The Role of Oncogenic Viruses in Head and Neck Cancers: Epidemiology, Pathogenesis, and Advancements in Detection Methods
Abstract
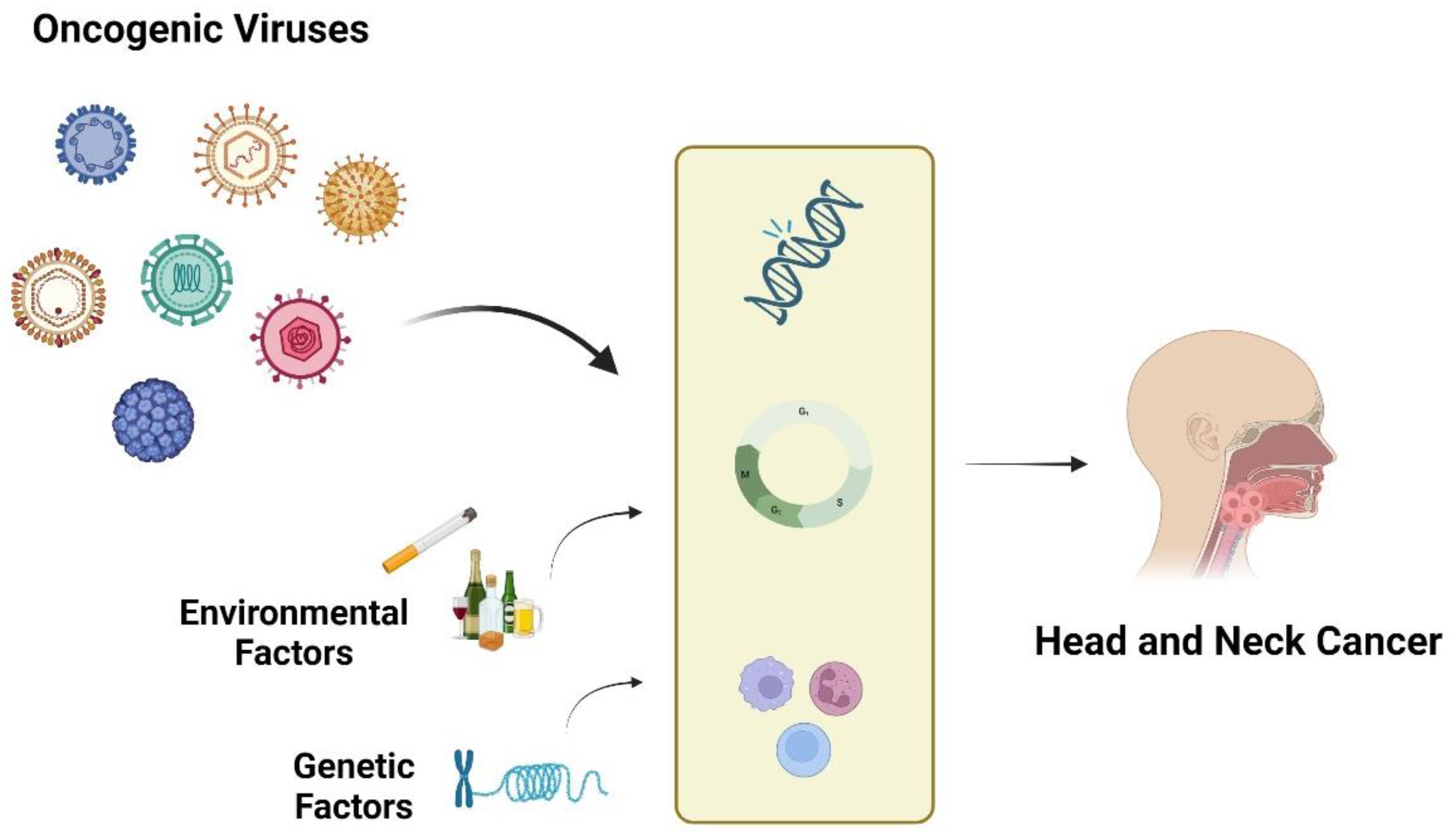
1. Introduction
2. Human Papillomavirus (HPV) in Head and Neck Cancers
2.1. HPV’s Association with Oropharyngeal Cancers: Mechanisms Underlying HPV-Induced Carcinogenesis in the Oropharynx
2.2. HPV Infection and Its Implications in Laryngeal Cancer
2.3. The Role of HPV Vaccination in Prevention Strategies for HPV-Associated HNCs
3. Beyond HPV: Emerging Oncogenic Viruses in HNCs
3.1. Epstein–Barr Virus (EBV): Its Role in Nasopharyngeal and Other Head and Neck Cancers
3.2. Human Herpesvirus 8 (HHV-8): The Second Most Prevalent Oncogenic Herpesvirus
4. Other Potentially Oncogenic Herpesviruses Influencing Head and Neck Cancers
5. Merkel Cell Polyomavirus (MCPV) and Its Role in Head and Neck Cancers
6. Human Bocavirus (HBoV) and Head and Neck Cancers: Exploring Its Role in Tonsil Squamous Cell Carcinomas
7. Epidemiology and Clinical Impact of Hepatitis Viruses in Head and Neck Cancers
8. The Intricacies of Co-Infections: Their Clinical Significance in Head and Neck Cancers
9. Innovations in Viral Detection and Screening for Head and Neck Cancers
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bhat, G.R.; Hyole, R.G.; Li, J. Head and neck cancer: Current challenges and future perspectives. Adv. Cancer Res. 2021, 152, 67–102. [Google Scholar]
- Kamiński, B. Lymphomas of the head-and-neck region. J. Cancer Res. Ther. 2021, 17, 1347–1350. [Google Scholar] [CrossRef]
- Li, Q.; Tie, Y.; Alu, A.; Ma, X.; Shi, H. Targeted therapy for head and neck cancer: Signaling pathways and clinical studies. Signal Transduct. Target. Ther. 2023, 8, 31. [Google Scholar] [CrossRef]
- Sabatini, M.E.; Chiocca, S. Human papillomavirus as a driver of head and neck cancers. Br. J. Cancer 2020, 122, 306–314. [Google Scholar] [CrossRef]
- Payaradka, R.; Ramesh, P.S.; Vyas, R.; Patil, P.; Rajendra, V.K.; Kumar, M.; Shetty, V.; Devegowda, D. Oncogenic viruses as etiological risk factors for head and neck cancers: An overview on prevalence, mechanism of infection and clinical relevance. Arch. Oral. Biol. 2022, 143, 105526. [Google Scholar] [CrossRef]
- Moore, P.S.; Chang, Y. Why do viruses cause cancer? Highlights of the first century of human tumor virology. Nat. Rev. Cancer 2010, 10, 878–889. [Google Scholar] [CrossRef]
- Miura, M.; Naito, T.; Saito, M. Current perspectives in human T-cell leukemia virus type 1 infection and its associated diseases. Front. Med. 2022, 9, 867478. [Google Scholar] [CrossRef]
- Tempera, I.; Lieberman, P.M. Oncogenic viruses as entropic drivers of cancer evolution. Front. Virol. 2021, 1, 753366. [Google Scholar] [CrossRef]
- Narisawa-Saito, M.; Kiyono, T. Basic mechanisms of high-risk human papillomavirus-induced carcinogenesis: Roles of E6 and E7 proteins. Cancer Sci. 2007, 98, 1505–1511. [Google Scholar] [CrossRef]
- Ndon, S.; Singh, A.; Ha, P.K.; Aswani, J.; Chan, J.Y.; Xu, M.J. Human papillomavirus-associated oropharyngeal cancer: Global epidemiology and public policy implications. Cancers 2023, 15, 4080. [Google Scholar] [CrossRef]
- Prabhu, S.R.; Wilson, D.F. Evidence of Epstein-Barr virus association with head and neck cancers: A review. J. Can. Dent. Assoc. 2016, 82, g2. [Google Scholar] [PubMed]
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. The Molecular Landscape of Head and Neck Cancer. Nat. Rev. Cancer 2018, 18, 269–282. [Google Scholar] [CrossRef]
