In Silico Analysis and Development of the Secretory Expression of D-Psicose-3-Epimerase in Escherichia coli
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Plasmids, and Culture Conditions
2.2. In Silico Analysis of DPEase and Signal Peptide Prediction for DPEase Localization
2.3. Construction of Plasmids and Molecular Cloning of DPEase Recombinants
2.4. Localization and Expression of DPEase and Preparation of Recombinant Enzymes from Various Compartments
2.5. Effect of Triton X-100 on the Localization and Expression of DPEase
2.6. Effect of Temperature on DPEase Expression
2.7. Enzyme Assay and Sugar Analysis
3. Results
3.1. In Silico Analysis of Signal Peptide for DPEase Localization
3.2. The DPEase Structure Analysis
3.3. Construction of DPEase Localization Constructs
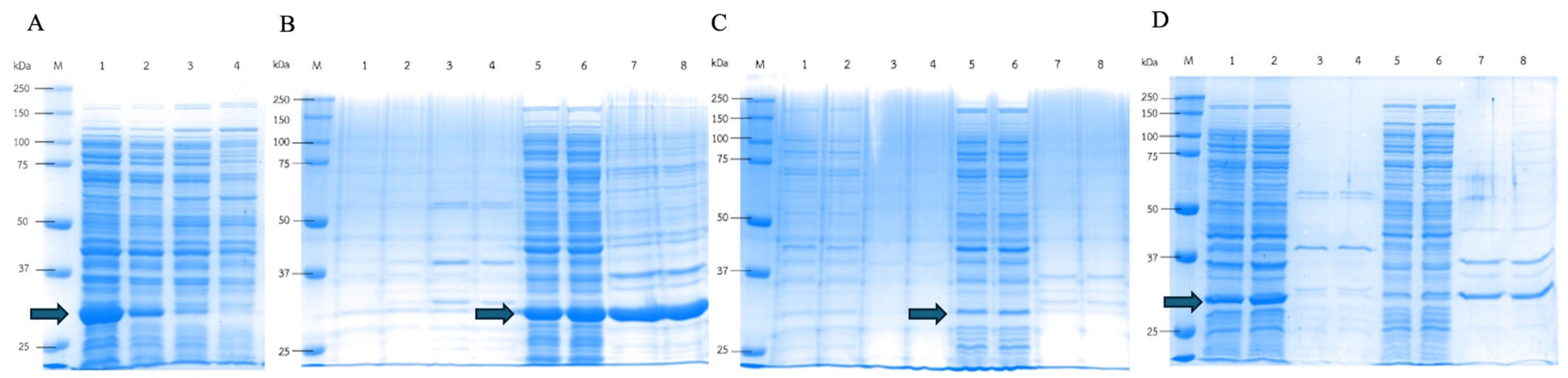
3.4. DPEase Localization Expression and Recombinant Enzymes from Various Compartments’ Activity Levels
3.5. Effect of Triton X-100 on the Localization Expression of DPEase
3.6. Effect of Low Temperature on DPEase Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Granström, T.B.; Takata, G.; Tokuda, M.; Izumori, K. Izumoring: A novel and complete strategy for bioproduction of rare sugars. J. Biosci. Bioeng. 2004, 97, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Roca, C.; Alves, V.D.; Freitas, F.; Reis, M.A.M. Exopolysaccharides enriched in rare sugars: Bacterial sources, production, and applications. Front. Microbiol. 2015, 6, 288. [Google Scholar] [CrossRef] [PubMed]
- Mijailovic, N.; Nesler, A.; Perazzolli, M.; Aït Barka, E.; Aziz, A. Rare Sugars: Recent Advances and Their Potential Role in Sustainable Crop Protection. Molecules 2021, 26, 1720. [Google Scholar] [CrossRef] [PubMed]
- Beerens, K.; Desmet, T.; Soetaert, W. Enzymes for the biocatalytic production of rare sugars. J. Ind. Microbiol. Biotechnol. 2012, 39, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Patra, F.; Patel, A.; Shah, N. Microbial Production of Low-Calorie Sugars. In Handbook of Food Bioengineering, Microbial Production of Food Ingredients and Additives, 1st ed.; Holban, A.M., Grumezescu, A.M., Eds.; Academic Press: London, UK, 2017; Volume 5, pp. 259–290. [Google Scholar] [CrossRef]
- Oshima, H.; Kimura, I.; Izumori, K. Psicose Contents in Various Food Products and its Origin. Food Sci. Technol. Res. 2006, 12, 137–143. [Google Scholar] [CrossRef]
- Lambré, C.; Barat Baviera, J.M.; Bolognesi, C.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lampi, E.; Mengelers, M.; Mortensen, A.; et al. Safety evaluation of the food enzyme d-psicose 3-epimerase from the genetically modified Corynebacterium glutamicum strain FIS002. EFSA J. 2021, 10, 19. [Google Scholar] [CrossRef]
- Matsuo, T.; Suzuki, H.; Hashiguchi, M.; Izumori, K. D-psicose is a rare sugar that provides no energy to growing rats. J. Nutr. Sci. Vitaminol. 2002, 48, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Moller, D.E.; Berger, J.P. Role of PPARs in the regulation of obesity-related insulin sensitivity and inflammation. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 17–21. [Google Scholar] [CrossRef]
