Enteropathogenic Providencia alcalifaciens: A Subgroup of P. alcalifaciens That Causes Diarrhea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolates
2.2. Genome Sequencing
2.3. Genome Assembly
2.4. TnPhoA Insertion Site
2.5. Bioinformatics and Data Resources
3. Results
3.1. Assembly and Annotation of P. alcalifaciens 2939/90
3.2. Assembly and Characterization of the Genome Sequences of TnPhoA Mutants
3.3. TnPhoA Insertions
3.4. TnPhoA Inserts in p2939_90_1 Have a Predicted Role in Type III Secretion
3.5. Two Type III Secretion Apparatus Loci in the P. alcalifaciens Strain 2939/90 Genome
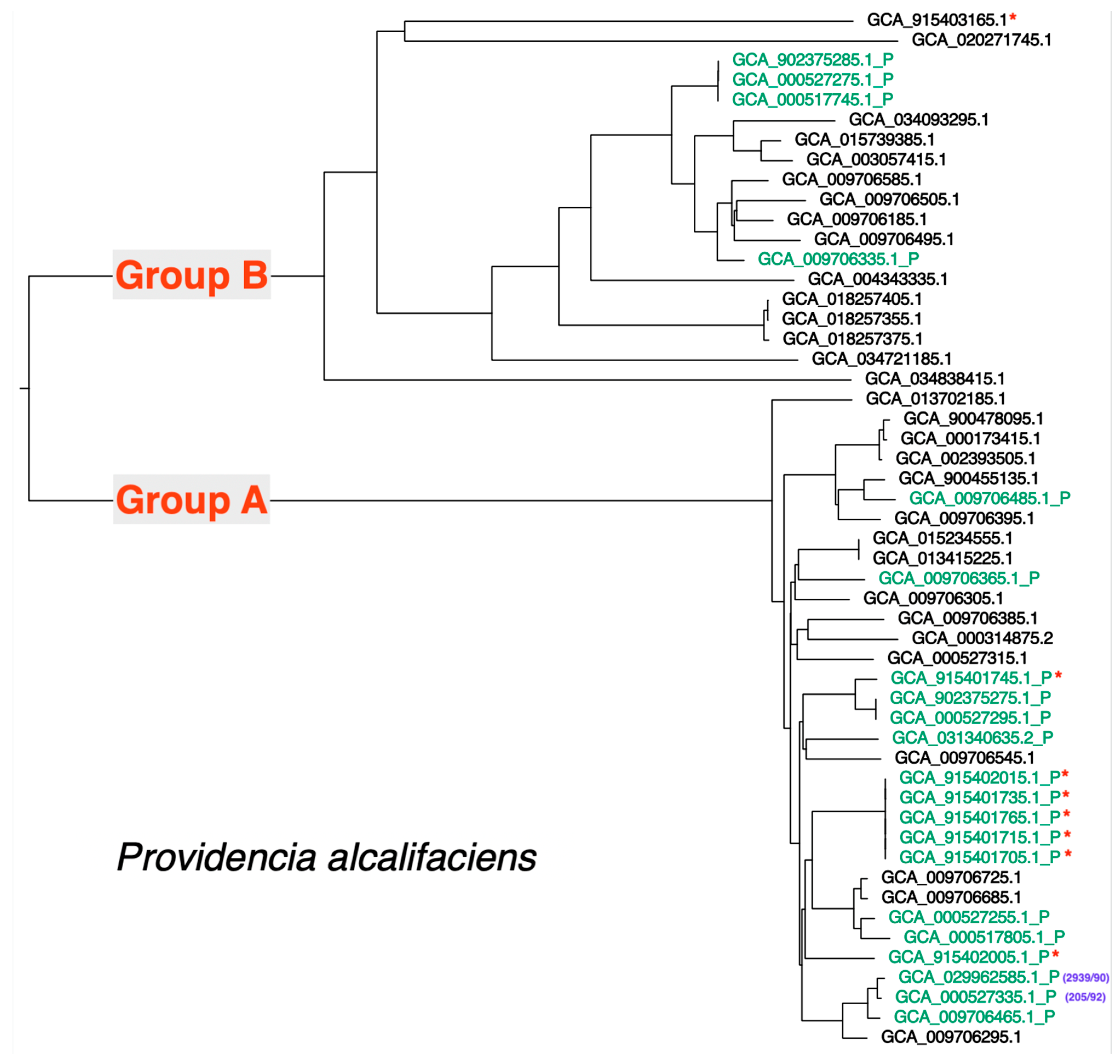
3.6. Distribution of the Type III Secretion Apparatus Loci in P. alcalifaciens
3.7. Type III Secreted Effector Protein Prediction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Janda, J.M.; Abbott, S. (Eds.) Genus Providencia. In Enterobacteria; ASM Press: Washington, DC, USA, 2006; pp. 279–299. [Google Scholar]
- Senior, B.W. Media for the detection and recognition of the enteropathogen Providencia alcalifaciens in faeces. J. Med. Microbiol. 1997, 46, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Yoh, M.; Matsuyama, J.; Ohnishi, M.; Takagi, K.; Miyagi, H.; Mori, K.; Park, K.S.; Ono, T.; Honda, T. Importance of Providencia species as a major cause of traveler’s diarrhoea. J. Med. Microbiol. 2005, 54, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, C.M.; Brenner, F.W.; Miller, J.M. Classification, identification, and clinical significance of Proteus, Providencia, and Morganella. Clin. Microbiol. Rev. 2000, 13, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Iiida, T.; Shiomi, Y.; Tagomori, K.; Akeda, Y.; Yanagihara, I.; Mushiake, S.; Ishiguro, F.; Honda, T. A large outbreak of foodborne infection attributed to Providencia alcalifaciens. J. Infect. Dis. 2001, 184, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.M.; Odoyo, E.; Larson, P.S.; Apondi, E.; Kathiiko, C.; Miringu, G.; Nakashima, M.; Ichinose, Y. First report of a foodborne Providencia alcalifaciens outbreak in Kenya. Am. J. Trop. Med. Hyg. 2015, 93, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Haynes, J.; Hawkey, P.M. Providencia alcalifaciens and travellers’ diarrhoea. Br. Med. J. 1989, 299, 94–95. [Google Scholar] [CrossRef]