- Swiecicki, P.L.; Brennan, J.R.; Mierzwa, M.; Spector, M.E.; Brenner, J.C. Head and neck squamous cell carcinoma detection and surveillance: Advances of liquid biomarkers. Laryngoscope 2019, 129, 1836–1843. [Google Scholar] [CrossRef]
- Mukherjee, S.; Fischbein, N.J.; Baugnon, K.L.; Policeni, B.A.; Raghavan, P. Contemporary Imaging and Reporting Strategies for Head and Neck Cancer: MRI, FDG PET/MRI, NI-RADS, and Carcinoma of Unknown Primary-AJR Expert Panel Narrative Review. AJR Am. J. Roentgenol. 2023, 220, 160–172. [Google Scholar] [CrossRef]
- Kobayashi, K.; Hisamatsu, K.; Suzui, N.; Hara, A.; Tomita, H.; Miyazaki, T. A Review of HPV-Related Head and Neck Cancer. J. Clin. Med. 2018, 7, 241. [Google Scholar] [CrossRef]
- de Sanjosé, S.; Brotons, M.; Pavón, M.A. The Natural History of Human Papillomavirus Infection. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 47, 2–13. [Google Scholar]
- Scheurer, M.E.; Tortolero-Luna, G.; Adler-Storthz, K. Human Papillomavirus Infection: Biology, Epidemiology, and Prevention. Int. J. Gynecol. Cancer 2005, 15, 727–746. [Google Scholar] [CrossRef] [PubMed]
- Doorbar, J. Model Systems of Human Papillomavirus-Associated Disease. J. Pathol. 2016, 238, 166–179. [Google Scholar] [CrossRef]
- Kajitani, N.; Satsuka, A.; Kawate, A.; Sakai, H. Productive Lifecycle of Human Papillomaviruses Dependent on Squamous Epithelial Differentiation. Front. Microbiol. 2012, 3, 152. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.V.; Galvão de Araújo, J.M.; de Medeiros Fernandes, T. Biology and Natural History of Human Papillomavirus Infection. Open Access J. Clin. Trials 2013, 5, 1–12. [Google Scholar] [CrossRef]
- Moody, C.A.; Laimins, L.A. Human Papillomavirus Oncoproteins: Pathways to Transformation. Nat. Rev. Cancer 2010, 10, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Vinokurova, S.; Wentzensen, N.; Kraus, I.; Klaes, R.; Driesch, C.; Melsheimer, P.; Kisseljov, F.; Dürst, M.; Schneider, A.; von Knebel Doeberitz, M. Type-Dependent Integration Frequency of Human Papillomavirus Genomes in Cervical Lesions. Cancer Res. 2008, 68, 307–313. [Google Scholar] [CrossRef]
- Pinatti, L.M.; Walline, H.M.; Carey, T.E. Human Papillomavirus Genome Integration and Head and Neck Cancer. J. Dent. Res. 2018, 97, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Akagi, K.; Li, J.; Broutian, T.R.; Padilla-Nash, H.; Xiao, W.; Jiang, B.; Rocco, J.W.; Teknos, T.N.; Kumar, B.; Wangsa, D.; et al. Genome-wide analysis of HPV integration in human cancers reveals recurrent, focal genomic instability. Genome Res. 2014, 24, 185–199. [Google Scholar] [CrossRef]
- Liberale, C.; Soloperto, D.; Marchioni, A.; Monzani, D.; Sacchetto, L. Updates on Larynx Cancer: Risk Factors and Oncogenesis. Int. J. Mol. Sci. 2023, 24, 12913. [Google Scholar] [CrossRef]
- Li, X.; Gao, L.; Li, H.; Gao, J.; Yang, Y.; Zhou, F.; Gao, C.; Li, M.; Jin, Q. Human papillomavirus infection and laryngeal cancer risk: A systematic review and meta-analysis. J. Infect. Dis. 2013, 207, 479–488. [Google Scholar] [CrossRef]
