Probiotics in miRNA-Mediated Regulation of Intestinal Immune Homeostasis in Pigs: A Physiological Narrative
Abstract
:1. Introduction
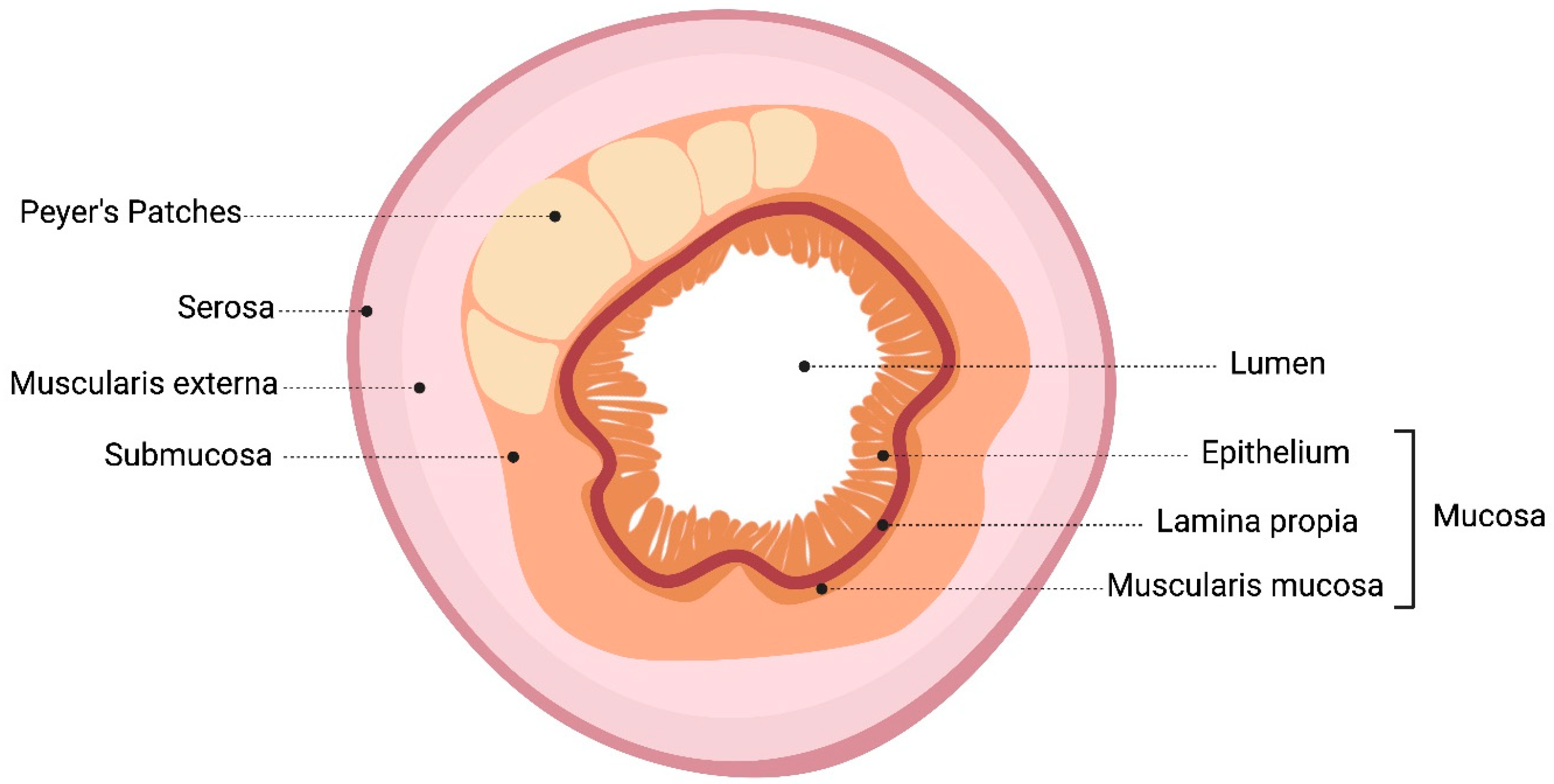
2. Intestinal Mucosal Immune System: Probiotics Pass through the Gastrointestinal Tract
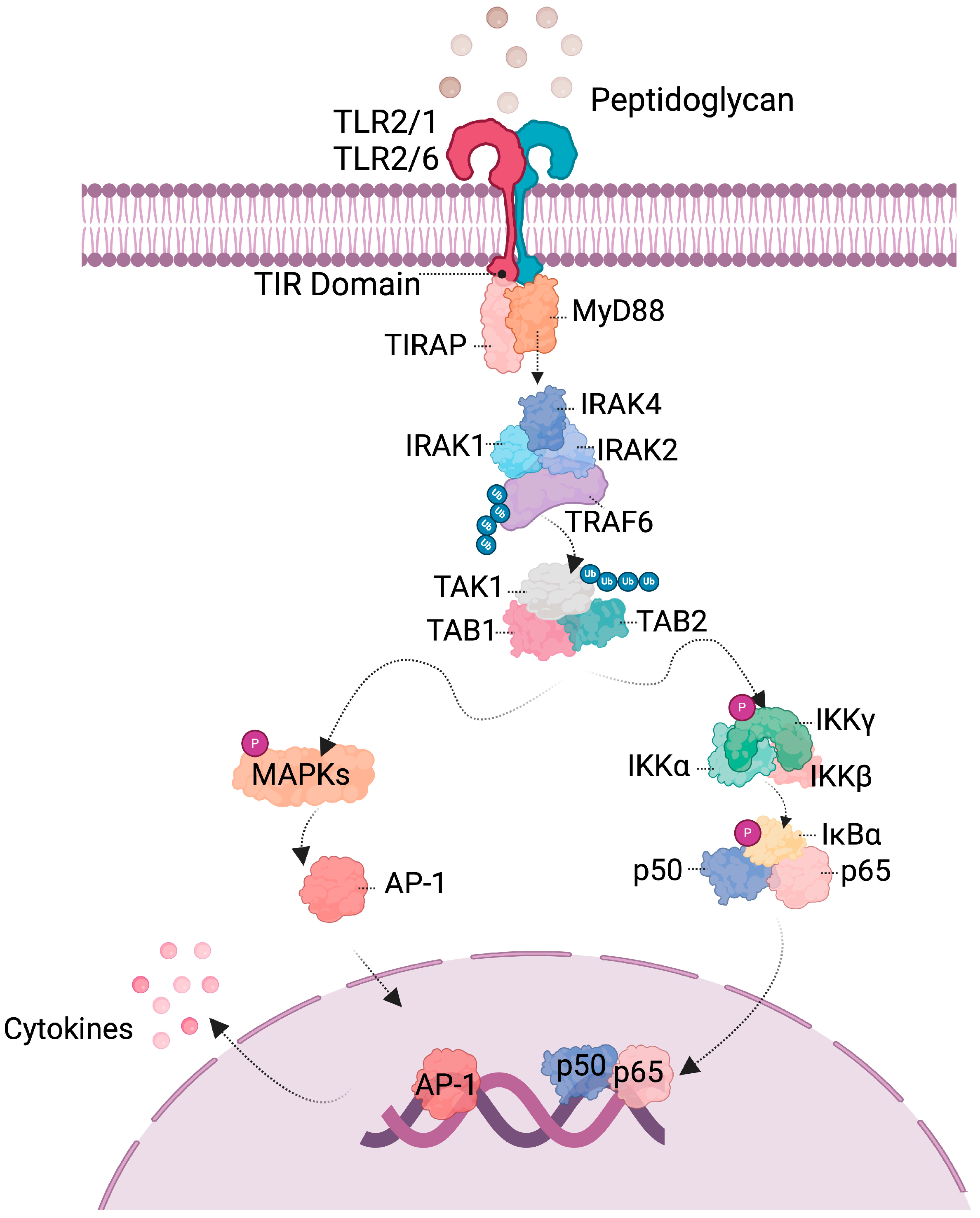
3. Probiotics Interact with Host Cell
| Probiotic | Effect | Model | Reference |
|---|---|---|---|
| B. coagulans | Increase level gene expression of IFN-α, IFN-γ, OAS1, MX2, IL-4, CCL-2 in ileum | Pig | [40] |
| B. animalis subsp. lactis | Induces IL-10 production in moDCs | Pig | [41] |
| B. animalis subsp. lactis | Reduces TNF-α production | Mice | [42] |
| B. breve | Induces CD4+ T cells in colon | Mice | [28] |
| B. subtilis | Increase IgA and IgM in serum | Pig | [43] |
| L. sobrius | Increase IgA in serum | Pig | [44] |
| L. acidophilus W55, B. lactis W51 | Induces FOXP3+ T reg, induces production of IL-10 in PBMCs | Human | [45] |
| L. rhamnosus GG, L. casei IMAU60214, L. helveticus IMAU70129 | Induces NF-κB p65 in macrophages | Human | [46] |
4. Probiotics Activate Signaling Pathways
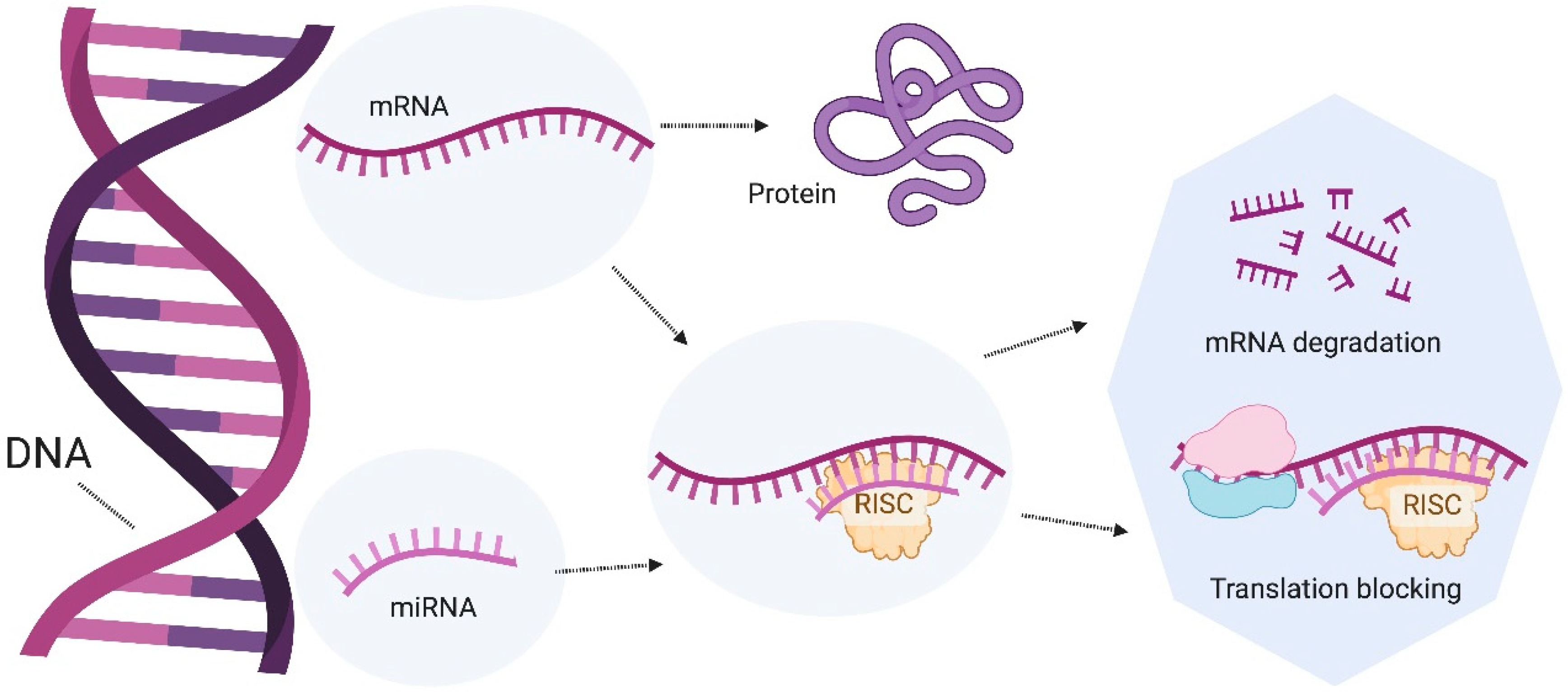
5. microRNAs as Regulators of the Intestinal Gut Microbiota
6. Role of microRNAs in the Intestine
7. Modulation of miRNAs by Probiotics
| Probiotic | miRNA Expression | Model | Reference |
|---|---|---|---|
| Lactiplantibacillus plantarum 299v | Increase in smiR-218b, miR-450a, miR-106a, miR-184, miR-9841-3p, miR-187. Decrease in miR-196a, miR-199a-3p, miR-218-5p, miR-194b-3p in ileum | Pig | [66] |
| Saccharomyces boulardii | Decrease in miR-155 y miR-223 in DSS-induced colitis | Mice | [58] |
| L. fermentum and L. salivarius | Decrease in miR-155 and miR-223 in DSS-induced colitis | Mice | [55] |
| L. acidophilus and B. bifidum | Decrease in miR-135b and miR-155, increase in miR-26b and miR-18a in AOM-induced colon cancer. | Mice | [67] |
| E. faecium | Increase in miR-149 and miR-1285 in jejunum. Increase in miR-423-5p and miR-1285 in lymph nodes. | Pig | [62] |
8. Perspectives and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut microbiota and immune system interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef] [PubMed]
- Hertli, S.; Zimmermann, P. Molecular interactions between the intestinal microbiota and the host. Mol. Microbiol. 2022, 117, 1297–1307. [Google Scholar] [CrossRef] [PubMed]
- Gieryńska, M.; Szulc-Dąbrowska, L.; Struzik, J.; Mielcarska, M.B.; Gregorczyk-Zboroch, K.P. Integrity of the Intestinal Barrier: The Involvement of Epithelial Cells and Microbiota—A Mutual Relationship. Animals 2022, 12, 145. [Google Scholar] [CrossRef] [PubMed]
