Growth and Yield Dynamics in Three Japanese Soybean Cultivars with Plant Growth-Promoting Pseudomonas spp. and Bradyrhizobium ottawaense Co-Inoculation
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Planting Material
2.2. Rhizobial Strains and Growth Conditions
2.3. PGPB Strains and Growth Conditions
2.4. Greenhouse Experiment
2.5. Measurements of Growth Parameters and Seed Yield
2.6. Chlorophyll Content and Net Photosynthesis Rate
2.7. N2-Fixation Measurement
2.8. Mineral Ion Analysis and N Uptake
2.9. Data Analysis
3. Results
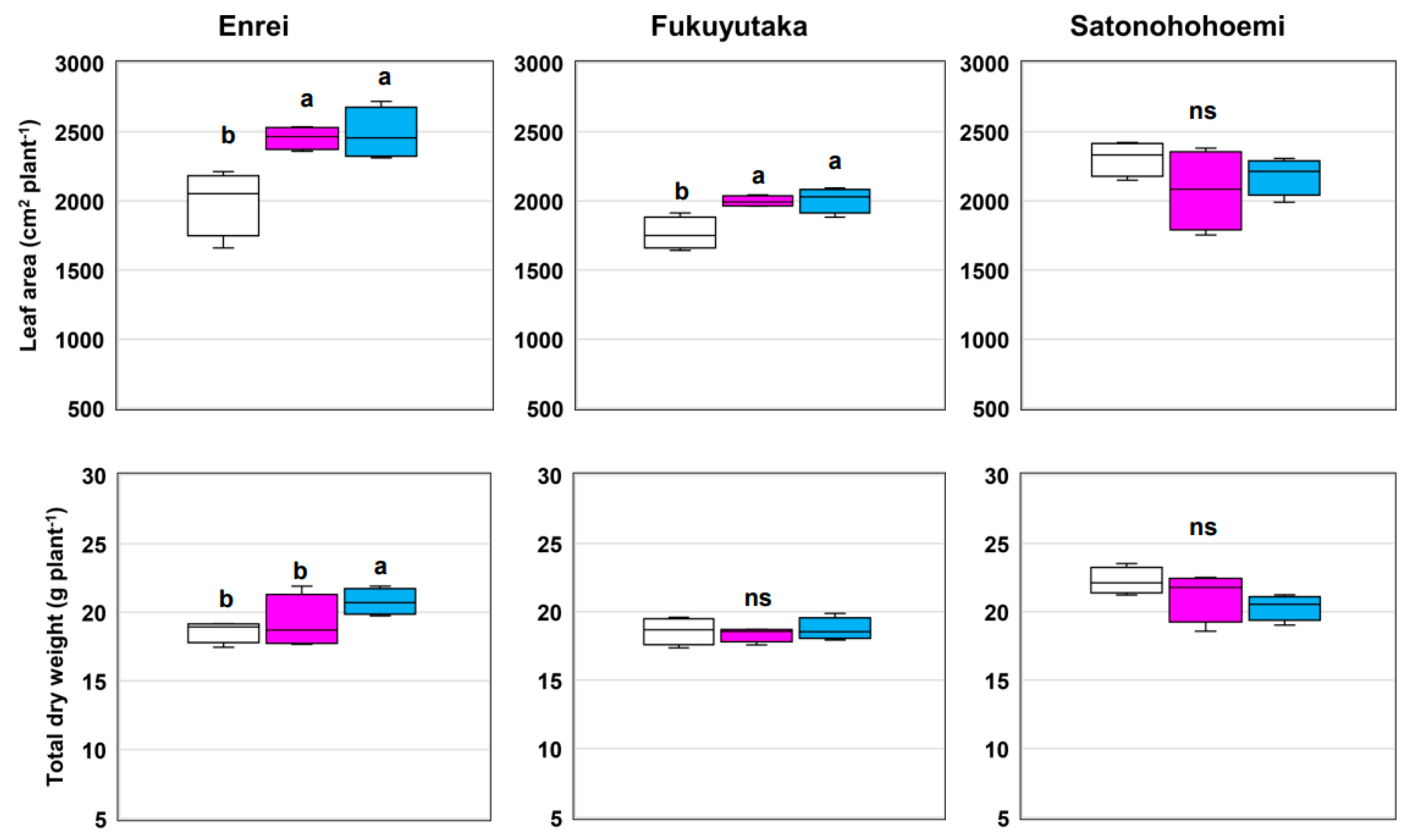
3.1. Growth and Leaf Physiology at 7 WAS
3.2. Nodulation and Biological N2 Fixation
3.3. Nutrient Uptake in Soybean Cultivars
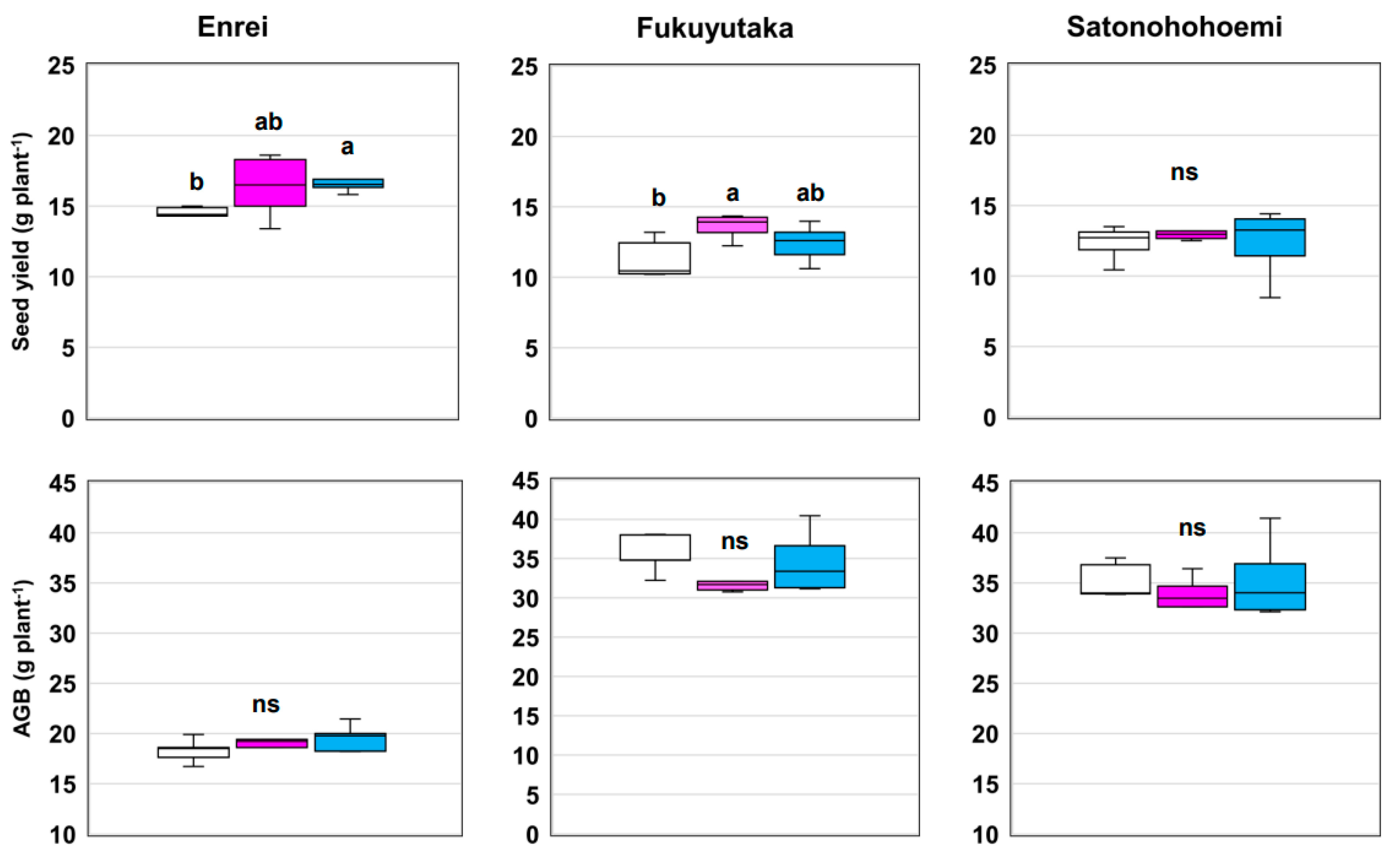
3.4. Yield and Aboveground Biomass
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jordan, D.C. NOTES: Transfer of Rhizobium japonicum Buchanan 1980 to Bradyrhizobium gen. nov., a genus of slow-growing, root nodule bacteria from leguminous plants. Int. J. Syst. Bacteriol. 1982, 32, 136–139. [Google Scholar] [CrossRef]
- Temesgen, D.; Assefa, F. Inoculation of native symbiotic effective Sinorhizobium spp. enhanced soybean [Glycine max (L.) Merr.] grain yield in Ethiopia. Environ. Syst. Res. 2020, 9, 38. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Jabborova, D.; Berg, G. Synergistic interactions between Bradyrhizobium japonicum and the endophyte Stenotrophomonas rhizophila and their effects on growth, and nodulation of soybean under salt stress. Plant Soil. 2016, 405, 35–45. [Google Scholar] [CrossRef]
- Zuffo, A.M.; De Rezende, P.M.; Bruzi, A.T.; Ribeiro, A.B.M.; Zambiazzi, E.V.; Soares, I.O.; Vilela, N.J.D.; Bianchi, M.C. Soybean cultivars agronomic performance and yield according to doses of Azospirillum brasilense applied to leaves. Aust. J. Crop Sci. 2016, 10, 579–583. [Google Scholar] [CrossRef]