- Gama, R.R.; Carvalho, A.L.; Longatto Filho, A.; Scorsato, A.P.; López, R.V.; Rautava, J.; Syrjänen, S.; Syrjänen, K. Detection of human papillomavirus in laryngeal squamous cell carcinoma: Systematic review and meta-analysis. Laryngoscope 2016, 126, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Erkul, E.; Yilmaz, I.; Narli, G.; Babayigit, M.A.; Gungor, A.; Demirel, D. The presence and prognostic significance of human papillomavirus in squamous cell carcinoma of the larynx. Eur. Arch. Otorhinolaryngol. 2017, 274, 2921–2926. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, Z.; Aria, H.; Ghaedrahmati, F.; Bakhtiari, T.; Azizi, M.; Bastan, R.; Hosseini, R.; Eskandari, N. An Update on Human Papilloma Virus Vaccines: History, Types, Protection, and Efficacy. Front. Immunol. 2022, 12, 805695. [Google Scholar] [CrossRef]
- Goldstone, S.E. Human papillomavirus (HPV) vaccines in adults: Learnings from long-term follow-up of quadrivalent HPV vaccine clinical trials. Hum. Vaccin. Immunother. 2023, 19, 2184760. [Google Scholar] [CrossRef]
- Zhou, J.Z.; Jou, J.; Cohen, E. Vaccine Strategies for Human Papillomavirus-Associated Head and Neck Cancers. Cancers 2021, 14, 33. [Google Scholar] [CrossRef]
- Nassif, S.J.; Steinwald, P.; Tracy, J.C. Human Papillomavirus Vaccine and Head and Neck Cancer. JAMA Otolaryngol. Head. Neck Surg. 2022, 148, 204. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Graubard, B.I.; Broutian, T.; Xiao, W.; Pickard, R.K.L.; Kahle, L.; Gillison, M.L. Prevalence of Oral HPV Infection in Unvaccinated Men and Women in the United States, 2009–2016. JAMA 2019, 322, 977–979. [Google Scholar] [CrossRef]
- Osazuwa-Peters, N.; Graboyes, E.M.; Khariwala, S.S. Expanding Indications for the Human Papillomavirus Vaccine: A Small Step Toward Head and Neck Cancer Prevention, with More to Achieve. JAMA Otolaryngol. Head. Neck Surg. 2020, 146, 1099–1101. [Google Scholar] [CrossRef]
- Giuliano, A.R.; Wilkin, T.; Bautista, O.M.; Cheon, K.; Connor, L.; Dubey, S.; Thomas Group; Luxembourg, A.; Rawat, S.; Shaw, A.; et al. Design of a phase III efficacy, immunogenicity, and safety study of 9-valent human papillomavirus vaccine in prevention of oral persistent infection in men. Contemp. Clin. Trials 2022, 115, 106592. [Google Scholar] [CrossRef]
- Epstein, M.A.; Achong, B.G.; Barr, Y.M. Virus particles in cultured lymphoblasts from Burkitt’s lymphma. Lancet 1964, 283, 702–703. [Google Scholar] [CrossRef]
- Damania, B.; Kenney, S.C.; Raab-Traub, N. Epstein-Barr virus: Biology and clinical disease. Cell 2022, 185, 3652–3670. [Google Scholar] [CrossRef]
- Bakkalci, D.; Jia, Y.; Winter, J.R.; Lewis, J.E.; Taylor, G.S.; Stagg, H.R. Risk factors for Epstein Barr virus-associated cancers: A systematic review, critical appraisal, and mapping of the epidemiological evidence. J. Glob. Health 2020, 10, 010405. [Google Scholar] [CrossRef]
- Scott, R.S. Epstein-Barr virus: A master epigenetic manipulator. Curr. Opin. Virol. 2017, 26, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Longnecker, R.; Kieff, E.; Cohen, J.I. Epstein-Barr Virus. In Fields Virology, 6th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; Volume 2, pp. 1898–1959. [Google Scholar]
- Liebowitz, D.; Kieff, E. Epstein-Barr Virus. In The Human Herpesvirus; Roizman, B., Whitley, R.J., Lopez, C., Eds.; Raven Press: New York, NY, USA, 1993; pp. 107–172. [Google Scholar]
- McKenzie, J.; El-Guindy, A. Epstein-Barr Virus Lytic Cycle Reactivation. Curr. Top. Microbiol. Immunol. 2015, 391, 237–261. [Google Scholar] [PubMed]
- Tsao, S.W.; Tsang, C.M.; Lo, K.W. Epstein-Barr virus infection and nasopharyngeal carcinoma. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160270. [Google Scholar] [CrossRef]
- Guidry, J.T.; Birdwell, C.E.; Scott, R.S. Epstein-Barr virus in the pathogenesis of oral cancers. Oral. Dis. 2018, 24, 497–508. [Google Scholar] [CrossRef]
