Optimization of H2O2 Production in Biological Systems for Design of Bio-Fenton Reactors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Microorganisms
2.2. Analytical Methods
2.3. The Characterization of Wastewater
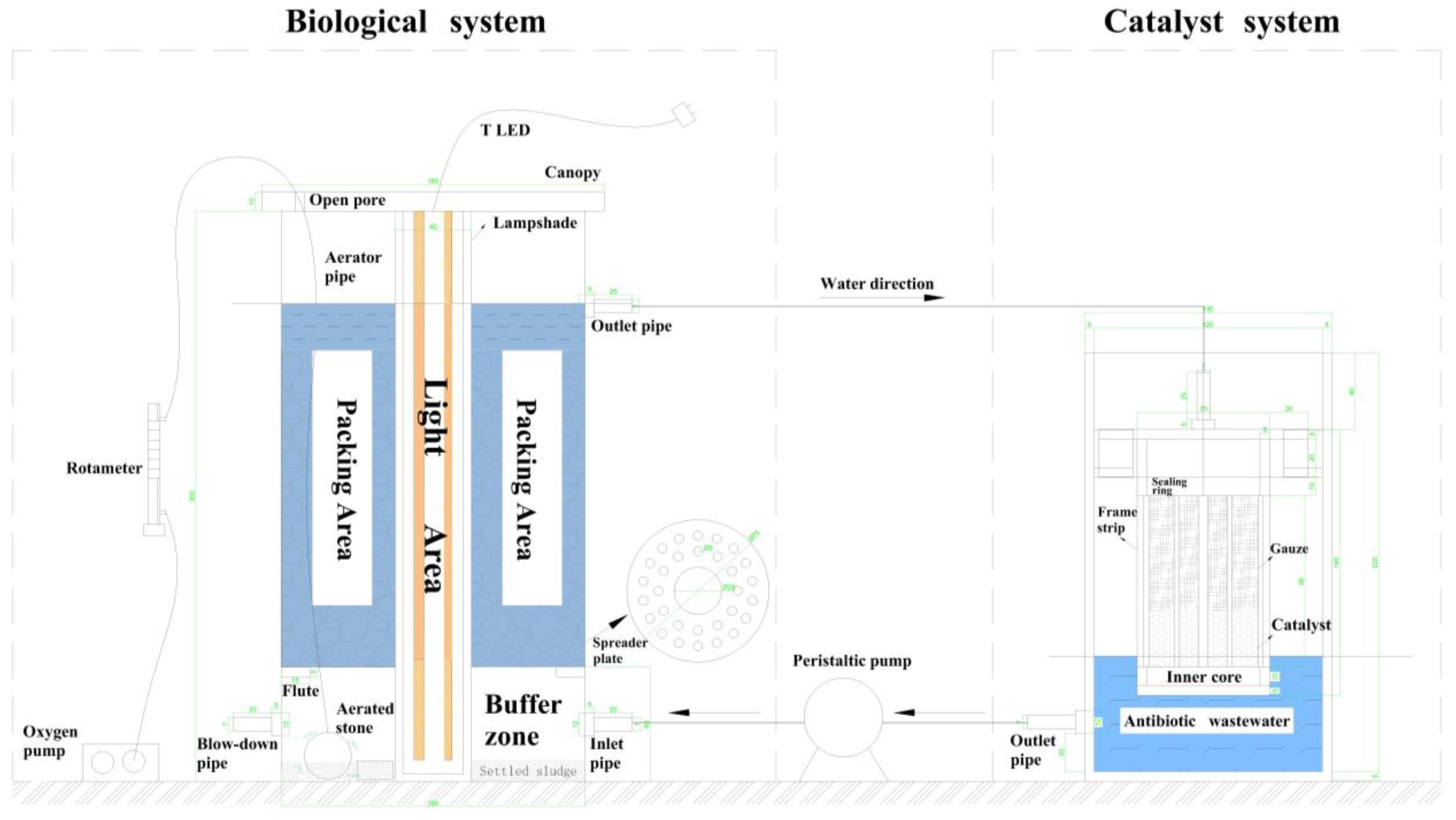
2.4. Dynamically Coupled Bio-Fenton Reactor
3. Results and Discussion
3.1. Characterization of Fillers and Microorganisms
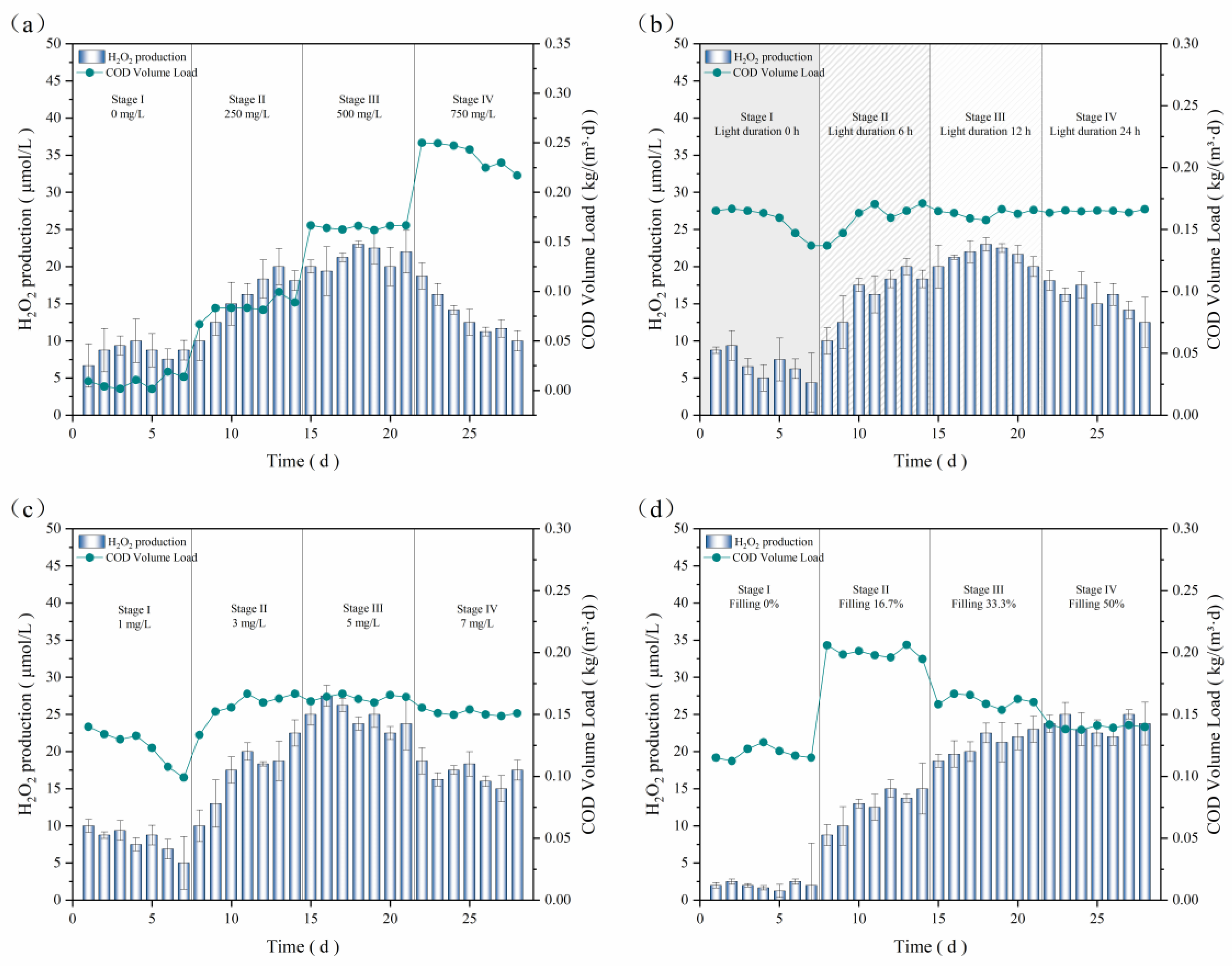
3.2. Production of H2O2 by Biological Systems
3.2.1. Pure Algae System
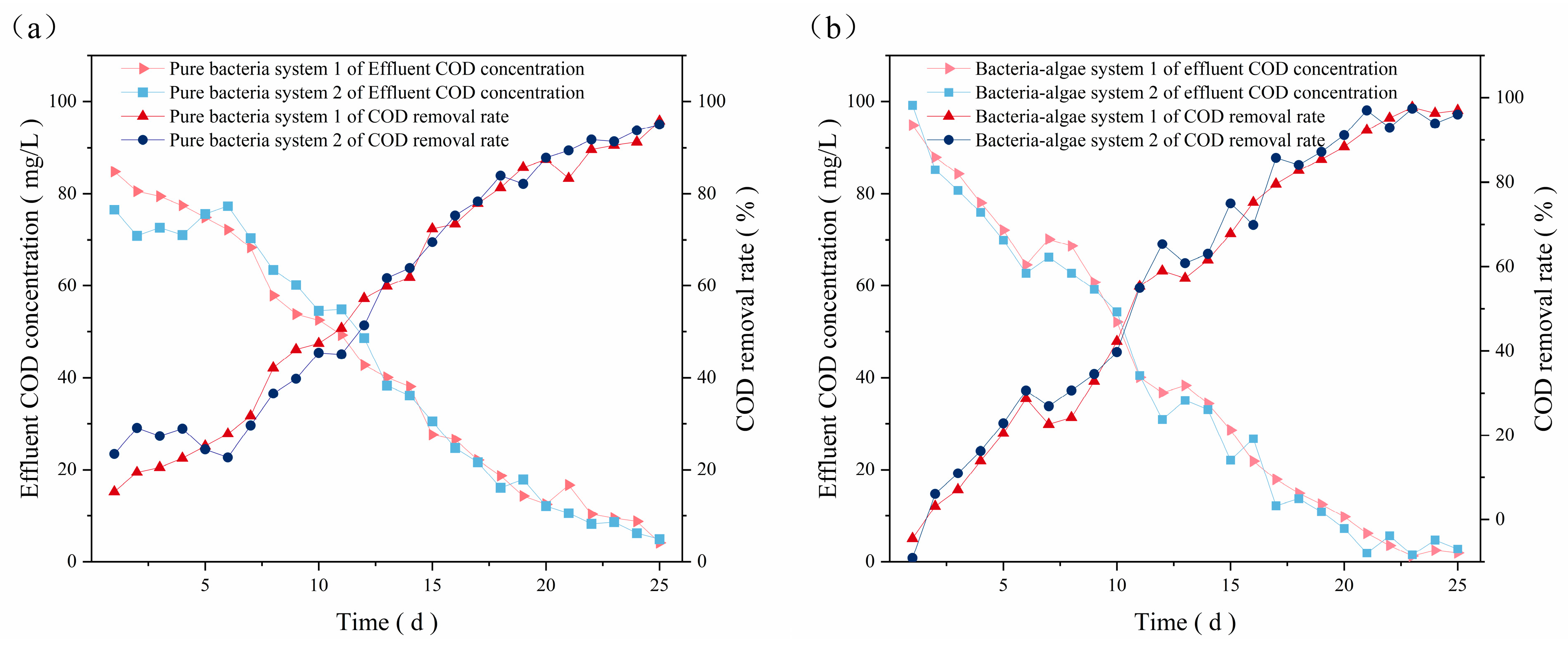
3.2.2. Pure Bacterial System
3.2.3. Bacteria–Algae System
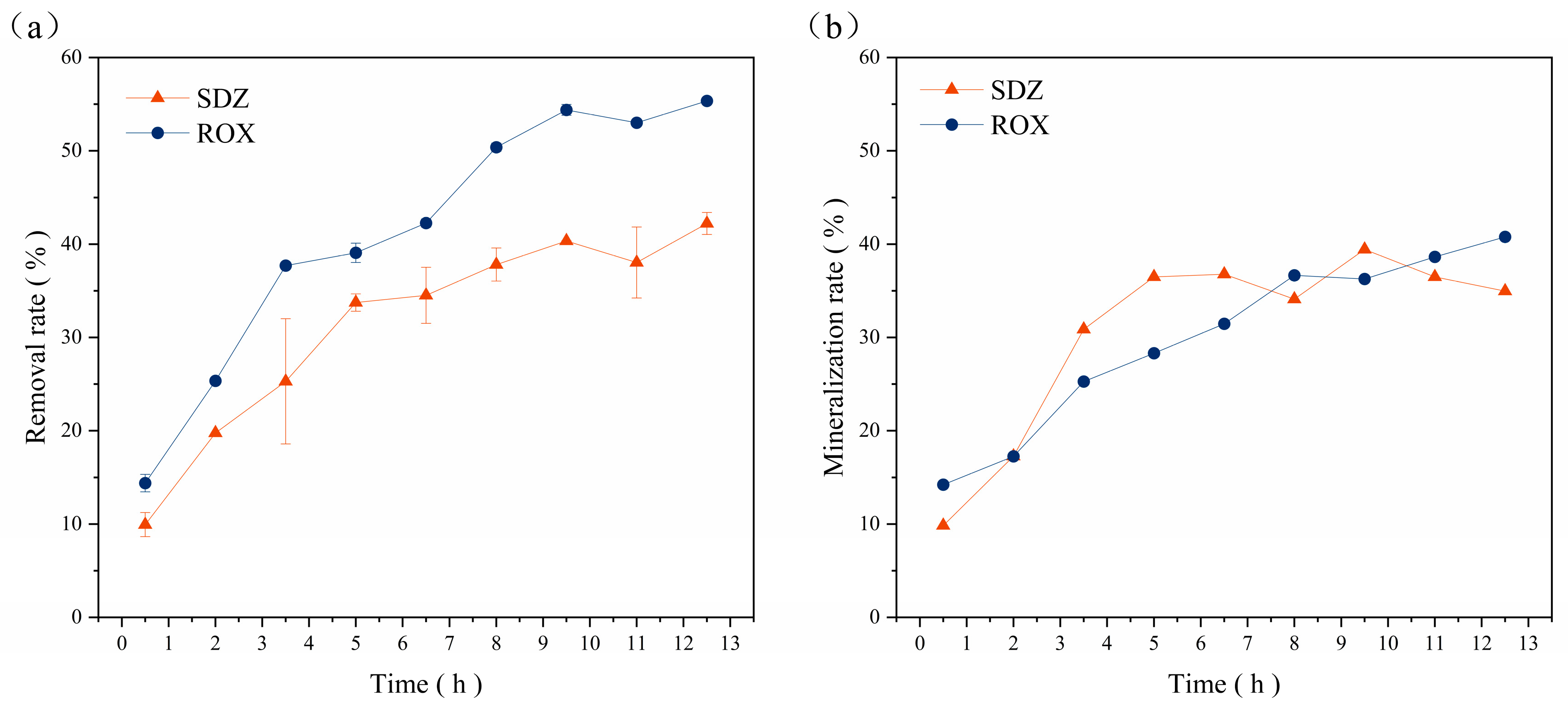
3.3. Dynamically Coupled Bio-Fenton Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Z.; Li, M.; Zhang, Z.; Li, P.; Zang, Y.; Liu, X. Antibiotics in Aquatic Environments of China: A Review and Meta-Analysis. Ecotoxicol. Environ. Saf. 2020, 199, 110668. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-Q.; Ying, G.-G.; Pan, C.-G.; Liu, Y.-S.; Zhao, J.-L. Comprehensive Evaluation of Antibiotics Emission and Fate in the River Basins of China: Source Analysis, Multimedia Modeling, and Linkage to Bacterial Resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Shi, Y.; Li, W.; Niu, H.; Liu, J.; Cai, Y. Occurrence of Antibiotics in Eight Sewage Treatment Plants in Beijing, China. Chemosphere 2012, 86, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Oberoi, A.S.; Jia, Y.; Zhang, H.; Khanal, S.K.; Lu, H. Insights into the Fate and Removal of Antibiotics in Engineered Biological Treatment Systems: A Critical Review. Environ. Sci. Technol. 