- Albert, M.J.; Faruque, A.S.G.; Mahalanabis, D. Association of Providencia alcalifaciens with diarrhea in children. J. Clin. Microbiol. 1998, 36, 1433–1435. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, H.J.; Valheim, M.; Sekse, C.; Bergsjo, B.A.; Wisloff, H.; Norstebo, S.F.; Scanke, E.; Lagesen, K.; Haaland, A.H.; Rodriguez-Campos, S.; et al. An official outbreak investigation of acute haemorrhagic diarrhoea in dogs in Norway points to Providencia alcalifaciens as a likely cause. Animals 2021, 11, 3201. [Google Scholar] [CrossRef] [PubMed]
- Spach, D.H.; Liles, W.C. Antimicrobial therapy for bacterial diseases. In Clinical Infectious Diseases: A Practical Approach; Root, R.K., Ed.; Oxford University Press, Inc.: New York, NY, USA, 1999; pp. 337–348. [Google Scholar]
- Swenson, J.M.; Hindler, J.A.; Peterson, L.R. Special phenotypic methods for detecting antibacterial resistance. In Manual of Clinical Microbiology, 7th ed.; Murray, P.R., Baron, E.J., Jorgenson, J.H., Pfaller, M.A., Yolken, R.H., Eds.; American Society for Microbiology: Washington, DC, USA, 1999; pp. 1563–1577. [Google Scholar]
- Albert, M.J.; Alam, K.; Ansaruzzaman, M.; Islam, M.M.; Rahman, A.S.; Haider, K.; Bhuiyan, N.A.; Nahar, S.; Ryan, N.; Montanaro, J. Pathogenesis of Providencia alcalifaciens-induced diarrhea. Infect. Immun. 1992, 60, 5017–5024. [Google Scholar] [CrossRef]
- Mathan, M.M.; Mathan, V.I.; Albert, M.J. Electron microscopic study of the attachment and penetration of rabbit intestinal epithelium by Providencia alcalifaciens. J. Pathol. 1993, 171, 67–71. [Google Scholar] [CrossRef]
- Albert, M.J.; Ansaruzzaman, M.; Bhuiyan, N.A.; Neogi, P.K.B.; Faruque, A.S.G. Characterics of invasion of HEp-2 cells by Providencia alcalifaciens. J. Med. Microbiol. 1995, 42, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Beatriz, E.; Guth, C.; Perrella, E. Prevalence of invasive ability and other virulence-associated characteristics in Providencia alcalifaciens strains isolated in Sao Palo, Brazil. J. Med. Microbiol. 1996, 45, 459–462. [Google Scholar]
- Janda, J.M.; Abbott, S.L.; Woodward, D.; Khashe, S. Invasion of HEp-2 and other eukaryotic cell lines by Providenciae: Further evidence supporting the role of Providencia alcalifaciens in bacterial gastroenteritis. Curr. Microbiol. 1998, 37, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Monira, S.; Nahar, S.; Ansaruzzaman, M.; Alam, K.; Alam, M.; Albert, M.J. TnphoA mutants of Providencia alcalifaciens with altered invasiveness of HEp-2 cells. J. Med. Microbiol. 2002, 51, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Jain, C.; Rodriguez-R, L.M.; Phillippy, A.M.; Konstantinidis, K.T.; Aluru, S. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat. Commun. 2018, 9, 5114. [Google Scholar] [CrossRef] [PubMed]