- Chung, Y.M.; Hyun Lee, J.; Youl Kim, D.; Hwang, S.H.; Hong, Y.H.; Kim, S.B.; Jin Lee, S.; Hye Park, C. Dietary D-psicose reduced visceral fat mass in high-fat diet-induced obese rats. J. Food Sci. 2012, 77, 53–58. [Google Scholar] [CrossRef]
- Matsuo, T.; Izumori, K. Effects of dietary D-psicose on diurnal variation in plasma glucose and insulin concentrations of rats. Biosci. Biotechnol. Biochem. 2006, 70, 2081–2085. [Google Scholar] [CrossRef]
- Kim, H.J.; Hyun, E.K.; Kim, Y.S.; Lee, Y.J.; Oh, D.K. Characterization of an Agrobacterium tumefaciens D-Psicose 3-Epimerase That Converts D-Fructose to D-Psicose. Appl. Environ. Microbiol. 2006, 72, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Ninchan, B.; Songbang, S.; Watthanasakphuban, N. Optimization and Comparative Methods for Efficient D-psicose Production Using Physicochemical and Enzymatic Processes. Sugar Tech. 2024, 1–12. [Google Scholar] [CrossRef]
- Li, C.; Lin, J.; Guo, Q.; Zhang, C.; Du, K.; Lin, H.; Lin, J. D-psicose 3-epimerase secretory overexpression, immobilization, and d-psicose biotransformation, separation and crystallization. J. Chem. Technol. Biotechnol. 2018, 93, 350–357. [Google Scholar] [CrossRef]
- Chen, J.; Wei, H.; Guo, Y.; Li, Q.; Wang, H.; Liu, J. Chaperone-mediated protein folding enhanced D-psicose 3-epimerase expression in engineered Bacillus subtilis. Process Biochem. 2021, 103, 65–70. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, Y.; Fu, G.; Song, Y.; Jin, Z.; Sun, Y.; Zhang, D. High-level intra- and extra-cellular production of d-psicose 3-epimerase via a modified xylose-inducible expression system in Bacillus subtilis. J. Ind. Microbiol. Biotechnol. 2016, 43, 1577–1591. [Google Scholar] [CrossRef] [PubMed]
- Westers, L.; Westers, H.; Quax, W.J. Bacillus subtilis as cell factory for pharmaceutical proteins: A biotechnological approach to optimize the host organism. Biochim. Biophys. Acta Mol. Cell Res. 2004, 1694, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Rettenbacher, L.A.; Arauzo-Aguilera, K.; Buscajoni, L.; Castillo-Corujo, A.; Ferrero-Bordera, B.; Kostopoulou, A.; Moran-Torres, R.; Núñez-Nepomuceno, D.; Öktem, A.; Palma, A.; et al. Microbial protein cell factories fight back? Trends Biotechnol. 2022, 40, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, D.W.; Ricker, N.; Barbieri, N.L.; Allen, H.K.; Nolan, L.K.; Logue, C.M. Outer membrane protein A (OmpA) of extraintestinal pathogenic Escherichia coli. BMC Res. Notes 2020, 13, 51. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Yang, K.; Shi, Y.; Zhang, J.; Han, Q.; Xia, X.; Song, Y. Outer membrane protein A (OmpA) may be used as a novel target to enrich and detect Escherichia coli in milk samples. J. Dairy Sci. 2022, 105, 2849–2857. [Google Scholar] [CrossRef]
- Scheller, D.; Twittenhoff, C.; Becker, F.; Holler, M.; Narberhaus, F. OmpA, a Common Virulence Factor, Is Under RNA Thermometer Control in Yersinia pseudotuberculosis. Front. Microbiol. 2021, 12, 687260. [Google Scholar] [CrossRef]