- Tonelli, M.L.; Magallanes-Noguera, C.; Fabra, A. Symbiotic performance and induction of systemic resistance against Cercospora sojina in soybean plants co-inoculated with Bacillus sp. CHEP5 and Bradyrhizobium japonicum E109. Arch. Microbiol. 2017, 199, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y. An inducible activator produced by a Serratia proteamaculans strain and its soybean growth-promoting activity under greenhouse conditions. J. Exp. Bot. 2002, 53, 1495–1502. [Google Scholar] [PubMed]
- Win, K.T.; Tanaka, F.; Okazaki, K.; Ohwaki, Y. The ACC deaminase expressing endophyte Pseudomonas spp. enhances NaCl stress tolerance by reducing stress-related ethylene production, resulting in improved growth, photosynthetic performance, and ionic balance in tomato plants. Plant Physiol. Biochem. 2018, 127, 599–607. [Google Scholar] [CrossRef]
- Mahmud, A.A.; Upadhyay, S.K.; Srivastava, A.K.; Bhojiya, A.A. Biofertilizers: A nexus between soil fertility and crop productivity under abiotic stress. Curr. Res. Environ. Sustain. 2021, 3, 100063. [Google Scholar] [CrossRef]
- Win, K.T.; Kobayashi, M.; Tanaka, F.; Takeuchi, K.; Oo, A.Z.; Jiang, C.-J. Identification of Pseudomonas strains for the biological control of soybean red crown root rot. Sci. Rep. 2022, 12, 14510. [Google Scholar] [CrossRef]
- Chaudhary, A.; Parveen, H.; Chaudhary, P.; Khatoon, H.; Bhatt, P. Rhizospheric microbes and their mechanism. In Microbial Technology for Sustainable Environment; Bhatt, P., Gangola, S., Udayanga, D., Kumar, G., Eds.; Springer: Singapore, 2021; pp. 79–93. [Google Scholar]
- Li, J.; Ovakim, D.H.; Charles, T.C.; Glick, B.R. An ACC deaminase minus mutant of Enterobacter cloacae UW4 no longer promotes root elongation. Curr. Microbiol. 2000, 41, 101–105. [Google Scholar] [CrossRef]
- Gravel, V.; Antoun, H.; Tweddell, R.J. Growth stimulation and fruit yield improvement of greenhouse tomato plants by inoculation with Pseudomonas putida or Trichoderma atroviride: Possible role of indole acetic acid (IAA). Soil Biol. Biochem. 2007, 39, 1968–1977. [Google Scholar] [CrossRef]
