Effect of Nitrogen on Microbial Communities of Purple Mudstone Weathering Products in Southwest China: A Column Experiment
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Experiment Design and Sampling
2.3. Measurement of Soil Chemical Properties
2.4. DNA Extraction and Sequencing
2.5. Statistical Analysis
3. Results
3.1. Soil Chemical Properties
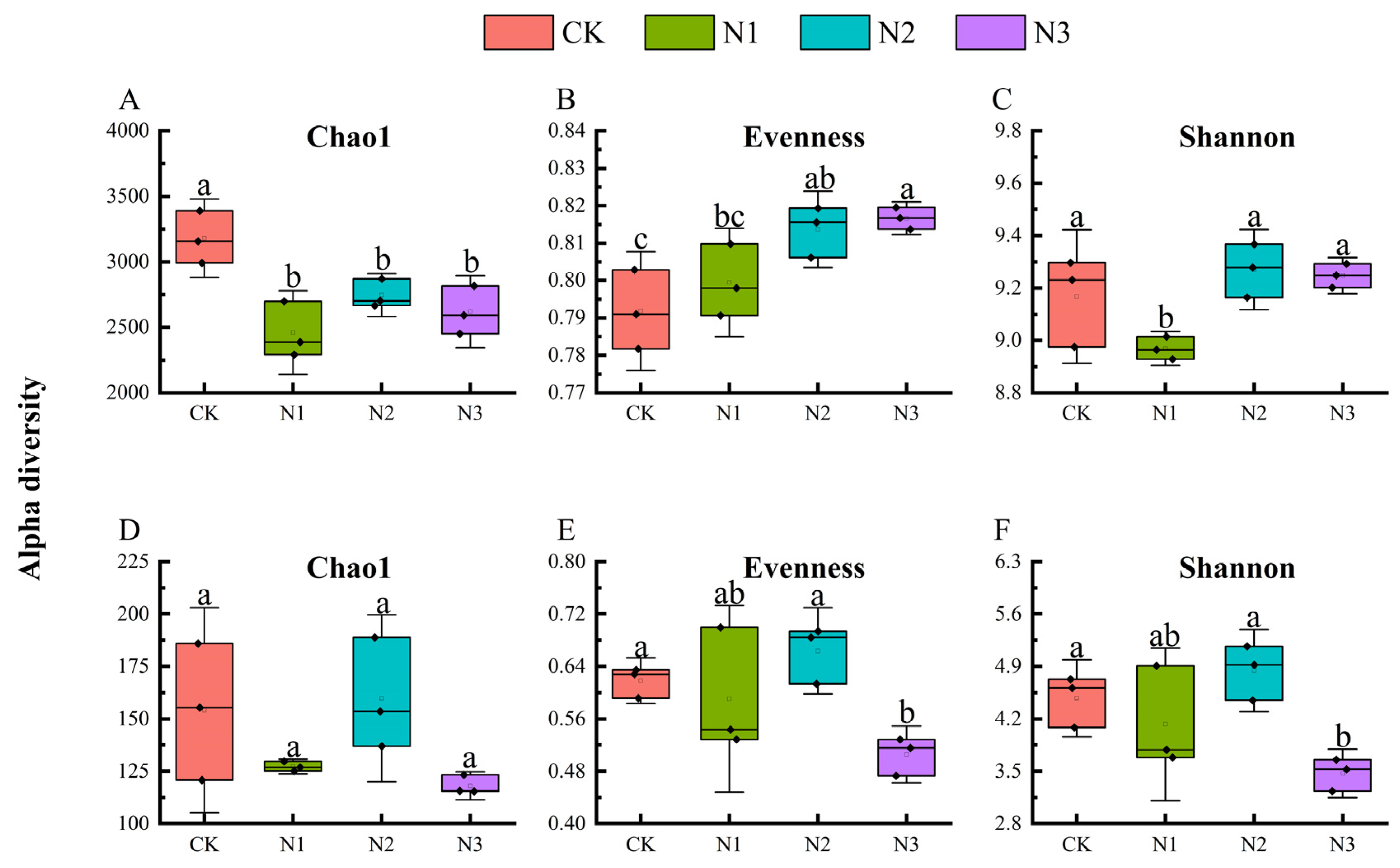
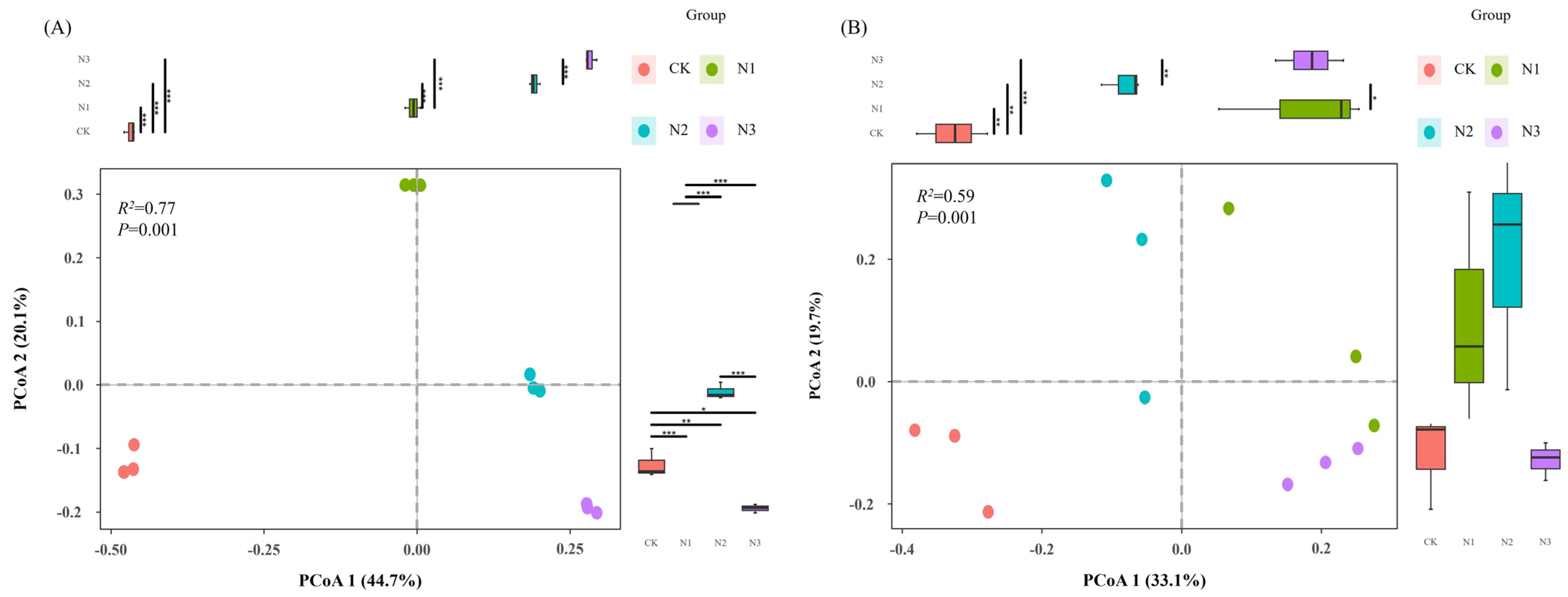
3.2. Diversity of Soil Microbial Communities
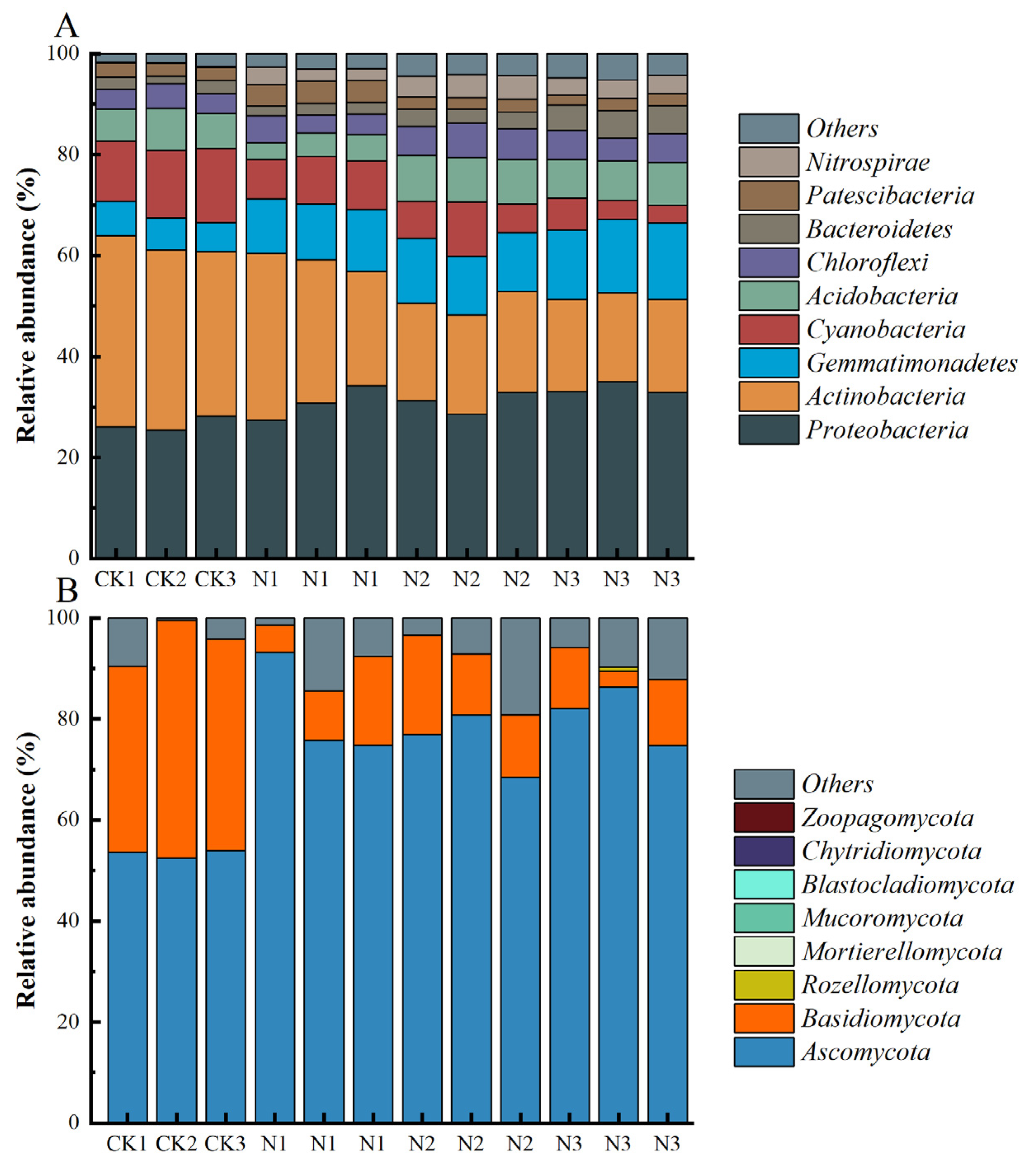
3.3. Composition of Soil Microbial Communities
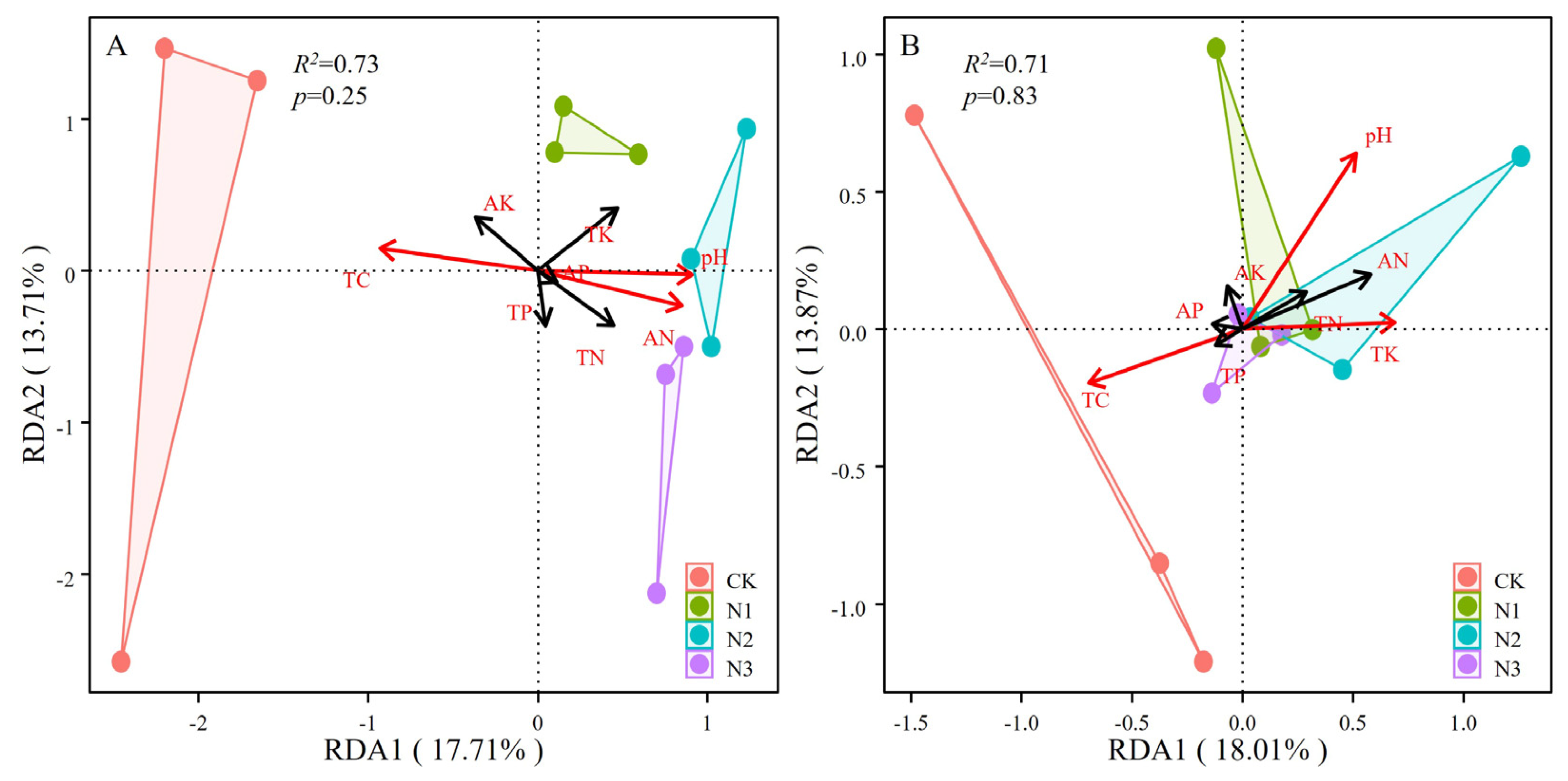
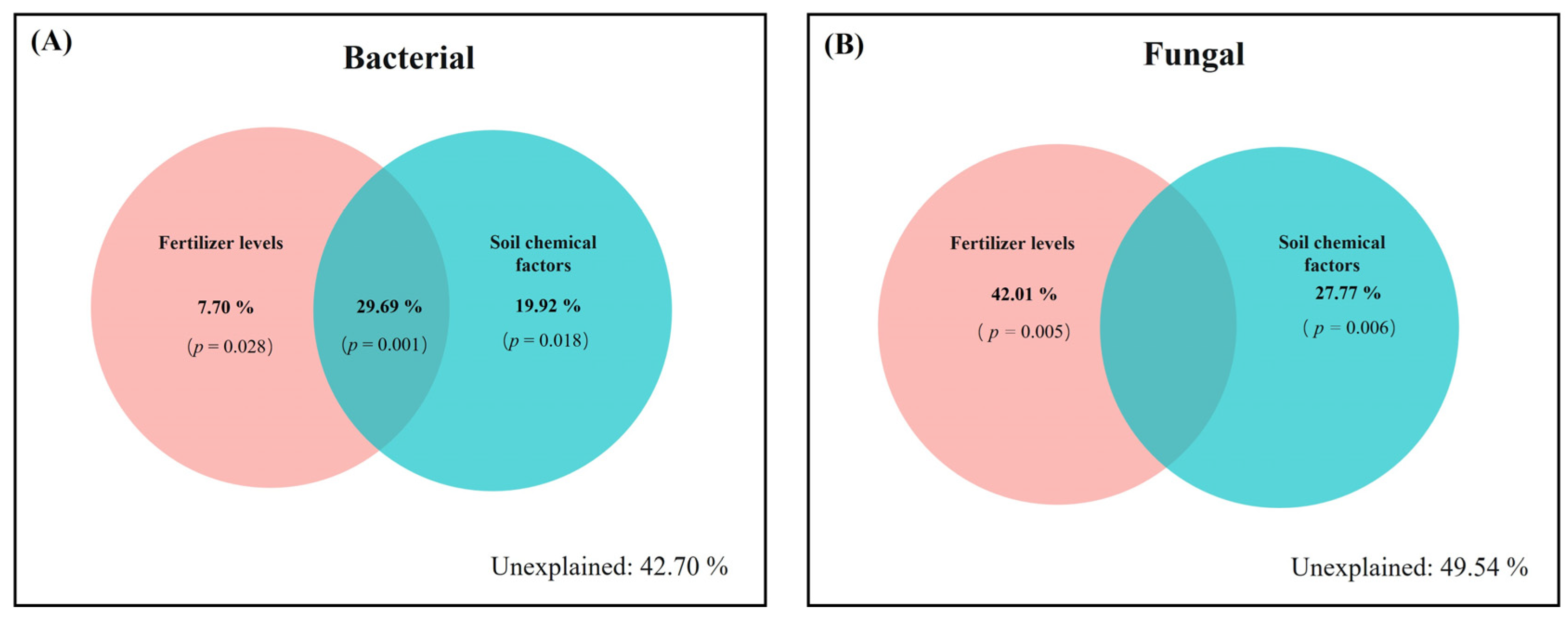
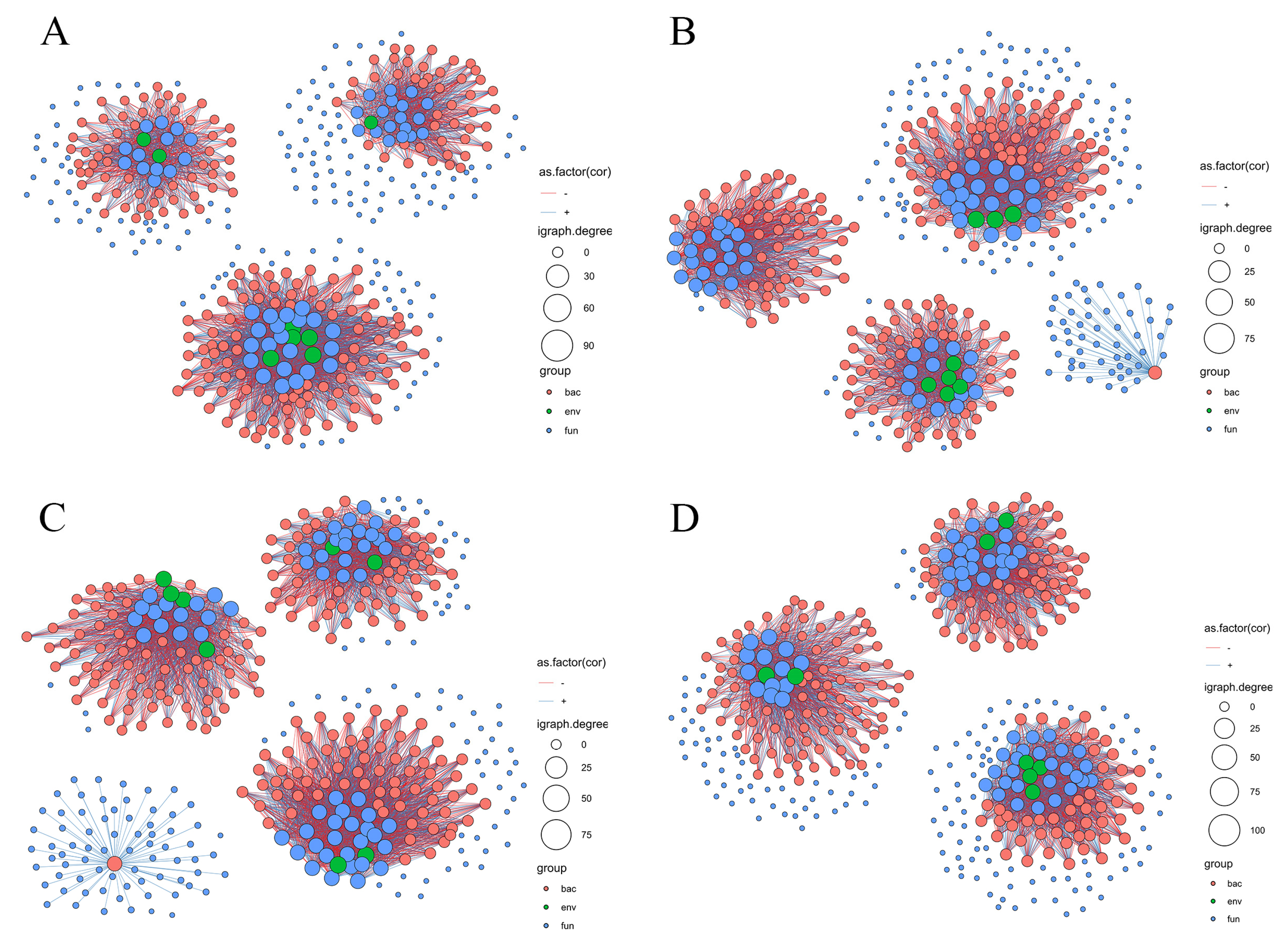
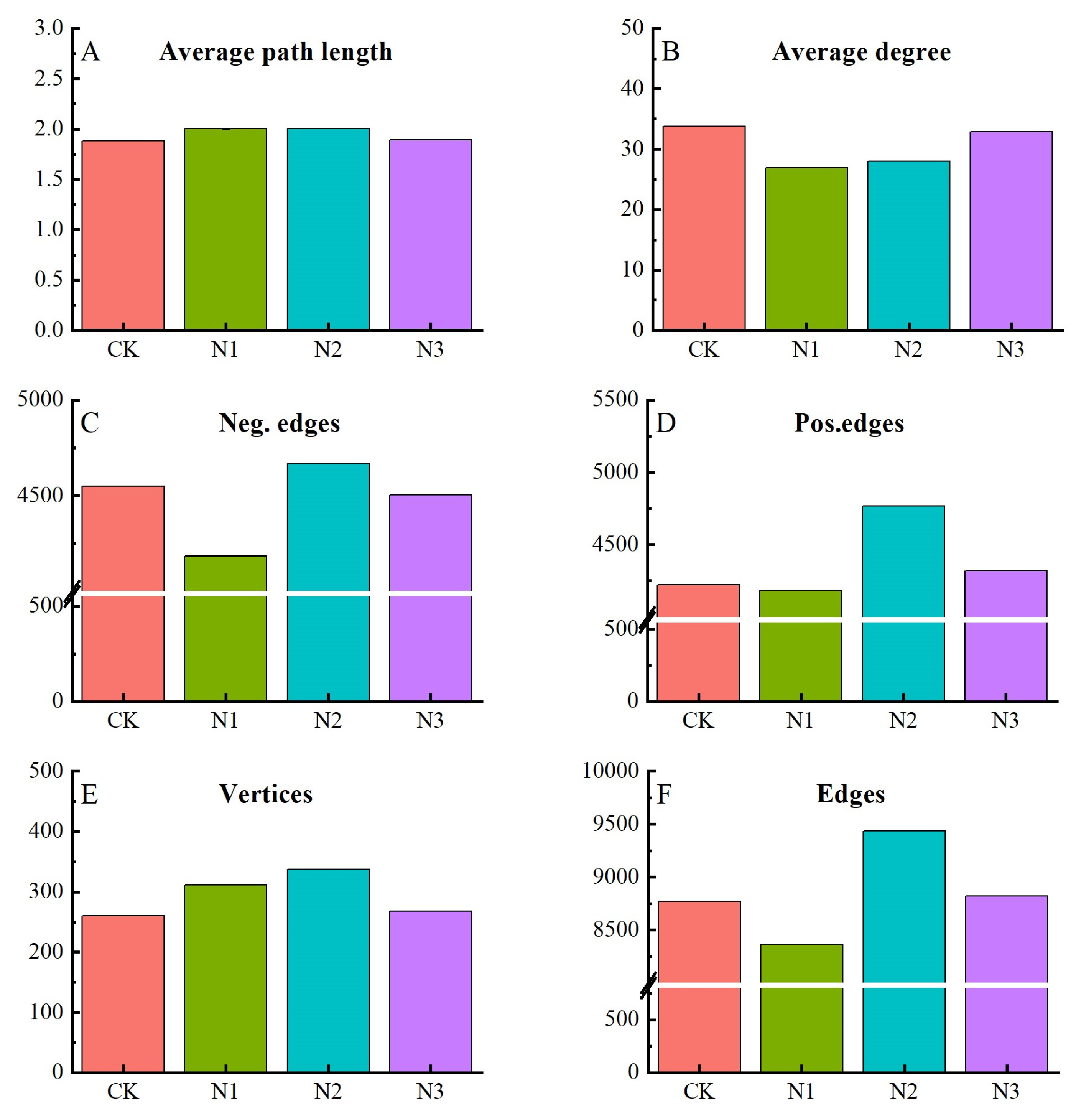
3.4. Relationship between Soil Chemical Properties and Soil Microbial Communities
4. Discussion
4.1. Effects of N Fertilizer Application on Soil Chemical Properties
4.2. Effects of N Fertilizer Application on Microbial Diversity and Composition
4.3. Relationship of Soil Chemical Properties and Microbial Community
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banerjee, S.; van der Heijden, M.G.A. Soil microbiomes and one health. Nat. Rev. Microbiol. 2023, 21, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Berdugo, M.; Campbell, C.D.; Singh, B.K. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 2016, 7, 10541. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Gong, X.; Dang, K.; Li, J.; Yang, P.; Gao, X.; Deng, X.; Feng, B. Linkages between nutrient ratio and the microbial community in rhizosphere soil following fertilizer management. Environ. Res. 2020, 184, 109261. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Huet, S.; Romdhane, S.; Breuil, M.-C.; Bru, D.; Mounier, A.; Spor, A.; Philippot, L. Experimental community coalescence sheds light on microbial interactions in soil and restores impaired functions. Microbiome 2023, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Robertson, G.P.; Vitousek, P.M. Nitrogen in agriculture: Balancing the cost of an essential resource. Annu. Rev. Env. Resour. 2009, 34, 97–125. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, X.; Song, L.; Lin, X.; Zhang, H.; Shen, C.; Chu, H. Nitrogen fertilization directly affects soil bacterial diversity and indirectly affects bacterial community composition. Soil Biol. Biochem. 2016, 92, 41–49. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2023, 22, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, W.; Yao, Y.; Li, H.; Wang, Q.; Niu, B. Microbial interactions within beneficial consortia promote soil health. Sci. Total Environ. 2023, 900, 165801. [Google Scholar] [CrossRef]
- Gui, H.; Breed, M.; Li, Y.; Xu, Q.; Yang, J.; Wanasinghe, D.N.; Li, Y.; Xu, J.; Mortimer, P. Continental-scale insights into the soil microbial co-occurrence networks of Australia and their environmental drivers. Soil Biol. Biochem. 2023, 186, 109177. [Google Scholar] [CrossRef]