- Brichácek, B.; Hirsch, I.; Síbl, O.; Vilikusová, E.; Vonka, V. Association of some supraglottic laryngeal carcinomas with EB virus. Int. J. Cancer 1983, 32, 193–197. [Google Scholar] [CrossRef]
- de Lima, M.A.P.; Silva, Á.D.L.; do Nascimento Filho, A.C.S.; Cordeiro, T.L.; Bezerra, J.P.S.; Rocha, M.A.B.; Pinheiro, S.F.L.; Pinheiro Junior, R.F.F.; Gadelha, M.D.S.V.; da Silva, C.G.L. Epstein-Barr Virus-Associated Carcinoma of the Larynx: A Systematic Review with Meta-Analysis. Pathogens 2021, 10, 1429. [Google Scholar] [CrossRef]
- Cathomas, G. Kaposi’s sarcoma-associated herpesvirus (KSHV)/human herpesvirus 8 (HHV-8) as a tumor virus. Herpes 2003, 10, 72–77. [Google Scholar]
- Stanberry, L.R. Herpes Viruses. In International Encyclopedia of Public Health; Elsevier: Amsterdam, The Netherlands, 2008; pp. 382–389. [Google Scholar]
- Dittmer, D.P.; Damania, B. Kaposi sarcoma-associated herpesvirus pathogenesis (KSHV)-an update. Curr. Opin. Virol. 2013, 3, 238–244. [Google Scholar] [CrossRef]
- Bhatt, A.P.; Damania, B. AKTivation of PI3K/AKT/mTOR signaling pathway by KSHV. Front. Immunol. 2013, 3, 401. [Google Scholar] [CrossRef]
- Nador, R.G.; Cesarman, E.; Chadburn, A.; Dawson, D.B.; Ansari, M.Q.; Sald, J.; Knowles, D.M. Primary effusion lymphoma: A distinct clinicopathologic entity associated with the Kaposi’s sarcoma-associated herpes virus. Blood 1996, 88, 645–656. [Google Scholar] [CrossRef]
- Huang, G.; Low, G. Human Herpes Virus-8-Associated Multicentric Castleman’s Disease in a Human Immunodeficiency Virus-Positive Patient with a Previous History of Kaposi’s Sarcoma. J. Clin. Imaging Sci. 2015, 5, 59. [Google Scholar] [CrossRef]
- Güvenç, M.G.; Midilli, K.; Ozdoğan, A.; Inci, E.; Tahamiler, R.; Enver, O.; Sirin, G.; Ergin, S.; Kuşkucu, M.; Divanoğlu, E.O.; et al. Detection of HHV-8 and HPV in laryngeal carcinoma. Auris Nasus Larynx 2008, 35, 357–362. [Google Scholar]
- Parker, T.M.; Smith, E.M.; Ritchie, J.M.; Haugen, T.H.; Vonka, V.; Turek, L.P.; Hamsikova, E. Head and neck cancer associated with herpes simplex virus 1 and 2 and other risk factors. Oral. Oncol. 2006, 42, 288–296. [Google Scholar] [CrossRef]
- Koivikko, T.; Rodrigues, P.C.; Vehviläinen, M.; Hyvönen, P.; Sundquist, E.; Arffman, R.K.; Al-Samadi, A.; Välimaa, H.; Salo, T.; Risteli, M. Detection of herpes simplex virus in oral tongue squamous cell carcinoma. Front. Pharmacol. 2023, 14, 1182152. [Google Scholar] [CrossRef]
- von Stebut, J.; Heiland, M.; Preissner, R.; Rendenbach, C.; Preissner, S. Association of Herpes simplex infection with significantly increased risk of head and neck cancer: Real-world evidence of about 500,000 patients. Int. J. Dermatol. 2024. [Google Scholar] [CrossRef]
- Trivic, A.; Milovanovic, J.; Kablar, D.; Tomic, A.; Folic, M.; Jotic, A.; Tomanovic, N.; Tomic, A.M.; Djoric, I.; Jankovic, M. Friend or Foe? Exploring the Role of Cytomegalovirus (HCMV) Infection in Head and Neck Tumors. Biomedicines 2024, 12, 872. [Google Scholar] [CrossRef]
- Wołącewicz, M.; Becht, R.; Grywalska, E.; Niedźwiedzka-Rystwej, P. Herpesviruses in Head and Neck Cancers. Viruses 2020, 12, 172. [Google Scholar] [CrossRef]
- Poluschkin, L.; Rautava, J.; Turunen, A.; Wang, Y.; Hedman, K.; Syrjänen, K.; Grenman, R.; Syrjänen, S. Polyomaviruses detectable in head and neck carcinomas. Oncotarget 2018, 9, 22642–22652. [Google Scholar] [CrossRef]