- Nie, D.; Hu, Y.; Chen, Z.; Li, M.; Hou, Z.; Luo, X.; Mao, X.; Xue, X. Outer membrane protein A (OmpA) as a potential therapeutic target for Acinetobacter baumannii infection. J. Biomed. Sci. 2020, 27, 26. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, H.; Suzue, K.; Takita, A.; Tomita, H. Roles of OmpA in Type III Secretion System-Mediated Virulence of Enterohemorrhagic Escherichia coli. Pathogens 2021, 10, 1496. [Google Scholar] [CrossRef] [PubMed]
- Vila-Farrés, X.; Parra-Millán, R.; Sánchez-Encinales, V.; Varese, M.; Ayerbe-Algaba, R.; Bayó, N.; Guardiola, S.; Pachón-Ibáñez, M.E.; Kotev, M.; García, J.; et al. Combating virulence of Gram-negative bacilli by OmpA inhibition. Sci. Rep. 2017, 7, 14683. [Google Scholar] [CrossRef]
- Ambrosi, C.; Pompili, M.; Scribano, D.; Zagaglia, C.; Ripa, S.; Nicoletti, M. Outer Membrane Protein A (OmpA): A New Player in Shigella flexneri Protrusion Formation and Inter-Cellular Spreading. PLoS ONE 2012, 7, 49625. [Google Scholar] [CrossRef] [PubMed]
- Watthanasakphuban, N.; Srila, P.; Pinmanee, P.; Sompinit, K.; Rattanaporn, K.; Peterbauer, C. Development of high cell density Limosilactobacillus reuteri KUB-AC5 for cell factory using oxidative stress reduction approach. Microb. Cell Fact. 2023, 22, 86. [Google Scholar] [CrossRef] [PubMed]
- Watthanasakphuban, N.; Virginia, L.J.; Haltrich, D.; Peterbauer, C. Analysis and Reconstitution of the Menaquinone Biosynthesis Pathway in Lactiplantibacillus plantarum and Lentilactibacillus buchneri. Microorganisms 2021, 9, 1476. [Google Scholar] [CrossRef] [PubMed]
- Hashemzaei, M.; Nezafat, N.; Ghoshoon, M.B.; Negahdaripour, M. In-silico selection of appropriate signal peptides for romiplostim secretory production in Escherichia coli. Inform. Med. Unlocked 2023, 36, 101146. [Google Scholar] [CrossRef]
- Yamabhai, M.; Emrat, S.; Sukasem, S.; Pesatcha, P.; Jaruseranee, N.; Buranabanyat, B. Secretion of recombinant Bacillus hydrolytic enzymes using Escherichia coli expression systems. J. Biotechnol. 2008, 133, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, H.J.; Oh, D.K.; Cha, S.S.; Rhee, S. Crystal Structure of d-Psicose 3-epimerase from Agrobacterium tumefaciens and its Complex with True Substrate d-Fructose: A Pivotal Role of Metal in Catalysis, an Active Site for the Non-phosphorylated Substrate, and its Conformational Changes. J. Mol. Biol. 2006, 361, 920–931. [Google Scholar] [CrossRef]
- Yang, L.; Xu, Y.; Chen, Y.; Ying, H. Efficient Extracellular Expression of Phospholipase D in Escherichia coli with an Optimized Signal Peptide. IOP Conf. Ser. Mater. Sci. Eng. 2018, 301, 012105. [Google Scholar] [CrossRef]
- Voulgaridou, G.P.; Mantso, T.; Chlichlia, K.; Panayiotidis, M.I.; Pappa, A. Efficient E. coli Expression Strategies for Production of Soluble Human Crystallin ALDH3A1. PLoS ONE 2013, 8, 56582. [Google Scholar] [CrossRef]
- Kleiner-Grote, G.R.M.; Risse, J.M.; Friehs, K. Secretion of recombinant proteins from E. coli. Eng. Life Sci. 2018, 18, 532–550. [Google Scholar] [CrossRef] [PubMed]
