Effects of Rehydration on Bacterial Diversity in the Rhizosphere of Broomcorn Millet (Panicum miliaceum L.) after Drought Stress at the Flowering Stage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Materials, Drought Stress Treatment and Sample Collection
2.2. DNA Extraction, PCR Amplification, and Illumina MiSeq Sequencing
2.3. Original Data Processing, Operation Classification Unit Division, and Diversity Analysis
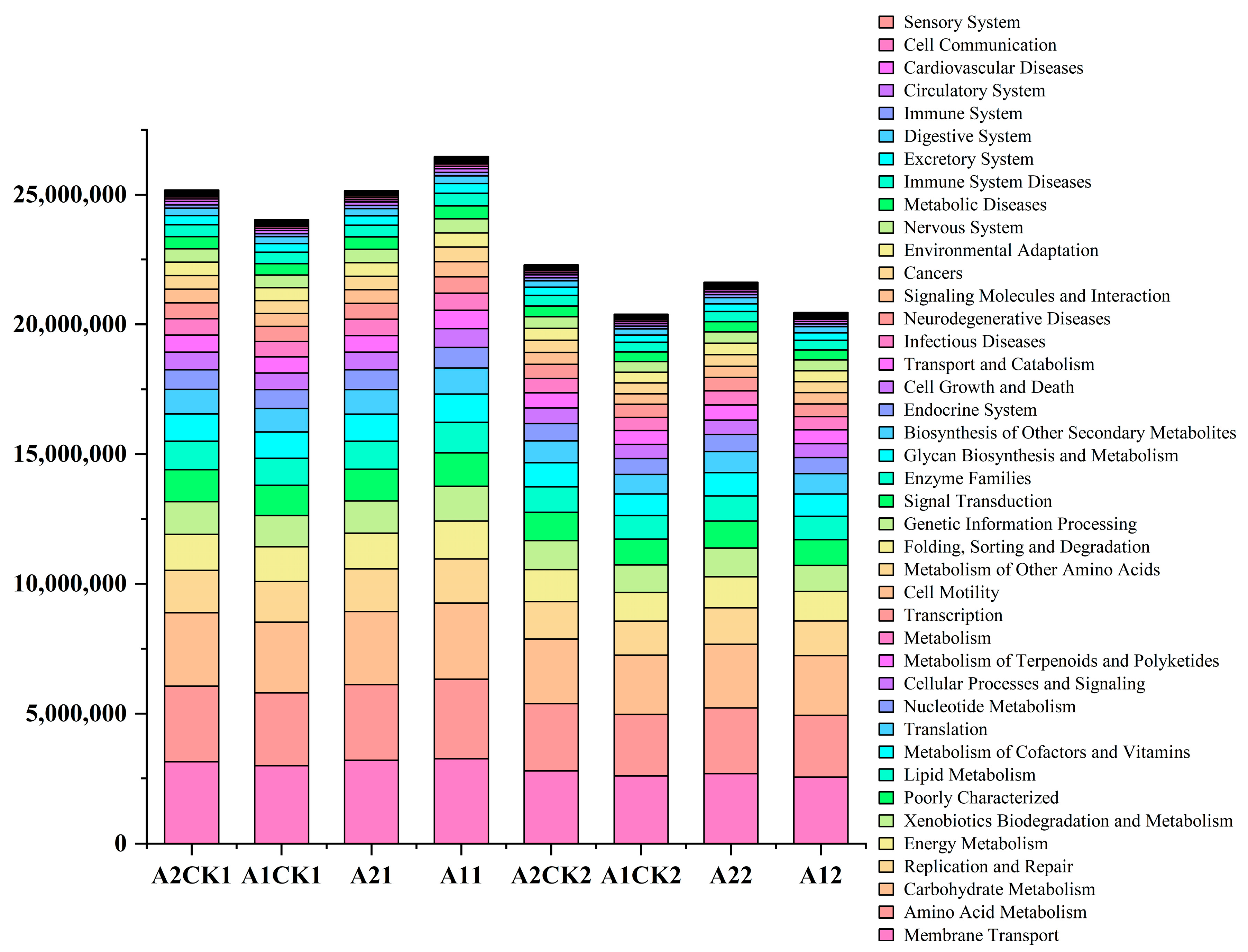
2.4. Prediction of the Metabolic Function of Microbial Community
2.5. Statistical Analysis
3. Results
3.1. Effects of Rewatering after Drought Stress on Bacterial Community Diversity in the Rhizosphere of Broomcorn Millet
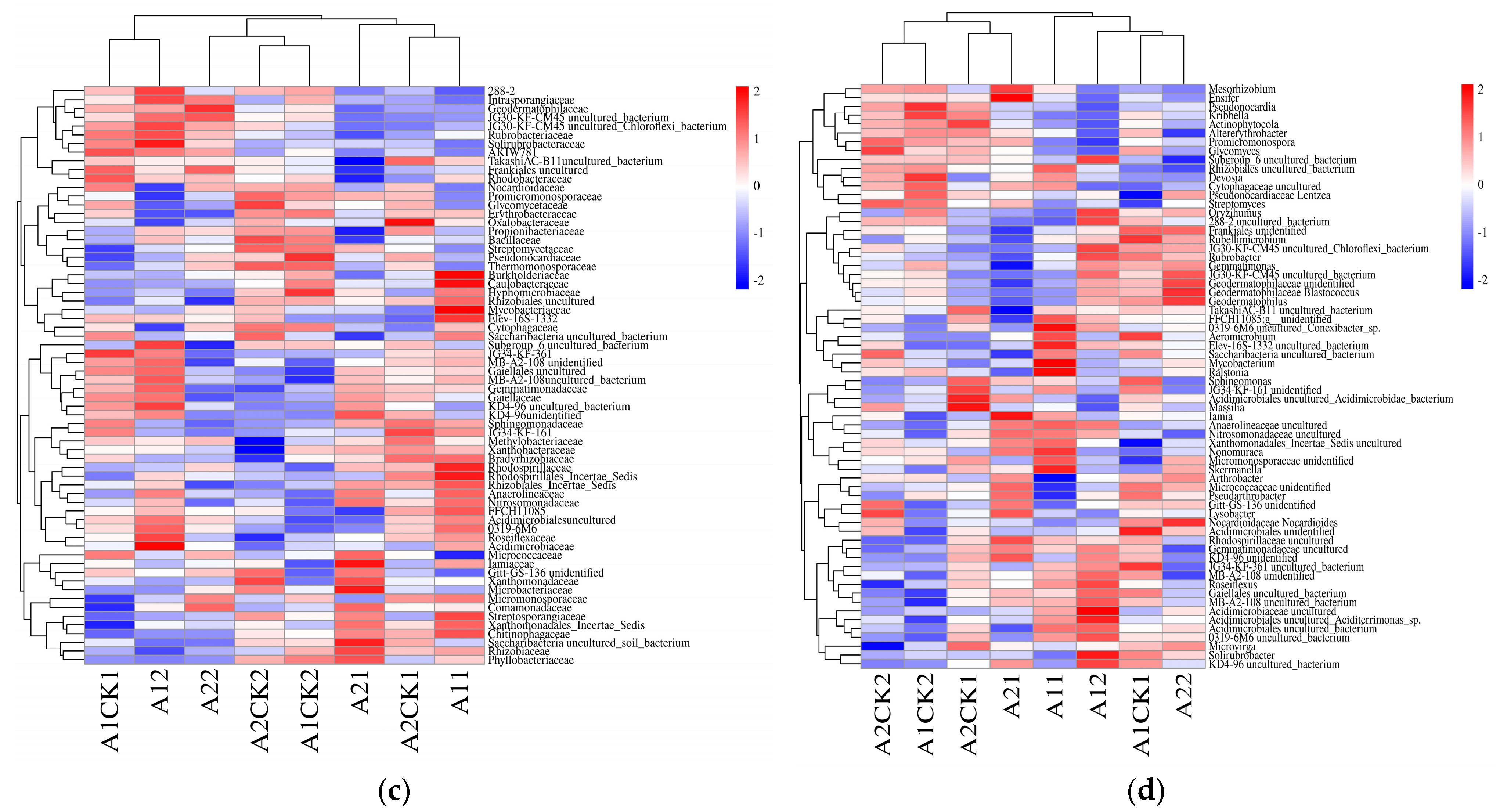
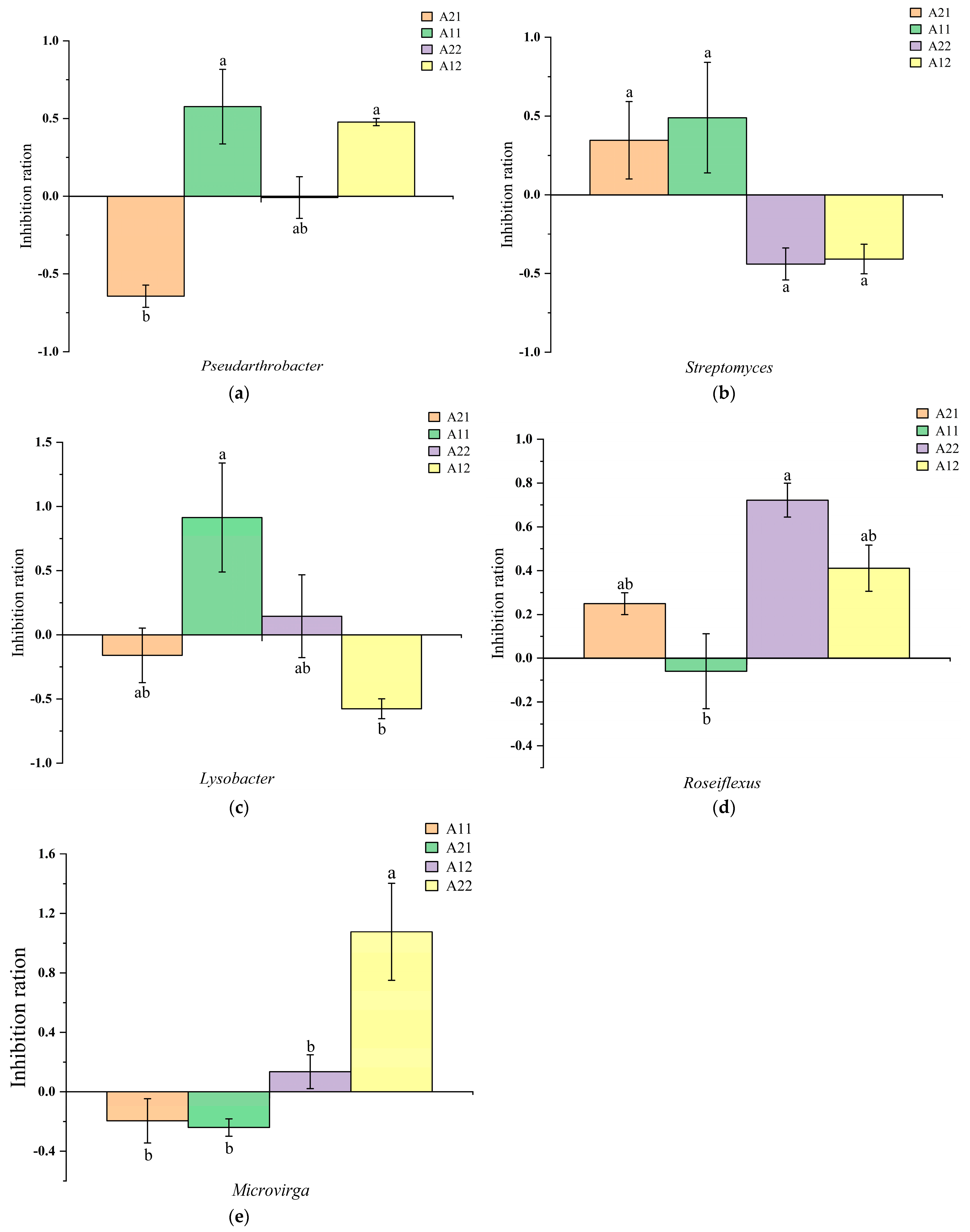
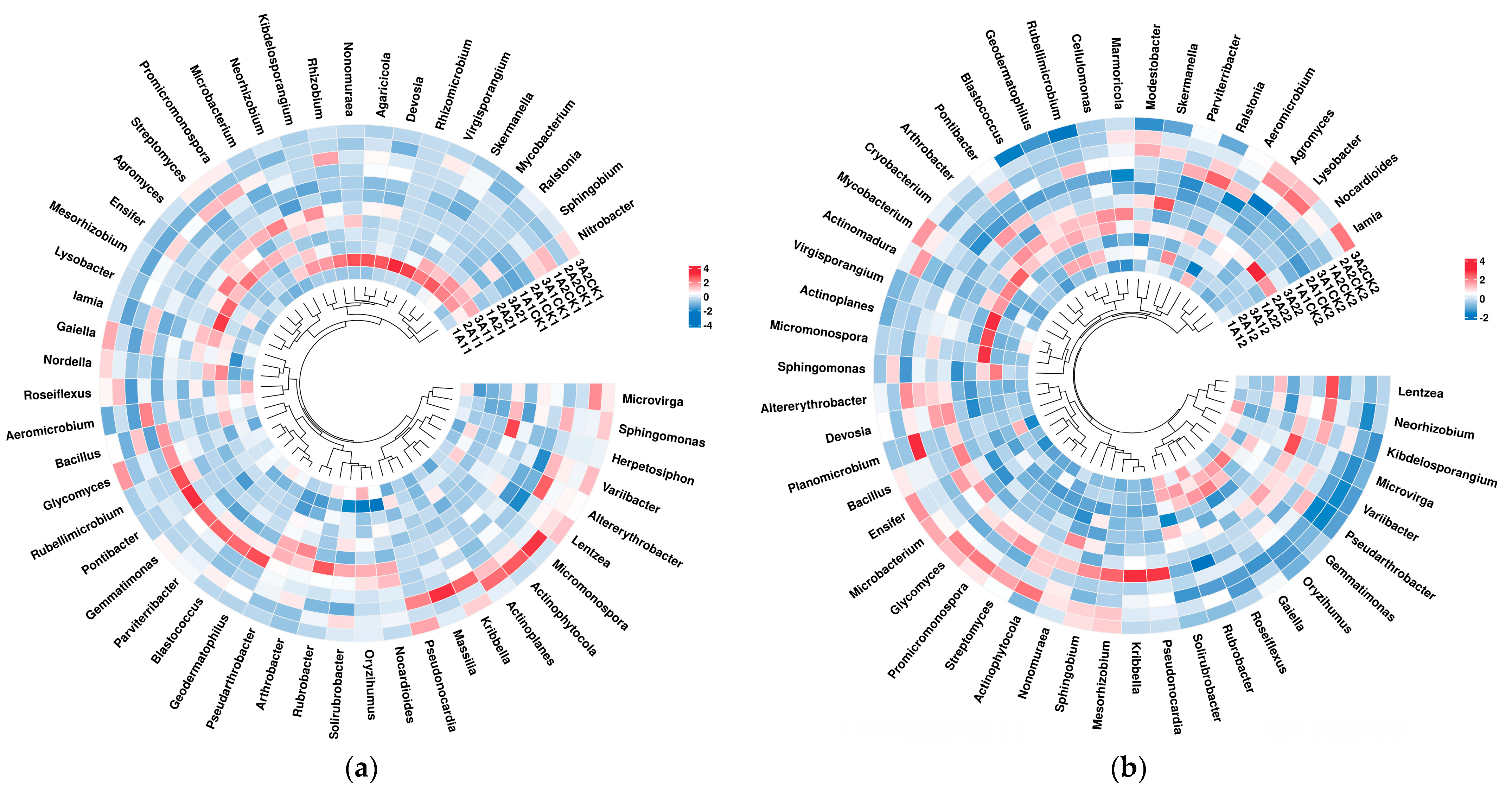
3.2. Changes in Bacterial Abundance
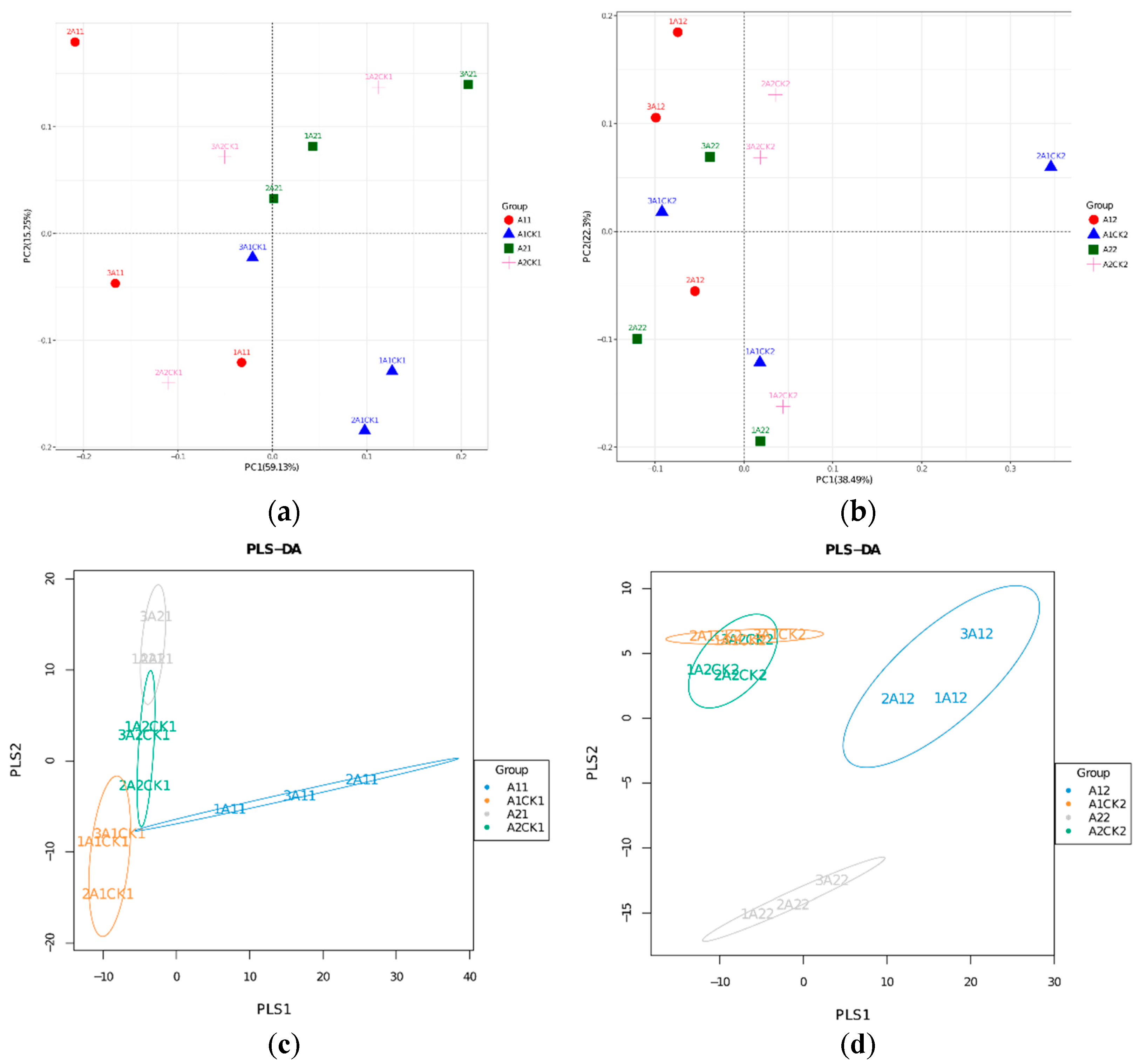
3.3. Changes in Beta Diversity of Rhizosphere Bacteria in Broomcorn Millet after Rewatering under Drought Stress
3.4. Effects of Rewatering after Drought Stress on the Metabolic Function of Rhizosphere Bacteria
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lamaoui, M.; Jemo, M.; Datla, R.; Bekkaoui, F. Heat and drought stresses in crops and approaches for their mitigation. Front. Chem. 2018, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Berdugo, M.; Delgado-Baquerizo, M.; Soliveres, S.; Hernández-Clemente, R.; Zhao, Y.; Gaitán, J.J.; Gross, N.; Saiz, H.; Maire, V.; Lehmann, A.; et al. Global ecosystem thresholds driven by aridity. Science 2020, 367, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; McDowell, N.G.; Fisher, R.A.; Wei, L.; Sevanto, S.; Christoffersen, B.O.; Weng, E.; Middleton, R.S. Increasing impacts of extreme droughts on vegetation productivity under climate change. Nat. Clim. Change 2019, 9, 948–953. [Google Scholar] [CrossRef]
- Koevoets, I.T.; Venema, J.H.; Elzenga, J.T.M.; Testerink, C. Roots Withstanding their Environment: Exploiting Root System Architecture Responses to Abiotic Stress to Improve Crop Tolerance. Front. Plant Sci. 2016, 7, 1335. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jaramillo, J.E.; Mendes, R.; Raaijmakers, J.M. Impact of plant domestication on rhizosphere microbiome assembly and functions. Plant Mol. Biol. 2016, 90, 635–644. [Google Scholar] [CrossRef]
- Ryan, P.R.; Dessaux, Y.; Thomashow, L.S.; Weller, D.M. Rhizosphere engineering and management for sustainable agriculture. Plant Soil 2009, 321, 363–383. [Google Scholar] [CrossRef]
- Sánchez-Blanco, M.J.; Ferrández, T.; Morales, M.A.; Morte, A.; Alarcón, J.J. Variations in water status, gas exchange, and growth in Rosmarinus officinalis plants infected with Glomus deserticola under drought conditions. J. Plant Physiol. 2004, 161, 675–682. [Google Scholar] [CrossRef]
