Soil Erosion Thickness and Seasonal Variations Together Drive Soil Nitrogen Dynamics at the Early Stage of Vegetation Restoration in the Dry-Hot Valley
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Establishment of the Simulated Erosion Thickness Plots
2.2.1. Principles of the Construction and Simulation Process of the Erosion Plots
2.2.2. Parent Materials Added
2.3. Soil and Plant Samples, Collection and Measurement
2.4. Soil Microbial DNA Extraction, PCR Amplification and Sequencing
2.5. Statistical Analysis
3. Results
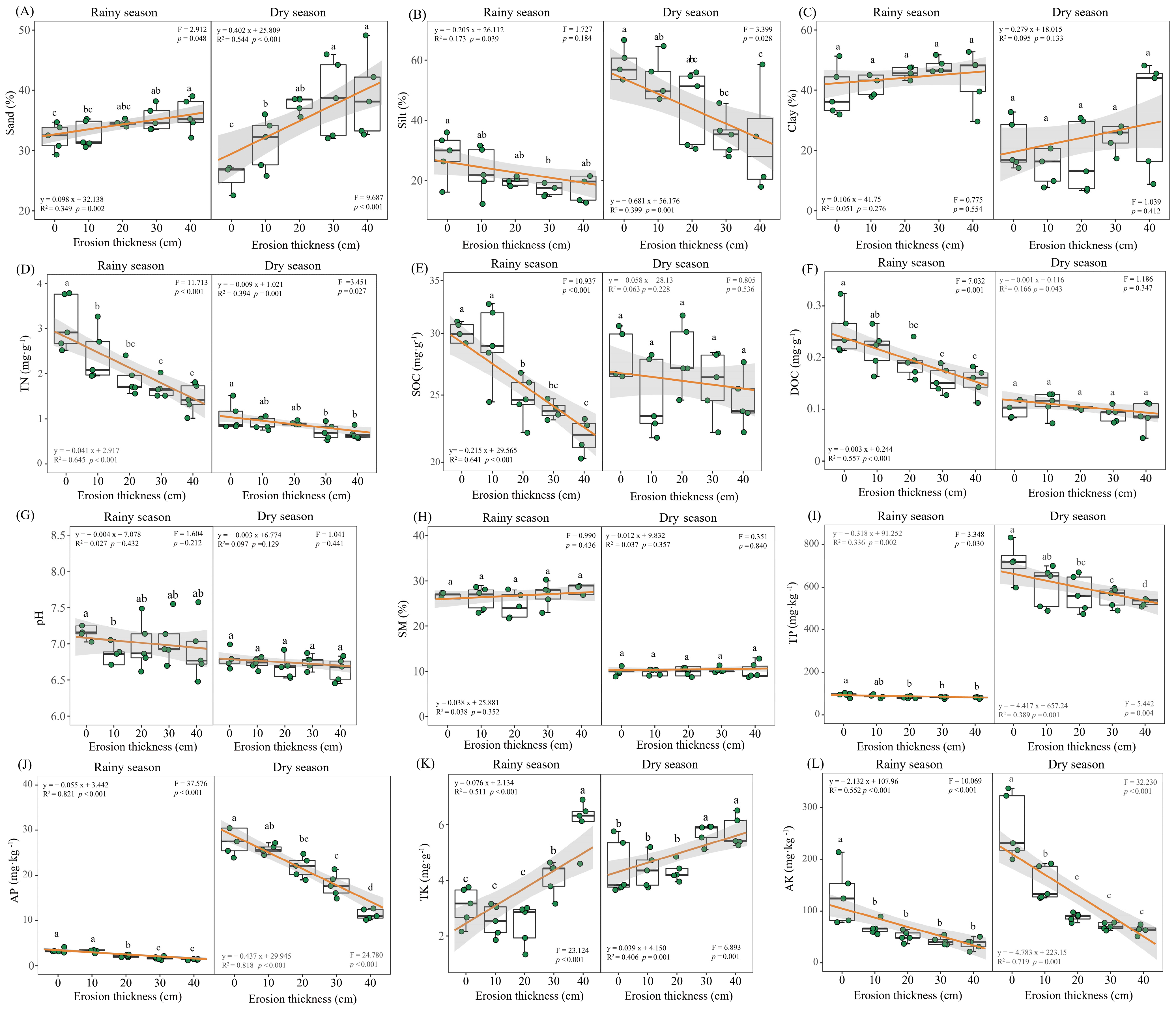
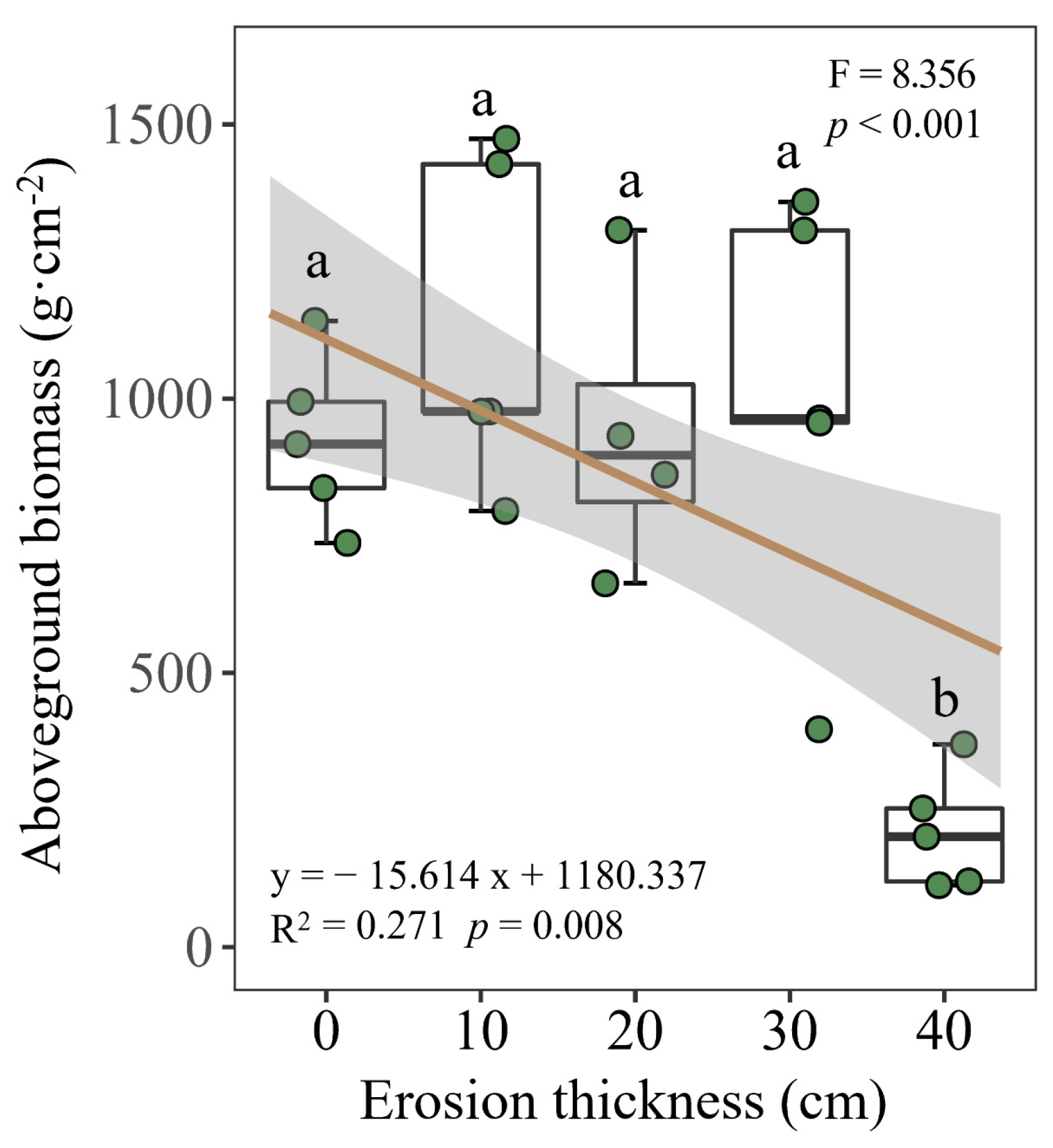
3.1. Soil Properties and Aboveground Biomass
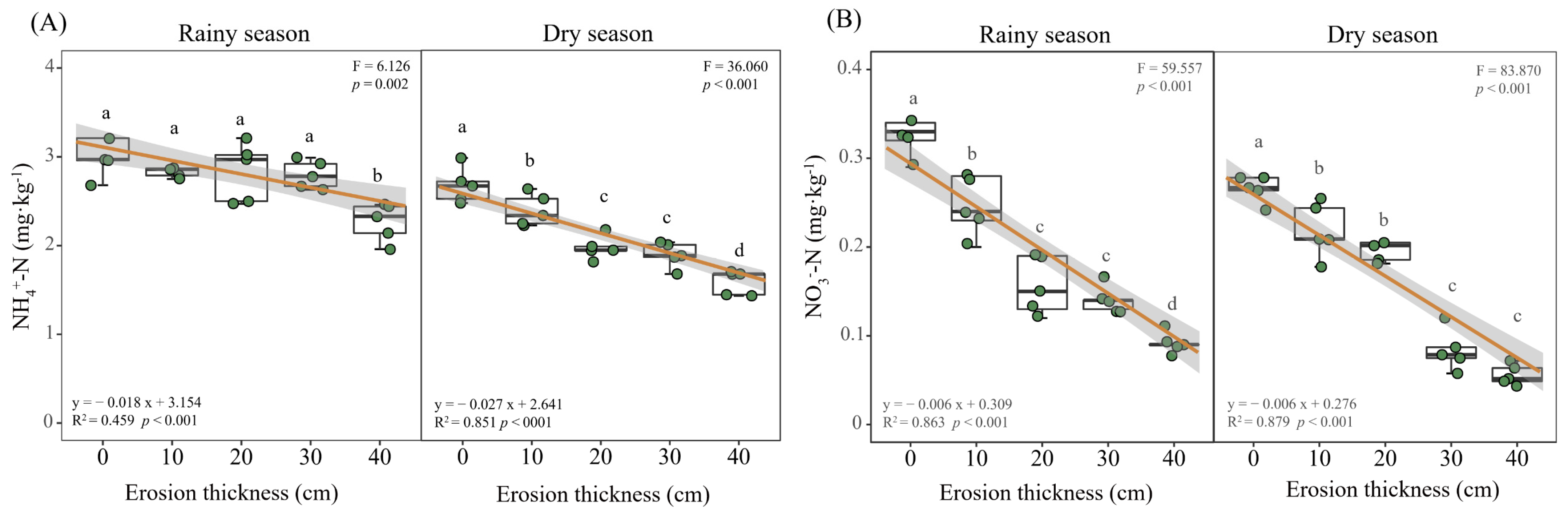
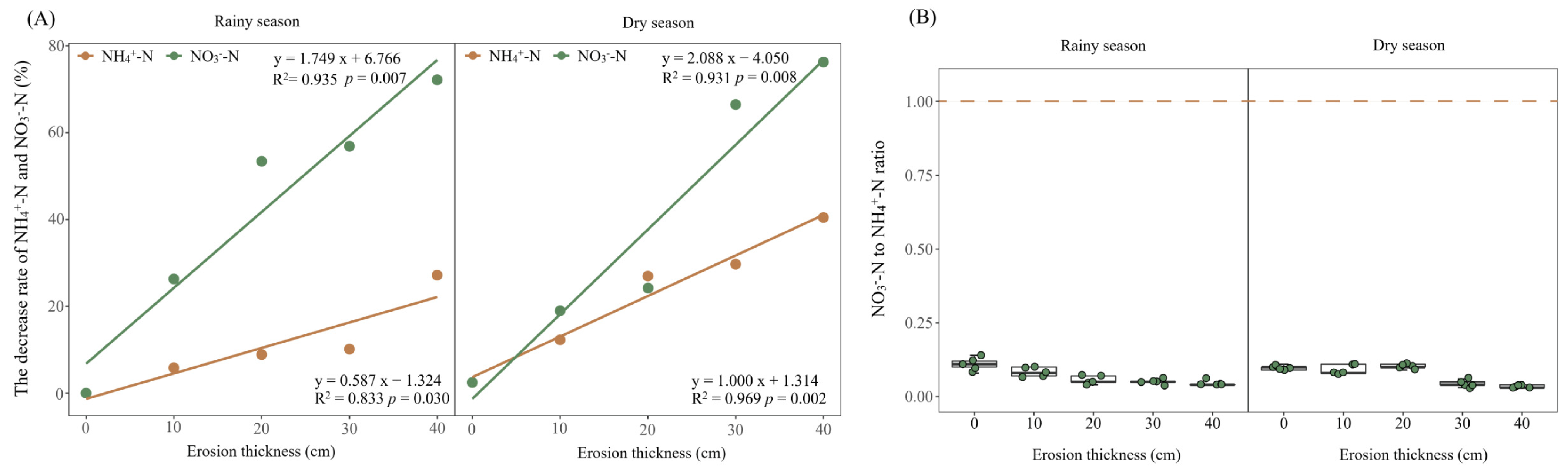
3.2. Soil Nitrogen Dynamics
3.3. Interaction Effects of Soil Erosion, Soil and Plant Properties on Nitrogen
4. Discussion
4.1. Effects of Soil Erosion Thickness on Soil Nitrogen Dynamics
4.2. Dominant Drivers of Soil Nitrogen
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. Proceedings of the Global Symposium on Soil Erosion; FAO Headquarters: Rome, Italy, 2019. [Google Scholar]
- Cao, W.; Qin, W.; Wang, D. Appropriate targets for soil erosion control at the national scale determined in China. River 2023, 2, 169–172. [Google Scholar] [CrossRef]
- Janeau, J.L.; Gillard, L.C.; Grellier, S.; Jouquet, P.; Le, T.P.Q.; Luu, T.N.M.; Ngo, Q.A.; Orange, D.; Pham, D.R.; Tran, D.T.; et al. Soil erosion, dissolved organic carbon and nutrient losses under different land use systems in a small catchment in northern Vietnam. Agric. Water Manag. 2014, 146, 314–323. [Google Scholar] [CrossRef]
- An, M.; Han, Y.; Zhao, C.; Qu, Z.; Xu, P.; Wang, X.; He, X. Effects of different wind directions on soil erosion and nitrogen loss processes under simulated wind-driven rain. Catena 2022, 217, 106423. [Google Scholar] [CrossRef]