- Bal, H.B.; Das, S.; Dangar, T.K.; Adhya, T.K. ACC deaminase and IAA producing growth promoting bacteria from the rhizosphere soil of tropical rice plants. J. Basic Microbiol. 2013, 53, 972–984. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2013, 169, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Duodu, S.; Bhuvaneswari, T.V.; Stokkermans, T.J.W.; Peters, N.K. A positive role for rhizobitoxine in Rhizobium-legume symbiosis. Mol. Plant-Microbe Interact. 1999, 12, 1082–1089. [Google Scholar] [CrossRef][Green Version]
- Nandwal, A.S.; Kukreja, S.; Kumar, N.; Sharma, P.K.; Jain, M.; Mann, A.; Singh, S. Plant water status, ethylene evolution, N2-fixing efficiency, antioxidant activity and lipid peroxidation in Cicer arietinum L. nodules as affected by short-term salinization and desalinization. J. Plant Physiol. 2007, 164, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Oldroyd, G.E.D. Positioning the nodule, the hormone dictum. Plant Signal. Behav. 2009, 4, 89–93. [Google Scholar] [CrossRef]
- Tittabutr, P.; Sripakdi, S.; Boonkerd, N.; Tanthanuch, W.; Minamisawa, K.; Teaumroong, N. Possible role of 1-Aminocyclopropane-1-Carboxylate (ACC) deaminase activity of Sinorhizobium sp. BL3 on symbiosis with mung bean and determinate nodule senescence. Microbes Environ. 2015, 30, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Alemneh, A.A.; Zhou, Y.; Ryder, M.H.; Denton, M.D. Mechanisms in plant growth-promoting rhizobacteria that enhance legume–rhizobial symbioses. J. Appl. Microbiol. 2020, 129, 1133–1156. [Google Scholar] [CrossRef]
- Spaepen, S.; Das, F.; Luyten, E.; Michiels, J.; Vanderleyden, J. Indole-3-acetic acid-regulated genes in Rhizobium etli CNPAF512. FEMS Microbiol. Lett. 2009, 291, 195–200. [Google Scholar] [CrossRef]
- Bolton, H.; Elliott, L.F.; Turco, R.F.; Kennedy, A.C. Rhizoplane colonization of pea seedlings by Rhizobium leguminosarum and a deleterious root colonizing Pseudomonas spp. and effects on plant growth. Plant Soil 1990, 123, 121–124. [Google Scholar] [CrossRef]
- Grimes, H.D.; Mount, M.S. Influence of Pseudomonas putida on nodulation of Phaseolus vulgaris. Soil Biol. Biochem. 1984, 16, 27–30. [Google Scholar] [CrossRef]