- Dimkpa, C.; Weinand, T.; Asch, F. Plant–rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ. 2009, 32, 1682–1694. [Google Scholar] [CrossRef]
- Cantabella, D.; Dolcet-Sanjuan, R.; Teixidó, N. Using plant growth-promoting microorganisms (PGPMs) to improve plant development under in vitro culture conditions. Planta 2022, 255, 117. [Google Scholar] [CrossRef]
- Saleem, A.R.; Brunetti, C.; Khalid, A.; Della Rocca, G.; Raio, A.; Emiliani, G.; De Carlo, A.; Mahmood, T.; Centritto, M. Drought response of Mucuna pruriens (L.) DC. inoculated with ACC deaminase and IAA producing rhizobacteria. PLoS ONE 2018, 13, e0191218. [Google Scholar] [CrossRef] [PubMed]
- Naheeda, B.; Muhammad, A.; Yunyun, S.; Yafang, L.; Nabil Sabet, A.M.; Parvaiz, A.; Lixin, Z. Improved drought tolerance by AMF inoculation in Maize (Zea mays) involves physiological and biochemical implications. Plants 2019, 8, 579. [Google Scholar] [CrossRef]
- Zou, Y.N.; Wang, P.; Liu, C.Y.; Ni, Q.D.; Zhang, D.J.; Wu, Q.S. Mycorrhizal trifoliate orange has greater root adaptation of morphology and phytohormones in response to drought stress. Sci. Rep. 2017, 7, 41134. [Google Scholar] [CrossRef]
- Wang, R.; Hunt, H.V.; Qiao, Z.; Wang, L.; Han, Y. Diversity and cultivation of broomcorn millet (Panicum miliaceum L.) in China: A review. Econ. Bot. 2016, 70, 332–342. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, L.; Gao, Y.; Yang, Q.; Dong, K.; Liu, T.; Feng, B. Comparative analysis of drought-responsive physiological and transcriptome in broomcorn millet (Panicum miliaceum L.) genotypes with contrasting drought tolerance. Ind. Crops Prod. 2022, 177, 114498. [Google Scholar] [CrossRef]
- Ren, J.; Liu, Y.; Mao, J.; Xu, Y.; Wang, M.; Hu, Y.; Wang, S.; Liu, S.; Qiao, Z.; Cao, X. Metabolomics and physiological methods revealed the effects of drought stress on the quality of broomcorn millet during the flowering stage. Agronomy 2024, 14, 236. [Google Scholar] [CrossRef]
- Cao, X.; Hu, Y.; Song, J.; Feng, H.; Wang, J.; Chen, L.; Wang, L.; Diao, X.; Wan, Y.; Liu, S.; et al. Transcriptome sequencing and metabolome analysis reveals the molecular mechanism of drought stress in millet. Int. J. Mol. Sci. 2022, 23, 10792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.P.; Feng, B.L.; Wang, P.K.; Dai, H.P.; Chai, Y. Leaf senescence and activities of antioxidant enzymes in different broomcorn millet (Panicum miliaceum L.) cultivars under simulated drought condition. J. Food Agric. Environ. 2012, 10, 438–444. [Google Scholar]
- Cao, X.; Wang, J.; Liu, S.; Chen, L.; Xiang, D.; Na, X.; Qiao, Z. Effect of different fertilizers on the bacterial community diversity in rhizosperic soil of broomcorn millet (Panicum miliaceum L.). Arch. Agron. Soil Sci. 2022, 68, 676–687. [Google Scholar] [CrossRef]
