Climate Change Stressors, Phosphate Limitation, and High Irradiation Interact to Increase Alexandrium minutum Toxicity and Modulate Encystment Rates
Abstract
:1. Introduction
2. Materials and Methods
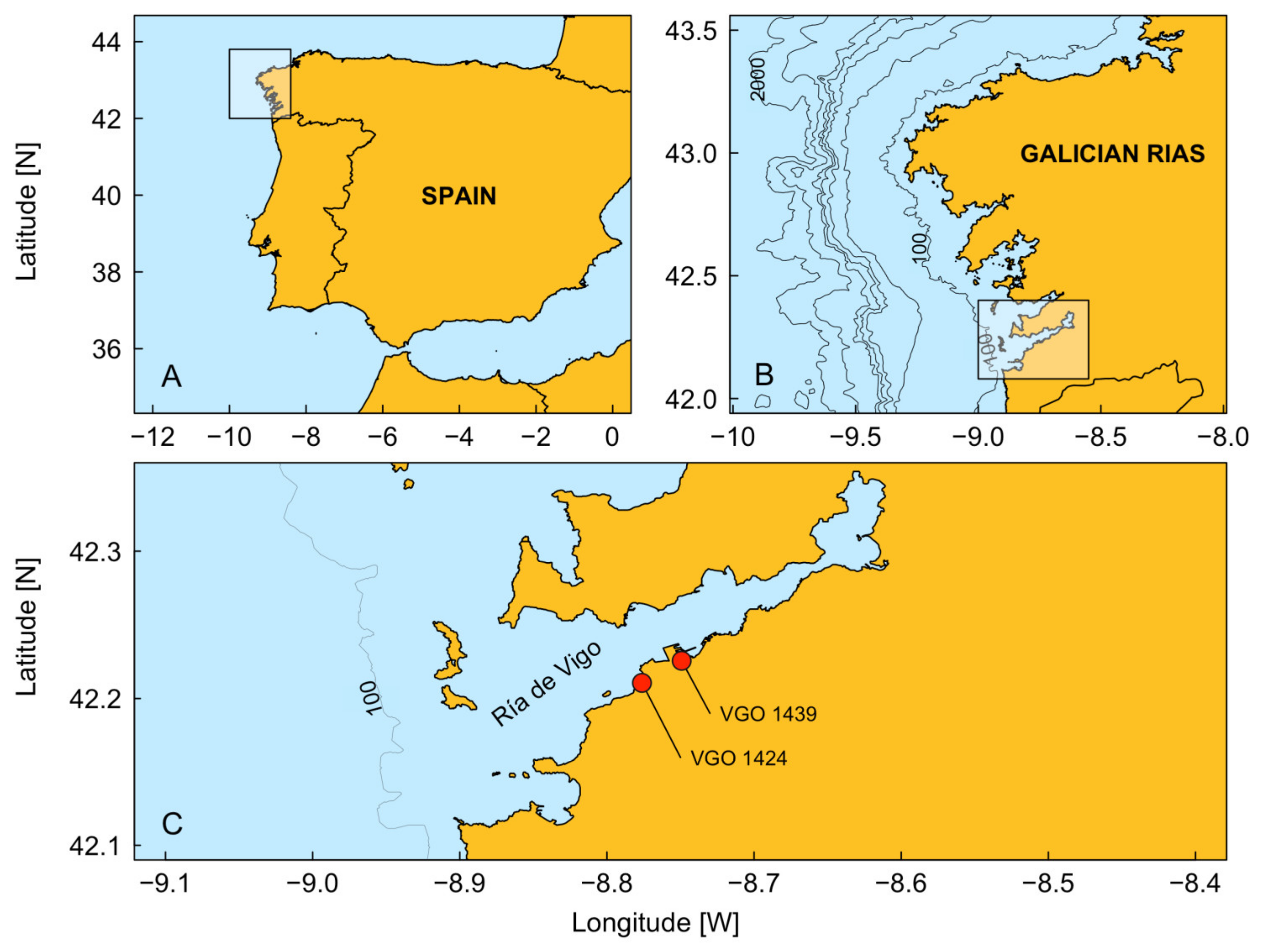
2.1. Stock Culture Conditions
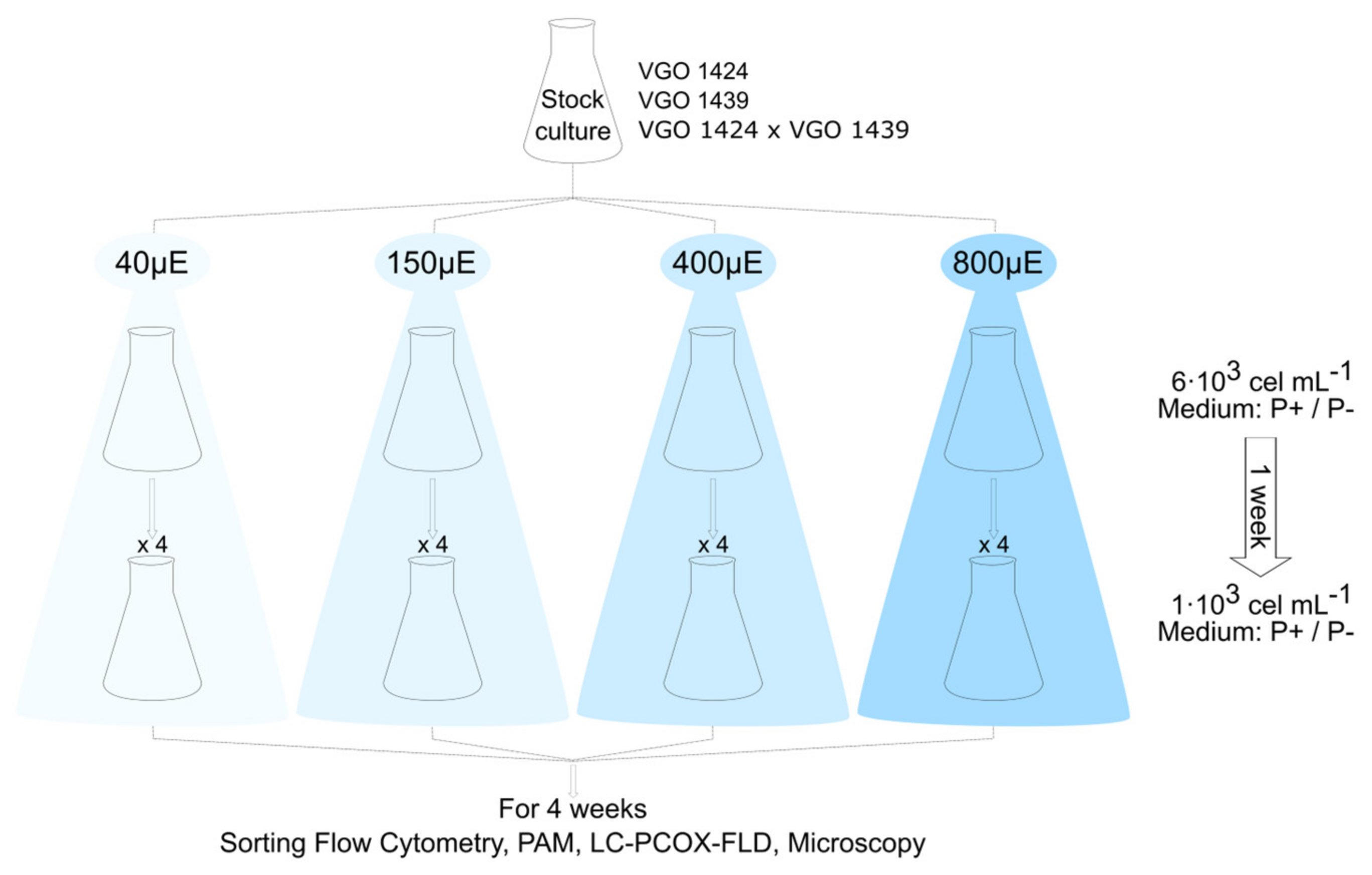
2.2. Light Experimental Design
2.3. Growth Rates and Cyst Production
2.4. Photosynthesis Efficiency
2.5. Toxin Analysis
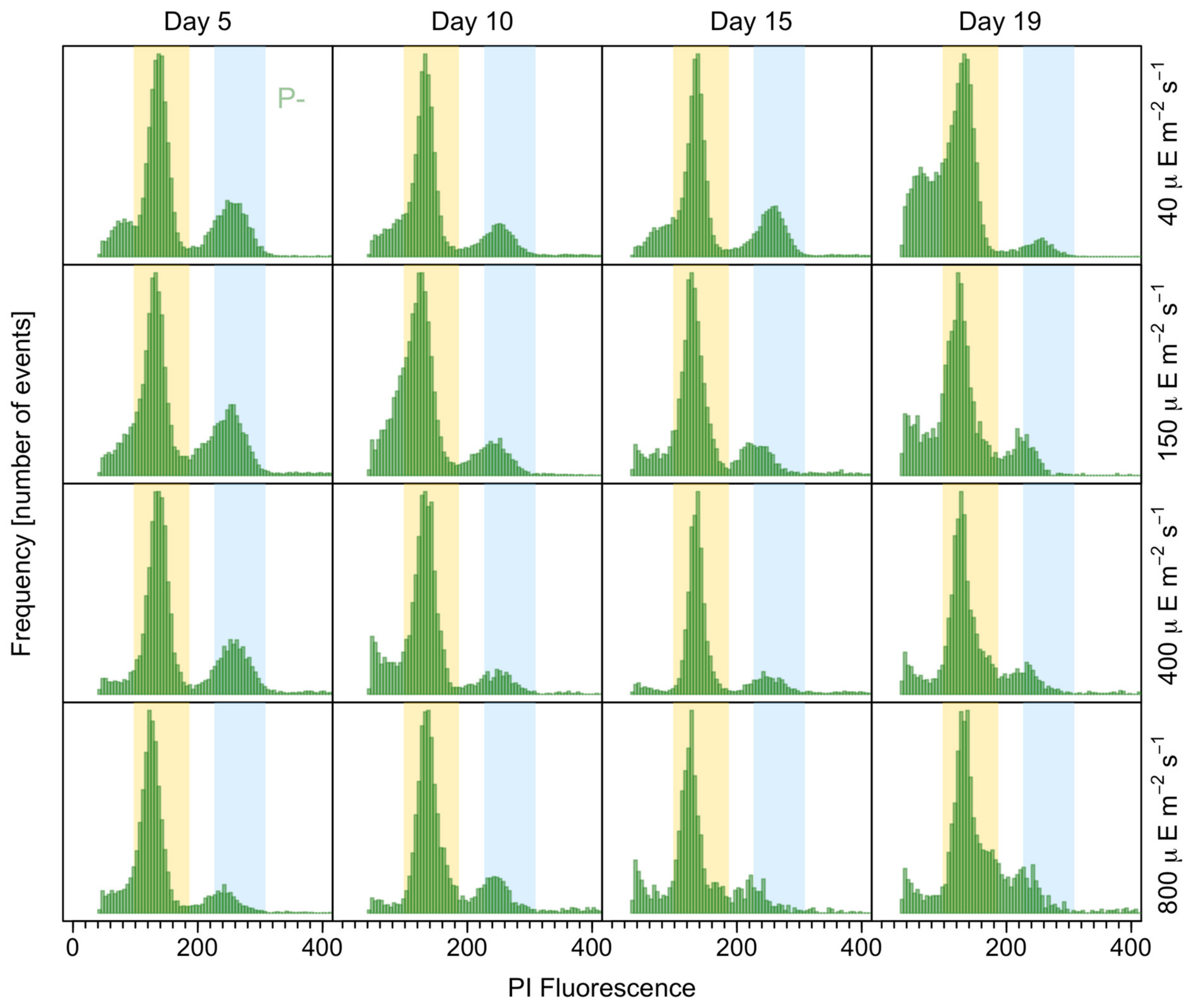
2.6. Sexuality and DNA Content Analyses
2.7. Statistical Analysis
3. Results
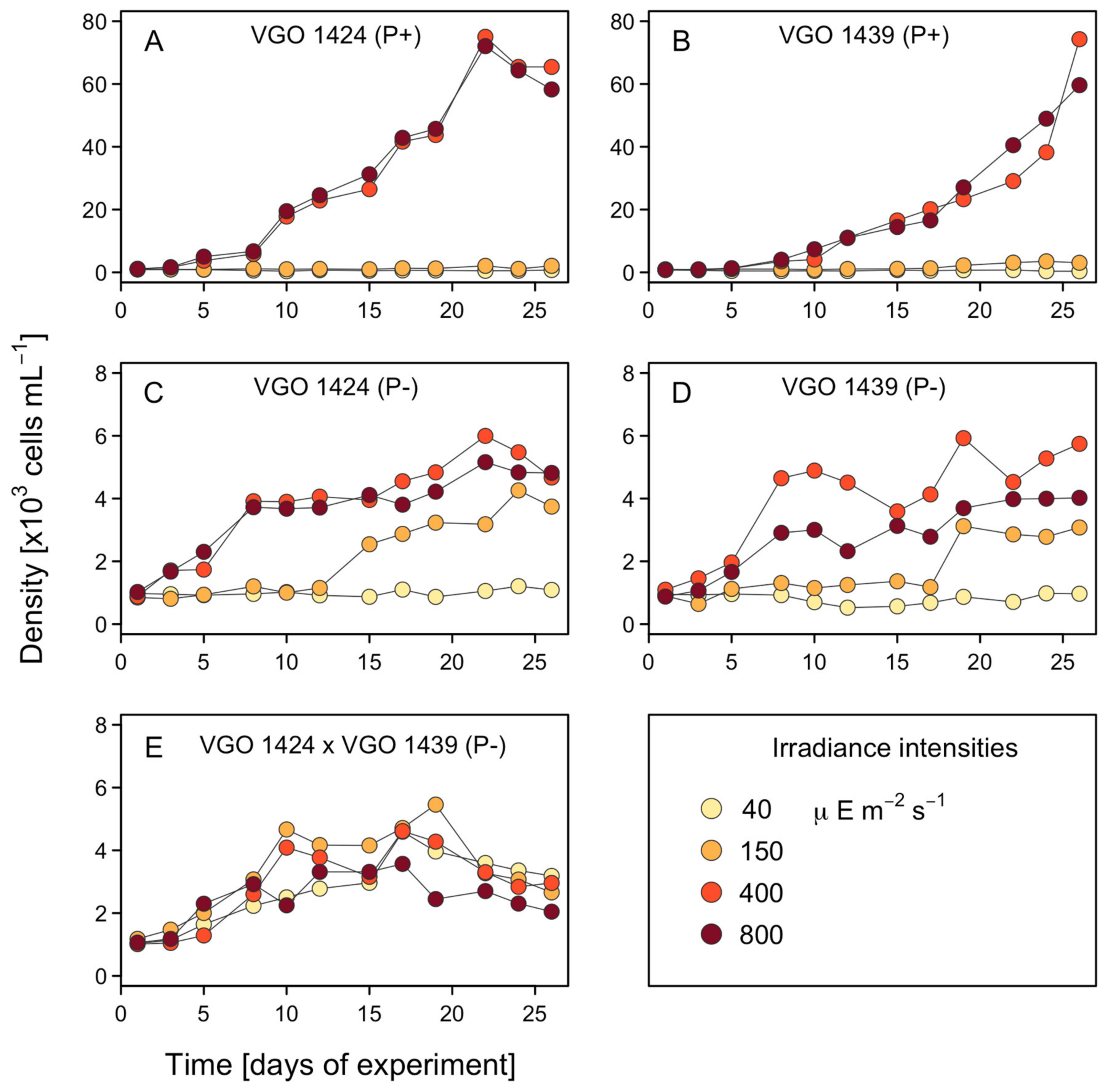
3.1. Growth Rates and Cell Size
3.2. Photosynthetic Efficiency
3.3. Toxin Production
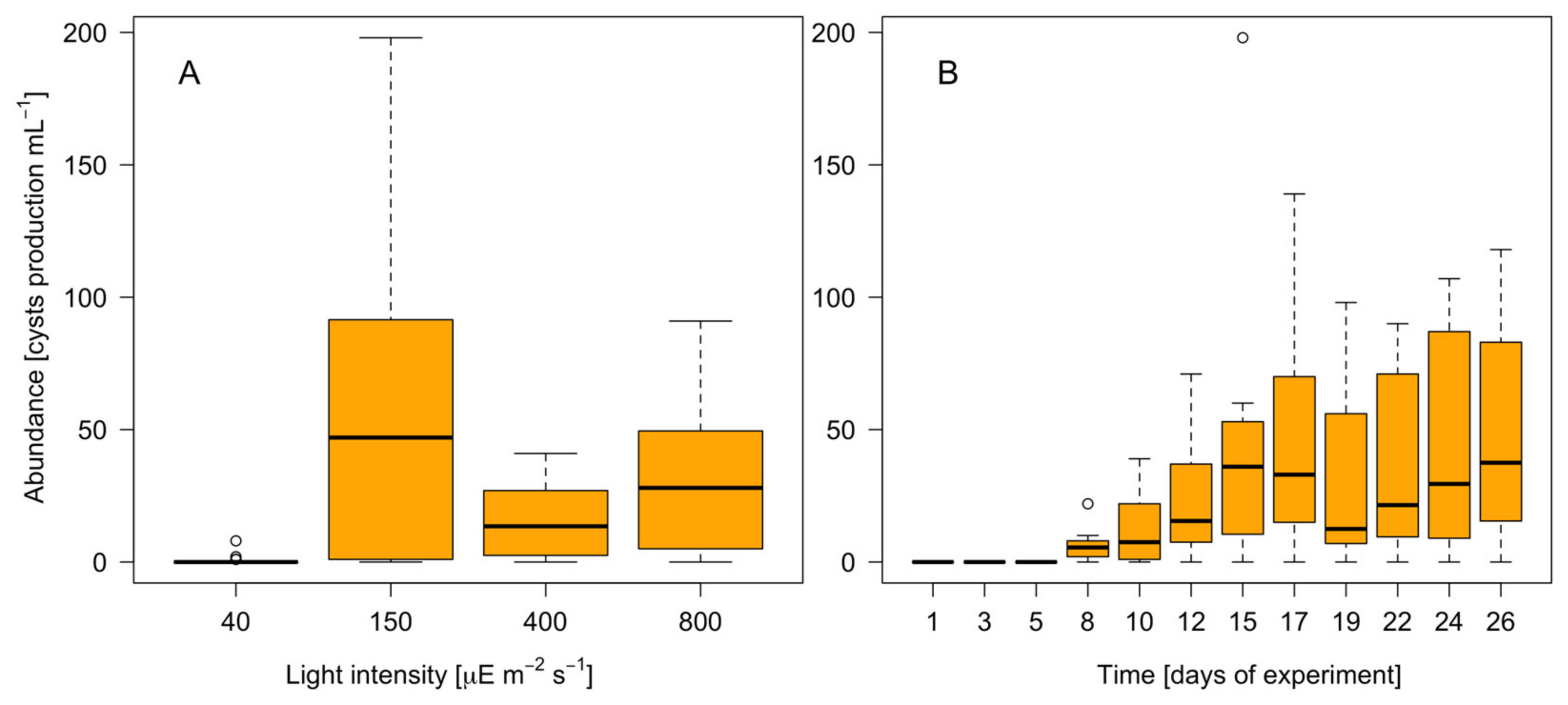
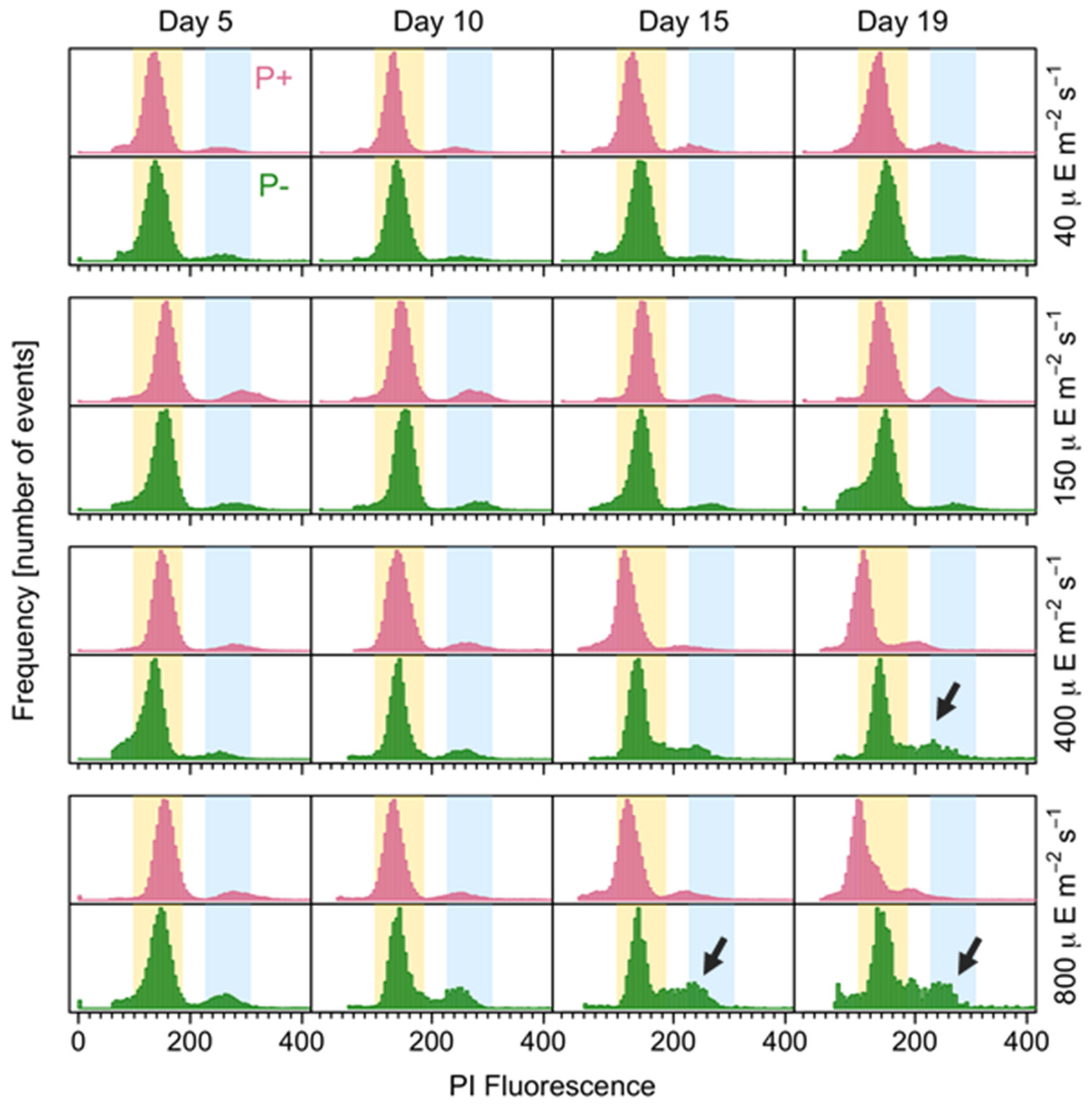
3.4. Resting Cyst Production and DNA Content
4. Discussion
4.1. Optimal Light Levels for A. minutum Growth
4.2. Toxicity Increases with Higher Light Levels and P Deficiency
4.3. Resting Cyst Formation Is Light-Dependent but Decreases at High Irradiances
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Figueroa, R.I.; Vázquez, J.A.; Massanet, A.; Murado, M.A.; Bravo, I. Interactive effects of salinity and temperature on planozygote and cyst formation on Alexandrium minutum (Dinophyceae). J. Phycol. 2011, 47, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Smayda, T.J. Novel and nuisance phytoplankton blooms in the sea: Evidence for a global epidemic. In Toxic Marine Phytoplankton; Graneli, E., Sundström, B., Edler, L., Anderson, D.M., Eds.; Elsevier: New York, NY, USA, 1990; pp. 29–40. [Google Scholar]
- Díaz, P.A.; Figueroa, R.I. Toxic algal bloom recurrence in the era of global change: Lessons from the Chilean Patagonian fjords. Microorganisms 2023, 11, 1874. [Google Scholar] [CrossRef]
- Wells, M.L.; Karlson, B.; Wulff, A.; Kudela, R.; Trick, C.; Asnaghi, V.; Berdalet, E.; Cochlan, W.; Davidson, K.; De Rijcke, M.; et al. Future HAB science: Directions and challenges in a changing climate. Harmful Algae 2020, 91, 101632. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.K.; Johnstone, J.A.; Banas, N.S.; Salathé, E.P. Present-day and future climate pathways affecting Alexandrium blooms in Puget Sound, WA, USA. Harmful Algae 2015, 48, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Salgado, X.A.; Labarta, U.; Fernández-Reiriz, M.J.; Figueiras, F.G.; Rosón, G.; Piedracoba, S.; Filgueira, R.; Cabanas, J.M. Renewal time and the impact of harmful algal blooms on the extensive mussel raft culture of the Iberian coastal upwelling system (SW Europe). Harmful Algae 2008, 7, 849–855. [Google Scholar] [CrossRef]
- Pérez, F.; Padín, X.A.; Pazos, Y.; Gilcoto, M.; Cabanas, J.M.; Pardo, P.C.; Doval, M.D.; Farina-Bustos, L. Plankton response to weakening of the Iberian coastal upwelling. Glob. Chang. Biol. 2010, 16, 1258–1267. [Google Scholar] [CrossRef]
- Detoni, A.M.S.; Navarro, G.; Padín, X.A.; Ramirez-Romero, E.; Zoffoli, A.L.; Pazos, Y.; Caballero, I. Potentially toxigenic phytoplankton patterns in the northwestern Iberian Peninsula. Front. Mar. Sci. 2024, 11, 1330090. [Google Scholar] [CrossRef]
- Nogueira, E.; Bravo, I.; Montero, P.; Díaz-Tapia, P.; Calvo, S.; Ben-Girirey, B.; Figueroa, R.I.; Garrido, J.L.; Ramilo, I.; Lluch, N.; et al. HABs in coastal upwelling systems: Insights from an exceptional red tide of the toxigenic dino agellate Alexandrium minutum. Ecol. Indic. 2022, 137, 108790. [Google Scholar] [CrossRef]
- Bravo, I.; Fraga, S.; Figueroa, R.I.; Pazos, Y.; Massanet, A.; Ramilo, I. Bloom dynamics and life cycle strategies of two toxic dinoflagellates in a coastal upwelling system (NW Iberian Peninsula). Deep Sea Res. I 2010, 57, 222–234. [Google Scholar] [CrossRef]
- Padin, X.A.; Babarro, J.M.; Otero, P.; Gilcoto, M.; Rellán, T.; Suárez, L.; Velo, A.; Peteiro, L.G. The declining availability of wild mussel seed for aquaculture in a coastal upwelling system. Front. Mar. Sci. 2024, 11, 1375269. [Google Scholar] [CrossRef]
- Avdelas, L.; Avdic-Mravlje, E.; Borges Marques, A.C.; Cano, S.; Capelle, J.J.; Carvalho, N.; Cozzolino, M.; Dennis, J.; Ellis, T.; Fernández Polanco, J.M.; et al. The decline of mussel aquaculture in the European Union: Causes, economic impacts and opportunities. Rev. Aquac. 2021, 13, 91–118. [Google Scholar] [CrossRef]
