sanders, J.G.; Valsta, L.; Brożyńska, M.; Zhu, Q.; et al. Combined effects of host genetics and diet on human gut microbiota and incident disease in a single population cohort. Nat. Genet. 2022, 54, 134–142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lopera-Maya, E.A.; Kurilshikov, A.; Van Der Graaf, A.; Hu, S.; Andreu-sánchez, S.; Chen, L.; Vila, A.V.; Gacesa, R.; Sinha, T.; Collij, V.; et al. Effect of Host Genetics on the Gut Microbiome in 7,738 Participants of the Dutch Microbiome Project. Nat. Genet. 2022, 54, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Nafee, T.; Watanabe, R.; Fregni, F. Multiple Sclerosis. In Clinical Trials in Neurology; Fregni, F., Ed.; Neuromethods; Humana Press: New York, NY, USA, 2018; Volume 138. [Google Scholar] [CrossRef]
- Pierce, B.L.; Ahsan, H.; Vanderweele, T.J. Power and Instrument Strength Requirements for Mendelian Randomization Studies Using Multiple Genetic Variants. Int. J. Epidemiol. 2011, 40, 740–752. [Google Scholar] [CrossRef] [PubMed]
- Hemani, G.; Tilling, K.; Smith, G.D. Orienting the Causal Relationship between Imprecisely Measured Traits Using GWAS Summary Data. PLoS Genet. 2017, 13, e1007081. [Google Scholar]
- Hemani, G.; Zheng, J.; Elsworth, B.; Wade, K.H.; Haberland, V.; Baird, D.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R.; et al. The MR-Base Platform Supports Systematic Causal Inference across the Human Phenome. eLife 2018, 7, e34408. [Google Scholar] [CrossRef] [PubMed]
- Johanson, D.M.; Goertz, J.E.; Marin, I.A.; Costello, J.; Overall, C.C.; Gaultier, A. Experimental Autoimmune Encephalomyelitis Is Associated with Changes of the Microbiota Composition in the Gastrointestinal Tract. Sci. Rep. 2020, 10, 15183. [Google Scholar] [CrossRef]
- Vacaras, V.; Muresanu, D.F.; Buzoianu, A.D.; Nistor, C.; Vesa, S.C.; Paraschiv, A.C.; Botos-Vacaras, D.; Vacaras, C.; Vithoulkas, G. The Role of Multiple Sclerosis Therapies on the Dynamic of Human Gut Microbiota. J. Neuroimmunol. 2023, 378, 578087. [Google Scholar] [CrossRef] [PubMed]
- Rajilić-Stojanović, M.; de Vos, W.M. The First 1000 Cultured Species of the Human Gastrointestinal Microbiota. FEMS Microbiol. Rev. 2014, 38, 996–1047. [Google Scholar] [CrossRef] [PubMed]
- Tremlett, H.; Fadrosh, D.W.; Faruqi, A.A.; Zhu, F.; Hart, J.; Roalstad, S.; Graves, J.; Lynch, S.; Waubant, E.; Aaen, G.; et al. Gut Microbiota in Early Pediatric Multiple Sclerosis: A Case−control Study. Eur. J. Neurol. 2016, 23, 1308–1321. [Google Scholar] [CrossRef] [PubMed]
- Elgendy, S.G.; Abd-Elhameed, R.; Daef, E.; Mohammed, S.M.; Hassan, H.M.; El-Mokhtar, M.A.; Nasreldein, A.; Khedr, E.M. Gut Microbiota in Forty Cases of Egyptian Relapsing Remitting Multiple Sclerosis. Iran. J. Microbiol. 2021, 13, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.G.; Sefik, E.; Geva-Zatorsky, N.; Kua, L.; Naskar, D.; Teng, F.; Pasman, L.; Ortiz-Lopez, A.; Jupp, R.; Wu, H.J.J.; et al. Identifying Species of Symbiont Bacteria from the Human Gut That, Alone, Can Induce Intestinal Th17 Cells in Mice. Proc. Natl. Acad. Sci. USA 2016, 113, E8141–E8150. [Google Scholar] [CrossRef]
- López, P.; Gueimonde, M.; Margolles, A.; Suárez, A. Distinct Bifidobacterium Strains Drive Different Immune Responses in Vitro. Int. J. Food Microbiol. 2010, 138, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Toghi, M.; Bitarafan, S.; Kasmaei, H.D.; Ghafouri-Fard, S. Bifidobacteria: A Probable Missing Puzzle Piece in the Pathogenesis of Multiple Sclerosis. Mult. Scler. Relat. Disord. 2019, 36, 101378. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.M.; Maghzi, A.H.; Liu, S.; Tankou, S.K.; Dhang, F.H.; Willocq, V.; Song, A.; Wasén, C.; Tauhid, S.; Chu, R.; et al. Gut Microbiome in Progressive Multiple Sclerosis. Ann. Neurol. 2021, 89, 1195–1211. [Google Scholar] [CrossRef] [PubMed]