- Tian, L.; Chen, P.; Gao, Z.; Gao, X.; Feng, B. Deciphering the distinct mechanisms shaping the broomcorn millet rhizosphere bacterial and fungal communities in a typical agricultural ecosystem of Northern China. Plant Soil 2022, 474, 469–484. [Google Scholar] [CrossRef]
- Tian, L.; Feng, Y.; Gao, Z.; Li, H.; Wang, B.; Huang, Y.; Gao, X.; Feng, B. Co-occurrence pattern and community assembly of broomcorn millet rhizosphere microbiomes in a typical agricultural ecosystem. Appl. Soil Ecol. 2022, 176, 104478. [Google Scholar] [CrossRef]
- Tian, L.; Yu, S.; Zhang, L.; Dong, K.; Feng, B. Mulching practices manipulate the microbial community diversity and network of root-associated compartments in the Loess Plateau. Soil Tillage Res. 2022, 223, 105476. [Google Scholar] [CrossRef]
- Cao, X.; Liu, S.; Wang, J.; Wang, H.; Chen, L.; Tian, X.; Zhang, L.; Chang, J.; Wang, L.; Mu, Z.; et al. Soil bacterial diversity changes in different broomcorn millet intercropping systems. J. Basic Microbiol. 2017, 57, 989–997. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, S.; Xia, J.; Zhan, C.; Cai, W.; Wang, W.; Luo, X.; Chen, N.; Li, J. Analysis of drought and flood alternation and its driving factors in the Yangtze River Basin under climate change. Atmos. Res. 2022, 270, 106087. [Google Scholar] [CrossRef]
- Ren, J.; Wang, W.; Wei, J.; Li, H.; Li, X.; Liu, G.; Chen, Y.; Ye, S. Evolution and prediction of drought-flood abrupt alternation events in Huang-Huai-Hai River Basin, China. Sci. Total Environ. 2023, 869, 161707. [Google Scholar] [CrossRef] [PubMed]
- Jun, W.; Yun, W.; Hai, W.; Ling, C.; Xiao, C.; Si, L.; Xiang, T.; Hui, Q.; Zhi, Q. Relation between rainfall and the yield of broomcorn millet in arid region. J. China Agric. Univ. 2019, 24, 11–14. (In Chinese) [Google Scholar]
- Wei, Z.; Cui, L.; Da, Z.; Yu, Z.; Qing, Y.; Xiao, D.; Bai, F. Compensation effexts of rewatering on root and shoot functions of broomcorn millet after water stress. J. Northwest AF Univ. (Nat. Sci. Ed.) 2016, 44, 45–52. (In Chinese) [Google Scholar]
- Fan, L.; Yin, H.; Guo, S.; Hong, Z.; Tian, L.; Bei, H. Isolation and analysis of genes induced by rehydration after serious drought in broomcorn millet (Panicum miliaceum L.) by Using SSH. J. China Agric. Univ. 2006, 14, 537–541. (In Chinese) [Google Scholar]
- Liu, Y.; Ren, J.; Hu, Y.; Wang, S.; Mao, J.; Xu, Y.; Wang, M.; Liu, S.; Qiao, Z.; Cao, X. Effects of drought stress during the flowering period on the rhizosphere fungal diversity of broomcorn millet (Panicum miliaceum L.). Agronomy 2023, 13, 2896. [Google Scholar] [CrossRef]