- Berhe, A.A.; Barnes, R.T.; Six, J.; Marín-Spiotta, E. Role of soil erosion in biogeochemical cycling of essential elements: Carbon, nitrogen, and phosphorus. Annu. Rev. Earth Planet. Sci. 2018, 46, 521–548. [Google Scholar] [CrossRef]
- Wang, L.; Yen, H.; Huang, C.; Wan, Y. Erosion and covered zones altered by surface coverage effects on soil nitrogen and carbon loss from an agricultural slope under laboratory-simulated rainfall events. Int. Soil Water Conserv. 2022, 10, 382–392. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Wu, J.; An, Z.; Suo, L.; Ding, J.; Li, S.; Wei, D.; Jin, L. Effects of the rainfall intensity and slope gradient on soil erosion and nitrogen loss on the sloping fields of miyun reservoir. Plants 2023, 12, 423. [Google Scholar] [CrossRef] [PubMed]
- Berhe, A.A.; Torn, M.S. Erosional redistribution of topsoil controls soil nitrogen dynamics. Biogeochemistry 2017, 132, 37–54. [Google Scholar] [CrossRef]
- Mihara, M. Nitrogen and phosphorus losses due to soil erosion during a typhoon, Japan. J. Agric. Eng. Res. 2001, 78, 209–216. [Google Scholar] [CrossRef]
- Fu, B.J.; Meng, Q.H.; Qiu, Y.; Zhao, W.W.; Zhang, Q.J.; Davidson, D.A. Effects of land use on soil erosion and nitrogen loss in the hilly area of the Loess Plateau, China. Land Degrad. Dev. 2003, 15, 87–96. [Google Scholar] [CrossRef]
- Qiu, L.; Zhu, H.; Liu, J.; Yao, Y.; Wang, X.; Rong, G.; Zhao, X.; Shao, M.; Wei, X. Soil erosion significantly reduces organic carbon and nitrogen mineralization in a simulated experiment. Agric. Ecosyst. Environ. 2021, 307, 107232. [Google Scholar] [CrossRef]
