Effects of Mint Oils on the Human Oral Microbiome: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Essential Oil Chemistry
2.2. Ethical Review and Informed Consent
2.3. Study Population
2.4. Treatment
2.5. Sampling
2.6. Oral DNA Extraction
2.7. Amplicon Library Preparation and Sequencing
2.8. Bioinformatic Analyses and Ecological Statistics
3. Results
3.1. Chemical Composition
3.2. Demographic Features of the Study Population
3.3. Microbiome Diversity and Composition
3.3.1. Sequencing Data Profiles
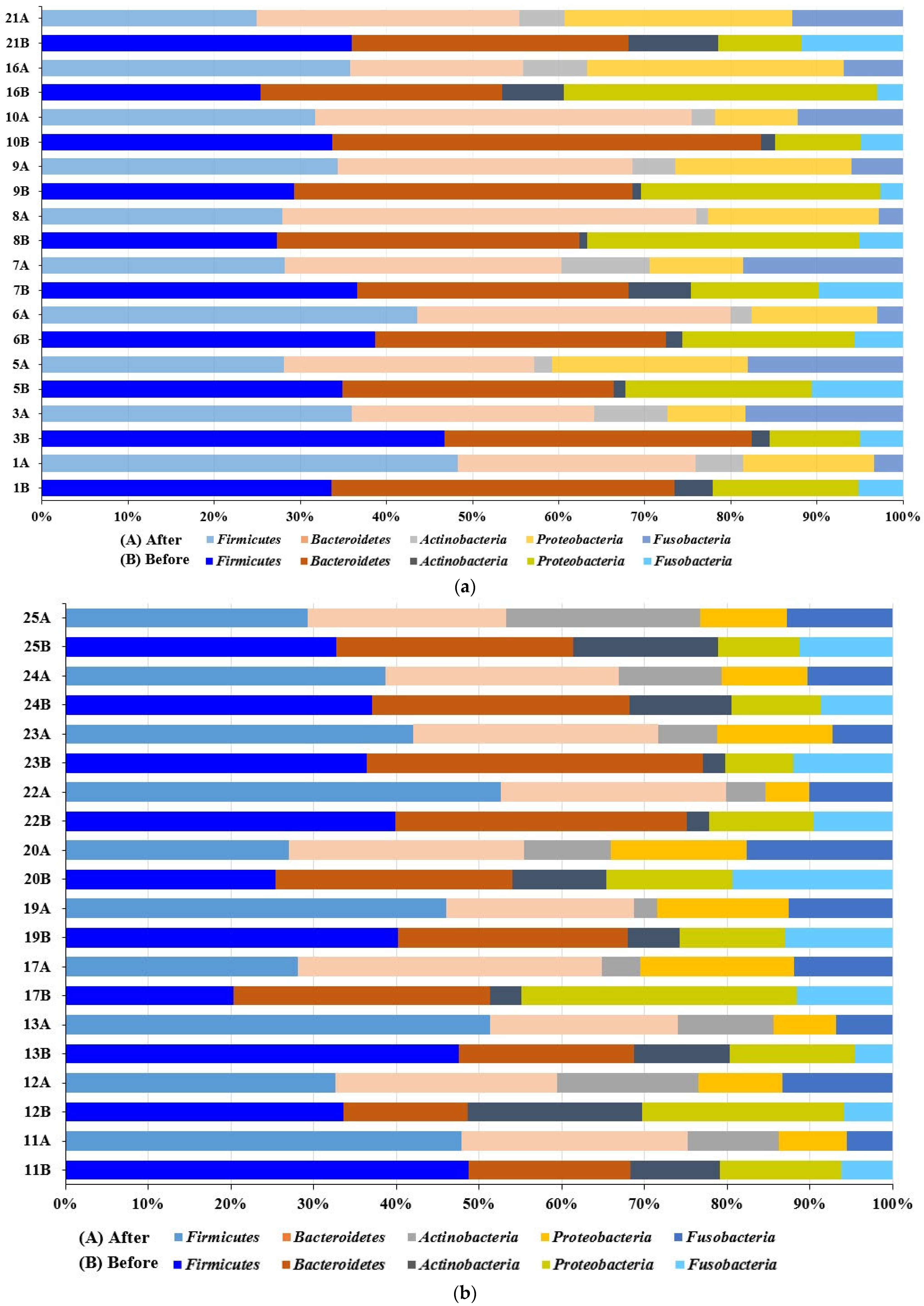
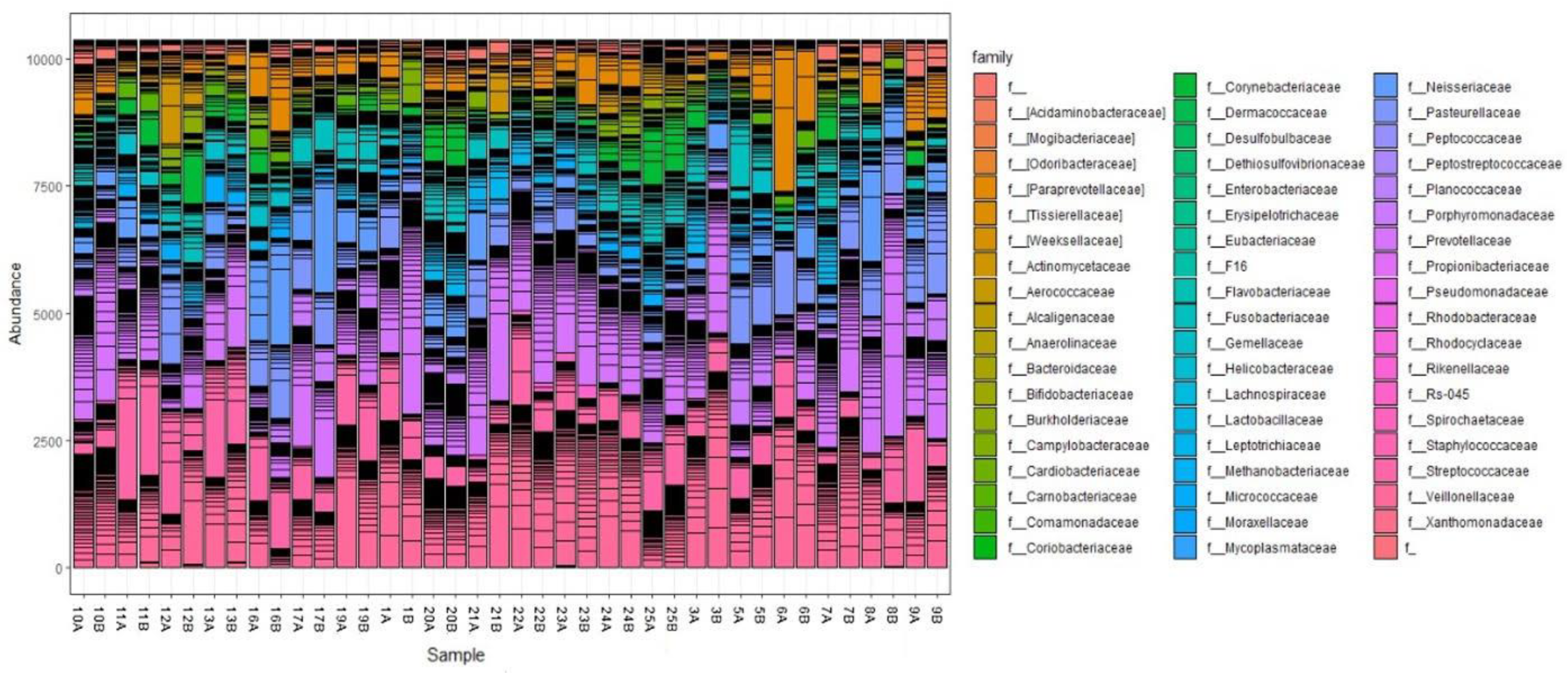
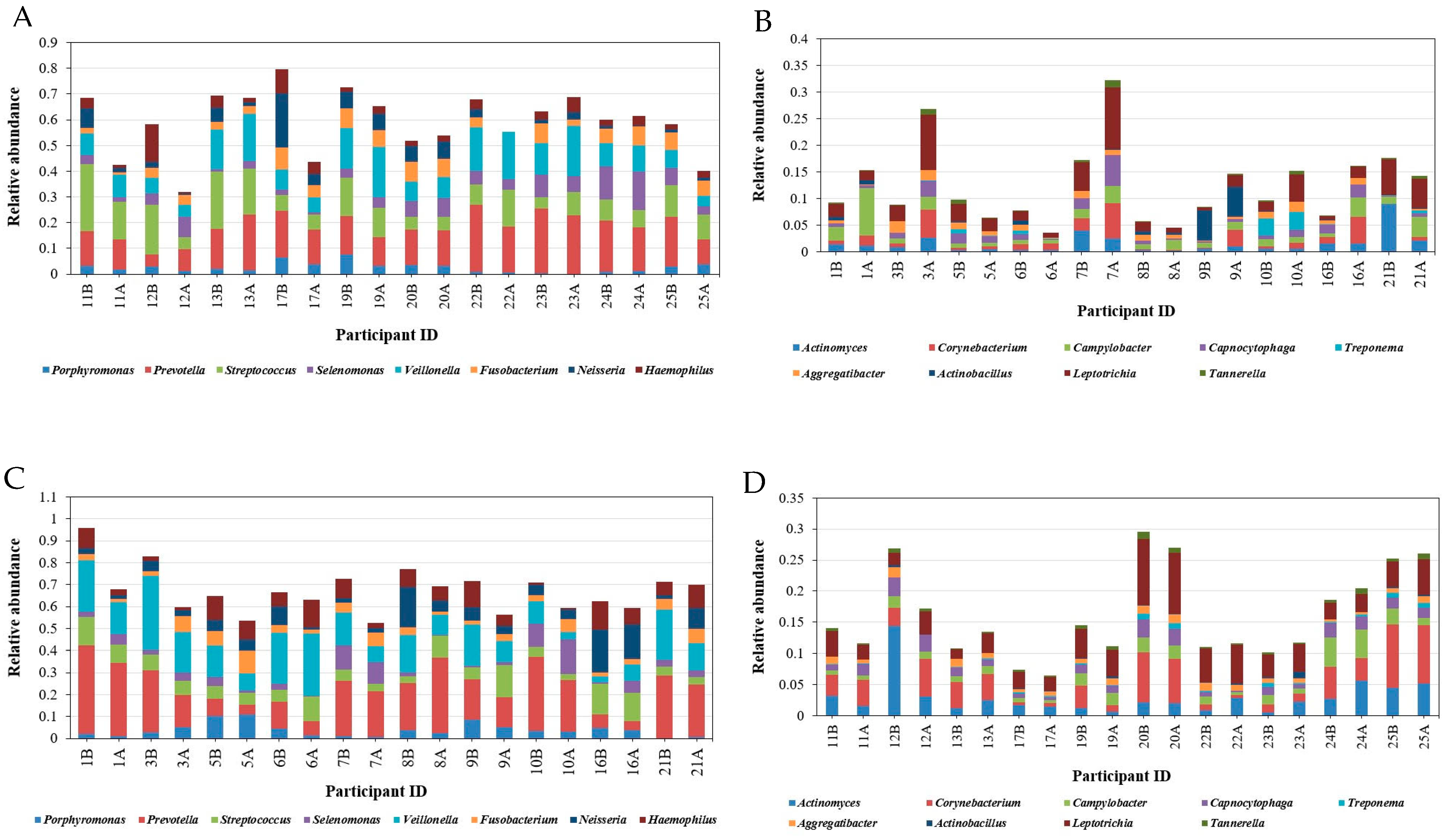
3.3.2. Taxonomical Classification
3.3.3. Alpha Diversity
3.3.4. Beta Diversity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ursell, L.K.; Metcalf, J.L.; Parfrey, L.W.; Knight, R. Defining the Human Microbiome. Nutr. Rev. 2012, 70, S38–S44. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The Healthy Human Microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Grice, E.A.; Segre, J.A. The Human Microbiome: Our Second Genome. Annu. Rev. Genom. Hum. Genet. 2012, 13, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Deo, P.N.; Deshmukh, R. Oral Microbiome: Unveiling the Fundamentals. J. Oral Maxillofac. Pathol. 2019, 23, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Sedghi, L.; DiMassa, V.; Harrington, A.; Lynch, S.V.; Kapila, Y.L. The Oral Microbiome: Role of Key Organisms and Complex Networks in Oral Health and Disease. Periodontol. 2000 2021, 87, 107–131. [Google Scholar] [CrossRef] [PubMed]
- Santonocito, S.; Giudice, A.; Polizzi, A.; Troiano, G.; Merlo, E.M.; Sclafani, R.; Grosso, G.; Isola, G. A Cross-Talk between Diet and the Oral Microbiome: Balance of Nutrition on Inflammation and Immune System’s Response during Periodontitis. Nutrients 2022, 14, 2426. [Google Scholar] [CrossRef] [PubMed]
- Maier, T. Oral Microbiome in Health and Disease: Maintaining a Healthy, Balanced Ecosystem and Reversing Dysbiosis. Microorganisms 2023, 11, 1453. [Google Scholar] [CrossRef] [PubMed]