- Na, X.; Cao, X.; Ma, C.; Ma, S.; Xu, P.; Liu, S.; Wang, J.; Wang, H.; Chen, L.; Qiao, Z. Plant stage, not drought stress, determines the effect of cultivars on bacterial community diversity in the rhizosphere of broomcorn millet (Panicum miliaceum L.). Front. Microbiol. 2019, 10, 828. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Chao, A. Nonparametric estimation of the number of classes in a population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Ramette, A. Multivariate analyses in microbial ecology. Fems Microbiol. Ecol. 2007, 62, 142–160. [Google Scholar] [CrossRef] [PubMed]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821.30. [Google Scholar] [CrossRef] [PubMed]
- Valliyodan, B.; Nguyen, H.T. Understanding regulatory networks and engineering for enhanced drought tolerance in plants. Curr. Opin. Plant Biol. 2006, 9, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Yamaguchi-Shinozaki, K.; Urao, T.; Iwasaki, T.; Hosokawa, D.; Shinozaki, K. Role of arabidopsis MYC and MYB homologs in drought- and abscisic acid-regulated gene expression. Plant Cell 1997, 9, 1859–1868. [Google Scholar] [PubMed]
- Vereyken, I.J.; Chupin, V.; Islamov, A.; Kuklin, A.; Hincha, D.K.; Kruijff, B.D. The effect of fructan on the phospholipid organization in the dry state. Biophys. J. 2003, 85, 3058–3065. [Google Scholar] [CrossRef] [PubMed]
- Taketani, R.G.; Lançoni, M.D.; Kavamura, V.N.; Durrer, A.; Andreote, F.D.; Melo, I.S. Dry season constrains bacterial phylogenetic diversity in a semi-arid rhizosphere system. Microb. Ecol. 2017, 73, 153–161. [Google Scholar] [CrossRef]
- Evans, S.E.; Wallenstein, M.D. Climate change alters ecological strategies of soil bacteria. Ecol. Lett. 2014, 17, 155–164. [Google Scholar] [CrossRef]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, J.; Lu, X.; Bai, Y.; Wang, G. Two diversities meet in the rhizosphere: Root specialized metabolites and microbiome. J. Genet. Genom. 2023, 23, S1673–S8527. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Peng, Y.; Xu, W. Crop root responses to drought stress: Molecular mechanisms, nutrient regulations, and interactions with microorganisms in the rhizosphere. Int. J. Mol. Sci. 2022, 23, 9310. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ling, Q.; Li, H.; Yan, L.; En, L.; Ling, H.; Yue, L. Variation characteristics of drought and rehydration on the growth of Hibiscus rosa-sinensis Linn. and soil microbial diversity in rhizosphere. Chin. J. Trop. Crops. 2020, 41, 401–408. (In Chinese) [Google Scholar]
- Doornbos, R.F.; Van Loon, L.C.; Bakker, P.A.H.M. Impact of root exudates and plant defense signaling on bacterial communities in the rhizosphere. A review. Agron. Sustain. Dev. 2012, 32, 227–243. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, W.; Wang, B.; Tang, J.; Chen, X. Secondary metabolites from the invasive Solidago canadensis L. accumulation in soil and contribution to inhibition of soil pathogen Pythium ultimum. Appl. Soil Ecol. 2011, 48, 280–286. [Google Scholar] [CrossRef]