- Eichinger, V.; Nussbaumer, T.; Platzer, A.; Jehl, M.-A.; Arnold, R.; Rattei, T. EffectiveDB—Updates and novel features for a better annotation of bacterial secreted proteins and type III, IV, VI secretion systems. Nucleic Acids Res. 2016, 44, D669–D674. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Goldborg, M.B. Recent insights into type-3 secretion system injectisome structure and mechanism of human enteric pathogens. Curr. Opin. Microbiol. 2023, 71, 102232. [Google Scholar] [CrossRef] [PubMed]
- Miras, I.; Hermant, D.; Arricau, N.; Popoff, M.Y. Nucletide sequence of iagA and iagB genes involved in invasion of HeLa cells by Salmonella enterica subsp. enterica ser. Typhi. Res. Microbiol. 1995, 146, 17–20. [Google Scholar] [CrossRef]
- Kim, J.S.; Eom, J.S.; Jang, J.I.; Kim, H.G.; Seo, D.W.; Bang, I.-S.; Bang, S.H.; Lee, I.S.; Park, Y.K. Role of Salmonella pathogenicity island 1 protein lacP in Salmonella enterica serovar Typhimurium pathogenesis. Infect. Immun. 2011, 79, 1440–1450. [Google Scholar] [CrossRef]
- Osiecki, J.C.; Barker, J.; Picking, W.L.; Serfis, A.B.; Berring, E.; Shah, S.; Harrington, A.; Picking, W.D. IpaC from Shigella and SipC from Salmonella possess similar biochemical properties but are functionally distinct. Mol. Microbiol. 2001, 42, 469–481. [Google Scholar] [CrossRef]
- Yu, D.; Yin, Z.; Jin, Y.; Zhou, J.; Ren, H.; Hu, M.; Li, B.; Zhou, W.; Liang, L.; Yue, J. Evolution of bopA gene in Burkholderia: A case of convergent evolution as a mechanism for bacterial autophagy evasion. Biomed. Res. Int. 2016, 2016, 6745028. [Google Scholar] [CrossRef]
- Dean, P.; Muhlen, S.; Quitard, S.; Kenny, B. The bacterial effectors EspG and EspG2 induce a destructive calpin activity that is kept in check by the co-delivered Tir effector. Cell Microbiol. 2010, 12, 1308–1321. [Google Scholar] [CrossRef]
- Alvin, J.W.; Lacy, D.B. Clostridium difficile toxin glucosyltransferase domains in complex with a non-hydrolyzable UDP-glucose analogue. J. Struct. Biol. 2017, 198, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Hensel, M.; Shea, J.E.; Gleeson, C.; Jones, M.D.; Dalton, E.; Holden, D.W. Simultaneous identification of bacterial virulence genes by negative selection. Science 1995, 269, 400–403. [Google Scholar] [CrossRef]
- Foultier, B.; Troisfontaines, P.; Muller, S.; Opperdoes, F.R.; Cornelis, G.R. Characterization of the ysa pathogenicity locus in the chromosome of Yersinia enterocolitica and phylogeny analysis of type III secretion systems. J. Mol. Evol. 2002, 55, 37–51. [Google Scholar] [CrossRef]