- Gupta, S.; Pandey, S. ACC Deaminase producing bacteria with multifarious plant growth promoting traits alleviates salinity stress in French Bean (Phaseolus vulgaris) plants. Front. Microbiol. 2019, 10, 1506. [Google Scholar] [CrossRef] [PubMed]
- Win, K.T.; Hara, S.; Tanaka, F.; Oo, A.Z.; Minamisawa, K.; Shimoda, S.; Imaizumi-Anraku, H. Synergistic N2-fixation and salt stress mitigation in soybean through dual inoculation of ACC deaminase-producing Pseudomonas and Bradyrhizobium. Sci. Rep. 2023, 13, 1705. [Google Scholar] [CrossRef] [PubMed]
- Zeffa, D.M.; Fantin, H.L.; Koltun, A.; Oliveira, A.L.M.; Nunes, M.P.B.A.; Canteri, M.G.; Gonçalves, L.S.A. Effects of plant growth-promoting rhizobacteria on co-inoculation with Bradyrhizobium in soybean crop: A meta-analysis of studies from 1987 to 2018. Peer J. 2020, 8, e7905. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.A.; Elkan, G.H. Transmissible resistance to penicillin G, neomycin, and chloramphenicol in Rhizobium japonicum. Antimicrob. Agents Chemother. 2020, 4, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Wasai-Hara, S.; Shintaro, H.; Morikawa, T.; Sugawara, M.; Takami, H.; Yoneda, J.; Tokunaga, T.; Minamisawa, K. Diversity of Bradyrhizobium in non-leguminous sorghum plants: B. ottawaense isolates unique in genes for N2O reductase and lack of the type VI secretion system. Microbes Environ. 2020, 35, ME19102. [Google Scholar] [CrossRef] [PubMed]
- Wasai-Hara, S.; Itakura, M.; Fernandes Siqueira, A.; Takemoto, D.; Sugawara, M.; Mitsui, H.; Sato, S.; Inagaki, N.; Yamazaki, T.; Imaizumi-Anraku, H.; et al. Bradyrhizobium ottawaense efficiently reduces nitrous oxide through high nosZ gene expression. Sci. Rep. 2023, 13, 18862. [Google Scholar] [CrossRef]
- Hardy, R.W.F.; Holsten, R.D.; Jackson, E.K.; Burns, R.C. The acetylene-ethylene assay for N2-fixation: Laboratory and field evaluation. Plant Physiol. 1968, 43, 1185–1207. [Google Scholar] [CrossRef] [PubMed]
- Imran, A.; Mirza, M.S.; Shah, T.M.; Malik, K.A.; Hafeez, F.Y. Differential response of kabuli and desi chickpea genotypes toward inoculation with PGPR in different soils. Front. Microbiol. 2015, 6, 859. [Google Scholar] [CrossRef]