- Mohebbi, E.; Noormohamadi, Z.; Sadeghi-Rad, H.; Sadeghi, F.; Yahyapour, Y.; Vaziri, F.; Rahimi, A.; Rahimi Jamnani, F.; Mehrabi, S.; Siadat, S.D.; et al. Low viral load of Merkel cell polyomavirus in Iranian patients with head and neck squamous cell carcinoma: Is it clinically important? J. Med. Virol. 2018, 90, 344–350. [Google Scholar] [CrossRef]
- Muñoz, J.P.; Blanco, R.; Osorio, J.C.; Oliva, C.; Diaz, M.J.; Carrillo-Beltrán, D.; Aguayo, R.; Castillo, A.; Tapia, J.C.; Calaf, G.M.; et al. Merkel cell polyomavirus detected in head and neck carcinomas from Chile. Infect. Agent. Cancer 2020, 15, 4. [Google Scholar] [CrossRef]
- Hasani Estalkhi, M.; Seyed Majidi, M.; Sadeghi, F.; Chehrazi, M.; Zebardast, A.; Hasanzadeh, A.; Yahyapour, Y. Prevalence of Merkel Cell Polyomavirus (MCPyV) in the Oral Cavity Biopsies in Northern Iran. Asian Pac. J. Cancer Prev. 2021, 22, 3927–3932. [Google Scholar] [CrossRef]
- Allander, T.; Tammi, M.T.; Eriksson, M.; Bjerkner, A.; Tiveljung-Lindell, A.; Andersson, B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc. Natl. Acad. Sci. USA 2005, 102, 12891–12896. [Google Scholar] [CrossRef]
- Schildgen, V.; Malecki, M.; Tillmann, R.L.; Brockmann, M.; Schildgen, O. The Human Bocavirus Is Associated with Some Lung and Colorectal Cancers and Persists in Solid Tumors. PLoS ONE 2013, 8, e68020. [Google Scholar] [CrossRef]
- Höpken, M.; Förster, I.; Maune, S.; Brockmann, M.; Schildgen, O.; Schildgen, V. Association of the Human Bocavirus with tonsil squamous cell carcinomas. Front. Microbiol. 2018, 9, 2450. [Google Scholar] [CrossRef]
- Schildgen, V.; Pieper, M.; Khalfaoui, S.; Arnold, W.H.; Schildgen, O. Human Bocavirus Infection of Permanent Cells Differentiated to Air-Liquid Interface Cultures Activates Transcription of Pathways Involved in Tumorigenesis. Cancers 2018, 10, 410. [Google Scholar] [CrossRef]
- Ivaska, L.E.; Silvoniemi, A.; Palomares, O.; Turunen, R.; Waris, M.; Mikola, E.; Puhakka, T.; Söderlund-Venermo, M.; Akdis, M.; Akdis, C.A.; et al. Persistent human bocavirus 1 infection and tonsillar immune responses. Clin. Transl. Allergy 2021, 11, e12030. [Google Scholar] [CrossRef]
- D’souza, S.; Lau, K.C.; Coffin, C.S.; Patel, T.R. Molecular mechanisms of viral hepatitis induced hepatocellular carcinoma. World J. Gastroenterol. 2020, 26, 5759–5783. [Google Scholar] [CrossRef]
- Kim, M.; Lee, Y.K.; Park, B.; Oh, D.J.; Choi, H.G. Hepatitis virus B and C infections are associated with an increased risk of non-Hodgkin lymphoma: A nested case-control study using a national sample cohort. J. Med. Virol. 2020, 92, 1214–1220. [Google Scholar] [CrossRef]
- Gondivkar, S.M.; Parikh, R.V.; Gadbail, A.R.; Solanke, V.; Chole, R.; Mankar, M.; Balsaraf, S. Involvement of viral factors with head and neck cancers. Oral. Oncol. 2012, 48, 195–199. [Google Scholar] [CrossRef]
- Hettmann, A.; Demcsák, A.; Decsi, G.; Bach, Á.; Pálinkó, D.; Rovó, L.; Nagy, K.; Takács, M.; Minarovits, J. Infectious Agents Associated with Head and Neck Carcinomas. Adv. Exp. Med. Biol. 2016, 897, 63–80. [Google Scholar]
- Tsai, K.N.; Kuo, C.F.; Ou, J.J. Mechanisms of Hepatitis B Virus Persistence. Trends Microbiol. 2018, 26, 33–42. [Google Scholar] [CrossRef]
- McGivern, D.R.; Lemon, S.M. Virus-specific mechanisms of carcinogenesis in hepatitis C virus associated liver cancer. Oncogene 2011, 30, 1969–1983. [Google Scholar] [CrossRef] [PubMed]
- Guntaka Ramareddy, V.; Padala Mythili, K. Interaction of Hepatitis C Viral Proteins with Cellular Oncoproteins in the Induction of Liver Cancer. Int. Sch. Res. Not. 2014, 351407. [Google Scholar] [CrossRef]