- Wijsman, J.; Troost, K.; Fang, J.; Roncarati, A. Global production of marine bivalves. Trends and challenges. In Goods and Services of Marine Bivalves; Smaal, A.C., Ferreira, J.G., Grant, J., Petersen, J.K., Strand, Ø., Eds.; Springer: Cham, Switzerland, 2018; pp. 7–26. [Google Scholar]
- Reguera, B.; Escalera, L.; Pazos, Y.; Moroño, A. Episodios de fitoplancton toxico en la Ría de Vigo. In La Ría de Vigo: Una Aproximación Integral al Ecosistema Marino de la Ría de Vigo; González-Garcés, A., Vilas, F., Alvarez-Salgado, X., Eds.; Instituto de Estudios Vigueses: Vigo, Spain, 2008; pp. 153–199. [Google Scholar]
- García-Moreiras, I.; Vila Costas, S.; García-Gil, S.; Muñoz Sobrino, C. Organic-walled dinoflagellate cyst assemblages in surface sediments of the Ría de Vigo (Atlantic margin of NW Iberia) in relation to environmental gradients. Mar. Micropaleontaol. 2023, 180, 102217. [Google Scholar] [CrossRef]
- Li, P.; Ma, Q.; Xu, S.; Liu, W.; Ma, Z.; Ni, G. Opposite growth responses of Alexandrium minutum and Alexandrium catenella to photoperiods and temperatures. Plants 2021, 10, 1058. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, B.; Song, X.; Jian, X.; Tang, C.; Campbell, D.A.; Lin, Q.; Li, G. High antioxidant capability interacts with respiration to mediate two Alexandrium species growth exploitation of photoperiods and light intensities. Harmful Algae 2019, 82, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Laabir, M.; Jauzein, C.; Genovesi, B.; Masseret, E.; Grzebyk, D.; Cecchi, P.; Vaquer, A.; Perrin, Y.; Collos, Y. Influence of temperature, salinity and irradiance on the growth and cell yield of the harmful red tide dinoflagellate Alexandrium catenella colonizing Mediterranean waters. J. Plankton Res. 2011, 33, 1550–1563. [Google Scholar] [CrossRef]
- Kwon, H.K.; Oh, S.J.; Yang, H.S.; Kim, D.M.; Kang, I.J.; Oshima, Y. Laboratory study for the phytoremediation of eutrophic coastal sediment using benthic microalgae and light emitting diode (LED). J. Fac. Agric. Kyushu Univ. 2013, 58, 417–425. [Google Scholar] [CrossRef]