- Hayashi, T.; Makino, K.; Ohnishi, M.; Kurokawa, K.; Ishii, K.; Yokoyama, K.; Han, C.-G.; Ohtsubo, E.; Nakayama, K.; Murata, T.; et al. Complete genome sequence of enterohemorrhagic Escherichia coli O157:H7 and genomic comparison with a laboratory strain K12. DNA Res. 2001, 8, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Ono, T.; Rokuda, M.; Jang, M.-H.; Okada, K.; Iida, T.; Honda, T. Functional characterization of two type III secretion systems of Vibrio parahaemoltyicus. Infect. Immun. 2004, 72, 6659–6665. [Google Scholar] [CrossRef] [PubMed]
- Rainbow, L.; Hart, C.A.; Winstanley, C. Distribution of type III secretion gene clusters in Burkholderia pseudomallei, B. thailandensis and B. mallei. J. Med. Microbiol. 2002, 51, 374–384. [Google Scholar] [CrossRef]
- Stevens, M.P.; Wood, M.W.; Taylor, L.A.; Monaghan, P.; Hawes, P.; Jones, P.W.; Wallis, T.S.; Galyov, E.E. An Inv/Mxi-Spa-like type III protein secretion system in Burkholderia pseudomallei modulates intracellular behaviour of the pathogen. Mol. Microbiol. 2002, 46, 649–659. [Google Scholar] [CrossRef]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L.T. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef] [PubMed]
| Strain/Mutant | 2939/90 | M-23 * | M-47 * | M-63 * | M-78 * |
|---|---|---|---|---|---|
| 2939/90 | 0 | 1 | 2 | 1 | 1 |
| M-23 | 1 | 0 | 1 | 0 | 0 |
| M-47 | 2 | 1 | 0 | 1 | 1 |
| M-63 | 1 | 0 | 1 | 0 | 0 |
| M-78 | 1 | 0 | 1 | 0 | 0 |
| Site | Left | Right | Replicon | Gene(s) | TnphoA Mutant Isolate |
|---|---|---|---|---|---|
| Site 1 | >site1 GCTTTGTTAGCACTAGCCAAAAAAC ATGGTTGGTCATTATCGAGAGAAAT | >site1 TAACAAAGCATCTTTCTGTTCTTTT GTTAAATAAATAAAAGCTCTTTCGT | p2939_90_4 | PO864_20965 to PO864_20995 (hypothetical protein) | M-78, M-23 |
| Site 2 | >site2 GCATAGATGAATGCTTGAATTATTT AGACATGAGTACATTAGGCAAACAA | >site2 CATCTATGCTTGTTGTTCTATCCTC AGAGCTAATAGATTTGTTGAGACTC | p2939_90_1 | PO864_RS20065 (SctG) | M-78, M-23 |
| Site 3 | >site3 CATTAAAGGTTACGAAAGAAGGCGG AGCTGATATTACCGATGACCCGTTA | >site3 CCTTTAATGCAACATCATTCGCATC AAACATTAACGGCTCAACAATCTCT | p2939_90_1 | PO864_19795 (SctC) | M-63 |
| Site 4 | >site4 ACGCAACACCAACCGCGGCCAATGC TAAACTCGCACCACCAGAGAATACG | p2939_90_1 | PO864_19725 (SctE) | M-63 | |
| Site 5 | >Site5 GTGTAATGGTGAACAATACGGGTGT TGATTTACTACCCACATTGGGTAAA | 2939/90 chromosome | PO864_08195 (fimbrial protein) | M-47 | |