2019, 53, 7234–7264. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhou, J.L. Occurrence and Behavior of Antibiotics in Water and Sediments from the Huangpu River, Shanghai, China. Chemosphere 2014, 95, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Xu, C.; Huang, Y.; Nie, H.; Wang, J. Tetracyclines, Sulfonamides and Quinolones and Their Corresponding Resistance Genes in the Three Gorges Reservoir, China. Sci. Total Environ. 2018, 631–632, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-H.; Liu, Y.; Du, P.-P.; Zeng, L.-J.; Mo, C.-H.; Li, Y.-W.; Lü, H.; Cai, Q.-Y. Occurrence and Distribution of Antibiotics and Antibiotic Resistant Genes in Water and Sediments of Urban Rivers with Black-Odor Water in Guangzhou, South China. Sci. Total Environ. 2019, 670, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Phoon, B.L.; Ong, C.C.; Mohamed Saheed, M.S.; Show, P.-L.; Chang, J.-S.; Ling, T.C.; Lam, S.S.; Juan, J.C. Conventional and Emerging Technologies for Removal of Antibiotics from Wastewater. J. Hazard. Mater. 2020, 400, 122961. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, Y.; Zhou, C.; Guo, C.; Wang, D.; Du, P.; Luo, Y.; Wan, J.; Meng, W. Distribution, Sources and Composition of Antibiotics in Sediment, Overlying Water and Pore Water from Taihu Lake, China. Sci. Total Environ. 2014, 497–498, 267–273. [Google Scholar] [CrossRef]
- Li, J.; Qiu, X.; Ren, S.; Liu, H.; Zhao, S.; Tong, Z.; Wang, Y. High Performance Electroactive Ultrafiltration Membrane for Antibiotic Resistance Removal from Wastewater Effluent. J. Membr. Sci. 2023, 672, 121429. [Google Scholar] [CrossRef]