- Natsuike, M.; Oikawa, H.; Matsuno, K.; Yamaguchi, A.; Imai, I. The physiological adaptations and toxin profiles of the toxic Alexandrium fundyense on the eastern Bering Sea and Chukchi Sea shelves. Harmful Algae 2017, 63, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Zmerli Triki, H.; Laabir, M.; Kéfi Daly-Yahia, O. Life history, excystment features, and growth characteristics of the Mediterranean harmful dinoflagellate Alexandrium pseudogonyaulax. J. Phycol. 2015, 51, 980–989. [Google Scholar] [CrossRef]
- Lim, P.T.; Leaw, C.P.; Usup, G.; Kobiyama, A.; Koike, K.; Ogata, T. Effects of light and temperature on growth, nitrate uptake, and toxin production of two tropical dinoflagellates: Alexandrium tamiyavanichii and Alexandrium minutum (Dinophyceae). J. Phycol. 2006, 42, 786–799. [Google Scholar] [CrossRef]
- Anderson, D.M.; Alpermann, T.J.; Cembella, A.; Collos, Y.; Masseret, E.; Montresor, M. The globally distributed genus Alexandrium: Multifaceted roles in marine ecosystems and impacts on human health. Harmful Algae 2012, 14, 10–35. [Google Scholar] [CrossRef]
- Seveno, J.; Even, Y.; Le Gac, M. Strong constitutive expression divergence among strains but no evidence of differential expression associated with sexual reproduction in Alexandrium minutum. Harmful Algae 2020, 100, 101940. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, R.I.; Dapena, C.; Bravo, I.; Cuadrado, A. The hidden sexuality of Alexandrium minutum: An example of overlooked sex in dinoflagellates. PLoS ONE 2015, 10, e0142667. [Google Scholar] [CrossRef] [PubMed]
- Brosnahan, M.L.; Fischer, A.D.; Lopez, C.B.; Moore, S.K.; Anderson, D.M. Cyst-forming dinoflagellates in a warming climate. Harmful Algae 2020, 91, 101728. [Google Scholar] [CrossRef] [PubMed]
- Liow, G.R.; Lau, W.L.S.; Law, I.K.; Gu, H.; Leaw, C.P.; Lim, P.T. Sexual processes and life cycle transitions of the tropical Pacific Alexandrium minutum (Dinophyceae). Phycol. Res. 2021, 69, 188–199. [Google Scholar] [CrossRef]
- Probert, I.P. Sexual Reproduction and Ecophysiology of the Marine Dinoflagellate Alexandrium Minutum Halim. Ph.D. Thesis, University of Westminster, London, UK, 1999. [Google Scholar]