- Peters, N.K.; Frost, J.W.; Long, S.R. A plant flavone, luteolin, induces expression of rhizobium meliloti nodulation genes. Science 1986, 233, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Bitas, V.; Kim, H.-S.; Bennett, J.W.; Kang, S. Sniffing on microbes: Diverse roles of microbial volatile organic compounds in plant health. Mol. Plant Microbe Interact. 2013, 26, 835–843. [Google Scholar] [CrossRef]
- Hunt, H.V.; Campana, M.G.; Lawes, M.C.; Park, Y.-J.; Bower, M.A.; Howe, C.J.; Jones, M.K. Genetic diversity and phylogeography of broomcorn millet (Panicum miliaceum L.) across Eurasia. Mol. Ecol. 2011, 20, 4756–4771. [Google Scholar] [CrossRef]
- Rajput, S.G.; Santra, D.K.; Schnable, J. Mapping QTLs for morpho-agronomic traits in proso millet (Panicum miliaceum L.). Mol. Breed. 2016, 36, 37. [Google Scholar] [CrossRef]
- Fuchslueger, L.; Bahn, M.; Fritz, K.; Hasibeder, R.; Richter, A. Experimental drought reduces the transfer of recently fixed plant carbon to soil microbes and alters the bacterial community composition in a mountain meadow. New Phytol. 2014, 201, 916–927. [Google Scholar] [CrossRef] [PubMed]
- Besserer, A.; Puech-Pagès, V.; Kiefer, P.; Gomez-Roldan, V.; Jauneau, A.; Roy, S.; Portais, J.-C.; Roux, C.; Bécard, G.; Séjalon-Delmas, N. Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. PLoS Biol. 2006, 4, e226. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, K.; Matsuzaki, K.; Hayashi, H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 2005, 435, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.J.; Buuren, M.L.V. A phosphate transporter from the mycorrhizal fungus Glomus versiforme. Nature 1995, 378, 626–629. [Google Scholar] [CrossRef] [PubMed]
- Van Rhijn, P.; Vanderleyden, J. The Rhizobium-plant symbiosis. Microbiol. Mol. Biol. Rev. 1995, 59, 124–142. [Google Scholar] [CrossRef]
- Jalmi, S.K.; Sinha, A.K. Ambiguities of PGPR-induced plant signaling and stress management. Front. Microbiol. 2022, 13, 899563. [Google Scholar] [CrossRef]
- Maghboli Balasjin, N.; Maki, J.S.; Schläppi, M.R.; Marshall, C.W. Plant growth-promoting activity of bacteria isolated from asian rice (Oryza sativa L.) depends on rice genotype. Microbiol. Spectr. 2022, 10, e02787-21. [Google Scholar] [CrossRef]
- Jiménez-Gómez, A.; Saati-Santamaría, Z.; Igual, J.M.; Rivas, R.; Mateos, P.F.; García-Fraile, P. Genome insights into the novel species microvirga brassicacearum, a rapeseed endophyte with biotechnological potential. Microorganisms 2019, 7, 354. [Google Scholar] [CrossRef]
- Puopolo, G.; Tomada, S.; Pertot, I. The impact of the omics era on the knowledge and use of Lysobacter species to control phytopathogenic microorganisms. J. Appl. Microbiol. 2018, 124, 15–27. [Google Scholar] [CrossRef]