- Jangi, S.; Gandhi, R.; Cox, L.M.; Li, N.; Von Glehn, F.; Yan, R.; Patel, B.; Mazzola, M.A.; Liu, S.; Glanz, B.L.; et al. Alterations of the Human Gut Microbiome in Multiple Sclerosis. Nat. Commun. 2016, 7, 12015. [Google Scholar] [CrossRef]
- Cani, P.D.; Depommier, C.; Derrien, M.; Everard, A.; de Vos, W.M. Akkermansia Muciniphila: Paradigm for next-Generation Beneficial Microorganisms. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 625–637. [Google Scholar] [CrossRef]
- Liu, S.; Rezende, R.M.; Moreira, T.G.; Tankou, S.K.; Cox, L.M.; Wu, M.; Song, A.; Dhang, F.H.; Wei, Z.; Costamagna, G.; et al. Oral Administration of miR-30d from Feces of MS Patients Suppresses MS-like Symptoms in Mice by Expanding Akkermansia Muciniphila. Cell Host Microbe 2019, 26, 779–794.e8. [Google Scholar] [CrossRef]
- Martin, R.; Sospedra, M.; Eiermann, T.; Olsson, T. Multiple Sclerosis: Doubling down on MHC. Trends Genet. 2021, 37, 784–797. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jelcic, I.; Mühlenbruch, L.; Haunerdinger, V.; Toussaint, N.C.; Zhao, Y.; Cruciani, C.; Faigle, W.; Naghavian, R.; Foege, M.; et al. HLA-DR15 Molecules Jointly Shape an Autoreactive T Cell Repertoire in Multiple Sclerosis. Cell 2020, 183, 1264–1281.e20. [Google Scholar] [CrossRef]
- Vallino, A.; Dos Santos, A.; Mathé, C.V.; Garcia, A.; Morille, J.; Dugast, E.; Shah, S.P.; Héry-Arnaud, G.; Guilloux, C.A.; Gleeson, P.J.; et al. Gut Bacteria Akkermansia Elicit a Specific IgG Response in CSF of Patients with MS. Neurol. Neuroimmunol. NeuroInflammation 2020, 7, e688. [Google Scholar] [CrossRef]
- Chen, J.; Chia, N.; Kalari, K.R.; Yao, J.Z.; Novotna, M.; Soldan, M.M.P.; Luckey, D.H.; Marietta, E.V.; Jeraldo, P.R.; Chen, X.; et al. Multiple Sclerosis Patients Have a Distinct Gut Microbiota Compared to Healthy Controls. Sci. Rep. 2016, 6, 28484. [Google Scholar] [CrossRef]
- Mirza, A.; Forbes, J.D.; Zhu, F.; Bernstein, C.N.; Van Domselaar, G.; Graham, M.; Waubant, E.; Tremlett, H. The Multiple Sclerosis Gut Microbiota: A Systematic Review. Mult. Scler. Relat. Disord. 2020, 37, 101427. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez-Rodriguez, A.; Roman, P.; Rueda-Ruzafa, L.; Campos-Rios, A.; Cardona, D. Changes in Gut Microbiota and Multiple Sclerosis: A Systematic Review. Int. J. Environ. Res. Public Health 2023, 20, 4624. [Google Scholar] [CrossRef]
- Shahi, S.K.; Jensen, S.N.; Murra, A.C.; Tang, N.; Guo, H.; Gibson-corley, K.N.; Zhang, J.; Karandikar, N.J.; Murray, J.A.; Mangalam, A.K.; et al. Human Commensal Prevotella Histicola Ameliorates Disease as Effectively as Interferon-Beta in the Experimental Autoimmune Encephalomyelitis. Front. Immunol. 2020, 11, 578648. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.M. The Immune Response to Prevotella Bacteria in Chronic Inflammatory Disease. Immunology 2017, 151, 363–374. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zancan, V.; Nasello, M.; Bigi, R.; Reniè, R.; Buscarinu, M.C.; Mechelli, R.; Ristori, G.; Salvetti, M.; Bellucci, G. Gut Microbiota Composition Is Causally Linked to Multiple Sclerosis: A Mendelian Randomization Analysis. Microorganisms 2024, 12, 1476. https://doi.org/10.3390/microorganisms12071476
Zancan V, Nasello M, Bigi R, Reniè R, Buscarinu MC, Mechelli R, Ristori G, Salvetti M, Bellucci G. Gut Microbiota Composition Is Causally Linked to Multiple Sclerosis: A Mendelian Randomization Analysis. Microorganisms. 2024; 12(7):1476. https://doi.org/10.3390/microorganisms12071476
Chicago/Turabian StyleZancan, Valeria, Martina Nasello, Rachele Bigi, Roberta Reniè, Maria Chiara Buscarinu, Rosella Mechelli, Giovanni Ristori, Marco Salvetti, and Gianmarco Bellucci. 2024. "Gut Microbiota Composition Is Causally Linked to Multiple Sclerosis: A Mendelian Randomization Analysis" Microorganisms 12, no. 7: 1476. https://doi.org/10.3390/microorganisms12071476