- Billen, B.; Vincke, C.; Hansen, R.; Devoogdt, N.; Muyldermans, S.; Adriaensens, P.; Guedens, W. Cytoplasmic versus periplasmic expression of site-specifically and bioorthogonally functionalized nanobodies using expressed protein ligation. Protein Expr. Purif. 2017, 133, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Goemans, C.; Denoncin, K.; Collet, J.F. Folding mechanisms of periplasmic proteins. Biochim. Biophys. Acta 2014, 1843, 1517–1528. [Google Scholar] [CrossRef] [PubMed]
- Goshisht, M.K. Machine Learning and Deep Learning in Synthetic Biology: Key Architectures, Applications, and Challenges. ACS Omega 2024, 9, 9921–9945. [Google Scholar] [CrossRef]
- Chang, C.C.H.; Song, J.; Tey, B.T.; Ramanan, R.N. Bioinformatics approaches for improved recombinant protein production in Escherichia coli: Protein solubility prediction. Brief. Bioinform. 2013, 15, 953–962. [Google Scholar] [CrossRef]
- Hon, J.; Marusiak, M.; Martinek, T.; Kunka, A.; Zendulka, J.; Bednar, D.; Damborsky, J. SoluProt: Prediction of soluble protein expression in Escherichia coli. Bioinformatics 2021, 37, 23–28. [Google Scholar] [CrossRef]
- Mahanta, P.; Bhardwaj, A.; Kumar, K.; Reddy, V.S.; Ramakumar, S. Structural insights into N-terminal to C-terminal interactions and implications for thermostability of a (β/α)8-triosephosphate isomerase barrel enzyme. FEBS J. 2015, 282, 3543–3555. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Liu, X.; Wang, Y.; Qin, X.; Wang, X.; Zhang, H.; Wang, Y.; Luo, H.; Yao, B.; Bai, Y.; et al. Fusion of a proline-rich oligopeptide to the C-terminus of a ruminal xylanase improves catalytic efficiency. Bioengineered 2022, 13, 10482–10492. [Google Scholar] [CrossRef]
- Bukhari, N.; Chor Leow, A.T.; Raja Abd Rahman, R.N.Z.; Shariff, F.M. Single residue substitution at N-terminal affects temperature stability and activity of L2 lipase. Molecules 2020, 25, 3433. [Google Scholar] [CrossRef]
- Cai, L.; Zhang, M.; Shao, T.; He, Y.; Li, J.; Ren, B.; Zhou, C. Effect of introducing disulfide bridges in c-terminal structure on the thermostability of xylanase xynzf-2 from Aspergillus niger. J. Gen. Appl. Microbiol. 2019, 65, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, G.; Zhang, Z.; Wang, S.; Chen, H. Terminal amino acids disturb xylanase thermostability and activity. J. Biol. Chem. 2011, 286, 44710–44715. [Google Scholar] [CrossRef]
- Notomista, E.; Catanzano, F.; Graziano, G.; Di Gaetano, S.; Barone, G.; Di Donato, A. Contribution of chain termini to the conformational stability and biological activity of onconase. Biochemistry 2001, 40, 9097–9103. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Huang, W.; Bakkalbasi, E.; Brown, N.G.; Adamski, C.J.; Rice, K.; Muzny, D.; Gibbs, R.A.; Palzkill, T. Deep sequencing of systematic combinatorial libraries reveals β-lactamase sequence constraints at high resolution. J. Mol. Biol. 2012, 424, 150–167. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, C.; Khan, M.R.I.; Wang, Y.; Ruan, Y.; Zhao, B.; Ma, X.; Zhang, K.; Zhao, X.; Ye, G.; et al. An Engineered Rare Codon Device for Optimization of Metabolic Pathways. Sci. Rep. 2016, 6, 20608. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.L.; Ceccarelli, E.A. Recombinant protein expression in Escherichia coli: Advances and challenges. Front. Microbiol. 2014, 5, 172. [Google Scholar] [CrossRef] [PubMed]