- Suárez, L.J.; Arboleda, S.; Angelov, N.; Arce, R.M. Oral Versus Gastrointestinal Mucosal Immune Niches in Homeo-stasis and Allostasis. Front. Immunol. 2021, 12, 705206. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-Y.; Zhu, C.; Zhu, M.; Yuan, L.; Li, S.; Gu, F.; Hu, P.; Chen, S.; Cai, D. Alternatives to antibiotics in pig production: Looking through the lens of immunophysiology. Stress Biol. 2024, 4, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.J.; Lee, S.I. MicroRNA expression profiling during the suckling-to-weaning transition in pigs. J. Anim. Sci. Technol. 2021, 63, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Wijtten, P.J.A.; van der Meulen, J.; Verstegen, M.W.A. Intestinal barrier function and absorption in pigs after weaning: A review. Br. J. Nutr. 2011, 105, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Duarte, M.E.; Kim, S.W. Dietary inclusion of multispecies probiotics to reduce the severity of post-weaning diarrhea caused by Escherichia coli F18+ in pigs. Anim. Nutr. 2021, 7, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health. Cells 2023, 12, 184. [Google Scholar] [CrossRef]
- Linares, D.M.; Gómez, C.; Renes, E.; Fresno, J.M.; Tornadijo, M.E.; Ross, R.P.; Stanton, C. Lactic Acid Bacteria and Bifidobacteria with Potential to Design Natural Biofunctional Health-Promoting Dairy Foods. Front. Microbiol. 2017, 8, 846. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Wu, K.-H.; Wu, H.-P. Unraveling the Complexities of Toll-like Receptors: From Molecular Mechanisms to Clinical Applications. Int. J. Mol. Sci. 2024, 25, 5037. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.; McCarthy, J.; O’driscoll, C.; Melgar, S. Pattern recognition receptors—Molecular orchestrators of inflammation in inflammatory bowel disease. Cytokine Growth Factor Rev. 2013, 24, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Di Sabatino, A.; Santacroce, G.; Rossi, C.M.; Broglio, G.; Lenti, M.V. Role of mucosal immunity and epithelial–vascular barrier in modulating gut homeostasis. Intern. Emerg. Med. 2023, 18, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Cao, W. Toll-Like Receptor Signaling Induces Nrf2 Pathway Activation through p62-Triggered Keap1 Degradation. Mol. Cell. Biol. 2015, 35, 2673–2683. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta -Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Parra, M.; Bárcenas-Preciado, V.; Reséndiz-Sandoval, M.; Hernández, J.; Mata-Haro, V. Downregulation of miR-671-5p promotes IL-10 mRNA increase in porcine moDCs stimulated with the probiotic BB12. Mol. Biol. Rep. 2023, 50, 919–925. [Google Scholar] [CrossRef] [PubMed]