- Zhu, L.; Luan, L.; Chen, Y.; Wang, X.; Zhou, S.; Zou, W.; Han, X.; Duan, Y.; Zhu, B.; Li, Y.; et al. Community assembly of organisms regulates soil microbial functional potential through dual mechanisms. Glob. Change Biol. 2024, 30, e17160. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Li, J.; Wang, J.; Zhang, C.; Liu, G.; Dong, Q. Diversity and co-occurrence network modularization of bacterial communities determine soil fertility and crop yields in arid fertigation agroecosystems. Biol. Fert. Soils 2021, 57, 809–824. [Google Scholar] [CrossRef]
- Hao, D.; Su, X.; Xie, H.; Bao, X.; Zhang, X.; Wang, L. Effects of tillage patterns and stover mulching on N2O production, nitrogen cycling genes and microbial dynamics in black soil. J. Environ. Manag. 2023, 345, 118458. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Cheng, L.; Che, L.; Su, Y.; Li, Y. Nutrients addition decreases soil fungal diversity and alters fungal guilds and co-occurrence networks in a semi-arid grassland in northern China. Sci. Total Environ. 2024, 926, 172100. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shi, Z.; Li, A.; Geng, T.; Liu, L.; Liu, W. Long-term nitrogen input reduces soil bacterial network complexity by shifts in life history strategy in temperate grassland. iMeta 2024, 3, e194. [Google Scholar] [CrossRef]
- Li, B.; Roley, S.S.; Duncan, D.S.; Guo, J.; Quensen, J.F.; Yu, H.; Tiedje, J.M. Long-term excess nitrogen fertilizer increases sensitivity of soil microbial community to seasonal change revealed by ecological network and metagenome analyses. Soil Biol. Biochem. 2021, 160, 108349. [Google Scholar] [CrossRef]