| Site 6 | >site6 ACTGAGCCTCGCTTCGTTCAGATAA AACAACAGAAATATTATCATATTCA | p2939_90_1 | PO864_20250 (SctJ) | M-47 |
| Chromosome | Unified Nomenclature ^ | Plasmid p2939_90_1 |
|---|---|---|
| PO864_RS07710 | SctA | PO864_RS19510 |
| PO864_RS07705 | SctB | PO864_RS19515 |
| PO864_RS07700 | SctE | PO864_RS19520 |
| PO864_RS07695 | chaperone | PO864_RS19525 |
| PO864_RS07690 | SctU | PO864_RS19530 |
| PO864_RS07685 | SctT | PO864_RS19535 |
| PO864_RS07680 | SctS | PO864_RS19540 |
| PO864_RS07675 | SctR | PO864_RS19545 |
| PO864_RS07670 | SctQ | PO864_RS19550 |
| PO864_RS07665 | SctP | PO864_RS19555 |
| PO864_RS07660 | SctO | PO864_RS19560 |
| PO864_RS07655 | SctN | PO864_RS19565 |
| PO864_RS07650 | chaperone | PO864_RS19570 |
| PO864_RS07645 | SctV | PO864_RS19575 |
| PO864_RS07640 | SctW | PO864_RS19580 |
| PO864_RS07635 | SctC | PO864_RS19585 |
| PO864_RS07630 | regulator | PO864_RS19590 |
| Absent | PO864_RS20070 a | |
| Absent | SctG | PO864_RS20065 |
| Absent | PO864_RS20060 b | |
| Absent | PO864_RS20055 c | |
| Absent | PO864_RS20050 d | |
| PO864_RS07625 | SctD | PO864_RS20045 |
| PO864_RS07620 | SctF | PO864_RS20040 |
| PO864_RS07615 | SctI | PO864_RS20035 |
| PO864_RS07610 | SctJ | PO864_RS20030 |
| PO864_RS07605 | SctK | PO864_RS20025 |
| PO864_RS07600 | SctL | PO864_RS20020 |
| PO864_RS07595 | Hypothetical protein | Absent |
| Absent | Effector (IpaC/SipC) | PO864_RS20015 |
| Absent | Effector (BopA) | PO864_RS19620 |
| Replicon | T3SS Effector (High) | T3SS Effector (Low) | Total CDS | Percent of CDS Predicted to Containt3ss Effectors |
|---|---|---|---|---|
| Chromosome | 427 | 209 | 3580 ^ | 17.8% |
| p2939_90_1 | 20 | 6 | 89 ^ | 29.2% |
| p2939_90_2 | 5 | 3 | 73 | 11.0% |
| p2939_90_3 | 3 | 4 | 47 | 14.9% |
| p2939_90_4 | 0 | 0 | 8 | 0.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulach, D.; Carter, G.P.; Albert, M.J. Enteropathogenic Providencia alcalifaciens: A Subgroup of P. alcalifaciens That Causes Diarrhea. Microorganisms 2024, 12, 1479. https://doi.org/10.3390/microorganisms12071479
Bulach D, Carter GP, Albert MJ. Enteropathogenic Providencia alcalifaciens: A Subgroup of P. alcalifaciens That Causes Diarrhea. Microorganisms. 2024; 12(7):1479. https://doi.org/10.3390/microorganisms12071479
Chicago/Turabian StyleBulach, Dieter, Glen P. Carter, and M. John Albert. 2024. "Enteropathogenic Providencia alcalifaciens: A Subgroup of P. alcalifaciens That Causes Diarrhea" Microorganisms 12, no. 7: 1479. https://doi.org/10.3390/microorganisms12071479