- Wang, Z.; Pan, S.; Lv, J.; Peng, Y.; Shi, J.; Wang, X. Erosion and deposition controlling redistribution and biodegradation of nitrogen fractions along a Mollisol agricultural landscape. J. Soils Sediments 2024, 24, 86–97. [Google Scholar] [CrossRef]
- Nie, X.; Zhang, H.; Su, Y. Soil carbon and nitrogen fraction dynamics affected by tillage erosion. Sci. Rep. 2019, 9, 16601. [Google Scholar] [CrossRef]
- Zhang, J.H.; Wang, Y.; Li, F.C. Soil organic carbon and nitrogen losses due to soil erosion and cropping in a sloping terrace landscape. Soil Res. 2015, 53, 87–96. [Google Scholar] [CrossRef]
- Zhang, R.; Rong, L.; Zhang, L. Soil nutrient variability mediates the effects of erosion on soil microbial communities: Results from a modified topsoil removal method in an agricultural field in Yunnan plateau, China. Environ. Sci. Pollut. Res. 2022, 29, 3659–3671. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, Q.; Zhu, H.; Reich, P.B.; Banerjee, S.; Heijden, M.G.A.; Sadowsky, M.J.; Ishii, S.; Jia, X.; Shao, M.; et al. Erosion reduces soil microbial diversity, network complexity and multifunctionality. ISME J. 2021, 15, 2474–2489. [Google Scholar] [CrossRef]
- Wen, L.; Li, D.; Yang, L.; Luo, P.; Chen, H.; Xiao, K.; Song, T.; Zhang, W.; He, X.; Chen, H.; et al. Rapid recuperation of soil nitrogen following agricultural abandonment in a karst area, southwest China. Biogeochemistry 2016, 129, 341–354. [Google Scholar] [CrossRef]
- Zhang, B.J.; Zhang, G.H.; Zhu, P.Z.; Yang, H.Y. Temporal variations in soil erodibility indicators of vegetation-restored steep gully slopes on the Loess Plateau of China. Agric. Ecosyst. Environ. 2019, 286, 106661. [Google Scholar] [CrossRef]