- González-Rascón, A.; Mata-Haro, V. MicroRNAs: Regulators of TLR2-Mediated Probiotic Immune Responses. MicroRNA 2016, 4, 168–174. [Google Scholar] [CrossRef]
- Sevignani, C.; Calin, G.A.; Siracusa, L.D.; Croce, C.M. Mammalian microRNAs: A small world for fine-tuning gene expression. Mamm. Genome 2006, 17, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Tan, H.; Kaiko, G.E. Role of the Intestinal Epithelium and Its Interaction with the Microbiota in Food Allergy. Front. Immunol. 2020, 11, 604054. [Google Scholar] [CrossRef]
- Han, S.; Lu, Y.; Xie, J.; Fei, Y.; Zheng, G.; Wang, Z.; Liu, J.; Lv, L.; Ling, Z.; Berglund, B.; et al. Probiotic Gastrointestinal Transit and Colonization After Oral Administration: A Long Journey. Front. Cell. Infect. Microbiol. 2021, 11, 609722. [Google Scholar] [CrossRef]
- Szabó, C.; Lugata, J.K.; Ortega, A.D.S.V. Gut Health and Influencing Factors in Pigs. Animals 2023, 13, 1350. [Google Scholar] [CrossRef]
- Judkins, T.C.; Archer, D.L.; Kramer, D.C.; Solch, R.J. Probiotics, Nutrition, and the Small Intestine. Curr. Gastroenterol. Rep. 2020, 22, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Welcome, M.O. Gastrointestinal physiology: Development, principles and mechanisms of regulation. In Gastrointestinal Physiology: Development, Principles and Mechanisms of Regulation; Springer: Berlin/Heidelberg, Germany, 2018; pp. 53–71. [Google Scholar]
- Peng, J.; Tang, Y.; Huang, Y. Gut health: The results of microbial and mucosal immune interactions in pigs. Anim. Nutr. 2021, 7, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Maroilley, T.; Berri, M.; Lemonnier, G.; Esquerré, D.; Chevaleyre, C.; Mélo, S.; Meurens, F.; Coville, J.L.; Leplat, J.J.; Rau, A.; et al. Immunome differences between porcine ileal and jejunal Peyer’s patches revealed by global transcriptome sequencing of gut-associated lymphoid tissues. Sci. Rep. 2018, 8, 9077. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Yan, W.; Ma, Y.; Fang, J. The impact of probiotics on gut health via alternation of immune status of monogastric animals. Anim. Nutr. 2021, 7, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Kotredes, K.P.; Thomas, B.; Gamero, A.M. The Protective Role of Type I Interferons in the Gastrointestinal Tract. Front. Immunol. 