- Bui, F.Q.; Almeida-da-Silva, C.L.C.; Huynh, B.; Trinh, A.; Liu, J.; Woodward, J.; Asadi, H.; Ojcius, D.M. Association between Periodontal Pathogens and Systemic Disease. Biomed. J. 2019, 42, 27–35. [Google Scholar] [CrossRef]
- Matayoshi, S.; Tojo, F.; Suehiro, Y.; Okuda, M.; Takagi, M.; Ochiai, M.; Kadono, M.; Mikasa, Y.; Okawa, R.; Nomura, R.; et al. Effects of Mouthwash on Periodontal Pathogens and Glycemic Control in Patients with Type 2 Diabetes Mellitus. Sci. Rep. 2024, 14, 2777. [Google Scholar] [CrossRef]
- Joshipura, K.J.; Muñoz-Torres, F.J.; Morou-Bermudez, E.; Patel, R.P. Over-the-Counter Mouthwash Use and Risk of Pre-Diabetes/Diabetes. Nitric Oxide 2017, 71, 14–20. [Google Scholar] [CrossRef]
- Wang, S.; Song, F.; Gu, H.; Wei, X.; Zhang, K.; Zhou, Y.; Luo, H. Comparative Evaluation of the Salivary and Buccal Mucosal Microbiota by 16S RRNA Sequencing for Forensic Investigations. Front. Microbiol. 2022, 13, 777882. [Google Scholar] [CrossRef] [PubMed]
- Wade, W.G.; Prosdocimi, E.M. Profiling of Oral Bacterial Communities. J. Dent. Res. 2020, 99, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.L.; Morton, J.T.; Dinis, M.; Alvarez, R.; Tran, N.C.; Knight, R.; Edlund, A. Deep Metagenomics Examines the Oral Microbiome during Dental Caries, Revealing Novel Taxa and Co-Occurrences with Host Molecules. Genome Res. 2021, 31, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Işcan, G.; Kirimer, N.; Kürkcüoğlu, M.; Başer, K.H.C.; Demirci, F. Antimicrobial Screening of Mentha piperita Essential Oils. J. Agric. Food Chem. 2002, 50, 3943–3946. [Google Scholar] [CrossRef] [PubMed]
- Inouye, S.; Yamaguchi, H.; Takizawa, T. Screening of the Antibacterial Effects of a Variety of Essential Oils on Respiratory Tract Pathogens, Using a Modified Dilution Assay Method. J. Infect. Chemother. 2001, 7, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Soković, M.; Glamočlija, J.; Marin, P.D.; Brkić, D.; van Griensven, L.J.L.D. Antibacterial Effects of the Essential Oils of Commonly Consumed Medicinal Herbs Using an In Vitro Model. Molecules 2010, 15, 7532–7546. [Google Scholar] [CrossRef]