- Donà, S.; Borsetto, D.; Fussey, J.; Biscaro, V.; Vian, E.; Spinato, G.; Menegaldo, A.; Da Mosto, M.C.; Rigoli, R.; Polesel, J.; et al. Association between hepatitis C and B viruses and head and neck squamous cell carcinoma. J. Clin. Virol. 2019, 121, 104209. [Google Scholar] [CrossRef]
- Nayyar, S.S.; Thiagarajan, S.; Malik, A.; D’Cruz, A.; Chaukar, D.; Patil, P.; Alahari, A.D.; Lashkar, S.G.; Prabhash, K. Head and neck squamous cell carcinoma in HIV, HBV and HCV seropositive patients-Prognosis and its predictors. J. Cancer Res. Ther. 2020, 16, 619–623. [Google Scholar] [CrossRef]
- Komori, M.F.; Kimura, T.; Kariya, S.; Onoda, T.; Takeda, S.; Mizukawa, N.; Iida, S.; Kimata, Y.; Nishizaki, K. Epidemiological Correlations Between Head and Neck Cancer and Hepatitis B Core Antibody Positivity. Anticancer Res. 2020, 40, 2393–2403. [Google Scholar] [CrossRef]
- Su, T.H.; Tseng, T.C.; Liu, C.J.; Chou, S.W.; Liu, C.H.; Yang, H.C.; Chen, P.J.; Chen, D.S.; Chen, C.L.; Kao, J.H. Antiviral therapy against chronic hepatitis C is associated with a reduced risk of oral cancer. Int. J. Cancer 2020, 147, 901–908. [Google Scholar] [CrossRef]
- Borsetto, D.; Fussey, J.; Fabris, L.; Bandolin, L.; Gaudioso, P.; Phillips, V.; Polesel, J.; Boscolo-Rizzo, P. HCV infection and the risk of head and neck cancer: A meta-analysis. Oral. Oncol. 2020, 109, 104869. [Google Scholar] [CrossRef]
- Hung, S.H.; Yang, T.H.; Cheng, Y.F.; Chen, C.S.; Lin, H.C. Associations of Head and Neck Cancer with Hepatitis B Virus and Hepatitis C Virus Infection. Cancers 2023, 15, 4510. [Google Scholar] [CrossRef]
- Tan, R.; Zhu, X.; Sun, Y.; Yang, S.L.; Peng, C.; Feng, X.; Chen, Z.; Yimamu, Y.; Liao, G.; Yang, L. The association of HBV infection and head and neck cancer: A systematic review and meta-analysis. BMC Cancer 2024, 24, 225. [Google Scholar] [CrossRef]
- Lai, C.L.; Lin, C.H.; Su, Y.C.; Shih, Y.H.; Wang, C.C.; Teng, C.J.; Chou, C.W. Survival outcomes of patients with head and neck squamous cell cancer with hepatitis B virus infection: An analysis from an endemic tertiary center. Cancer Med. 2023, 12, 6802–6810. [Google Scholar] [CrossRef] [PubMed]
- Beachler, D.C.; DʼSouza, G. Oral human papillomavirus infection and head and neck cancers in HIV-infected individuals. Curr. Opin. Oncol. 2013, 25, 503–510. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Madeleine, M.M.; Biggar, R.J.; Engels, E.A. Risk of human papillomavirus-associated cancers among persons with AIDS. J. Natl. Cancer Inst. 2009, 101, 1120–1130. [Google Scholar] [CrossRef] [PubMed]
- Salahuddin, S.; Cohen, O.; Wu, M.; Irizarry, J.P.; Vega, T.; Gan, G.; Deng, Y.; Isaeva, N.; Schalper, K.A.; Mehra, S.; et al. Impact of HIV infection on clinical outcomes among people diagnosed with head and neck cancer. J. Clin. Oncol. 2022, 40, e18080. [Google Scholar] [CrossRef]
- Yusuf, K.; Sampath, V.; Umar, S. Bacterial infections and cancer: Exploring this association and its implications for cancer patients. Int. J. Mol. Sci. 2023, 24, 3110. [Google Scholar] [CrossRef] [PubMed]
- Wroblewski, L.E.; Peek, R.M., Jr.; Wilson, K.T. Helicobacter pylori and gastric cancer: Factors that modulate disease risk. Clin. Microbiol. Rev. 2010, 4, 713–739. [Google Scholar] [CrossRef] [PubMed]
