Characterising Eastern Grey Kangaroos (Macropus giganteus) as Hosts of Coxiella burnetii
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Site and Study Population
2.2. Sample Collection
2.3. Serology
2.4. Nucleic Acid Extraction
2.5. PCR Detection
2.6. Histopathology and Immunohistochemistry
2.7. Statistical Analysis
3. Results
3.1. Kangaroos Sampled
3.2. Serology
3.3. PCR Detection of C. burnetii
3.4. Histopathology and Immunohistochemistry
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haydon, D.T.; Cleaveland, S.; Taylor, L.H.; Laurenson, M.K. Identifying reservoirs of infection: A conceptual and practical challenge. Emerg. Infect. Dis. 2002, 8, 1468–1473. [Google Scholar] [CrossRef] [PubMed]
- Viana, M.; Mancy, R.; Biek, R.; Cleaveland, S.; Cross, P.C.; Lloyd-Smith, J.O.; Haydon, D.T. Assembling evidence for identifying reservoirs of infection. Trends Ecol. Evol. 2014, 29, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Ashford, R.W. What it takes to be a reservoir host. Belg. J. Zool. 1997, 127, 85–90. [Google Scholar]
- Tolpinrud, A.; Chaber, A.-L.; Devlin, J.M.; Stenos, J.; Stevenson, M.A. Animal Reservoirs of Q Fever—A Scoping Review; University of Melbourne: Melbourne, Australia, 2024; Manuscript in preparation. [Google Scholar]
- Maurin, M.; Raoult, D. Q fever. Clin. Microbiol. Rev. 1999, 12, 518–553. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.; Souriau, A.; Buendia, A.J.; Arricau-Bouvery, N.; Martinez, C.M.; Salinas, J.; Rodolakis, A.; Navarro, J.A. Experimental Coxiella burnetii infection in pregnant goats: A histopathological and immunohistochemical study. J. Comp. Pathol. 2006, 135, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Joulie, A.; Laroucau, K.; Bailly, X.; Prigent, M.; Gasqui, P.; Lepetitcolin, E.; Blanchard, B.; Rousset, E.; Sidi-Boumedine, K.; Jourdain, E. Circulation of Coxiella burnetii in a naturally infected flock of dairy sheep: Shedding dynamics, environmental contamination, and genotype diversity. Appl. Environ. Microbiol. 2015, 81, 7253–7260. [Google Scholar] [CrossRef] [PubMed]
- Astobiza, I.; Barandika, J.F.; Hurtado, A.; Juste, R.A.; Garcia-Perez, A.L. Kinetics of Coxiella burnetii excretion in a commercial dairy sheep flock after treatment with oxytetracycline. Vet. J. 2010, 184, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Guatteo, R.; Beaudeau, F.; Berri, M.; Rodolakis, A.; Joly, A.; Seegers, H. Shedding routes of Coxiella burnetii in dairy cows: Implications for detection and control. Vet. Res. 2006, 37, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Eldin, C.; Mélenotte, C.; Mediannikov, O.; Ghigo, E.; Million, M.; Edouard, S.; Mege, J.-L.; Maurin, M.; Raoult, D. From Q fever to Coxiella burnetii infection: A paradigm change. Clin. Microbiol. Rev. 2017, 30, 115–190. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Barrio, D.; Ruiz-Fons, F. Coxiella burnetii in wild mammals: A systematic review. Transbound. Emerg. Dis. 2019, 66, 662–671. [Google Scholar] [CrossRef]