- Farzadfar, S.; Knight, J.D.; Congreves, K.A. Soil organic nitrogen: An overlooked but potentially significant contribution to crop nutrition. Plant Soil 2021, 462, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Henry, H.A.L.; Jefferies, R.L. Plant amino acid uptake, soluble N turnover and microbial N capture in soils of a grazed Arctic salt marsh. J. Ecol. 2003, 91, 627–636. [Google Scholar] [CrossRef]
- Jones, D.L.; Healey, J.R.; Willett, V.B.; Farrar, J.F.; Hodge, A. Dissolved organic nitrogen uptake by plants-an important N uptake pathway? Soil Biol. Biochem. 2005, 37, 413–423. [Google Scholar] [CrossRef]
- Miller, A.E.; Bowman, W.D. Alpine plants show species-level differences in the uptake of organic and inorganic nitrogen. Plant Soil 2003, 250, 283–292. [Google Scholar] [CrossRef]
- Robertson, G.P.; Groffman, P.M. Nitrogen Transformations, 4th ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 421–446. [Google Scholar]
- Feyissa, A.; Yang, F.; Wu, J.; Chen, Q.; Zhang, D.; Cheng, X. Soil nitrogen dynamics at a regional scale along a precipitation gradient in secondary grassland of China. Sci. Total Environ. 2021, 781, 146736. [Google Scholar] [CrossRef]
- Liu, T.; Chen, Z.; Rong, L.; Duan, X. Land-use driven changes in soil microbial community composition and soil fertility in the dry-Hot Valley region of southwestern China. Microorganisms 2022, 10, 956. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Cui, P.; Ge, Y.; Chen, C.; Wang, D.; Wu, C.; Li, J.; Yu, W.; Zhang, G.; Lin, H. The succession characteristics of soil erosion during different vegetation succession stages in dry-hot river valley of Jinsha River, upper reaches of Yangtze River. Ecol. Eng. 2014, 62, 13–26. [Google Scholar] [CrossRef]
- Duan, X.; Bai, Z.; Rong, L.; Li, Y.; Ding, J.; Tao, Y.; Li, J.; Li, J.; Wang, W. Investigation method for regional soil erosion based on the Chinese Soil Loss Equation and high-resolution spatial data: Case study on the mountainous Yunnan Province, China. Catena 2020, 184, 104237. [Google Scholar] [CrossRef]