- Rambabu, K.; Banat, F.; Pham, Q.M.; Ho, S.-H.; Ren, N.-Q.; Show, P.L. Biological Remediation of Acid Mine Drainage: Review of Past Trends and Current Outlook. Environ. Sci. Ecotechnol. 2020, 2, 100024. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.J.; Janssen, F.; Polerecky, L.; Herlory, O.; De Beer, D. Phototrophic Biofilm Activity and Dynamics of Diurnal Cd Cycling in a Freshwater Stream. Environ. Sci. Technol. 2009, 43, 7245–7251. [Google Scholar] [CrossRef] [PubMed]
- Wimpenny, J. Ecological Determinants of Biofilm Formation. Biofouling 1996, 10, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.D.; De Bruyn, W.J.; Hirsch, C.M.; Jakubowski, S.D. Hydrogen Peroxide Measurements in Recreational Marine Bathing Waters in Southern California, USA. Water Res. 2010, 44, 2203–2210. [Google Scholar] [CrossRef] [PubMed]
- Fujii, M.; Dang, T.C.; Rose, A.L.; Omura, T.; Waite, T.D. Effect of Light on Iron Uptake by the Freshwater Cyanobacterium Microcystis aruginosa. Environ. Sci. Technol. 2011, 45, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Reczek, C.R.; Chandel, N.S. ROS-Dependent Signal Transduction. Curr. Opin. Cell Biol. 2015, 33, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Kahoush, M.; Behary, N.; Cayla, A.; Nierstrasz, V. Bio-Fenton and Bio-Electro-Fenton as Sustainable Methods for Degrading Organic Pollutants in Wastewater. Process Biochem. 2018, 64, 237–247. [Google Scholar] [CrossRef]