2017, 8, 410. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.G.; Kayama, H.; Ueda, Y.; Takahashi, T.; Asahara, T.; Tsuji, H.; Tsuji, N.M.; Kiyono, H.; Ma, J.S.; Kusu, T.; et al. Probiotic Bifidobacterium breve Induces IL-10-Producing Tr1 Cells in the Colon. PLoS Pathogens 2012, 8, e1002714. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E.; Aumiller, T.; Spindler, H.K.; Rosenfelder, P.; Eklund, M.; Witzig, M.; Jørgensen, H.; Knudsen, K.E.B.; Mosenthin, R. Wheat and barley differently affect porcine intestinal microbiota. J. Sci. Food Agric. 2016, 96, 2230–2239. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Markowiak-Kopeć, P.; Śliżewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef]
- Tedelind, S.; Westberg, F.; Kjerrulf, M.; Vidal, A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: A study with relevance to inflammatory bowel disease. World J. Gastroenterol. 2007, 13, 2826–2832. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K.; Vasquez, R.; Kim, S.H.; Hwang, I.-C.; Song, J.H.; Park, J.H.; Kim, I.H.; Kang, D.-K. Multispecies probiotics alter fecal short-chain fatty acids and lactate levels in weaned pigs by modulating gut microbiota. J. Anim. Sci. Technol. 2021, 63, 1142–1158. [Google Scholar] [CrossRef] [PubMed]
- Barkhidarian, B.; Roldos, L.; Iskandar, M.M.; Saedisomeolia, A.; Kubow, S. Probiotic Supplementation and Micronutrient Status in Healthy Subjects: A Systematic Review of Clinical Trials. Nutrients 2021, 13, 3001. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.M.; Versalovic, J. Probiotics-host communication modulation of signaling pathways in the intestine. Gut Microbes 2010, 1, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health Benefits of Probiotics: A Review. Int. Sch. Res. Not. 2013, 2013, 481651. [Google Scholar] [CrossRef] [PubMed]
- Kaji, R.; Kiyoshima-Shibata, J.; Tsujibe, S.; Nanno, M.; Shida, K. Short communication: Probiotic induction of interleukin-10 and interleukin-12 production by macrophages is modulated by co-stimulation with microbial components. J. Dairy Sci. 2018, 101, 2838–2841. [Google Scholar] [CrossRef] [PubMed]
- Yeşilyurt, N.; Yılmaz, B.; Ağagündüz, D.; Capasso, R. Involvement of Probiotics and Postbiotics in the Immune System Modulation. Biologics 2021, 1, 89–110. [Google Scholar] [CrossRef]