- Irani, S.; Barati, I.; Badiei, M. Periodontitis and oral cancer—Current concepts of the etiopathogenesis. Oncol. Rev. 2020, 14, 465. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, I.; Verma, M.; Panda, M. Role of oral microbiome signatures in diagnosis and prognosis of oral cancer. Technol. Cancer Res. Treat. 2019, 18, 1533033819867354. [Google Scholar] [CrossRef]
- Baima, G.; Minoli, M.; Michaud, D.S.; Aimetti, M.; Sanz, M.; Loos, B.G.; Romandini, M. Periodontitis and risk of cancer: Mechanistic evidence. Periodontology 2000 2023. [Google Scholar] [CrossRef] [PubMed]
- Polz-Gruszka, D.; Stec, A.; Dworzański, J.; Polz-Dacewicz, M. EBV, HSV, CMV and HPV in laryngeal and oropharyngeal carcinoma in Polish patients. Anticancer Res. 2015, 35, 1657–1661. [Google Scholar] [PubMed]
- Vanshika, S.; Preeti, A.; Sumaira, Q.; Vijay, K.; Shikha, T.; Shivanjali, R.; Shankar, S.U.; Mati, G.M. Incidence OF HPV and EBV in oral cancer and their clinico-pathological correlation- a pilot study of 108 cases. J. Oral. Biol. Craniofac. Res. 2021, 11, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Blanco, R.; Carrillo-Beltrán, D.; Corvalán, A.H.; Aguayo, F. High-Risk Human Papillomavirus and Epstein-Barr Virus Coinfection: A Potential Role in Head and Neck Carcinogenesis. Biology 2021, 10, 1232. [Google Scholar] [CrossRef]
- Migliaro, M.; Massuh, D.; Infante, M.F.; Brahm, A.M.; San Martín, M.T.; Ortuño, D. Role of Epstein-Barr virus and human papilloma virus in the development of oropharyngeal cancer: A literature review. Int. J. Dent. 2022, 2022, 3191569. [Google Scholar] [CrossRef] [PubMed]
- Al-Thawadi, H.; Gupta, I.; Jabeen, A.; Skenderi, F.; Aboulkassim, T.; Yasmeen, A.; Malki, M.I.; Batist, G.; Vranic, S.; Al Moustafa, A.E. Co-presence of human papillomaviruses and Epstein-Barr virus is linked with advanced tumor stage: A tissue microarray study in head and neck cancer patients. Cancer Cell Int. 2020, 20, 361. [Google Scholar] [CrossRef] [PubMed]
- Ursu, R.G.; Luchian, I.; Ghetu, N.; Costan, V.V.; Stamatin, O.; Palade, O.D.; Damian, C.; Iancu, L.S.; Porumb-Andrese, E. Emerging Oncogenic Viruses in Head and Neck Cancers from Romanian Patients. Appl. Sci. 2021, 11, 9356. [Google Scholar] [CrossRef]
- Mulder, F.J.; Klufah, F.; Janssen, F.M.E.; Farshadpour, F.; Willems, S.M.; de Bree, R.; Zur Hausen, A.; van den Hout, M.F.C.M.; Kremer, B.; Speel, E.M. Presence of human papillomavirus and Epstein-Barr virus, but absence of merkel cell polyomavirus, in head and neck cancer of non-smokers and non-drinkers. Front. Oncol. 2021, 10, 560434. [Google Scholar] [CrossRef] [PubMed]
- Schindele, A.; Holm, A.; Nylander, K.; Allard, A.; Olofsson, K. Mapping human papillomavirus, Epstein-Barr virus, cytomegalovirus, adenovirus, and p16 in laryngeal cancer. Discov. Oncol. 2022, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Solis, R.N.; Silverman, D.A.; Birkeland, A.C. Current trends in precision medicine and next-generation sequencing in head and neck cancer. Curr. Treat. Options Oncol. 2022, 23, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Dongre, H.N.; Haave, H.; Fromreide, S.; Erland, F.A.; Moe, S.E.E.; Dhayalan, S.M.; Riis, R.K.; Sapkota, D.; Costea, D.E.; Aarstad, H.J.; et al. Targeted next-generation sequencing of cancer-related genes in a Norwegian patient cohort with head and neck squamous cell carcinoma reveals novel actionable mutations and correlations with pathological parameters. Front. Oncol. 2021, 11, 734134. [Google Scholar] [CrossRef] [PubMed]
- Faden, D.L. Liquid biopsy for the diagnosis of HPV-associated head and neck cancer. Cancer Cytopathol. 2022, 130, 12–15. [Google Scholar] [CrossRef]