- Nehring, S. Mechanisms for recurrent nuisance algal blooms in coastal zones: Resting cyst formation as life-strategy of dinoflagellates. In Interdisciplinary Discussion of Coastal Research and Coastal Managment Issues and Problems; Sterr, H., Hofstade, J., Plag, H.-P., Eds.; Peter Lang: Frankfurt, Germany, 1993; pp. 454–467. [Google Scholar]
- Bravo, I.; Figueroa, R.I. Towards an ecological understanding of dinoflagellate cyst functions. Microorganisms 2014, 2, 11–32. [Google Scholar] [CrossRef] [PubMed]
- Dapena, C.; Bravo, I.; Cuadrado, A.; Figueroa, R.I. Nuclear and cell morphological changes during the cell cycle and growth of the toxic dinoflagellate Alexandrium minutum. Protist 2015, 166, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Sgrosso, S.; Esposito, F.; Montresor, M. Temperature and daylength regulate encystment in calcareous cyst-forming dinoflagellates. Mar. Ecol. Prog. Ser. 2001, 211, 77–87. [Google Scholar] [CrossRef]
- Li, M.; Shi, X.; Guo, C.; Lin, S. Phosphorus deficiency inhibits cell division but not growth in the dinoflagellate Amphidinium carterae. Front. Microbiol. 2016, 7, 826. [Google Scholar] [CrossRef]
- Huang, K.; Zhuang, Y.; Wang, Z.; Ou, L.; Cen, J.; Lu, S.; Qi, Y. Bioavailability of organic phosphorus compounds to the harmful dinoflagellate Karenia mikimotoi. Microorganisms 2021, 9, 1961. [Google Scholar] [CrossRef]
- Kalinina, V.; Berdieva, M.; Aksenov, N.; Skarlato, S. Phosphorus deficiency induces sexual repro-duction in the dinoflagellate Prorocentrum cordatum. Sci. Rep. 2023, 13, 14191. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.X.; García, N.S.; Hutchins, D.A. CO2 and phosphate availability control the toxicity of the harmful bloom dinoflagellate Karlodinium veneficum. Aquat. Microb. Ecol. 2010, 59, 55–65. [Google Scholar] [CrossRef]
- Boyer, G.L.; Sullivan, J.J.; Andersen, R.J.; Harrison, P.J.; Taylor, F.J.R. Effects of nutrient limitation on toxin production and composition in the marine dinoflagellate Protogonyaulax tamarensis. Mar. Biol. 1987, 96, 123–128. [Google Scholar] [CrossRef]
- Frangópulos, M.; Guisande, C.; deBlas, E.; Maneiro, I. Toxin production and competitive abilities under phosphorus limitation of Alexandrium species. Harmful Algae 2004, 3, 131–139. [Google Scholar] [CrossRef]