- Santos, G.O.S.; Cordeiro-Junior, P.J.M.; Sánchez-Montes, I.; Souto, R.S.; Kronka, M.S.; Lanza, M.R.V. Recent Advances in H2O2 Electrosynthesis Based on the Application of Gas Diffusion Electrodes: Challenges and Opportunities. Curr. Opin. Electrochem. 2022, 36, 101124. [Google Scholar] [CrossRef]
- Kurc, L.; Páter, M.; Červený, L. Activity of Basic Catalysts in Oxidation of 2-Ethyl-5,6,7,8-Tetrahydro-9,10-Anthrahydroquinone. J. Mol. Catal. A Chem. 2003, 202, 327–332. [Google Scholar] [CrossRef]
- Hu, S.; Sun, X.; Zhao, Y.; Li, W.; Wang, H.; Wu, G. The Effective Photocatalytic Water Splitting to Simultaneously Produce H2 and H2O2 over Pt Loaded K-g-C3N4 Catalyst. J. Taiwan Inst. Chem. Eng. 2020, 107, 129–138. [Google Scholar] [CrossRef]
- Shi, K.; Wang, J.; Yin, L.; Xu, Y.; Kong, D.; Li, H.; Zhang, Y.; He, H.; Yang, S.; Ni, L.; et al. Photocatalysis Combined with Microalgae to Promote the Degradation and Detoxification of Tetracycline Hydrochloride. Bull. Environ. Contam. Toxicol. 2023, 110, 43. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Watanabe, M.; Nakayasu, Y.; Kohata, K. Changes in O2- and H2O2 Production by Chattonella Antiqua during Diel Vertical Migration under Nutrient Stratification. Aquat. Microb. Ecol. 2005, 39, 183–191. [Google Scholar] [CrossRef]
- Ghatge, S.; Yang, Y.; Ko, Y.; Yoon, Y.; Ahn, J.-H.; Kim, J.J.; Hur, H.-G. Degradation of Sulfonated Polyethylene by a Bio-Photo-Fenton Approach Using Glucose Oxidase Immobilized on Titanium Dioxide. J. Hazard. Mater. 2022, 423, 127067. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Xie, Y.; Mao, W.; Lu, Y.; Wang, Y.; Li, H.; Zhang, C. Efficient Biodegradation of Tris-(2-Chloroisopropyl) Phosphate by a Novel Strain Amycolatopsis Sp. FT-1: Process Optimization, Mechanism Studies and Toxicity Changes. J. Hazard. Mater. 