- Pope, J.H.; Scott, W.; Dwyer, R. Coxiella burneti in kangaroos and kangaroo ticks in Western Queensland. Aust. J. Exp. Biol. Med. Sci. 1960, 38, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Banazis, M.J.; Bestall, A.S.; Reid, S.A.; Fenwick, S.G. A survey of Western Australian sheep, cattle and kangaroos to determine the prevalence of Coxiella burnetii. Vet. Microbiol. 2010, 143, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Potter, A.S.; Banazis, M.J.; Yang, R.; Reid, S.A.; Fenwick, S.G. Prevalence of Coxiella burnetii in western grey kangaroos (Macropus fuliginosus) in Western Australia. J. Wildl. Dis. 2011, 47, 821–828. [Google Scholar] [CrossRef]
- Cooper, A.; Barnes, T.; Potter, A.; Ketheesan, N.; Govan, B. Determination of Coxiella burnetii seroprevalence in macropods in Australia. Vet. Microbiol. 2012, 155, 317–323. [Google Scholar] [CrossRef]
- Cooper, A.; Goullet, M.; Mitchell, J.; Ketheesan, N.; Govan, B. Serological evidence of Coxiella burnetii exposure in native marsupials and introduced animals in Queensland, Australia. Epidemiol. Infect. 2012, 140, 1304–1308. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.; Stephens, J.; Ketheesan, N.; Govan, B. Detection of Coxiella burnetii DNA in wildlife and ticks in Northern Queensland, Australia. Vector Borne Zoonotic Dis. 2013, 13, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Graves, S.R.; Islam, A. Endemic Q fever in New South Wales, Australia: A case series (2005–2013). Am. J. Trop. Med. Hyg. 2016, 95, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Sivabalan, P.; Saboo, A.; Yew, J.; Norton, R. Q fever in an endemic region of north Queensland, Australia: A 10 year review. One Health 2017, 3, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Queensland Health. Q Fever Notifications in Queensland, 2016; Communicable Diseases Branch, Department of Health: Brisbane, QLD, Australia, 2019. [Google Scholar]
- Tozer, S.; Wood, C.; Si, D.; Nissen, M.; Sloots, T.; Lambert, S. The improving state of Q fever surveillance. A review of Queensland notifications, 2003–2017. Commun. Dis. Intell. 2020, 44, 20210048086. [Google Scholar] [CrossRef] [PubMed]
- Clark, N.J.; Tozer, S.; Wood, C.; Firestone, S.M.; Stevenson, M.; Caraguel, C.; Chaber, A.-L.; Heller, J.; Magalhaes, R.J.S. Unravelling animal exposure profiles of human Q fever cases in Queensland, Australia, using natural language processing. Transbound. Emerg. Dis. 2020, 67, 2133–2145. [Google Scholar] [CrossRef]
- Shapiro, A.; Bosward, K.; Mathews, K.; Vincent, G.; Stenos, J.; Tadepalli, M.; Norris, J. Molecular detection of Coxiella burnetii in raw meat intended for pet consumption. Zoonoses Public Health 2020, 67, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Bureau of Meteorology. Wind: Wind Roses for Selected Locations in Australia. 2019. Available online: http://www.bom.gov.au/climate/averages/wind/selection_map.shtml (accessed on 21 November 2023).
- Brandimarti, M.E.; Gray, R.; Silva, F.R.O.; Herbert, C.A. Kangaroos at maximum capacity: Health assessment of free-ranging eastern grey kangaroos on a coastal headland. J. Mammal. 2021, 102, 837–851. [Google Scholar] [CrossRef] [PubMed]
- Herbert, C.A.; Snape, M.A.; Wimpenny, C.E.; Coulson, G. Kangaroos in peri-urban areas: A fool’s paradise? Ecol. Manag. Restor. 2021, 22, 167–175. [Google Scholar] [CrossRef]