- Garrido, C.; Frangópulos, M.; Varela, D. Efecto de diferentes proporciones de nitrógeno/fósforo en el crecimiento y toxicidad de Alexandrium catenella (Dinoflagellata). An. Inst. Patagon. 2012, 40, 113–123. [Google Scholar] [CrossRef]
- Guisande, C.; Frangópulos, M.; Maneiro, I.; Vergara, A.; Riveiro, I. Ecological advantages of toxin production by the dinoflagellate Alexandrium minutum under phosphorus limitation. Mar. Ecol. Prog. Ser. 2002, 225, 169–176. [Google Scholar] [CrossRef]
- Hardison, D.R.; Sunda, W.G.; Shea, D.; Litaker, R.W. Increased toxicity of Karenia brevis during phosphate limited growth: Ecological and evolutionary implications. PLoS ONE 2013, 8, e58545. [Google Scholar] [CrossRef] [PubMed]
- Guillard, R.R.L. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals; Smith, W.L., Chanley, M.H., Eds.; Plenum Press: New York, NY, USA, 1975; pp. 29–60. [Google Scholar]
- Rourke, W.A.; Murphy, C.J.; Pitcher, G.; van de Riet, J.M.; Burns, B.G.; Thomas, K.M.; Quilliam, M.A. Rapid postcolumn methodology for determination of paralytic shellfish toxins in shellfish tissue. J. AOAC Int. 2008, 91, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Salgado, P.; Riobó, P.; Rodríguez, F.; Franco, J.M.; Bravo, I. Differences in the toxin profiles of Alexandrium ostenfeldii (Dinophyceae) strains isolated from different geographic origins: Evidence of paralytic toxin, spirolide, and gymnodimine. Toxicon 2015, 103, 85–98. [Google Scholar] [CrossRef] [PubMed]
- McCullagh, P.; Nelder, J.A. Generalized Linear Models; Chapman and Hall: London, UK, 1989; Volume 37, p. 258. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002; p. 495. [Google Scholar]
- Gotelli, N.J.; Ellison, A.M. A Primer of Ecological Statistics; Sinauer Associates, Inc.: Sunderland, MA, USA, 2004; p. 510. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S-Plus, 2nd ed.; Springer: New York, NY, USA, 1998; p. 548. [Google Scholar]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. 2021. Available online: https://CRAN.R-project.org/package=DHARMa (accessed on 10 March 2024).