- Wu, C.; Yan, B.; Wei, F.; Wang, H.; Gao, L.; Ma, H.; Liu, Q.; Liu, Y.; Liu, G.; Wang, G. Long-term application of nitrogen and phosphorus fertilizers changes the process of community construction by affecting keystone species of crop rhizosphere microorganisms. Sci. Total Environ. 2023, 897, 165239. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Han, Z.; Du, J.; Ci, E.; Ni, J.; Xie, D.; Wei, C. Relationships between the lithology of purple rocks and the pedogenesis of purple soils in the Sichuan Basin, China. Sci. Rep. 2019, 9, 13272. [Google Scholar] [CrossRef]
- He, Y. Purple Soils in China; China Science Press: Beijing, China, 1991; ISBN 7-03-011231-8. [Google Scholar]
- Zhao, J.; Lu, C.; Deng, L.; Liu, G. Impacts of simulated acid solution on the disintegration and cation release of purple rock (mudstone) in Southwest China. Geomorphology 2018, 316, 35–43. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, A.; Zhao, J.; Lu, C.; Liu, G. Quantitative model prediction of the combined effect of moisture content and temperature on purple mudstone decay in south-western China. Geomorphology 2017, 295, 656–661. [Google Scholar] [CrossRef]
- Bao, S. Soil and Agricultural Chemistry Analysis; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Forester, B.R.; Lasky, J.R.; Wagner, H.H.; Urban, D.L. Comparing methods for detecting multilocus adaptation with multivariate genotype-environment associations. Mol. Ecol. 2018, 27, 2215–2233. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Xie, P.; Yang, S.; Niu, G.; Liu, X.; Ding, Z.; Xue, C.; Liu, Y.; Shen, Q.; Yuan, J. ggClusterNet: An R package for microbiome network analysis and modularity-based multiple network layouts. iMeta 2022, 1, e32. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Mao, Q.; Gilliam, F.S.; Luo, Y.; Mo, J. Nitrogen deposition contributes to soil acidification in tropical ecosystems. Glob. Change Biol. 2014, 20, 3790–3801. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Ros, G.H.; Zhu, Q.; Xu, M.; Wen, S.; Cai, Z.; Zhang, F.; de Vries, W. Major drivers of soil acidification over 30 years differ in paddy and upland soils in China. Sci. Total Environ. 2024, 916, 170189. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, L.; Xiao, Q.; Huang, Y.; Liu, K.; Wu, Y.; Li, D.; Duan, Y.; Zhang, W. Long-term manuring enhances soil gross nitrogen mineralization and ammonium immobilization in subtropical area. Agric. Ecosyst. Environ. 2023, 348, 108439. [Google Scholar] [CrossRef]
- Xu, T.; Xu, M.; Zhang, M.; Letnic, M.; Wang, J.; Wang, L. Spatial effects of nitrogen deposition on soil organic carbon stocks in patchy degraded saline-alkaline grassland. Geoderma 2023, 432, 116408. [Google Scholar] [CrossRef]
- Wang, X.; Feng, J.; Ao, G.; Qin, W.; Han, M.; Shen, Y.; Liu, M.; Chen, Y.; Zhu, B. Globally nitrogen addition alters soil microbial community structure, but has minor effects on soil microbial diversity and richness. Soil Biol. Biochem. 2023, 179, 108982. [Google Scholar] [CrossRef]