- Hanson, G.; Coller, J. Codon optimality, bias and usage in translation and mRNA decay. Nat. Rev. Mol. Cell Biol. 2018, 19, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Lee, S.Y. Secretory and extracellular production of recombinant proteins using Escherichia coli. Appl. Microbiol. Biotechnol. 2004, 64, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Samant, S.; Gupta, G.; Karthikeyan, S.; Haq, S.F.; Nair, A.; Sambasivam, G.; Sukumaran, S. Effect of codon-optimized E. coli signal peptides on recombinant Bacillus stearothermophilus maltogenic amylase periplasmic localization, yield and activity. J. Ind. Microbiol. Biotechnol. 2014, 41, 1435–1442. [Google Scholar] [CrossRef]
- Han, S.J.; Machhi, S.; Berge, M.; Xi, G.; Linke, T.; Schoner, R. Novel signal peptides improve the secretion of recombinant Staphylococcus aureus Alpha toxinH35L in Escherichia coli. AMB Express 2017, 7, 93. [Google Scholar] [CrossRef]
- Jonet, M.A.; Mahadi, N.M.; Murad, A.M.A.; Rabu, A.; Bakar, F.D.A.; Rahim, R.A.; Low, K.O.; Illias, R.M. Optimization of a Heterologous Signal Peptide by Site-Directed Mutagenesis for Improved Secretion of Recombinant Proteins in Escherichia coli. Microb. Physiol. 2012, 22, 48–58. [Google Scholar] [CrossRef] [PubMed]
- de Souza, G.A.; Leversen, N.A.; Målen, H.; Wiker, H.G. Bacterial proteins with cleaved or uncleaved signal peptides of the general secretory pathway. J. Proteom. 2011, 75, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Misal, S.A.; Ovhal, S.D.; Li, S.; Karty, J.A.; Tang, H.; Radivojac, P.; Reilly, J.P. Non-Specific Signal Peptidase Processing of Extracellular Proteins in Staphylococcus aureus N315. Proteomes 2023, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Sharma, L.; Kulothungan, S.R.; Adkar, B.V.; Prajapati, R.S.; Ali, P.S.S.; Krishnan, B.; Varadarajan, R. Effect of Signal Peptide on Stability and Folding of Escherichia coli Thioredoxin. PLoS ONE 2013, 8, 63442. [Google Scholar] [CrossRef] [PubMed]
- Baneyx, F.; Mujacic, M. Recombinant protein folding and misfolding in Escherichia coli. Nat. Biotechnol. 2004, 22, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Sevastsyanovich, Y.R.; Alfasi, S.N.; Cole, J.A. Sense and nonsense from a systems biology approach to microbial recombinant protein production. Biotechnol. Appl. Biochem. 2010, 55, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Le, Y.; Peng, J.; Wu, H.; Sun, J.; Shao, W. An Approach to the Production of Soluble Protein from a Fungal Gene Encoding an Aggregation-Prone Xylanase in Escherichia coli. PLoS ONE 2011, 6, 18489. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.M.; Panda, A.K. Solubilization and refolding of bacterial inclusion body proteins. J. Biosci. Bioeng. 2005, 99, 303–310. [Google Scholar] [CrossRef]