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; ISBN 3-900051-07-0. Available online: http://www.r-project.org/ (accessed on 10 March 2024).
- Ben-Gigirey, B.; Rossignoli, A.E.; Riobó, P.; Rodríguez, F. First report of paralytic shellfish toxins in marine invertebrates and fish in Spain. Toxins 2020, 12, 723. [Google Scholar] [CrossRef] [PubMed]
- Tilstone, G.H.; Figueiras, F.G.; Fermin, E.G.; Arbones, B. Significance of nanophytoplankton photosynthesis and primary production in a coastal upwelling system (Ria deVigo, NW Spain). Mar. Ecol. Prog. Ser. 1999, 183, 13–27. [Google Scholar] [CrossRef]
- Arnone, R.A.; Ladner, S.; La Violette, P.E.; Brock, J.C.; Rochford, P.A. Seasonal and interannual varibility of surface photosynthetically available radiation in the Arabian Sea. J. Geophys. Res. 1998, 103, 7735–7748. [Google Scholar] [CrossRef]
- Cullen, J.J.; Neale, P.J. Quantifying the effects of ultravioletradiation on aquatic photosynthesis. In Photosynthetic Responses to the Environment; Yamamoto, H., Smith, C.M., Eds.; The American Society of Plant Biologists: Washington, DC, USA, 1993; pp. 45–60. [Google Scholar]
- Rijstenbil, J. Assessment of oxidative stress in the planktonic diatom Thalassiosira psedonana in response to UVA and UVB radiation. J. Plankton Res. 2002, 24, 1277–1288. [Google Scholar] [CrossRef]
- Cooney, E.C.; Fredrickson, K.A.; Bright, K.J.; Strom, S.L. Contrasting effects of high-intensity photosynthetically active radiation on two bloom-forming dinoflagellates. J. Phycol. 2019, 55, 1082–1095. [Google Scholar] [CrossRef] [PubMed]
- John, E.; Flynn, K. Modelling changes in paralytic shellfish toxin content of dinoflagellates in response to nitrogen and phosphorus supply. Mar. Ecol. Prog. Ser. 2002, 225, 147–160. [Google Scholar] [CrossRef]
- Lim, P.T.; Leaw, C.P.; Kobiyama, A.; Ogata, T. Growth and toxin production of tropical Alexandrium minutum Halim (Dinophyceae) under various nitrogen to phosphorus ratios. J. Appl. Physiol. 2010, 22, 203–210. [Google Scholar] [CrossRef]
- Hii, K.S.; Lim, P.T.; Kon, N.F.; Takata, Y.; Usup, G.; Leaw, C.P. Physiological and transcriptional responses to inorganic nutrition in a tropical Pacific strain of Alexandrium minutum: Implications for the saxitoxin genes and toxin production. Harmful Algae 2016, 59, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.; Kulis, D.M.; Sullivan, J.J.; Hall, S.; Lee, C. Dynamics and physiology of saxitoxin production by the dinoflagellates Alexandrium spp. Mar. Biol. 1990, 104, 511–524. [Google Scholar] [CrossRef]
- Duhamel, S.; Díaz, J.M.; Adams, J.C.; Djaoudi, K.; Steck, V.; Waggoner, E.M. Phosphorus as an integral component of global marine biogeochemistry. Nat. Geosci. 2021, 14, 359–368. [Google Scholar] [CrossRef]
- Figueroa, R.I.; Estrada, M.; Garcés, E. Life histories of microalgal species causing harmful blooms: Haploids, diploids and the relevance of benthic stages. Harmful Algae 2018, 73, 44–57. [Google Scholar] [CrossRef]
- Figueroa, R.I.; Garces, E.; Bravo, I. Comparative study of the life cycles of Alexandrium tamutum and Alexandrium minutum (Gonyaulacales, Dinophyceae) in culture. J. Phycol. 2007, 43, 1039–1053. [Google Scholar] [CrossRef]