- Inouye, S.; Takizawa, T.; Yamaguchi, H. Antibacterial Activity of Essential Oils and Their Major Constituents against Respiratory Tract Pathogens by Gaseous Contact. J. Antimicrob. Chemother. 2001, 47, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Mandava, K.; Batchu, U.R.; Kakulavaram, S.; Repally, S.; Chennuri, I.; Bedarakota, S.; Sunkara, N. Design and Study of Anticaries Effect of Different Medicinal Plants against S. mutans Glucosyltransferase. BMC Complement. Altern. Med. 2019, 19, 197. [Google Scholar] [CrossRef]
- Fitsiou, E.; Mitropoulou, G.; Spyridopoulou, K.; Tiptiri-Kourpeti, A.; Vamvakias, M.; Bardouki, H.; Panayiotidis, M.; Galanis, A.; Kourkoutas, Y.; Chlichlia, K.; et al. Phytochemical Profile and Evaluation of the Biological Activities of Essential Oils Derived from the Greek Aromatic Plant Species Ocimum basilicum, Mentha spicata, Pimpinella anisum and Fortunella margarita. Molecules 2016, 21, 1069. [Google Scholar] [CrossRef]
- Imai, H.; Osawa, K.; Yasuda, H.; Hamashima, H.; Arai, T.; Sasatsu, M. Inhibition by the Essential Oils of Peppermint and Spearmint of the Growth of Pathogenic Bacteria. Microbios 2001, 106 (Suppl. S1), 31–39. [Google Scholar]
- Mimica-Dukić, N.; Bozin, B.; Soković, M.; Mihajlović, B.; Matavulj, M. Antimicrobial and Antioxidant Activities of Three Mentha Species Essential Oils. Planta Med. 2003, 69, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Boren, K.; Crown, A.; Carlson, R. Multidrug and Pan-Antibiotic Resistance—The Role of Antimicrobial and Synergistic Essential Oils: A Review. Nat. Prod. Commun. 2020, 15, 1934578X2096259. [Google Scholar] [CrossRef]