- Duan, X.; Liu, B.; Gu, Z.; Rong, L.; Feng, D. Quantifying soil erosion effects on soil productivity in the dry-hot valley, southwestern China. Environ. Earth Sci. 2016, 75, 1164. [Google Scholar] [CrossRef]
- Ji, Z.; Fang, H.; Yang, Y.; Pan, Z.; Sha, Y. Assessment of system functions after vegetation restoration of the degraded ecosystem in arid-hot valleys of Jinsha River: A case study on small watershed of Yuanmou. Ecol. Environ. Sci. 2009, 18, 1383–1389. [Google Scholar] [CrossRef]
- Liu, G.; Deng, W.; Wen, A.; Sha, Y.; Ji, Z.; Xiong, D.; Fang, H. A review on the essentiality and subject of orientation of establishing gully erosion and collapse experimental station in jinsha river valley. J. Mt. Sci. Engl. 2010, 28, 333–340. [Google Scholar] [CrossRef]
- Xiong, D.H.; Zhou, H.Y.; Yang, Z.; Zhang, X.B. Slope lithologic property, soil moisture condition and revegetation in dry-hot valley of Jinsha River. Chin. Geogr. Sci. 2005, 15, 186–192. [Google Scholar] [CrossRef]
- Gu, Z.; Duan, X.; Shi, Y.; Li, Y.; Pan, X. Spatiotemporal variation in vegetation coverage and its response to climatic factors in the Red River Basin, China. Ecol. Indic. 2018, 93, 54–64. [Google Scholar] [CrossRef]
- Huang, Z. Changes of dry-wet climate in the dry season in Yunnan (1961–2007). Adv. Clim. Change Res. 2011, 2, 49–54. [Google Scholar] [CrossRef]
- Gong, Z.T.; Zhang, G.L.; Chen, Z.C. Soil Classification, 1st ed.; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Soil Survey Staff in USDA. Keys to Soil Taxonomy, 12th ed.; United States Department of Agriculture, Natural Resources Conservation Service: Washington, DC, USA, 2014.