- Ferrandino, R.M.; Chen, S.; Kappauf, C.; Barlow, J.; Gold, B.S.; Berger, M.H.; Westra, W.H.; Teng, M.S.; Khan, M.N.; Posner, M.R.; et al. Performance of liquid biopsy for diagnosis and surveillance of human papillomavirus-associated oropharyngeal cancer. JAMA Otolaryngol. Head. Neck Surg. 2023, 149, 971–977. [Google Scholar] [CrossRef]
- Aye, L.; Bryan, M.E.; Das, D.; Hirayama, S.; Al-Inaya, Y.; Mendel, J.; Naegele, S.; Faden, D. Multi-feature next-generation liquid biopsy for diagnosis and prognosis in HPV-associated head and neck cancer. J. Clin. Oncol. 2024, 42, 16. [Google Scholar] [CrossRef]
- Bhambhani, C.; Kang, Q.; Hovelson, D.H.; Sandford, E.; Olesnavich, M.; Dermody, S.M.; Wolfgang, J.; Tuck, K.L.; Brummel, C.; Bhangale, A.D.; et al. ctDNA transiting into urine is ultrashort and facilitates noninvasive liquid biopsy of HPV+ oropharyngeal cancer. JCI Insight 2024, 9, e177759. [Google Scholar] [CrossRef] [PubMed]
| Virus | Genome Type | Associated Cancers in Head and Neck Region |
|---|---|---|
| Human Papillomavirus (HPV) | DNA | Oropharyngeal, laryngeal, oral cavity |
| Epstein–Barr Virus (EBV) | DNA | Nasopharyngeal, Burkitt & Hodgkin lymphomas |
| Human Herpesvirus 8 (HHV-8) | DNA | Kaposi’s sarcoma, primary effusion lymphoma, multicentric Castleman disease, laryngeal |
| Merkel Cell Polyomavirus (MCPV) | DNA | Merkel cell carcinoma, head & neck squamous cell, oral squamous cell carcinomas |
| Hepatitis B Virus (HBV) | DNA | Head & neck squamous cell carcinoma |
| Hepatitis C Virus (HCV) | RNA | Head & neck squamous cell carcinoma, oral cavity |
| Human Herpesvirus 1 (HHV-1) | DNA | Correlation with lip, oral cavity & pharynx |
| Cytomegalovirus (CMV) | DNA | Pro-oncogenic link with nasopharyngeal, protective effect with tumors of the lip/oral region & salivary glands |
| Human Herpesviruses 2, 3, 6, 7 (HHV-2, HHV-3, HHV-6, HHV-7) | DNA | Potential tumorigenic properties—not fully elucidated |
| Human Bocavirus (HBoV) | DNA | Potential involvement in tonsil squamous cell carcinoma |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samara, P.; Athanasopoulos, M.; Mastronikolis, S.; Kyrodimos, E.; Athanasopoulos, I.; Mastronikolis, N.S. The Role of Oncogenic Viruses in Head and Neck Cancers: Epidemiology, Pathogenesis, and Advancements in Detection Methods. Microorganisms 2024, 12, 1482. https://doi.org/10.3390/microorganisms12071482
Samara P, Athanasopoulos M, Mastronikolis S, Kyrodimos E, Athanasopoulos I, Mastronikolis NS. The Role of Oncogenic Viruses in Head and Neck Cancers: Epidemiology, Pathogenesis, and Advancements in Detection Methods. Microorganisms. 2024; 12(7):1482. https://doi.org/10.3390/microorganisms12071482
Chicago/Turabian StyleSamara, Pinelopi, Michail Athanasopoulos, Stylianos Mastronikolis, Efthymios Kyrodimos, Ioannis Athanasopoulos, and Nicholas S. Mastronikolis. 2024. "The Role of Oncogenic Viruses in Head and Neck Cancers: Epidemiology, Pathogenesis, and Advancements in Detection Methods" Microorganisms 12, no. 7: 1482. https://doi.org/10.3390/microorganisms12071482
APA StyleSamara, P., Athanasopoulos, M., Mastronikolis, S., Kyrodimos, E., Athanasopoulos, I., & Mastronikolis, N. S. (2024). The Role of Oncogenic Viruses in Head and Neck Cancers: Epidemiology, Pathogenesis, and Advancements in Detection Methods. Microorganisms, 12(7), 1482. https://doi.org/10.3390/microorganisms12071482