- Henderson, T.; Rajaratnam, R.; Vernes, K. Population density of eastern grey kangaroos (Macropus giganteus) in a periurban matrix at Coffs Harbour, New South Wales. Austral Mammal. 2017, 40, 312–314. [Google Scholar] [CrossRef]
- Tolpinrud, A.; Stenos, J.; Chaber, A.L.; Devlin, J.M.; Herbert, C.; Pas, A.; Dunowska, M.; Stevenson, M.A.; Firestone, S.M. Validation of an indirect immunofluorescence assay and commercial Q fever enzyme-linked immunosorbent assay for use in macropods. J. Clin. Microbiol. 2022, 60, e0023622. [Google Scholar] [CrossRef]
- Levy, P.S.; Lemeshow, S. Sampling of Populations: Methods and Applications, 3rd ed.; Wiley: London, UK, 1999. [Google Scholar]
- Tolpinrud, A.; Tadepalli, M.; Stenos, J.; Lignereux, L.; Chaber, A.L.; Devlin, J.M.; Caraguel, C.; Stevenson, M.A. Tissue distribution of Coxiella burnetii and antibody responses in macropods co-grazing with livestock in Queensland, Australia. PLoS ONE 2024, 19, e0303877. [Google Scholar] [CrossRef]
- Schneeberger, P.M.; Hermans, M.H.A.; van Hannen, E.J.; Schellekens, J.J.A.; Leenders, A.C.A.P.; Wever, P.C. Real-time PCR with serum samples is indispensable for early diagnosis of acute Q fever. Clin. Vaccine Immunol. 2010, 17, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, M.G.; Graves, S.R.; Banazis, M.J.; Fenwick, S.G.; Stenos, J. A comparison of methods for extracting DNA from Coxiella burnetii as measured by a duplex qPCR assay. Lett. Appl. Microbiol. 2011, 52, 514–520. [Google Scholar] [CrossRef]
- Bond, K.A.; Vincent, G.; Wilks, C.R.; Franklin, L.; Sutton, B.; Stenos, J.; Cowan, R.; Lim, K.; Athan, E.; Harris, O.; et al. One Health approach to controlling a Q fever outbreak on an Australian goat farm. Epidemiol. Infect. 2016, 144, 1129–1141. [Google Scholar] [CrossRef]
- Rodriguez-Lazaro, D.; Hernandez, M.; Scortti, M.; Esteve, T.; Vazquez-Boland, J.A.; Pla, M. Quantitative detection of Listeria monocytogenes and Listeria innocua by real-time PCR: Assessment of hly, iap, and lin02483 targets and AmpliFluor technology. Appl. Environ. Microbiol. 2004, 70, 1366–1377. [Google Scholar] [CrossRef] [PubMed]
- Muleme, M.; Devlin, J.M.; Campbell, A.; Vincent, G.; Benham, P.J.; Sprohnle, C.; Stent, A.; Cameron, A.; Islam, A.; Graves, S.; et al. A randomised controlled trial of the immunogenicity and safety of a formaldehyde-inactivated Coxiella burnetii vaccine in 8-week-old goats. Vet. Immunol. Immunopathol. 2021, 236, 110253. [Google Scholar] [CrossRef] [PubMed]
- Rogan, W.J.; Gladen, B. Estimating prevalence from the results of a screening test. Am. J. Epidemiol. 1978, 107, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, M.; Sergeant, E.; Heuer, C.; Nunes, T.; Marshall, J.; Sanchez, J.; Thornton, R.; Reiczigel, J.; Robison-Cox, J.; Sebastiani, P.; et al. epiR: Tools for the Analysis of Epidemiological Data, R package version 2.0.73; 2024. Available online: https://CRAN.R-project.org/package=epiR (accessed on 20 March 2024).
- R Core Team. R: The R Project for Statistical Computing. Available online: http://www.R-project.org (accessed on 1 January 2024).