- Muntean, D.; Licker, M.; Alexa, E.; Popescu, I.; Jianu, C.; Buda, V.; Dehelean, C.A.; Ghiulai, R.; Horhat, F.; Horhat, D.; et al. Evaluation of Essential Oil Obtained from Mentha×piperita L. against Multidrug-Resistant Strains. Infect. Drug Resist. 2019, 12, 2905–2914. [Google Scholar] [CrossRef] [PubMed]
- Jeyakumar, E.; Lawrence, R.; Pal, T. Comparative Evaluation in the Efficacy of Peppermint (Mentha piperita) Oil with Standards Antibiotics against Selected Bacterial Pathogens. Asian Pac. J. Trop. Biomed. 2011, 1, S253–S257. [Google Scholar] [CrossRef]
- Kotan, R.; Kordali, S.; Cakir, A. Screening of Antibacterial Activities of Twenty-One Oxygenated Monoterpenes. Z. Naturforschung C 2007, 62, 507–513. [Google Scholar] [CrossRef]
- Iraji, A.; Yazdanpanah, S.; Alizadeh, F.; Mirzamohammadi, S.; Ghasemi, Y.; Pakshir, K.; Yang, Y.; Zomorodian, K. Screening the Antifungal Activities of Monoterpenes and Their Isomers against Candida Species. J. Appl. Microbiol. 2020, 129, 1541–1551. [Google Scholar] [CrossRef] [PubMed]
- Haghgoo, R.; Abbasi, F. Evaluation of the Use of a Peppermint Mouth Rinse for Halitosis by Girls Studying in Tehran High Schools. J. Int. Soc. Prev. Community Dent. 2013, 3, 29. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential Oils in Food Preservation: Mode of Action, Synergies, and Interactions with Food Matrix Components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Park, S.J.; Yang, W.M. Inhalation of Essential Oil from Mentha piperita Ameliorates PM10-Exposed Asthma by Targeting IL-6/JAK2/STAT3 Pathway Based on a Network Pharmacological Analysis. Pharmaceuticals 2020, 14, 2. [Google Scholar] [CrossRef]
- Ghodrati, M.; Farahpour, M.R.; Hamishehkar, H. Encapsulation of Peppermint Essential Oil in Nanostructured Lipid Carriers: In-Vitro Antibacterial Activity and Accelerative Effect on Infected Wound Healing. Colloids Surf. A Physicochem. Eng. Asp. 2019, 564, 161–169. [Google Scholar] [CrossRef]