- Yu, G.; Chen, J.; Yu, M.; Li, A.; Wang, Y.; He, X.; Tang, X.; Liu, H.; Jiang, J.; Mo, J.; et al. Eighteen-year nitrogen addition does not increase plant phosphorus demand in a nitrogen-saturated tropical forest. J. Ecol. 2023, 111, 1545–1560. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, L.; Yang, S.; Wang, Z.; Tian, R.; Peng, Z.; Chen, Y.; Zhang, X.; Kuang, J.; Ling, N.; et al. Critical transition of soil bacterial diversity and composition triggered by nitrogen enrichment. Ecology 2020, 101, e03053. [Google Scholar] [CrossRef]
- Dai, Z.; Su, W.; Chen, H.; Barberán, A.; Zhao, H.; Yu, M.; Yu, L.; Brookes, P.C.; Schadt, C.W.; Chang, S.X.; et al. Long-term nitrogen fertilization decreases bacterial diversity and favors the growth of Actinobacteria and Proteobacteria in agro-ecosystems across the globe. Glob. Change Biol. 2018, 24, 3452–3461. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, H.; Wang, J.; Wang, J.; Zhang, Y. Long-term fertilization with high nitrogen rates decreased diversity and stability of diazotroph communities in soils of sweet potato. Appl. Soil Ecol. 2022, 170, 104266. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, C.; Luo, Y. Meta-analysis of the impacts of global change factors on soil microbial diversity and functionality. Nat. Commun. 2020, 11, 3072. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Kuzyakov, Y. Soil organic matter priming: The pH effects. Glob. Change Biol. 2024, 30, e17349. [Google Scholar] [CrossRef] [PubMed]