- Duan, X.; Zou, C.; Wu, J. Triton X-100 enhances the solubility and secretion ratio of aggregation-prone pullulanase produced in Escherichia coli. Bioresour. Technol. 2015, 194, 137–143. [Google Scholar] [CrossRef]
- Lopes, A.M.; Magalhães, P.O.; Mazzola, P.G.; Rangel-Yagui, C.O.; De Carvalho, J.C.M.; Penna, T.C.; Pessoa, A., Jr. LPS removal from an E. coli fermentation broth using aqueous two-phase micellar system. Biotechnol. Prog. 2010, 26, 1644–1653. [Google Scholar] [CrossRef]
- Dinarello, C.A. Infection, fever, and exogenous and endogenous pyrogens: Some concepts have changed. J. Endotoxin Res. 2004, 10, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Sweadner, K.J.; Forte, M.; Nelsen, L.L. Filtration removal of endotoxin (pyrogens) in solution in different states of aggregation. Appl. Environ. Microbiol. 1977, 34, 382–385. [Google Scholar] [CrossRef] [PubMed]
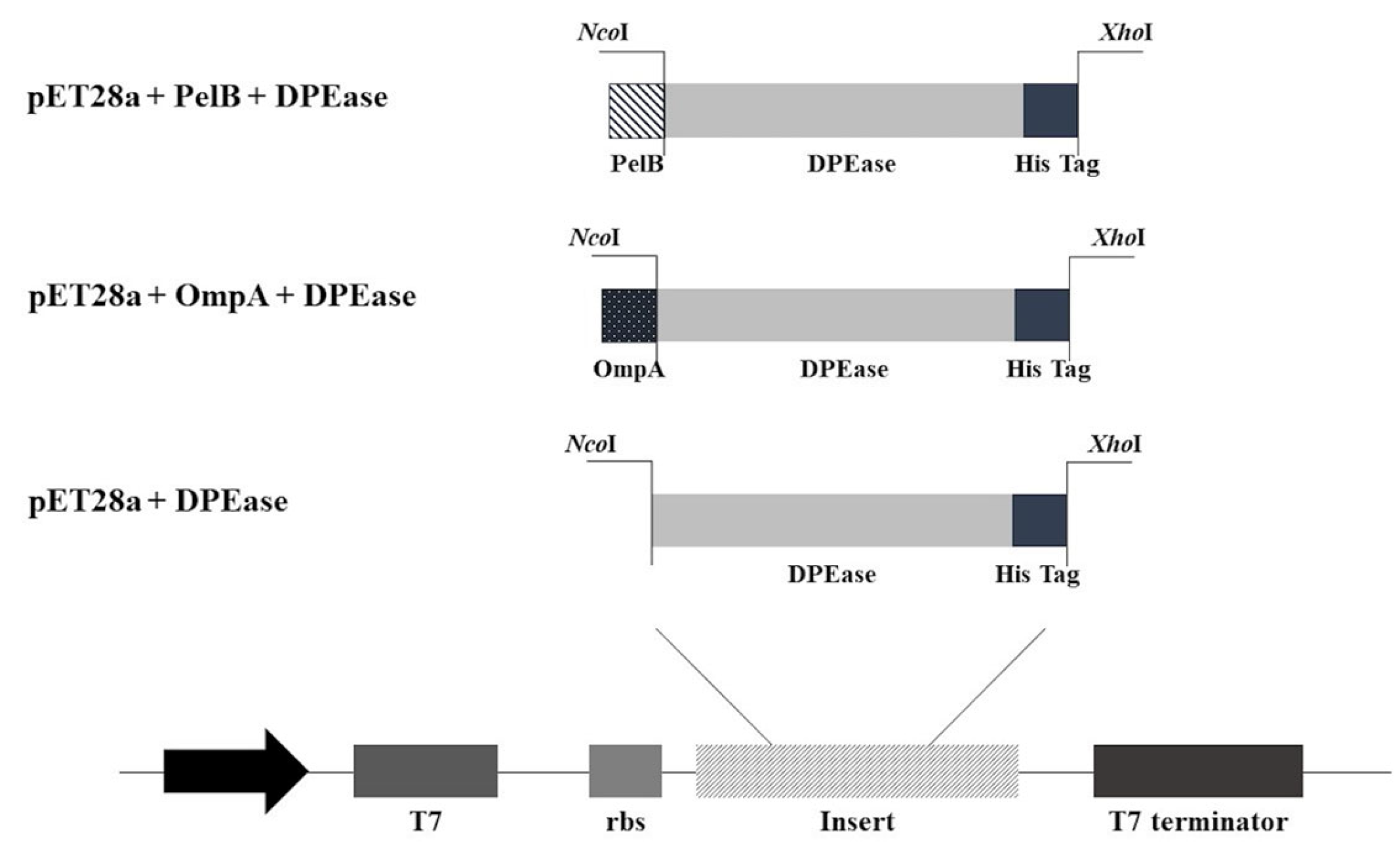

| Strain and Plasmid | Relevant Features | Source |
|---|---|---|
| Strain | ||
| Escherichia coli BL21(DE3) | Expression host | Lab stock |
| Neb5α | Cloning host | NEB |
| Psicose | E. coli BL21 harboring pET28a + DPEase | This work |
| OmpA_Psicose | E. coli BL21 harboring pET28a + OmpA + DPEase | This work |
| PelB_Psicose | E. coli BL21 harboring pET28a + PelB + DPEase | This work |
| Plasmid | ||
| pET28a | Kanr; T7 lac | Novagen |
| pET28a + DPEase | Kanr; pET28a derivative; D-psicose-3-epimerase biosynthesis gene | This work |
| pET28a + OmpA + DPEase | Kanr; pET28a + OmpA derivative; D-psicose-3-epimerase biosynthesis gene | This work |
| pET28a + PelB + DPEase | Kanr; pET28a + PelB derivative; D-psicose-3-epimerase biosynthesis gene | This work |
| Gene Construct | Secretion Pathway | Amino Acid Cleavage Position | Probability | |||
|---|---|---|---|---|---|---|
| Other | Sec/SPI | Sec/SPII | Tat/SPI | |||
| DPEase | 1.000000 | 0.000000 | 0.000000 | 0.000000 | nd | nd |
| draA_DPEase | 0.000249 | 0.999048 | 0.000181 | 0.000173 | 21–22 | 0.9779 |
| faeG_DPEase | 0.000277 | 0.999000 | 0.000183 | 0.000177 | 21–22 | 0.9727 |
| fedA_DPEase | 0.000268 | 0.999026 | 0.000198 | 0.000165 | 21–22 | 0.9714 |
| flgI_DPEase | 0.000194 | 0.999163 | 0.000146 | 0.000153 | 20–21 | 0.9767 |
| malE_DPEase | 0.000265 | 0.999029 | 0.000175 | 0.000178 | 26–27 | 0.9735 |
| OmpA_DPEase | 0.000235 | 0.999069 | 0.000182 | 0.000177 | 21–22 | 0.9777 |
| pbpG_DPEase | 0.00021 | 0.99914 | 0.000162 | 0.000167 | 25–26 | 0.9754 |
| PelB_DPEase | 0.000312 | 0.99895 | 0.000185 | 0.000180 | 22–23 | 0.9785 |
| xylF_DPEase | 0.000245 | 0.999111 | 0.00017 | 0.000158 | 23–24 | 0.9762 |
| yncJ_DPEase | 0.000255 | 0.999044 | 0.000186 | 0.000168 | 22–23 | 0.9759 |
| zraP_DPEase | 0.000234 | 0.999058 | 0.000168 | 0.000178 | 26–27 | 0.9737 |
| ampC_DPEase | 0.000729 | 0.617677 | 0.380953 | 0.000254 | 19–20 | 0.5919 |
| OmpC_DPEase | 0.00022 | 0.999148 | 0.000161 | 0.000160 | 21–22 | 0.9732 |
| STII_DPEase | 0.000259 | 0.99905 | 0.000171 | 0.000166 | 23–24 | 0.9771 |