- Wang, Z.; Liu, B.; Wang, X.; Gao, X.; Liu, G. Erosion effect on the productivity of black soil in Northeast China. Sci. China Ser. D-Earth Sci. 2009, 52, 1005–1021. [Google Scholar] [CrossRef]
- Hou, S.; Xin, M.; Wang, L.; Jiang, H.; Li, N.; Wang, Z. The effects of erosion on the microbial populations and enzyme activity in black soil of northeastern China. Acta Ecol. Sin. 2014, 34, 295–301. [Google Scholar] [CrossRef]
- Ma, S.; Song, Y.; Liu, J.; Kang, X.; Yue, Z.Q. Extended wet sieving method for determination of complete particle size distribution of general soils. J. Rock Mech. Geotech. 2024, 16, 242–257. [Google Scholar] [CrossRef]
- United States Department of Agriculture. Soil Survey Manual; Government Printing Office: Washington, DC, USA, 1951.
- NY-T 1121.3-2006; Soil Testing-Part 3: Method for Determination of Soil Mechanical Composition. Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2006.
- Wu, C.; Liu, G.; Huang, C.; Liu, Q. Soil quality assessment in Yellow River Delta: Establishing a minimum data set and fuzzy logic model. Geoderma 2019, 334, 82–89. [Google Scholar] [CrossRef]
- Lumini, E.; Orgiazzi, A.; Borriello, R.; Bonfante, P.; Bianciotto, V. Disclosing arbuscular mycorrhizal fungal biodiversity in soil through a land-use gradient using a pyrosequencing approach. Environ. Microbiol. 2010, 12, 2165–2179. [Google Scholar] [CrossRef]
- Poly, F.; Monrozier, L.J.; Bally, R. Improvement in the RFLP procedure for studying the diversity of nifH genes in communities of nitrogen fixers in soil. Res. Microbiol. 2001, 152, 95–103. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef]
- Mao, X.; Zheng, J.; Yu, W.; Guo, X.; Xu, K.; Zhao, R.; Xiao, L.; Wang, M.; Jiang, Y.; Zhang, S.; et al. Climate-induced shifts in composition and protection regulate temperature sensitivity of carbon decomposition through soil profile. Soil Biol. Biochem. 2022, 172, 108743. [Google Scholar] [CrossRef]
- Gao, X.; Chen, H.; Govaert, L.; Wang, W.; Yang, J. Responses of zooplankton body size and community trophic structure to temperature change in a subtropical reservoir. Ecol. Evol. 2019, 9, 12544–12555. [Google Scholar] [CrossRef]