- Palumbo, J.D.; Yuen, G.Y.; Jochum, C.C.; Tatum, K.; Kobayashi, D.Y. Mutagenesis of β-1,3-glucanase genes in Lysobacter enzymogenes strain C3 results in reduced biological control activity toward bipolaris leaf spot of tall fescue and pythium damping-off of sugar beet. Phytopathology 2005, 95, 701–707. [Google Scholar] [CrossRef]
- Li, S.; Jochum, C.C.; Yu, F.; Zaleta-Rivera, K.; Du, L.; Harris, S.D.; Yuen, G.Y. An antibiotic complex from Lysobacter enzymogenes strain C3: Antimicrobial activity and role in plant disease control. Phytopathology 2008, 98, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Yang, Z.; Tao, M.; Shen, D.; Cui, C.; Wang, P.; Wang, L.; Jing, M.; Qian, G.; Shao, X. Lysobacter enzymogenes prevents phytophthora infection by inhibiting pathogen growth and eliciting plant immune responses. Front. Plant Sci. 2023, 14, 1116147. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Wang, H.; Yang, B.; Jiang, W.; Jing, M.; Li, H.; Xia, Y.; Xu, Y.; Hu, Q.; Wang, F.; et al. Phytophthora sojae effector PsAvh240 inhibits host aspartic protease secretion to promote infection. Mol. Plant 2019, 12, 552–564. [Google Scholar] [CrossRef] [PubMed]
- Asaf, S.; Khan, A.L.; Khan, M.A.; Al-Harrasi, A.; Lee, I.-J. Complete genome sequencing and analysis of endophytic Sphingomonas sp. LK11 and its potential in plant growth. 3 Biotech 2018, 8, 389. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Jiang, W.; Cheng, Z.; Heikkila, J.J.; Glick, B.R. The complete genome sequence of the plant growth-promoting Bacterium Pseudomonas sp. UW4. PLoS ONE 2013, 8, e58640. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.K.; Kim, J.-K.; Owens, T.G.; Ranwala, A.P.; Choi, Y.D.; Kochian, L.V.; Wu, R.J. Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Proc. Natl. Acad. Sci. USA 2002, 99, 15898–15903. [Google Scholar] [CrossRef] [PubMed]
- Lamark, T.; Røkenes, T.P.; McDougall, J.; Strøm, A.R. The complex bet promoters of Escherichia coli: Regulation by oxygen (ArcA), choline (BetI), and osmotic stress. J. Bacteriol. 1996, 178, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Chen, N.; Shi, M.; Xian, M.; Song, Y.; Liu, J. The metabolism and biotechnological application of betaine in microorganism. Appl. Microbiol. Biotechnol. 2016, 100, 3865–3876. [Google Scholar] [CrossRef]
- Wang, F.; Wei, Y.; Yan, T.; Wang, C.; Chao, Y.; Jia, M.; An, L.; Sheng, H. Sphingomonas sp. Hbc-6 alters physiological metabolism and recruits beneficial rhizosphere bacteria to improve plant growth and drought tolerance. Front. Plant Sci. 2022, 13, 1002772. [Google Scholar] [CrossRef]
- Finkel, O.M.; Salas-González, I.; Castrillo, G.; Conway, J.M.; Law, T.F.; Teixeira, P.J.P.L.; Wilson, E.D.; Fitzpatrick, C.R.; Jones, C.D.; Dangl, J.L. A single bacterial genus maintains root growth in a complex microbiome. Nature 2020, 587, 103–108. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, X.; Zhao, W.; Cao, Y.; Guo, T.; He, X.; Ni, H.; Tang, X. Phosphate-solubilizing Pseudomonas sp. strain P34-L promotes wheat growth by colonizing the wheat rhizosphere and improving the wheat root system and soil phosphorus nutritional status. J. Plant Growth Regul. 2019, 38, 1314–1324. [Google Scholar] [CrossRef]