| Ompf_DPEase | 0.000262 | 0.999009 | 0.000171 | 0.000186 | 22–23 | 0.9786 |
| lamB_DPEase | 0.000262 | 0.999078 | 0.000165 | 0.000188 | 25–26 | 0.9694 |
| araF_DPEase | 0.000268 | 0.999022 | 0.000180 | 0.000171 | 23–24 | 0.9767 |
| nmpc_DPEase | 0.000248 | 0.999052 | 0.000167 | 0.000173 | 23–24 | 0.9788 |
| ppiA_DPEase | 0.000221 | 0.999077 | 0.000163 | 0.000178 | 24–25 | 0.9728 |
| yaaI_DPEase | 0.000481 | 0.954444 | 0.044384 | 0.000234 | 23–24 | 0.9212 |
| glnH_DPEase | 0.00024 | 0.999078 | 0.000160 | 0.000169 | 22–23 | 0.9795 |
| rna_DPEase | 0.00029 | 0.999048 | 0.000184 | 0.000160 | 23–24 | 0.9723 |
| DsbC_DPEase | 0.000237 | 0.999039 | 0.000214 | 0.000164 | 20–21 | 0.9773 |
| rbsB_DPEase | 0.000212 | 0.999116 | 0.000161 | 0.000185 | 25–26 | 0.9008 |
| gfcA_DPEase | 0.000299 | 0.998936 | 0.000200 | 0.000179 | 21–22 | 0.9765 |
| Gene Construct | Cell Wall & Surface | Extra-Cellular | Intra-Cellular | Cytoplasmic Membrane | Outer Membrane | Periplasm | Solubility |
|---|---|---|---|---|---|---|---|
| DPEase | 0 | 0.0002 | 0.9936 | 0.0002 | 0.0053 | 0.0008 | 0.919 |
| draA_DPEase | 0 | 0.0006 | 0.0232 | 0.0002 | 0.0458 | 0.9302 | 0.834 |
| faeG_DPEase | 0 | 0.0035 | 0.0088 | 0.0001 | 0.0248 | 0.9627 | 0.823 |
| fedA_DPEase | 0 | 0.0019 | 0.0286 | 0.0004 | 0.0516 | 0.9175 | 0.802 |
| flgI_DPEase | 0 | 0.0011 | 0.0374 | 0.0007 | 0.0319 | 0.9289 | 0.829 |
| malE_DPEase | 0 | 0.0010 | 0.0015 | 0.0001 | 0.0072 | 0.9903 | 0.798 |
| OmpA_DPEase | 0 | 0.0026 | 0.0115 | 0.0002 | 0.0283 | 0.9575 | 0.797 |
| pbpG_DPEase | 0 | 0.0021 | 0.0306 | 0.0005 | 0.0272 | 0.9396 | 0.799 |
| PelB_DPEase | 0 | 0.0015 | 0.0143 | 0.0002 | 0.0106 | 0.9919 | 0.890 |
| xylF_DPEase | 0 | 0.0016 | 0.0055 | 0.0002 | 0.0163 | 0.9764 | 0.867 |
| yncJ_DPEase | 0 | 0.0020 | 0.0157 | 0.0004 | 0.0261 | 0.9557 | 0.809 |
| zraP_DPEase | 0 | 0.0015 | 0.0008 | 0.0001 | 0.0057 | 0.9733 | 0.807 |
| ampC_DPEase | 0.0001 | 0.0053 | 0.0185 | 0.0003 | 0.0533 | 0.9225 | 0.835 |
| OmpC_DPEase | 0 | 0.0022 | 0.0149 | 0.0003 | 0.0339 | 0.9486 | 0.842 |
| STII_DPEase | 0.0001 | 0.0018 | 0.0310 | 0.0015 | 0.0820 | 0.8836 | 0.816 |
| Ompf_DPEase | 0 | 0.0013 | 0.0348 | 0.0002 | 0.0269 | 0.9368 | 0.818 |
| lamB_DPEase | 0 | 0.0025 | 0.0143 | 0.0002 | 0.0240 | 0.9589 | 0.832 |
| araF_DPEase | 0 | 0.0006 | 0.0064 | 0.0001 | 0.0185 | 0.9745 | 0.802 |
| nmpc_DPEase | 0 | 0.0007 | 0.0035 | 0.0001 | 0.0154 | 0.9803 | 0.800 |
| ppiA_DPEase | 0 | 0.0003 | 0.0041 | 0.0001 | 0.007 | 0.9885 | 0.767 |
| yaaI_DPEase | 0 | 0.0016 | 0.0256 | 0.0004 | 0.0163 | 0.9562 | 0.822 |
| glnH_DPEase | 0 | 0.0012 | 0.0044 | 0.0002 | 0.0287 | 0.9656 | 0.868 |
| rna_DPEase | 0 | 0.0015 | 0.0414 | 0.0004 | 0.0405 | 0.9162 | 0.864 |
| DsbC_DPEase | 0.0001 | 0.0016 | 0.0725 | 0.0006 | 0.0824 | 0.8429 | 0.850 |
| rbsB_DPEase | 0 | 0.0012 | 0.0065 | 0.0002 | 0.0261 | 0.9659 | 0.827 |
| gfcA_DPEase | 0 | 0.0007 | 0.0200 | 0.0002 | 0.0164 | 0.9627 | 0.800 |
| Sample | Fraction | Protein Conc. (mg/mL) | DPEase Activity (unit/mL) | Specific Activity (unit/mg) |