- Schmidt, J.; Messmer, M.; Wilbois, K.-P. Beneficial microorganisms for soybean (Glycine max (L.) Merr), with a focus on low root-zone temperatures. Plant Soil 2015, 397, 411–445. [Google Scholar] [CrossRef]
- Yadav, M.R.; Kumar, R.; Parihar, C.M.; Yadav, R.K.; Jat, S.L.; Ram, H.; Meena, R.K.; Singh, M.; Birbal; Verma, A.P.; et al. Strategies for improving nitrogen use efficiency: A review. Agric. Rev. 2017, 38, 29–40. [Google Scholar] [CrossRef]
- Mathesius, U.; Schlaman, H.R.M.; Spaink, H.P.; Sautter, C.; Rolfe, B.G.; Djordjevic, M.A. Auxin transport inhibition precedes root nodule formation in white clover roots and is regulated by flavonoids and derivatives of chitin oligosaccharides. Plant J. 1998, 14, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, H.; Minakawa, Y.; Akao, S.; Minamisawa, K. The involvement of indole-3-acetic acid produced by Bradyrhizobium elkanii in nodule formation. Plant Cell Physiol. 1994, 35, 1261–1265. [Google Scholar] [CrossRef]
- Matsuoka, H.; Ohwaki, Y.; Terakado-Tonooka, J.; Tanaka, F. Changes in volatiles in carrots inoculated with ACC deaminase-producing bacteria isolated from organic crops. Plant Soil 2016, 407, 173–186. [Google Scholar] [CrossRef]
- Oldroyd, G.E.D.; Engstrom, E.M.; Long, S.R. Ethylene inhibits the Nod factor signal transduction pathway of Medicago truncatula. Plant Cell 2001, 13, 1835–1849. [Google Scholar] [CrossRef] [PubMed]
- Reid, D.; Liu, H.; Kelly, S.; Kawaharada, Y.; Mun, T.; Andersen, S.U.; Desbrosses, G.; Stougaarda, J. Dynamics of ethylene production in response to compatible Nod factor. Plant Physiol. 2018, 176, 1764–1772. [Google Scholar] [CrossRef] [PubMed]
- Madhaiyan, M.; Poonguzhali, S.; Sa, T. Regulation of ethylene levels in canola (Brassica campestris) by 1-aminocyclopropane1-carboxylate deaminase-containing Methylobacterium fujisawaense. Planta 2006, 224, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Remans, R.; Ramaekers, L.; Schelkens, S.; Hernandez, G.; Garcia, A.; Reyes, J.L.; Mendez, N.; Toscano, V.; Mulling, M.; Galvez, L. Effect of Rhizobium–Azospirillum coinoculation on nitrogen fixation and yield of two contrasting Phaseolus vulgaris L. genotypes cultivated across different environments in Cuba. Plant Soil 2008, 312, 25–37. [Google Scholar] [CrossRef]