- Jorge, G.L.; Kisiala, A.; Morrison, E.; Aoki, M.; Nogueira, A.P.O.; Emery, R.J.N. Endosymbiotic Methylobacterium oryzae mitigates the impact of limited water availability in lentil (Lens culinaris Medik.) by increasing plant cytokinin levels. Environ. Exp. Bot. 2019, 162, 525–540. [Google Scholar] [CrossRef]
- Kumar, M.; Kour, D.; Yadav, A.N.; Saxena, R.; Rai, P.K.; Jyoti, A.; Tomar, R.S. Biodiversity of methylotrophic microbial communities and their potential role in mitigation of abiotic stresses in plants. Biologia 2019, 74, 287–308. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, F.; Huang, Y.; Zhou, M.; Gao, J.; Yan, T.; Sheng, H.; An, L. Sphingomonas sp. Cra20 increases plant growth rate and alters rhizosphere microbial community structure of Arabidopsis thaliana under drought stress. Front. Microbiol. 2019, 10, 1221. [Google Scholar] [CrossRef] [PubMed]
- Hanada, S.; Takaichi, S.; Matsuura, K.; Nakamura, K. Roseiflexus castenholzii gen. nov., sp. nov., a thermophilic, filamentous, photosynthetic bacterium that lacks chlorosomes. Int. J. Syst. Evol. Microbiol. 2002, 52, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Xin, Y.; Wang, C.; Blankenship, R.E.; Sun, F.; Xu, X. Cryo-EM structures of the air-oxidized and dithionite-reduced photosynthetic alternative complex III from Roseiflexus castenholzii. Sci. Adv. 2020, 6, eaba2739. [Google Scholar] [CrossRef] [PubMed]
- Ham, S.H.; Yoon, A.R.; Oh, H.E.; Park, Y.G. Plant growth-promoting microorganism Pseudarthrobacter sp. NIBRBAC000502770 enhances the growth and flavonoid content of Geum aleppicum. Microorganisms 2022, 10, 1241. [Google Scholar] [CrossRef]
- Hartwig, U.A.; Phillips, D.A. Release and modification of nod-gene-inducing flavonoids from alfalfa seeds. Plant Physiol. 1991, 95, 804–807. [Google Scholar] [CrossRef] [PubMed]
- Cesco, S.; Neumann, G.; Tomasi, N.; Pinton, R.; Weisskopf, L. Release of plant-borne flavonoids into the rhizosphere and their role in plant nutrition. Plant Soil 2010, 329, 1–25. [Google Scholar] [CrossRef]
- Jones, S.E.; Elliot, M.A. Streptomyces exploration: Competition, volatile communication and new bacterial behaviours. Trends Microbiol. 2017, 25, 522–531. [Google Scholar] [CrossRef]
- Farré-Armengol, G.; Filella, I.; Llusia, J.; Peñuelas, J. Bidirectional interaction between phyllospheric microbiotas and plant volatile emissions. Trends Plant Sci. 2016, 21, 854–860. [Google Scholar] [CrossRef]
- Schulz-Bohm, K.; Gerards, S.; Hundscheid, M.; Melenhorst, J.; de Boer, W.; Garbeva, P. Calling from distance: Attraction of soil bacteria by plant root volatiles. ISME J. 2018, 12, 1252–1262. [Google Scholar] [CrossRef]