- González-Barrio, D.; Maio, E.; Vieira-Pinto, M.; Ruiz-Fons, F. European rabbits as reservoir for Coxiella burnetii. Emerg. Infect. Dis. 2015, 21, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- Selmi, R.; Mamlouk, A.; Ben Yahia, H.; Abdelaali, H.; Ben Said, M.; Sellami, K.; Daaloul-Jedidi, M.; Jemli, M.H.; Messadi, L. Coxiella burnetii in Tunisian dromedary camels (Camelus dromedarius): Seroprevalence, associated risk factors and seasonal dynamics. Acta Trop. 2018, 188, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Lyoo, K.; Kim, D.; Jang, H.; Lee, S.; Park, M.; Hahn, T. Prevalence of antibodies against Coxiella burnetii in Korean native cattle, dairy cattle, and dogs in South Korea. Vector Borne Zoonotic Dis. 2017, 17, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Meredith, A.L.; Cleaveland, S.C.; Denwood, M.J.; Brown, J.K.; Shaw, D.J. Coxiella burnetii (Q-fever) seroprevalence in prey and predators in the United Kingdom: Evaluation of infection in wild rodents, foxes and domestic cats using a modified ELISA. Transbound. Emerg. Dis. 2015, 62, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Gardner, B.R.; Stenos, J.; Hufschmid, J.; Arnould, J.P.Y.; McIntosh, R.R.; Tadepalli, M.; Tolpinrud, A.; Marenda, M.; Lynch, M.; Stent, A. An old pathogen in a new environment—Implications of Coxiella burnetii in Australian fur seals (Arctocephalus pusillus doriferus). Front. Mar. Sci. 2022, 9, 809075. [Google Scholar] [CrossRef]
- Gillespie, J.H.; Baker, J.A. Experimental Q fever in cats. Am. J. Vet. Res. 1952, 13, 91–94. [Google Scholar] [PubMed]
- Ac, P.; Rehacek, J.; Brezina, R. Elimination of Coxiella burneti in faeces and the transmission of this agent to small rodents by the peroral route. Bull. World Health Organ. 1968, 39, 973–974. [Google Scholar]
- Řeháček, J.; Urvolgyi, J.; Brezina, R.; Kazar, J.; Kovacova, E. Experimental infection of hare (Lepus europaeus) with Coxiella burnetti and Rickettsia slovaca. Acta Virol. 1978, 22, 417–425. [Google Scholar]
- Nagaoka, H.; Sugieda, M.; Akiyama, M.; Nishina, T.; Akahane, S.; Fujiwara, K. Isolation of Coxiella burnetii from the vagina of feline clients at veterinary clinics. J. Vet. Med. Sci. 1998, 60, 251–252. [Google Scholar] [CrossRef] [PubMed]
- To, H.; Sakai, R.; Shirota, K.; Kano, C.; Abe, S.; Sugimoto, T.; Takehara, K.; Morita, C.; Takashima, I.; Maruyama, T.; et al. Coxiellosis in domestic and wild birds from Japan. J. Wildl. Dis. 1998, 34, 310–316. [Google Scholar] [CrossRef] [PubMed]