2023, 443, 130149. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Li, M.; Su, Y.; Dong, D.; Guo, Z.; Liang, D. The Degradation of Linear Alkylbenzene Sulfonate (LAS) in the Presence of Light and Natural Biofilms: The Important Role of Photosynthesis. J. Hazard. Mater. 2012, 229–230, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.-Y.; Gao, F.; Yang, H.-L.; Wu, H.-W.-J.; Li, C.; Lu, M.-M.; Yang, Z.-Y. Simultaneous Removal of Nutrient and Sulfonamides from Marine Aquaculture Wastewater by Concentrated and Attached Cultivation of Chlorella Vulgaris in an Algal Biofilm Membrane Photobioreactor (BF-MPBR). Sci. Total Environ. 2020, 725, 138524. [Google Scholar] [CrossRef] [PubMed]
- Vadakke Pariyarath, R.; Inagaki, Y.; Sakakibara, Y. Phycoremediation of Tetracycline via Bio-Fenton Process Using Diatoms. J. Water Process Eng. 2021, 40, 101851. [Google Scholar] [CrossRef]
- Yang, Y.; Ghatge, S.; Ko, Y.; Yoon, Y.; Ahn, J.-H.; Kim, J.J.; Hur, H.-G. Non-Specific Degradation of Chloroacetanilide Herbicides by Glucose Oxidase Supported Bio-Fenton Reaction. Chemosphere 2022, 292, 133417. [Google Scholar] [CrossRef] [PubMed]
- Eskandarian, M.; Mahdizadeh, F.; Ghalamchi, L.; Naghavi, S. Bio-Fenton Process for Acid Blue 113 Textile Azo Dye Decolorization: Characteristics and Neural Network Modeling. Desalin. Water Treat. 2014, 52, 4990–4998. [Google Scholar] [CrossRef]