- Uroz, S.; Calvaruso, C.; Turpault, M.P.; Frey-Klett, P. Mineral weathering by bacteria: Ecology, actors and mechanisms. Trends Microbiol. 2009, 17, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Tao, F.; Huang, Y.; Hungate, B.A.; Manzoni, S.; Frey, S.D.; Schmidt, M.W.I.; Reichstein, M.; Carvalhais, N.; Ciais, P.; Jiang, L.; et al. Microbial carbon use efficiency promotes global soil carbon storage. Nature 2023, 618, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, T.; Shi, Z.; Chiariello, N.R.; Docherty, K.; Field, C.B.; Gutknecht, J.; Gao, Q.; Gu, Y.; Guo, X.; et al. Long-term nitrogen deposition enhances microbial capacities in soil carbon stabilization but reduces network complexity. Microbiome 2022, 10, 112. [Google Scholar] [CrossRef]
- Ji, L.; Shen, F.; Liu, Y.; Yang, Y.; Wang, J.; Purahong, W.; Yang, L. Contrasting altitudinal patterns and co-occurrence networks of soil bacterial and fungal communities along soil depths in the cold-temperate montane forests of China. Catena 2022, 209, 105844. [Google Scholar] [CrossRef]
- Silva-Sánchez, A.; Soares, M.; Rousk, J. Testing the dependence of microbial growth and carbon use efficiency on nitrogen availability, pH, and organic matter quality. Soil Biol. Biochem. 2019, 134, 25–35. [Google Scholar] [CrossRef]
| Treatments | CK | N1 | N2 | N3 |
|---|---|---|---|---|
| pH | 7.92 ± 0.08 b | 8.22 ± 0.01 a | 8.23 ± 0.03 a | 8.19 ± 0.02 a |
| TC (g kg−1) | 2.41 ± 0.00 a | 1.62 ± 0.11 b | 0.77 ± 0.11 d | 1.20 ± 0.10 c |
| TN (g kg−1) | 1.84 ± 0.02 b | 1.95 ± 0.08 b | 1.89 ± 0.10 b | 2.24 ± 0.09 a |
| TP (g kg−1) | 11.53 ± 0.05 ab | 10.83 ± 0.76 b | 11.11 ± 0.03 ab | 12.46 ± 0.35 a |