- Beltran-Medina, J.I.; Romero-Perdomo, F.; Molano-Chavez, L.; Silva, A.M.M.; Estrada-Bonilla, G.A. Differential plant growth promotion under reduced phosphate rates in two genotypes of maize by a Rhizobial phosphate-solubilizing strain. Front. Sustain. Food Syst. 2022, 6, 955473. [Google Scholar] [CrossRef]
- Sánchez, A.C.; Gutiérrez, R.T.; Santana, R.C.; Urrutia, A.R.; Fauvart, M.; Michiels, J.; Vanderleyden, J. Effects of co-inoculation of native Rhizobium and Pseudomonas strains on growth parameters and yield of two contrasting Phaseolus vulgaris L. genotypes under Cuban soil conditions. Eur. J. Soil Biol. 2014, 62, 105–112. [Google Scholar] [CrossRef]


| ANOVA (p) | |||
|---|---|---|---|
| Traits | Variety | PGPB | Variety × PGPB |
| Leaf area | 0.0004 | 0.0307 | 0.0086 |
| SPAD values | 0.0000 | 0.0000 | 0.1130 |
| Photosynthesis rate (Asat) | 0.0000 | 0.0000 | 0.0026 |
| Total biomass | 0.0032 | 0.7314 | 0.0312 |
| Nodule number | 0.0003 | 0.0009 | 0.0985 |
| Nodule dry weight | 0.0155 | 0.7012 | 0.0875 |
| Acetylene reduction assay (ARA) | 0.0002 | 0.0989 | 0.0000 |
| Seed yield | 0.0003 | 0.0168 | 0.4800 |
| Aboveground biomass | 0.0000 | 0.1404 | 0.4840 |
| Cultivar | Treatment | N | P | K | Ca | Mg | S | Cu | Fe |
|---|---|---|---|---|---|---|---|---|---|
| Enrei | SG09 | 256.9 ± 28.7 | 1584.1 ± 361.8 | 25,692.0 ± 5312.4 | 17,884.3 ± 3394.9 | 4793.9 ± 842.8 | 2051.9 ± 463.4 | 2.8 ± 0.6 | 85.7 ± 3.0 |
| SG09 + OFT2 | 319.8 ± 14.0 ** | 1990.1 ± 161.7 | 29,209.9 ± 2282.4 | 20,732.5 ± 1802.0 | 5412.5 ± 389.8 | 2381.6 ± 176.4 | 3.2 ± 0.10 | 85.2 ± 5.5 | |
| SG09 + OFT5 | 348.1 ± 12.7 ** | 2156.8 ± 83.4 * | 32,155.4 ± 397.8 | 24,364.6 ± 362.0 * | 6018.4 ± 448.2 * | 2698.6 ± 95.2 * | 3.6 ± 0.4 | 101.3 ± 7.5 * | |
| Fukuyutaka | SG09 | 286.1 ± 18.9 | 2007.5 ± 41.8 | 25,688.9 ± 2589.2 | 21,899.5 ± 730.2 | 4957.7 ± 48.4 | 2507.8 ± 149.2 | 2.9 ± 0.1 | 88.0 ± 19.6 |