- González-Barrio, D.; Martín-Hernando, M.P.; Ruiz-Fons, F. Shedding patterns of endemic Eurasian wild boar (Sus scrofa) pathogens. Res. Vet. Sci. 2015, 102, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Gardner, B.R.; Hufschmid, J.; Stenos, J.; Tadepalli, M.; Sutton, G.; Fromant, A.; Eizenberg, Y.; Geeson, J.J.; Arnould, J.P.Y. Pacific Gulls (Larus pacificus) as Potential Vectors of Coxiella burnetii in an Australian Fur Seal Breeding Colony. Pathogens 2023, 12, 122. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Barrio, D.; Hagen, F.; Tilburg, J.J.; Ruiz-Fons, F. Coxiella burnetii Genotypes in Iberian Wildlife. Microb. Ecol. 2016, 72, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.O. The Role of Australian Native Wildlife in Q Fever; University of Sydney: Sydney, Australia, 2022. [Google Scholar]
- Jones, R.M.; Hertwig, S.; Pitman, J.; Vipond, R.; Aspan, A.; Bolske, G.; McCaughey, C.; McKenna, J.P.; van Rotterdam, B.J.; de Bruin, A.; et al. Interlaboratory comparison of real-time polymerase chain reaction methods to detect Coxiella burnetii, the causative agent of Q fever. J. Vet. Diagn. Investig. 2011, 23, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Marchette, N.J.; Sidwell, R.W.; Nicholes, P.S.; Bushman, J.B. Studies on infectious diseases in wild animals in Utah. III. Experimental Q fever in wild vertebrates. Zoonoses Res 1962, 1, 321–339. [Google Scholar] [PubMed]
- Shannon, J.G.; Heinzen, R.A. Adaptive immunity to the obligate intracellular pathogen Coxiella burnetii. Immunol. Res. 2009, 43, 138–148. [Google Scholar] [CrossRef]
- Derrick, E.H.; Smith, D.J.W.; Brown, H.E. Studies in the epidemiology of Q fever. 6. The susceptibility of various animals to Q fever. Aust. J. Exp. Biol. Med. Sci. 1940, 18, 409–413. [Google Scholar] [CrossRef]
- Smith, D.J.W. Studies in the Epidemiology of Q Fever., 10. The Transmission of Q Fever by the Tick Ixodes holocyclus (with Notes on Tick-paralysis in Bandicoots.). Aust. J. Exp. Biol. Med. Sci. 1942, 20, 213–217. [Google Scholar] [CrossRef]
- Sobëslavsky, O.; Syrucek, L. Transovular Transmission of C. burneti in the Domestic Fowl (Gallus gallus domesticus). J. Hyg. Epidemiol. Microbiol. Immunol. 1959, 3, 458–464. [Google Scholar] [PubMed]
- Babudieri, B.; Moscovici, C. Experimental and natural infection of birds by Coxiella burneti. Nature 1952, 169, 195–196. [Google Scholar] [CrossRef] [PubMed]
- Marmion, B.P.; Storm, P.A.; Ayres, J.G.; Semendric, L.; Mathews, L.; Winslow, W.; Turra, M.; Harris, R.J. Long-term persistence of Coxiella burnetii after acute primary Q fever. QJM 2005, 98, 7–20. [Google Scholar] [CrossRef]