- Ravi, S.; Lonappan, L.; Touahar, I.; Fonteneau, É.; Vaidyanathan, V.K.; Cabana, H. Evaluation of Bio-Fenton Oxidation Approach for the Remediation of Trichloroethylene from Aqueous Solutions. J. Environ. Manag. 2020, 270, 110899. [Google Scholar] [CrossRef]
- Lukes, P.; Appleton, A.T.; Locke, B.R. Hydrogen Peroxide and Ozone Formation in Hybrid Gas–Liquid Electrical Discharge Reactors. IEEE Trans. Ind. Applicat. 2004, 40, 60–67. [Google Scholar] [CrossRef]
- Li, W.; Lyu, B.; Li, J.; Korshin, G.V.; Zhang, M.; Zhang, Y.; Li, P.; Han, J. Phototransformation of Roxithromycin in the Presence of Dissolved Organic Matter: Characteriazation of the Degradation Products and Toxicity Evaluation. Sci. Total Environ. 2020, 733, 139348. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, C.; Palaska, G.; Lazaridou, M.; Samaras, P.; Sakellaropoulos, G.P. The Effects of Toxic Substances on the Activated Sludge Microfauna. Desalination 2007, 211, 177–191. [Google Scholar] [CrossRef]
- Curds, C.R. The Ecology and Role of Protozoa in Aerobic Sewage Treatment Processes. Annu. Rev. Microbiol. 1982, 36, 27–28. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xu, M.; Cao, H.; Zhu, J.; Zhou, K.; Xu, J.; Yang, X.; Gan, Y.; Liu, W.; Zhai, J.; et al. The Activated-Sludge Fauna and Performance of Five Sewage Treatment Plants in Beijing, China. Eur. J. Protistol. 2004, 40, 147–152. [Google Scholar] [CrossRef]
- Lu, I.-F.; Sung, M.-S.; Lee, T.-M. Salinity Stress and Hydrogen Peroxide Regulation of Antioxidant Defense System in Ulva Fasciata. Mar. Biol. 2006, 150, 1–15. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Mittler, R. ROS-Induced ROS Release in Plant and Animal Cells. Free Radic. Biol. Med. 2018, 122, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Rasdi, N.W.; Qin, J.G. Effect of N:P Ratio on Growth and Chemical Composition of Nannochloropsis Oculata and Tisochrysis Lutea. J. Appl. Phycol. 2015, 27, 2221–2230. [Google Scholar] [CrossRef]
- Ralph, P.J.; Gademann, R. Rapid Light Curves: A Powerful Tool to Assess Photosynthetic Activity. Aquat. Bot. 2005, 82, 222–237. [Google Scholar] [CrossRef]
- Kesaano, M.; Sims, R.C. Algal Biofilm Based Technology for Wastewater Treatment. Algal Res. 2014, 5, 231–240. [Google Scholar] [CrossRef]
- Zhang, H.; Gong, W.; Bai, L.; Chen, R.; Zeng, W.; Yan, Z.; Li, G.; Liang, H. Aeration-Induced CO2 Stripping, Instead of High Dissolved Oxygen, Have a Negative Impact on Algae–Bacteria Symbiosis (ABS) System Stability and Wastewater Treatment Efficiency. Chem. Eng. J. 2020, 382, 122957. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, P.; Yang, L.; Yang, W.; Hou, J.; Liao, Z. Optimization of H2O2 Production in Biological Systems for Design of Bio-Fenton Reactors. Microorganisms 2024, 12, 1488. https://doi.org/10.3390/microorganisms12071488
Zhou P, Yang L, Yang W, Hou J, Liao Z. Optimization of H2O2 Production in Biological Systems for Design of Bio-Fenton Reactors. Microorganisms. 2024; 12(7):1488. https://doi.org/10.3390/microorganisms12071488
Chicago/Turabian StyleZhou, Peiguo, Liping Yang, Wenjing Yang, Jiaxin Hou, and Ziqiao Liao. 2024. "Optimization of H2O2 Production in Biological Systems for Design of Bio-Fenton Reactors" Microorganisms 12, no. 7: 1488. https://doi.org/10.3390/microorganisms12071488