- Modarresi, M.; Farahpour, M.-R.; Baradaran, B. Topical Application of Mentha piperita Essential Oil Accelerates Wound Healing in Infected Mice Model. Inflammopharmacology 2019, 27, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Wahab, N.; Warikoo, R. Bioefficacy of Mentha piperita Essential Oil against Dengue Fever Mosquito Aedes aegypti L. Asian Pac. J. Trop. Biomed. 2011, 1, 85–88. [Google Scholar] [CrossRef]
- Asadollahi, A.; Khoobdel, M.; Zahraei-Ramazani, A.; Azarmi, S.; Mosawi, S.H. Effectiveness of Plant-Based Repellents against Different Anopheles Species: A Systematic Review. Malar. J. 2019, 18, 436. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.K.; Malik, A. Antimicrobial Action of Essential Oil Vapours and Negative Air Ions against Pseudomonas Fluorescens. Int. J. Food Microbiol. 2010, 143, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Scartazzini, L.; Tosati, J.V.; Cortez, D.H.C.; Rossi, M.J.; Flôres, S.H.; Hubinger, M.D.; Di Luccio, M.; Monteiro, A.R. Gelatin Edible Coatings with Mint Essential Oil (Mentha arvensis): Film Characterization and Antifungal Properties. J. Food Sci. Technol. 2019, 56, 4045–4056. [Google Scholar] [CrossRef]
- Verma, S.K.; Goswami, P.; Verma, R.S.; Padalia, R.C.; Chauhan, A.; Singh, V.R.; Darokar, M.P. Chemical Composition and Antimicrobial Activity of Bergamot-Mint (Mentha citrata Ehrh.) Essential Oils Isolated from the Herbage and Aqueous Distillate Using Different Methods. Ind. Crops Prod. 2016, 91, 152–160. [Google Scholar] [CrossRef]
- Snoussi, M.; Noumi, E.; Trabelsi, N.; Flamini, G.; Papetti, A.; De Feo, V. Mentha spicata Essential Oil: Chemical Composition, Antioxidant and Antibacterial Activities against Planktonic and Biofilm Cultures of Vibrio Spp. Strains. Molecules 2015, 20, 14402–14424. [Google Scholar] [CrossRef]
- Gamal-AbdelNaser, A.; Mohammed, W.S.; ElHefnawi, M.; AbdAllah, M.; Elsharkawy, A.; Zahran, F.M. The Oral Microbiome of Treated and Untreated Chronic HCV Infection: A Preliminary Study. Oral Dis. 2023, 29, 843–852. [Google Scholar] [CrossRef]