- Latif, A.; Shehzad, A.; Niazi, S.; Zahid, A.; Ashraf, W.; Iqbal, M.W.; Rehman, A.; Riaz, T.; Aadil, R.M.; Khan, I.M.; et al. Probiotics: Mechanism of action, health benefits and their application in food industries. Front. Microbiol. 2023, 14, 1216674. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, Y.; Lv, Y.; Li, P.; Yi, D.; Wang, L.; Zhao, D.; Chen, H.; Gong, J.; Hou, Y. Beneficial Impact and Molecular Mechanism of Bacillus coagulans on Piglets’ Intestine. Int. J. Mol. Sci. 2018, 19, 2084. [Google Scholar] [CrossRef]
- Tian, Y.; Xu, J.; Li, Y.; Zhao, R.; Du, S.; Lv, C.; Wu, W.; Liu, R.; Sheng, X.; Song, Y.; et al. MicroRNA-31 Reduces Inflammatory Signaling and Promotes Re-generation in Colon Epithelium, and Delivery of Mimics in Microspheres Reduces Colitis in Mice. Gastroenterology 2019, 156, 2281–2296. [Google Scholar] [CrossRef]
- Chae, J.M.; Heo, W.; Cho, H.T.; Lee, D.H.; Kim, J.H.; Rhee, M.S.; Park, T.-S.; Kim, Y.K.; Lee, J.H.; Kim, Y.J. Effects of Orally-Administered Bifidobacterium animalis subsp. lactis Strain BB12 on Dextran Sodium Sulfate-Induced Colitis in Mice. J. Microbiol. Biotechnol. 2018, 28, 1800–1805. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Zhang, N.; Zhou, M.; Tu, Y.; Deng, K.; Diao, Q. Effects of dietary probiotics on growth performance, faecal microbiota and serum profiles in weaned piglets. Anim. Prod. Sci. 2014, 54, 616–621. [Google Scholar] [CrossRef]
- Konstantinov, S.R.; Smidt, H.; Akkermans, A.D.L.; Casini, L.; Trevisi, P.; Mazzoni, M.; De Filippi, S.; Bosi, P.; De Vos, W.M. Feeding of Lactobacillus sobrius reduces Escherichia coli F4 levels in the gut and promotes growth of infected piglets. FEMS Microbiol. Ecol. 2008, 66, 599–607. [Google Scholar] [CrossRef] [PubMed]
- De Roock, S.; Van Elk, M.; Van Dijk, M.E.A.; Timmerman, H.M.; Rijkers, G.T.; Prakken, B.J.; Hoekstra, M.O.; De Kleer, I.M. Lactic acid bacteria differ in their ability to induce functional regulatory T cells in humans. Clin. Exp. Allergy 2010, 40, 103–110. [Google Scholar] [CrossRef]
- Rocha-Ramírez, L.M.; Pérez-Solano, R.A.; Castañón-Alonso, S.L.; Guerrero, S.S.M.; Pacheco, A.R.; Garibay, M.G.; Eslava, C. Probiotic Lactobacillus Strains Stimulate the Inflammatory Response and Activate Human Macrophages. J. Immunol. Res. 2017, 2017, 4607491. [Google Scholar] [CrossRef] [PubMed]
- El-Zayat, S.R.; Sibaii, H.; Mannaa, F.A. Toll-like receptors activation, signaling, and targeting: An overview. Bull. Natl. Res. Cent. 2019, 43, 1. [Google Scholar] [CrossRef]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef] [PubMed]