| SG09 + OFT2 | 334.6 ± 7.0 * | 1879.8 ± 203.4 | 26,233.9 ± 2363.0 | 19,079.9 ± 1909.8 | 4293.4 ± 479.6 | 2434.1 ± 264.1 | 2.8 ± 0.2 | 87.8 ± 8.3 | |
| SG09 + OFT5 | 306.8 ± 16.5 | 1901.7 ± 104.6 | 28,183.5 ± 353.0 | 18,786.0 ± 476.7 ** | 5000.3 ± 76.6 | 2506.6 ± 68.6 | 3.1 ± 0.1 * | 91.8 ± 12.1 | |
| Satonohohoemi | SG09 | 290.1 ± 19.2 | 1981.4 ± 144.4 | 32,187.1 ± 1547.8 | 25,138.2 ± 807.7 | 6431.2 ± 497.9 | 2898.4 ± 13.2 | 3.4 ± 0.2 | 89.3 ± 10.8 |
| SG09 + OFT2 | 279.5 ± 37.0 | 2110.0 ± 103.5 | 31,852.4 ± 4623.6 | 23,278.8 ± 3835.5 | 6090.6 ± 1151.2 | 2749.1 ± 279.5 | 3.4 ± 0.6 | 87.7 ± 17.2 | |
| SG09 + OFT5 | 280.5 ± 26.9 | 1892.5 ± 195.7 | 31,628.1 ± 573.3 | 24,891.4 ± 652.5 | 6298.3 ± 194.7 | 2846.7 ± 285.1 | 3.5 ± 0.4 | 108.4 ± 10.9 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Win, K.T.; Tanaka, F.; Minamisawa, K.; Imaizumi-Anraku, H. Growth and Yield Dynamics in Three Japanese Soybean Cultivars with Plant Growth-Promoting Pseudomonas spp. and Bradyrhizobium ottawaense Co-Inoculation. Microorganisms 2024, 12, 1478. https://doi.org/10.3390/microorganisms12071478
Win KT, Tanaka F, Minamisawa K, Imaizumi-Anraku H. Growth and Yield Dynamics in Three Japanese Soybean Cultivars with Plant Growth-Promoting Pseudomonas spp. and Bradyrhizobium ottawaense Co-Inoculation. Microorganisms. 2024; 12(7):1478. https://doi.org/10.3390/microorganisms12071478
Chicago/Turabian StyleWin, Khin Thuzar, Fukuyo Tanaka, Kiwamu Minamisawa, and Haruko Imaizumi-Anraku. 2024. "Growth and Yield Dynamics in Three Japanese Soybean Cultivars with Plant Growth-Promoting Pseudomonas spp. and Bradyrhizobium ottawaense Co-Inoculation" Microorganisms 12, no. 7: 1478. https://doi.org/10.3390/microorganisms12071478
APA StyleWin, K. T., Tanaka, F., Minamisawa, K., & Imaizumi-Anraku, H. (2024). Growth and Yield Dynamics in Three Japanese Soybean Cultivars with Plant Growth-Promoting Pseudomonas spp. and Bradyrhizobium ottawaense Co-Inoculation. Microorganisms, 12(7), 1478. https://doi.org/10.3390/microorganisms12071478