- Jiang, Y.; Yong, Z.; Xiao, F.; Peng, L.; Hai, W. Effect of drought stress and rewatering on physiological characteristics of roots in different proso millet varieties. Acta Bot. Boreali-Occident. Sin. 2012, 32, 0348–0354. (In Chinese) [Google Scholar]
- Deng, Y.; Kong, W.; Zhang, X.; Zhu, Y.; Xie, T.; Chen, M.; Zhu, L.; Sun, J.; Zhang, Z.; Chen, C.; et al. Rhizosphere microbial community enrichment processes in healthy and diseased plants: Implications of soil properties on biomarkers. Front. Microbiol. 2024, 15, 1333076. [Google Scholar] [CrossRef]
- Xu, J.; Cheng, Y. Linking plant secondary metabolites and plant microbiomes: A review. Front. Plant Sci. 2021, 12, 621276. [Google Scholar]
- Mukherjee, S.; Bassler, B.L. Bacterial quorum sensing in complex and dynamically changing environments. Nat. Rev. Microbiol. 2019, 17, 371–382. [Google Scholar] [CrossRef]


| Factor | OTU | Shannon Index | ||
|---|---|---|---|---|
| F | p | F | p | |
| Cultivar | 1.404 | 0.253 | 1.887 | 0.188 |
| Treatment | 1.009 | 0.330 | 2.791 | 0.114 |
| Period | 10.041 | <0.01 | 0.233 | 0.636 |
| Cultivar × Treatment | 1.606 | 0.223 | 3.045 | 0.100 |
| Cultivar × Period | 0.001 | 0.982 | 0.112 | 0.743 |
| Treatment × Period | 0.168 | 0.687 | 0.647 | 0.433 |
| Cultivar × Treatment × Period | 0.340 | 0.568 | 0.430 | 0.521 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Mao, J.; Xu, Y.; Ren, J.; Wang, M.; Wang, S.; Liu, S.; Wang, R.; Wang, L.; Wang, L.; et al. Effects of Rehydration on Bacterial Diversity in the Rhizosphere of Broomcorn Millet (Panicum miliaceum L.) after Drought Stress at the Flowering Stage. Microorganisms 2024, 12, 1534. https://doi.org/10.3390/microorganisms12081534
Liu Y, Mao J, Xu Y, Ren J, Wang M, Wang S, Liu S, Wang R, Wang L, Wang L, et al. Effects of Rehydration on Bacterial Diversity in the Rhizosphere of Broomcorn Millet (Panicum miliaceum L.) after Drought Stress at the Flowering Stage. Microorganisms. 2024; 12(8):1534. https://doi.org/10.3390/microorganisms12081534
Chicago/Turabian StyleLiu, Yuhan, Jiao Mao, Yuanmeng Xu, Jiangling Ren, Mengyao Wang, Shu Wang, Sichen Liu, Ruiyun Wang, Lun Wang, Liwei Wang, and et al. 2024. "Effects of Rehydration on Bacterial Diversity in the Rhizosphere of Broomcorn Millet (Panicum miliaceum L.) after Drought Stress at the Flowering Stage" Microorganisms 12, no. 8: 1534. https://doi.org/10.3390/microorganisms12081534
APA StyleLiu, Y., Mao, J., Xu, Y., Ren, J., Wang, M., Wang, S., Liu, S., Wang, R., Wang, L., Wang, L., Qiao, Z., & Cao, X. (2024). Effects of Rehydration on Bacterial Diversity in the Rhizosphere of Broomcorn Millet (Panicum miliaceum L.) after Drought Stress at the Flowering Stage. Microorganisms, 12(8), 1534. https://doi.org/10.3390/microorganisms12081534