- Paveljšek, D.; Ivičak-Kocjan, K.; Treven, P.; Benčina, M.; Jerala, R.; Rogelj, I. Distinctive probiotic features share commonTLR2-dependent signalling in intestinal epithelial cells. Cell. Microbiol. 2021, 23, e13264. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-like Receptor Signaling Pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, C.-Z.; Wan, J.-Y.; Yao, H.; Yuan, C.-S. Dissecting the Interplay Mechanism between Epigenetics and Gut Microbiota: Health Maintenance and Disease Prevention. Int. J. Mol. Sci. 2021, 22, 6933. [Google Scholar] [CrossRef]
- Fardi, F.; Khasraghi, L.B.; Shahbakhti, N.; Naseriyan, A.S.; Najafi, S.; Sanaaee, S.; Alipourfard, I.; Zamany, M.; Karamipour, S.; Jahani, M.; et al. An interplay between non-coding RNAs and gut microbiota in human health. Diabetes Res. Clin. Pract. 2023, 201, 110739. [Google Scholar] [CrossRef]
- Bi, K.; Zhang, X.; Chen, W.; Diao, H. MicroRNAs Regulate Intestinal Immunity and Gut Microbiota for Gastrointestinal Health: A Comprehensive Review. Genes 2020, 11, 1075. [Google Scholar] [CrossRef] [PubMed]
- Algieri, F.; Garrido-Mesa, J.; Vezza, T.; Rodríguez-Sojo, M.J.; Rodríguez-Cabezas, M.E.; Olivares, M.; García, F.; Gálvez, J.; Morón, R.; Rodríguez-Nogales, A. Intestinal anti-inflammatory effects of probiotics in DNBS-colitis via modulation of gut microbiota and microRNAs. Eur. J. Nutr. 2021, 60, 2537–2551. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Nogales, A.; Algieri, F.; Garrido-Mesa, J.; Vezza, T.; Utrilla, M.P.; Chueca, N.; Garcia, F.; Olivares, M.; Rodríguez-Cabezas, M.E.; Gálvez, J. Differential intestinal anti-inflammatory effects of Lactobacillus fermentum and Lactobacillus salivarius in DSS mouse colitis: Impact on microRNAs expression and microbiota composition. Mol. Nutr. Food Res. 2017, 61, 1700144. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, W.; Yuan, T.; Fu, L.; Zhou, L.; Lin, G.; Zhao, S.; Zhou, H.; Wu, G.; Wang, J. MicroRNA-29a mediates the impairment of intestinal epithelial integrity induced by intrauterine growth restriction in pig. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G434–G442. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Feng, T.; Yao, S.; Wolf, K.J.; Liu, C.-G.; Liu, X.; Elson, C.O.; Cong, Y. Microbiota Downregulates Dendritic Cell Expression of miR-10a, Which Targets IL-12/IL-23p40. J. Immunol. 2011, 187, 5879–5886. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Nogales, A.; Algieri, F.; Garrido-Mesa, J.; Vezza, T.; Utrilla, M.P.; Chueca, N.; García, F.; Rodríguez-Cabezas, M.E.; Gálvez, J. Intestinal anti-inflammatory effect of the probiotic Saccharomyces boulardii in DSS-induced colitis in mice: Impact on mi-croRNAs expression and gut microbiota composition. J. Nutr. Biochem. 