- Elghannam, M.T.; Hassanien, M.H.; Ameen, Y.A.; Turky, E.A.; Elattar, G.M.; ElRay, A.A.; Eltalkawy, M.D. Oral Microbiota and Liver Diseases. Clin. Nutr. ESPEN 2023, 54, 68–72. [Google Scholar] [CrossRef]
- Freire, M.; Moustafa, A.; Harkins, D.M.; Torralba, M.G.; Zhang, Y.; Leong, P.; Saffery, R.; Bockmann, M.; Kuelbs, C.; Hughes, T.; et al. Longitudinal Study of Oral Microbiome Variation in Twins. Sci. Rep. 2020, 10, 7954. [Google Scholar] [CrossRef]
- Decarlo, A.; Johnson, S.; Ouédraogo, A.; Dosoky, N.S.; Setzer, W.N. Chemical Composition of the Oleogum Resin Essential Oils of Boswellia dalzielii from Burkina Faso. Plants 2019, 8, 223. [Google Scholar] [CrossRef] [PubMed]
- Brzychczy-Sroka, B.; Talaga-Ćwiertnia, K.; Sroka-Oleksiak, A.; Gurgul, A.; Zarzecka-Francica, E.; Ostrowski, W.; Kąkol, J.; Drożdż, K.; Brzychczy-Włoch, M.; Zarzecka, J. Standardization of the Protocol for Oral Cavity Examination and Collecting of the Biological Samples for Microbiome Research Using the Next-Generation Sequencing (NGS): Own Experience with the COVID-19 Patients. Sci. Rep. 2024, 14, 3717. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.J.S.; Parmar, V.; Blaser, M.J. Assessing Saliva Microbiome Collection and Processing Methods. NPJ Biofilms Microbiomes 2021, 7, 81. [Google Scholar] [CrossRef] [PubMed]
- Caselli, E.; Fabbri, C.; D’Accolti, M.; Soffritti, I.; Bassi, C.; Mazzacane, S.; Franchi, M. Defining the Oral Microbiome by Whole-Genome Sequencing and Resistome Analysis: The Complexity of the Healthy Picture. BMC Microbiol. 2020, 20, 120. [Google Scholar] [CrossRef] [PubMed]
- Illumina 16S Metagenomic Sequencing Library Preparation: Preparing 16S Ribosomal RNA Gene Amplicons for the Illumina MiSeq System. Part No. 15044223 Rev B. Illumina, San Diego, CA, USA. Available online: https://www.illumina.com/content/dam/illumina-support/documents/documentation/chemistry_documentation/16s/16s-metagenomic-library-prep-guide-15044223-b.pdf (accessed on 5 December 2020).