- Quinton, J.N.; Govers, G.; Oost, K.V.; Bardgett, R.D. The impact of agricultural soil erosion on biogeochemical cycling. Nat. Geosci. 2010, 3, 311–314. [Google Scholar] [CrossRef]
- Vilakazi, B.S.; Zengeni, R.; Mafongoya, P. The effects of different tillage techniques and N fertilizer rates on Nitrogen and Phosphorus in dry land Agriculture. Agronomy 2022, 12, 2389. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; Yang, Y.; Shi, Y.; Li, H. Degradation reduces the diversity of nitrogen-fixing bacteria in the alpine wetland on the Qinghai-Tibet Plateau. Front. Plant Sci. 2022, 13, 939762. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, N. Effect of edaphic factors and seasonal variation on spore density and root colonization of arbuscular mycorrhizal fungi in sugarcane fields. Ann. Microbiol. 2012, 63, 151–160. [Google Scholar] [CrossRef]
- Liang, M.; Wu, Y.; Zhao, Q.; Jiang, Y.; Sun, W.; Liu, G.; Ma, L.; Xue, S. Secondary vegetation succession on the Loess Plateau altered the interaction between arbuscular mycorrhizal fungi and nitrogen-fixing bacteria. For. Ecol. Manag. 2003, 530, 120744. [Google Scholar] [CrossRef]
- Zhu, C.; Tian, G.; Luo, G.; Kong, Y.; Guo, J.; Wang, M.; Guo, S.; Ling, N.; Shen, Q. N-fertilizer-driven association between the arbuscular mycorrhizal fungal community and diazotrophic community impacts wheat yield. Agric. Ecosyst. Environ. 2018, 254, 191–201. [Google Scholar] [CrossRef]
- Yu, H.; Liu, X.; Yang, C.; Peng, Y.; Yu, X.; Gu, H.; Zheng, X.; Wang, C.; Xiao, F.; Shu, L.; et al. Co-symbiosis of arbuscular mycorrhizal fungi (AMF) and diazotrophs promote biological nitrogen fixation in mangrove ecosystems. Soil Biol. Biochem. 2021, 161, 108382. [Google Scholar] [CrossRef]
- Oehl, F.; Sieverding, E.; Ineichen, K.; Ris, E.A.; Boller, T.; Wiemken, A. Community structure of arbuscular mycorrhizal fungi at different soil depths in extensively and intensively managed agroecosystems. New Phytol. 2005, 165, 273–283. [Google Scholar] [CrossRef]