- Mathews, F. Zoonoses in wildlife integrating ecology into management. Adv. Parasitol. 2009, 68, 185–209. [Google Scholar] [CrossRef] [PubMed]
- Suzán, G.; Esponda, F.; Carrasco-Hernández, R.; Aguirre, A.A. Habitat fragmentation and infectious disease ecology. In New Directions in Conservation Medicine: Applied Cases of Ecological Health; Aguirre, A.A., Ostfeld, R.S., Daszak, P., Eds.; Oxford University Press: New York, NY, USA, 2012; pp. 135–150. [Google Scholar]
- Plowright, R.K.; Ahmed, A.N.; Coulson, T.; Crowther, T.W.; Ejotre, I.; Faust, C.L.; Frick, W.F.; Hudson, P.J.; Kingston, T.; Nameer, P.O.; et al. Ecological countermeasures to prevent pathogen spillover and subsequent pandemics. Nat. Commun. 2024, 15, 2577. [Google Scholar] [CrossRef]
- Hawker, J.I.; Ayres, J.G.; Blair, I.; Evans, M.R.; Smith, D.L.; Smith, E.G.; Burge, P.S.; Carpenter, M.J.; Caul, E.O.; Coupland, B.; et al. A large outbreak of Q fever in the West Midlands: Windborne spread into a metropolitan area? Commun. Dis. Public Health 1998, 1, 180–187. [Google Scholar]
- Tissot-Dupont, H.; Amadei, M.A.; Nezri, M.; Raoult, D. Wind in November, Q fever in December. Emerg. Infect. Dis. 2004, 10, 1264–1269. [Google Scholar] [CrossRef]
- Tissot-Dupont, H.; Torres, S.; Nezri, M.; Raoult, D. Hyperendemic focus of Q fever related to sheep and wind. Am. J. Epidemiol. 1999, 150, 67–74. [Google Scholar] [CrossRef]
- O’Connor, B.A.; Tribe, I.G.; Givney, R. A windy day in a sheep saleyard: An outbreak of Q fever in rural South Australia. Epidemiol. Infect. 2015, 143, 391–398. [Google Scholar] [CrossRef]
- Nusinovici, S.; Frossling, J.; Widgren, S.; Beaudeau, F.; Lindberg, A. Q fever infection in dairy cattle herds: Increased risk with high wind speed and low precipitation. Epidemiol. Infect. 2015, 143, 3316–3326. [Google Scholar] [CrossRef] [PubMed]
| Strata | Total Tested | PCR | IFA | ||
|---|---|---|---|---|---|
| pos | % pos (95% CI) | pos | % pos (95% CI) | ||
| Sex: | |||||
| Male | 19 | 15 | 79 (54, 94) | 14 | 74 (49, 91) |
| Female | 21 | 11 | 52 (30, 74) | 19 | 90 (70, 99) |
| Age group: | |||||
| Adult | 22 | 12 | 55 (32, 76) | 17 | 77 (55, 92) |
| Subadult | 18 | 14 | 78 (52, 94) | 16 | 89 (65, 99) |
| Total | 40 | 26 | 65 (48, 79) | 33 | 82 (67, 93) |
| 2017/2018 | 2021 | ||||
|---|---|---|---|---|---|
| Kangaroo ID | Phase I | Phase II | Phase I | Phase II | Trend |
| 306 | Negative | Negative | Negative | Negative | No change |