2018, 61, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Souba, W.W.; Croce, C.M.; Verne, G.N. MicroRNA-29a regulates intestinal membrane permeability in patients with irritable bowel syndrome. Gut 2010, 59, 775–784. [Google Scholar] [CrossRef]
- Zou, L.; Xiong, X.; Yang, H.; Wang, K.; Zhou, J.; Lv, D.; Yin, Y. Identification of microRNA transcriptome reveals that miR-100 is involved in the renewal of porcine intestinal epithelial cells. Sci. China Life Sci. 2019, 62, 816–828. [Google Scholar] [CrossRef]
- Tao, X.; Liu, S.; Men, X.; Xu, Z. Over-expression of miR-146b and its regulatory role in intestinal epithelial cell viability, proliferation, and apoptosis in piglets. Biol. Direct 2017, 12, 27. [Google Scholar] [CrossRef]
- Kreuzer-Redmer, S.; Bekurtz, J.C.; Arends, D.; Bortfeldt, R.; Kutz-Lohroff, B.; Sharbati, S.; Einspanier, R.; Brockmann, G.A. Feeding of Enterococcus faecium NCIMB 10415 Leads to Intestinal miRNA-423-5p-Induced Regulation of Immune-Relevant Genes. Appl. Environ. Microbiol. 2016, 82, 2263–2269. [Google Scholar] [CrossRef] [PubMed]
- Alivernini, S.; Gremese, E.; McSharry, C.; Tolusso, B.; Ferraccioli, G.; McInnes, I.B.; Kurowska-Stolarska, M. MicroRNA-155—At the Critical Interface of Innate and Adaptive Immunity in Arthritis. Front. Immunol. 2018, 8, 1932. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, Q.; Wang, J.; Qiu, X.; Qi, R.; Huang, J. Identification of differentially expressed miRNAs after Lactobacillus reuteri treatment in the ileum mucosa of piglets. Genes Genom. 2020, 42, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Parra, M.; Arenas-Padilla, M.; Bárcenas-Preciado, V.; Hernández, J.; Mata-Haro, V. The Probiotic BB12 Induces MicroRNAs Involved in Antigen Processing and Presentation in Porcine Monocyte-Derived Dendritic Cells. Int. J. Mol. Sci. 2020, 21, 687. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, Q.; Wang, J.; Qiu, X.; Qi, R.; Huang, J. Lactobacillus Plantarum 299v Changes miRNA Expression in the Intestines of Piglets and Leads to Downregulation of LITAF by Regulating ssc-miR-450a. Probiotics Antimicrob. Proteins 2021, 13, 1093–1105. [Google Scholar] [CrossRef]