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, A.; Park, J.; Chen, Y.-A.; Kawashima, H.; Mizuguchi, K. Impact of Quality Trimming on the Efficiency of Reads Joining and Diversity Analysis of Illumina Paired-End Reads in the Context of QIIME1 and QIIME2 Microbiome Analysis Frameworks. BMC Bioinform. 2019, 20, 581. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a Chimera-Checked 16S RRNA Gene Database and Workbench Compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-High-Throughput Microbial Community Analysis on the Illumina HiSeq and MiSeq Platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, W. Application of High-Throughput Sequencing in Understanding Human Oral Microbiome Related with Health and Disease. Front. Microbiol. 2014, 5, 508. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Yin, J.; Zhao, J.; Ma, S.; Wang, H.; Wang, M.; Chen, W.; Wei, W. Microbial Similarity and Preference for Specific Sites in Healthy Oral Cavity and Esophagus. Front. Microbiol. 2018, 9, 1603. [Google Scholar] [CrossRef] [PubMed]
- Shade, A.; Handelsman, J. Beyond the Venn Diagram: The Hunt for a Core Microbiome. Environ. Microbiol. 2012, 14, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Zaura, E.; Keijser, B.J.; Huse, S.M.; Crielaard, W. Defining the Healthy “Core Microbiome” of Oral Microbial Communities. BMC Microbiol. 2009, 9, 259. [Google Scholar] [CrossRef] [PubMed]
- Avila, M.; Ojcius, D.M.; Yilmaz, Ö. The Oral Microbiota: Living with a Permanent Guest. DNA Cell Biol. 2009, 28, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the Normal Bacterial Flora of the Oral Cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef] [PubMed]
- Crielaard, W.; Zaura, E.; Schuller, A.A.; Huse, S.M.; Montijn, R.C.; Keijser, B.J. Exploring the Oral Microbiota of Children at Various Developmental Stages of Their Dentition in the Relation to Their Oral Health. BMC Med. Genom. 2011, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Kong, J.; Jia, P.; Wei, C.; Wang, Y.; Pan, Z.; Huang, W.; Li, L.; Chen, H.; Xiang, C. Analysis of Oral Microbiota in Children with Dental Caries by PCR-DGGE and Barcoded Pyrosequencing. Microb. Ecol. 2010, 60, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Musić, L.; Par, M.; Peručić, J.; Badovinac, A.; Plančak, D.; Puhar, I. Relationship Between Halitosis and Periodontitis: A Pilot Study. Acta Stomatol. Croat. 2021, 55, 198–206. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Hong, J.-Y. Oral Microbiome as a Co-Mediator of Halitosis and Periodontitis: A Narrative Review. Front. Oral Health 2023, 4, 1229145. [Google Scholar] [CrossRef]
- Persson, S.; Edlund, M.; Claesson, R.; Carlsson, J. The Formation of Hydrogen Sulfide and Methyl Mercaptan by Oral Bacteria. Oral Microbiol. Immunol. 1990, 5, 195–201. [Google Scholar] [CrossRef]
- Al-Bayati, F.A. Isolation and Identification of Antimicrobial Compound from Mentha longifolia L. Leaves Grown Wild in Iraq. Ann. Clin. Microbiol. Antimicrob. 2009, 8, 20. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, C.; Zhang, N.; Peng, Y.; Ma, Y.; Gu, K.; Liu, X.; Liu, X.; Liu, X.; Liu, Y.; et al. Menthone Exerts Its Antimicrobial Activity against Methicillin Resistant Staphylococcus aureus by Affecting Cell Membrane Properties and Lipid Profile. Drug Des. Devel Ther. 2023, 17, 219–236. [Google Scholar] [CrossRef] [PubMed]