- Llavería, G.; Figueroa, R.I.; Garcés, E.; Berdalet, E. Cell cycle and cell mortality of Alexandrium minutum (Dinophyceae) under small-scale turbulence conditions. J. Phycol. 2009, 45, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Balzer, I.; Hardeland, R. Effects of indoleamines and short photoperiods on the encystment of Gonyaulax polyedra. Chronobiol. Int. 1992, 9, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Villegas, C.; Figueroa, R.I.; Baldrich, A.; Pérez-Santos, I.; Díaz, M.; Tomasetti, S.J.; Seguel, M.; Álvarez, G.; Salgado, P.; Díaz, P.A. Small and patchy is enough: An example about how toxic HAB events can spread through low resting cyst loads. Harmful Algae 2023, 129, 102495. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, R.I.; Garcés, E.; Camp, J. Reproductive plasticity and local adaptation in the host-parasite system formed by the toxic Alexandrium minutum and the dinoflagellate parasite Parvilucifera sinerae. Harmful Algae 2010, 10, 56–63. [Google Scholar] [CrossRef]
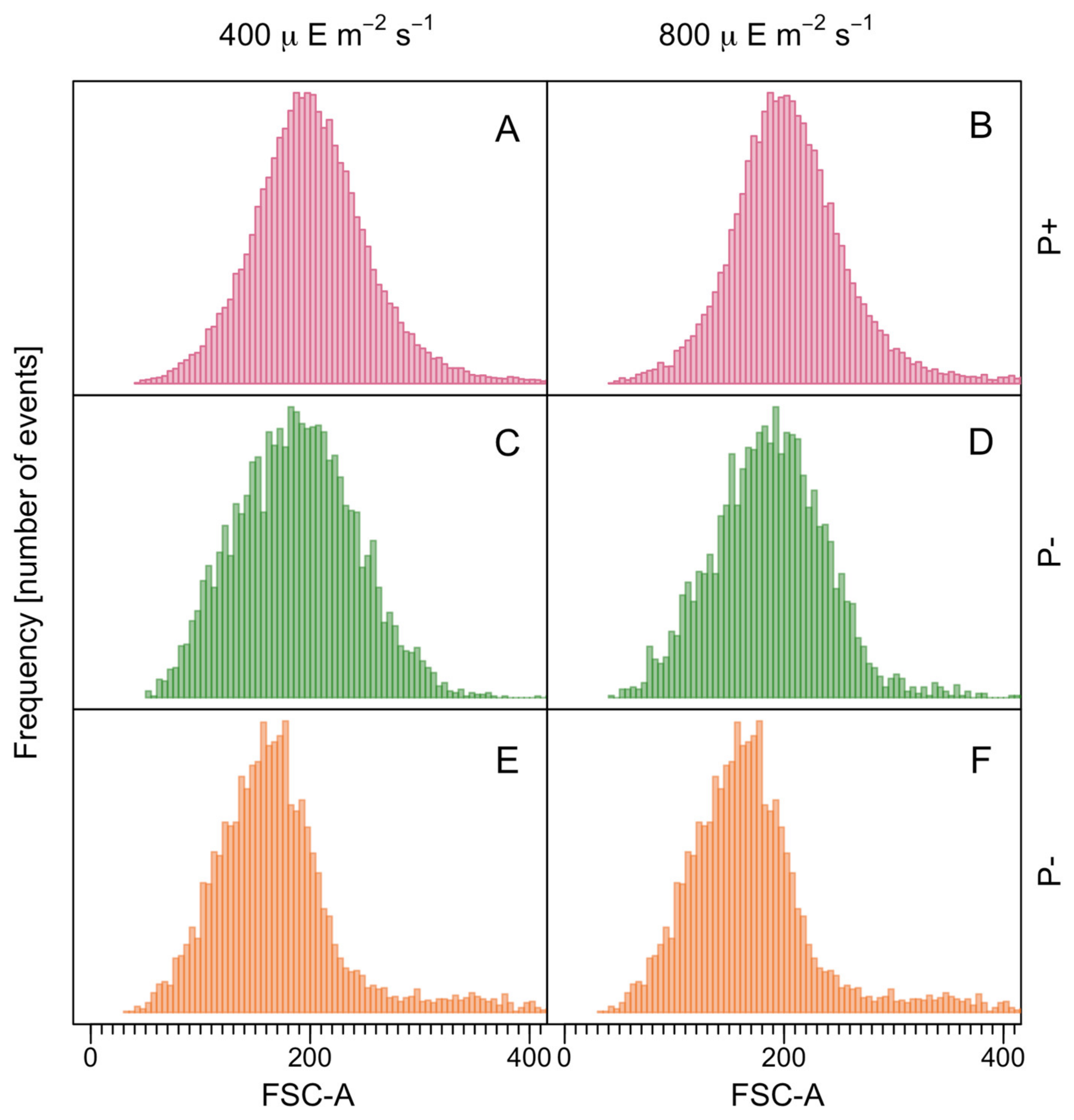


| Light Intensity (µE m−2 s−1) | Clonal | Clonal-P | Cross-P |
|---|---|---|---|
| 40 | 830 | 1095 | 4595 |
| 150 | 2580 | 3521 | 5458 |
| 400 | 69,856 | 3580 | 4613 |
| 800 | 58,950 | 4572 | 3570 |
| Light Intensity (µE m−2 s−1) | Clonal Fv/Fm | Clonal-P Fv/Fm | Cross-P Fv/Fm |
|---|---|---|---|
| 40 | 0.298 | 0.705 | 0.722 |
| 150 | 0.473 | 0.723 | 0.583 |
| 400 | 0.592 | 0.591 | 0.564 |
| 800 | 0.427 | 0.353 | 0.302 |
| Light Intensity | Clonal vs. Clonal-P | Clonal-P vs. Cross-P |
|---|---|---|
| 40 | 0.005 | 0.402 |
| 150 | 0.005 | 0.065 |
| 400 | 0.818 | 0.429 |
| 800 | 0.261 | 0.643 |
| % 2C | SD (% 2C) | % 4C | SD (% 4C) | Cysts (Cyst mL−1) | SD Cyst (Cyst mL−1) | |
|---|---|---|---|---|---|---|
| VGO 1424 (P−) | 7.96 | 1.66 | 3.04 | 0.72 | 0 | 0 |
| VGO 1439 (P−) | 9.63 | 3.19 | 1.79 | 0.52 | 0 | 0 |
| Cross (P−) | 13.95 | 3.99 | 4.49 | 1.24 | 198 | 39.73 |
| Predictive Variable | Degrees of Freedom (Df) | Deviance | Residual Df | Residual Deviance | p (>X2) |
|---|---|---|---|---|---|
| Null | 95 | 274.23 | |||
| Light intensity | 3 | 110.706 | 92 | 163.53 | <0.0001 * |
| Days of the experiment | 1 | 61.682 | 91 | 101.84 | <0.0001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sixto, M.; Riobó, P.; Rodríguez, F.; Díaz, P.A.; Figueroa, R.I. Climate Change Stressors, Phosphate Limitation, and High Irradiation Interact to Increase Alexandrium minutum Toxicity and Modulate Encystment Rates. Microorganisms 2024, 12, 1480. https://doi.org/10.3390/microorganisms12071480
Sixto M, Riobó P, Rodríguez F, Díaz PA, Figueroa RI. Climate Change Stressors, Phosphate Limitation, and High Irradiation Interact to Increase Alexandrium minutum Toxicity and Modulate Encystment Rates. Microorganisms. 2024; 12(7):1480. https://doi.org/10.3390/microorganisms12071480
Chicago/Turabian StyleSixto, Marta, Pilar Riobó, Francisco Rodríguez, Patricio A. Díaz, and Rosa I. Figueroa. 2024. "Climate Change Stressors, Phosphate Limitation, and High Irradiation Interact to Increase Alexandrium minutum Toxicity and Modulate Encystment Rates" Microorganisms 12, no. 7: 1480. https://doi.org/10.3390/microorganisms12071480