- Heydari, Z.; Rahaie, M.; Alizadeh, A.M.; Agah, S.; Khalighfard, S.; Bahmani, S. Effects of Lactobacillus acidophilus and Bifidobacterium bifidum Probiotics on the Expression of MicroRNAs 135b, 26b, 18a and 155, and Their Involving Genes in Mice Colon Cancer. Probiotics Antimicrob. Proteins 2019, 11, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Arenas-Padilla, M.; González-Rascón, A.; Hernández-Mendoza, A.; Calderón de la Barca, A.M.; Hernández, J.; Ma-ta-Haro, V. Immunomodulation by Bifidobacterium animalis subsp. lactis Bb12: Integrative Analysis of miRNA Expression and TLR2 Pathway–Related Target Proteins in Swine Monocytes. Probiotics Antimicrob. Proteins 2022, 14, 510–522. [Google Scholar] [CrossRef]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef]
- Heckman, C.A.; Mehew, J.W.; Boxer, L.M. NF-kB activates Bcl-2 expression in t(14;18) lymphoma cells. Oncogene 2002, 21, 3898–3908. [Google Scholar] [CrossRef]
- Weissman, R.; Diamond, E.L.; Haroche, J.; Durham, B.H.; Cohen, F.; Buthorn, J.; Amoura, Z.; Emile, J.-F.; Mazor, R.D.; Shomron, N.; et al. MicroRNA-15a-5p acts as a tumor suppressor in histiocytosis by mediating CXCL10-ERK-LIN28a-let-7 axis. Leukemia 2022, 36, 1139–1149. [Google Scholar] [CrossRef]
- Jurj, A.; Zanoaga, O.; Raduly, L.; Morhan, V.; Papi, Z.; Ciocan, C.; Pop, L.-A.; Berindan-Neagoe, I.; Braicu, C. Discovering the Biological Significance and Therapeutic Potential of miR-29b-3p in Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2023, 24, 5048. [Google Scholar] [CrossRef] [PubMed]
- Asehnoune, K.; Strassheim, D.; Mitra, S.; Kim, J.Y.; Abraham, E. Involvement of PKCα/β in TLR4 and TLR2 dependent activation of NF-κB. Cell Signal. 2005, 17, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Ninsuwon, J.; Waiyamitra, P.; Roongsitthichai, A.; Surachetpong, W. Expressions of miR-155 and miR-181 and pre-dictions of their structures and targets in pigs (Sus scrofa). Vet. World 2020, 13, 1667. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bárcenas-Preciado, V.; Mata-Haro, V. Probiotics in miRNA-Mediated Regulation of Intestinal Immune Homeostasis in Pigs: A Physiological Narrative. Microorganisms 2024, 12, 1606. https://doi.org/10.3390/microorganisms12081606
Bárcenas-Preciado V, Mata-Haro V. Probiotics in miRNA-Mediated Regulation of Intestinal Immune Homeostasis in Pigs: A Physiological Narrative. Microorganisms. 2024; 12(8):1606. https://doi.org/10.3390/microorganisms12081606
Chicago/Turabian StyleBárcenas-Preciado, Valeria, and Verónica Mata-Haro. 2024. "Probiotics in miRNA-Mediated Regulation of Intestinal Immune Homeostasis in Pigs: A Physiological Narrative" Microorganisms 12, no. 8: 1606. https://doi.org/10.3390/microorganisms12081606