| TK (g kg−1) | 18.65 ± 0.24 a | 19.09 ± 0.14 a | 19.17 ± 0.00 a | 18.74 ± 0.14 a |
| AN (mg kg−1) | 10.45 ± 0.00 c | 18.11 ± 3.11 b | 35.98 ± 0.99 a | 35.41 ± 3.17 a |
| AP (mg kg−1) | 0.39 ± 0.10 b | 0.19 ± 0.07 c | 0.29 ± 0.08 c | 0.72 ± 0.17 a |
| AK (g kg−1) | 0.21 ± 0.00 a | 0.22 ± 0.00 a | 0.20 ± 0.00 ab | 0.19 ± 0.01 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Li, W.; Xu, P.; Wang, X.; Tang, J.; Liu, G.; Wang, T.; Zhao, J. Effect of Nitrogen on Microbial Communities of Purple Mudstone Weathering Products in Southwest China: A Column Experiment. Microorganisms 2024, 12, 1548. https://doi.org/10.3390/microorganisms12081548
Li C, Li W, Xu P, Wang X, Tang J, Liu G, Wang T, Zhao J. Effect of Nitrogen on Microbial Communities of Purple Mudstone Weathering Products in Southwest China: A Column Experiment. Microorganisms. 2024; 12(8):1548. https://doi.org/10.3390/microorganisms12081548
Chicago/Turabian StyleLi, Chunpei, Wanting Li, Peng Xu, Xuan Wang, Jialiang Tang, Gangcai Liu, Ting Wang, and Jixia Zhao. 2024. "Effect of Nitrogen on Microbial Communities of Purple Mudstone Weathering Products in Southwest China: A Column Experiment" Microorganisms 12, no. 8: 1548. https://doi.org/10.3390/microorganisms12081548
APA StyleLi, C., Li, W., Xu, P., Wang, X., Tang, J., Liu, G., Wang, T., & Zhao, J. (2024). Effect of Nitrogen on Microbial Communities of Purple Mudstone Weathering Products in Southwest China: A Column Experiment. Microorganisms, 12(8), 1548. https://doi.org/10.3390/microorganisms12081548