|---|---|---|---|---|
| Psicose | Fermentation broth | 0.297 ± 0.004 | ND | ND |
| Cell lysate | 2.572 ± 0.114 | 1.608 ± 0.043 | 0.625 | |
| Periplasm fraction | 0.085 ± 0.002 | ND | ND | |
| OmpA_Psicose | Fermentation broth | 1.390 ± 0.043 | ND | ND |
| Cell lysate | 1.371 ± 0.051 | 1.243 ± 0.138 | 0.907 | |
| Periplasm fraction | 0.231 ± 0.006 | ND | ND | |
| PelB_Psicose | Fermentation broth | 1.709 ± 0.031 | ND | ND |
| Cell lysate | 0.835 ± 0.015 | 1.379 ± 0.034 | 1.651 | |
| Periplasm fraction | 0.041 ± 0.005 | ND | ND |
| Sample | Fraction | Protein Conc. (mg/mL) | DPEase Activity (unit/mL) | Specific Activity (unit/mg) |
|---|---|---|---|---|
| Psicose-X | Fermentation broth | 1.341 ± 0.054 | ND | ND |
| Cell lysate | 2.531 ± 0.068 | 1.019 ± 0.048 | 0.403 | |
| Periplasm fraction | 0.069 ± 0.002 | ND | ND | |
| OmpA_Psicose-X | Fermentation broth | 1.709 ± 0.031 | ND | ND |
| Cell lysate | 1.835 ± 0.015 | 1.609 ± 0.073 | 0.877 | |
| Periplasm fraction | 0.041 ± 0.005 | ND | ND | |
| PelB_Psicose-X | Fermentation broth | 1.999 ± 0.030 | 0.163 ± 0.047 | 0.082 |
| Cell lysate | 1.438 ± 0.184 | 1.545 ± 0.085 | 1.074 | |
| Periplasm fraction | 0.062 ± 0.024 | ND | ND |
| Sample | Fraction | Protein Conc. (mg/mL) | DPEase Activity (unit/mL) | Specific Activity (unit/mg) |
|---|---|---|---|---|
| Psicose-X | Fermentation broth | 0.748 ± 0.028 | ND | ND |
| Cell lysate | 2.490 ± 0.132 | 1.897 ± 0.053 | 0.75 | |
| Periplasm fraction | 0.074 ± 0.009 | ND | ND | |
| OmpA_Psicose-X | Fermentation broth | 0.923 ± 0.060 | ND | ND |
| Cell lysate | 1.109.0 ± 0.058 | 1.309 ± 0.031 | 1.18 | |
| Periplasm fraction | 0.02 ± 0.001 | ND | ND | |
| PelB_Psicose-X | Fermentation broth | 1.936 ± 0.017 | 0.512 ± 0.047 | 0.26 |
| Cell lysate | 1.157 ± 0.038 | 1.362 ± 0.078 | 1.18 | |
| Periplasm fraction | 0.030 ± 0.008 | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watthanasakphuban, N.; Ninchan, B.; Pinmanee, P.; Rattanaporn, K.; Keawsompong, S. In Silico Analysis and Development of the Secretory Expression of D-Psicose-3-Epimerase in Escherichia coli. Microorganisms 2024, 12, 1574. https://doi.org/10.3390/microorganisms12081574
Watthanasakphuban N, Ninchan B, Pinmanee P, Rattanaporn K, Keawsompong S. In Silico Analysis and Development of the Secretory Expression of D-Psicose-3-Epimerase in Escherichia coli. Microorganisms. 2024; 12(8):1574. https://doi.org/10.3390/microorganisms12081574
Chicago/Turabian StyleWatthanasakphuban, Nisit, Boontiwa Ninchan, Phitsanu Pinmanee, Kittipong Rattanaporn, and Suttipun Keawsompong. 2024. "In Silico Analysis and Development of the Secretory Expression of D-Psicose-3-Epimerase in Escherichia coli" Microorganisms 12, no. 8: 1574. https://doi.org/10.3390/microorganisms12081574
APA StyleWatthanasakphuban, N., Ninchan, B., Pinmanee, P., Rattanaporn, K., & Keawsompong, S. (2024). In Silico Analysis and Development of the Secretory Expression of D-Psicose-3-Epimerase in Escherichia coli. Microorganisms, 12(8), 1574. https://doi.org/10.3390/microorganisms12081574