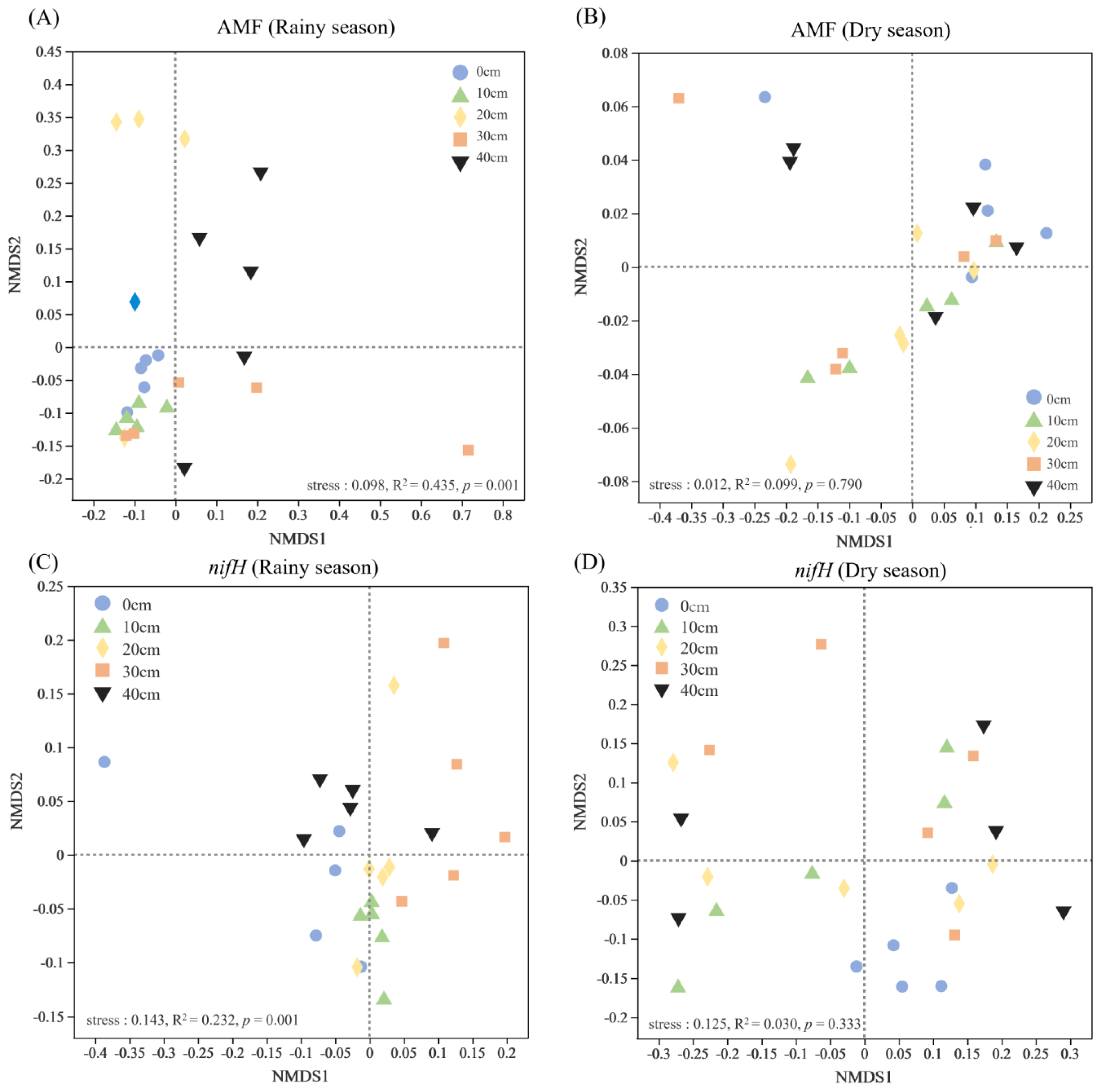

| AMF | nifH Genes | |||||||
|---|---|---|---|---|---|---|---|---|
| Rainy Season | Dry Season | Rainy Season | Dry Season | |||||
| Vs_Group | R2 | p | R2 | p | R2 | p | R2 | p |
| 0–10 | 0.400 | 0.021 | 0.098 | 0.373 | 0.249 | 0.012 | 0.251 | 0.024 |
| 0–20 | 0.437 | 0.034 | 0.148 | 0.300 | 0.164 | 0.139 | 0.153 | 0.284 |
| 0–30 | 0.252 | 0.095 | 0.118 | 0.282 | 0.336 | 0.004 | 0.305 | 0.060 |
| 0–40 | 0.404 | 0.007 | 0.068 | 0.447 | 0.207 | 0.014 | 0.163 | 0.245 |
| 10–20 | 0.512 | 0.039 | 0.009 | 0.911 | 0.152 | 0.183 | 0.041 | 0.784 |
| 10–30 | 0.216 | 0.217 | 0.131 | 0.375 | 0.374 | 0.008 | 0.110 | 0.465 |
| 10–40 | 0.489 | 0.009 | 0.052 | 0.640 | 0.359 | 0.009 | 0.059 | 0.678 |
| 20–30 | 0.293 | 0.031 | 0.129 | 0.379 | 0.167 | 0.192 | 0.083 | 0.536 |
| 20–40 | 0.273 | 0.044 | 0.066 | 0.561 | 0.131 | 0.348 | 0.052 | 0.752 |
| 30–40 | 0.204 | 0.080 | 0.042 | 0.750 | 0.246 | 0.038 | 0.043 | 0.812 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Chen, Z.; Rong, L.; Duan, X.; Qin, Y.; Chun, Z.; Liu, X.; Wu, J.; Wang, Z.; Liu, T. Soil Erosion Thickness and Seasonal Variations Together Drive Soil Nitrogen Dynamics at the Early Stage of Vegetation Restoration in the Dry-Hot Valley. Microorganisms 2024, 12, 1546. https://doi.org/10.3390/microorganisms12081546
Liu W, Chen Z, Rong L, Duan X, Qin Y, Chun Z, Liu X, Wu J, Wang Z, Liu T. Soil Erosion Thickness and Seasonal Variations Together Drive Soil Nitrogen Dynamics at the Early Stage of Vegetation Restoration in the Dry-Hot Valley. Microorganisms. 2024; 12(8):1546. https://doi.org/10.3390/microorganisms12081546
Chicago/Turabian StyleLiu, Wenxu, Zhe Chen, Li Rong, Xingwu Duan, Yuhong Qin, Zhenjie Chun, Xuening Liu, Jia Wu, Zihao Wang, and Taicong Liu. 2024. "Soil Erosion Thickness and Seasonal Variations Together Drive Soil Nitrogen Dynamics at the Early Stage of Vegetation Restoration in the Dry-Hot Valley" Microorganisms 12, no. 8: 1546. https://doi.org/10.3390/microorganisms12081546