| 307 | Negative | Negative | Negative | Negative | No change |
| 309 | 1:2048 | Negative | 1:4096 | 1:512 | Increasing |
| 323 | 1:32 | Negative | 1:1024 | 1:256 | Increasing |
| 341 | 1:16,384 | 1:8192 | 1:16,384 | 1:8192 | No change |
| IFA + | IFA − | Total | |
|---|---|---|---|
| PCR + | 20 | 6 | 26 |
| PCR − | 13 | 1 | 14 |
| Total | 33 | 7 | 40 |
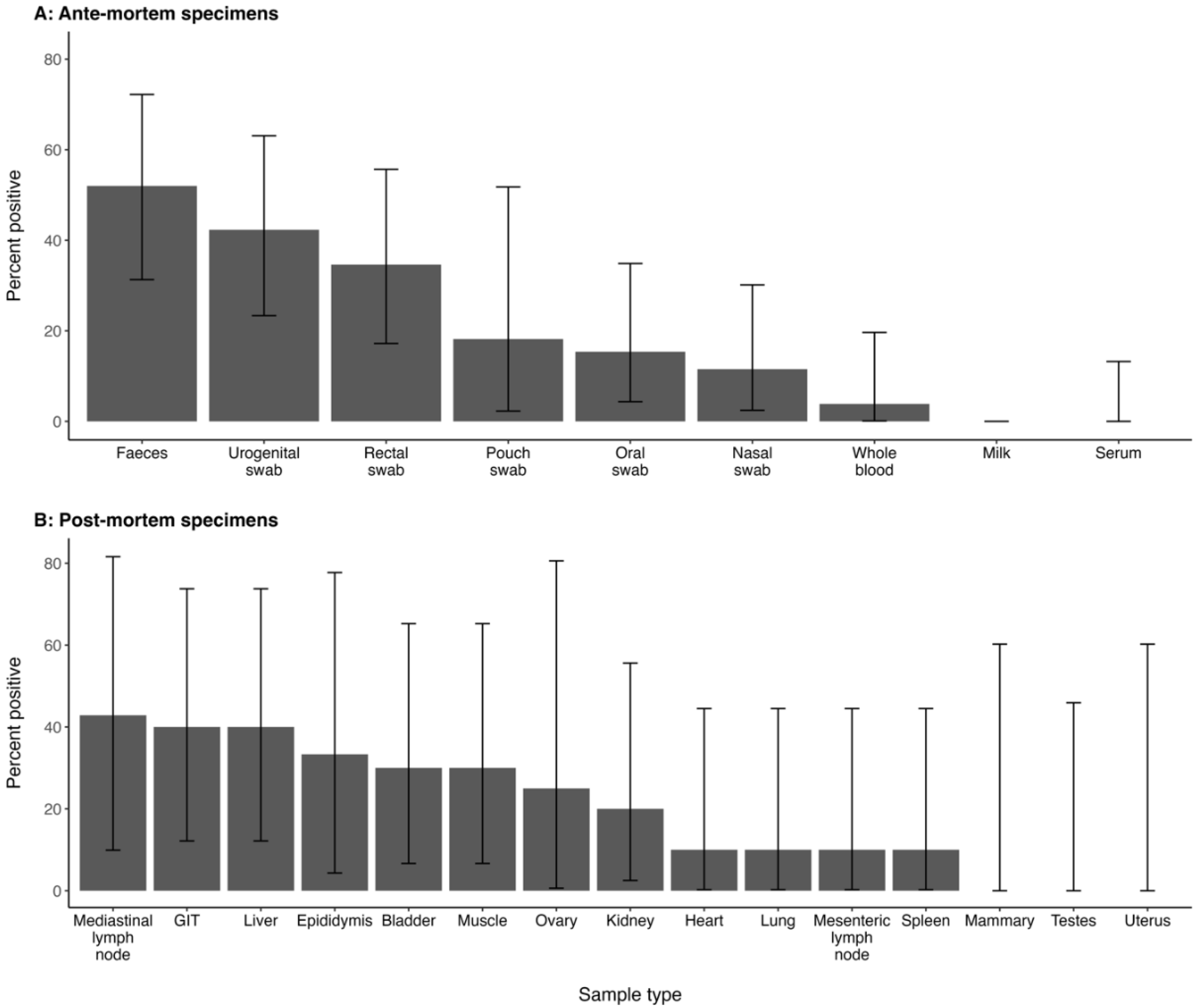
| Sample Type | Total Tested | pos | % pos (95% CI) |
|---|---|---|---|
| Whole blood | 40 | 1 | 2.5 (0.1, 13) |
| Serum | 40 | 0 | 0 (0, 8.8) |
| Nasal swab | 40 | 3 | 7.5 (1.6, 20) |
| Oral swab | 40 | 4 | 10 (2.8, 24) |
| Urogenital swab | 40 | 11 | 28 (15, 44) |
| Rectal swab | 40 | 9 | 23 (11, 39) |
| Faeces | 35 | 13 | 37 (22, 55) |
| Pouch swab | 21 | 2 | 9.5 (1.2, 30) |
| Milk | 3 | 0 | 0 (0, 71) |
| Total kangaroos | 40 | 21 * | 52 (36, 68) |
| Tissue—Sample Type | Total Tested | pos | % pos (95% CI) |
|---|---|---|---|
| Heart | 12 | 1 | 8.3 (0.2, 38) |
| Lung | 12 | 1 | 8.3 (0.2, 38) |
| Mediastinal lymph node | 7 | 3 | 43 (10, 82) |
| Mesenteric lymph node | 12 | 1 | 8.3 (0.2, 38) |
| Spleen | 12 | 1 | 8.3 (0.2, 38) |
| Liver | 12 | 4 | 33 (10, 65) |
| Kidneys | 12 | 2 | 17 (2.1, 48) |
| Gastrointestinal tract | 12 | 4 | 33 (10, 65) |
| Skeletal muscle | 12 | 3 | 25 (5.5, 57) |
| Bladder | 12 | 3 | 25 (5.5, 57) |
| Mammary gland | 6 | 0 | 0 (0, 46) |
| Ovaries | 6 | 1 | 17 (0.4, 64) |
| Uterus | 6 | 0 | 0 (0, 46) |
| Testes | 6 | 0 | 0 (0, 46) |
| Epididymis | 6 | 2 | 33 (4.3, 78) |
| Total kangaroos | 12 | 10 * | 83 (52, 98) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tolpinrud, A.; Dobson, E.; Herbert, C.A.; Gray, R.; Stenos, J.; Chaber, A.-L.; Devlin, J.M.; Stevenson, M.A. Characterising Eastern Grey Kangaroos (Macropus giganteus) as Hosts of Coxiella burnetii. Microorganisms 2024, 12, 1477. https://doi.org/10.3390/microorganisms12071477
Tolpinrud A, Dobson E, Herbert CA, Gray R, Stenos J, Chaber A-L, Devlin JM, Stevenson MA. Characterising Eastern Grey Kangaroos (Macropus giganteus) as Hosts of Coxiella burnetii. Microorganisms. 2024; 12(7):1477. https://doi.org/10.3390/microorganisms12071477
Chicago/Turabian StyleTolpinrud, Anita, Elizabeth Dobson, Catherine A. Herbert, Rachael Gray, John Stenos, Anne-Lise Chaber, Joanne M. Devlin, and Mark A. Stevenson. 2024. "Characterising Eastern Grey Kangaroos (Macropus giganteus) as Hosts of Coxiella burnetii" Microorganisms 12, no. 7: 1477. https://doi.org/10.3390/microorganisms12071477