- Dobler, D.; Runkel, F.; Schmidts, T. Effect of Essential Oils on Oral Halitosis Treatment: A Review. Eur. J. Oral Sci. 2020, 128, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Torres-Morales, J.; Mark Welch, J.L.; Dewhirst, F.E.; Borisy, G.G. Site-Specialization of Human Oral Gemella Species. J. Oral Microbiol. 2023, 15, 2225261. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, T.; Oge, S.; Nakata, S.; Ueno, Y.; Ukita, H.; Kousaka, R.; Miura, Y.; Yoshinari, N.; Yoshida, A. Gemella Haemolysans Inhibits the Growth of the Periodontal Pathogen Porphyromonas Gingivalis. Sci. Rep. 2021, 11, 11742. [Google Scholar] [CrossRef]
- Mark Welch, J.L.; Rossetti, B.J.; Rieken, C.W.; Dewhirst, F.E.; Borisy, G.G. Biogeography of a Human Oral Microbiome at the Micron Scale. Proc. Natl. Acad. Sci. USA 2016, 113, E791–E800. [Google Scholar] [CrossRef] [PubMed]
- Esberg, A.; Barone, A.; Eriksson, L.; Lif Holgerson, P.; Teneberg, S.; Johansson, I. Corynebacterium Matruchotii Demography and Adhesion Determinants in the Oral Cavity of Healthy Individuals. Microorganisms 2020, 8, 1780. [Google Scholar] [CrossRef] [PubMed]
- Kreth, J.; Helliwell, E.; Treerat, P.; Merritt, J. Molecular Commensalism: How Oral Corynebacteria and Their Extracellular Membrane Vesicles Shape Microbiome Interactions. Front. Oral Health 2024, 5, 1410786. [Google Scholar] [CrossRef]





| Participant ID | Gender | Age | No. of Teeth | Oral Hygiene Devices at Home | City of Residency |
|---|---|---|---|---|---|
| 1 | Male | 23 | 32 | None | Ismailia |
| 3 | Male | 23 | 32 | Floss | Cairo |
| 5 | Male | 24 | 32 | None | Cairo |
| 6 | Male | 23 | 30 | None | Ismailia |
| 7 | Male | 23 | 32 | None | Sinai |
| 8 | Male | 22 | 32 | None | Ismailia |
| 9 | Male | 22 | 31 | None | Ismailia |
| 10 | Male | 23 | 31 | None | Ismailia |
| 11 | Female | 21 | 32 | None | Eltour |
| 12 | Female | 21 | 32 | None | Suez |
| 13 | Female | 21 | 32 | None | Suez |
| 16 | Male | 21 | 32 | None | Suez |
| 17 | Female | 21 | 32 | None | Suez |
| 19 | Female | 21 | 31 | None | Suez |
| 20 | Female | 21 | 32 | None | Suez |
| 21 | Male | 23 | 32 | Floss | Ismailia |
| 22 | Female | 25 | 32 | None | Ismailia |
| 23 | Female | 22 | 32 | None | Ismailia |
| 24 | Female | 25 | 31 | Floss | Suez |
| 25 | Female | 33 | 32 | Floss | Ismailia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelrahman, S.M.; El Samak, M.; El-Baz, L.M.F.; Hanora, A.M.S.; Satyal, P.; Dosoky, N.S. Effects of Mint Oils on the Human Oral Microbiome: A Pilot Study. Microorganisms 2024, 12, 1538. https://doi.org/10.3390/microorganisms12081538
Abdelrahman SM, El Samak M, El-Baz LMF, Hanora AMS, Satyal P, Dosoky NS. Effects of Mint Oils on the Human Oral Microbiome: A Pilot Study. Microorganisms. 2024; 12(8):1538. https://doi.org/10.3390/microorganisms12081538
Chicago/Turabian StyleAbdelrahman, Samar M., Manar El Samak, Lamis M. F. El-Baz, Amro M. S. Hanora, Prabodh Satyal, and Noura S. Dosoky. 2024. "Effects of Mint Oils on the Human Oral Microbiome: A Pilot Study" Microorganisms 12, no. 8: 1538. https://doi.org/10.3390/microorganisms12081538






