Comparing the Environmental Influences and Community Assembly of Protist Communities in Two Anthropogenic Coastal Areas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Areas and Sampling Collection
2.2. Measurement of Environmental Variables
2.3. DNA Extraction, PCR Amplification, and Sequencing
2.4. Bioinformatics Processing
2.5. Statistical Analysis
3. Results
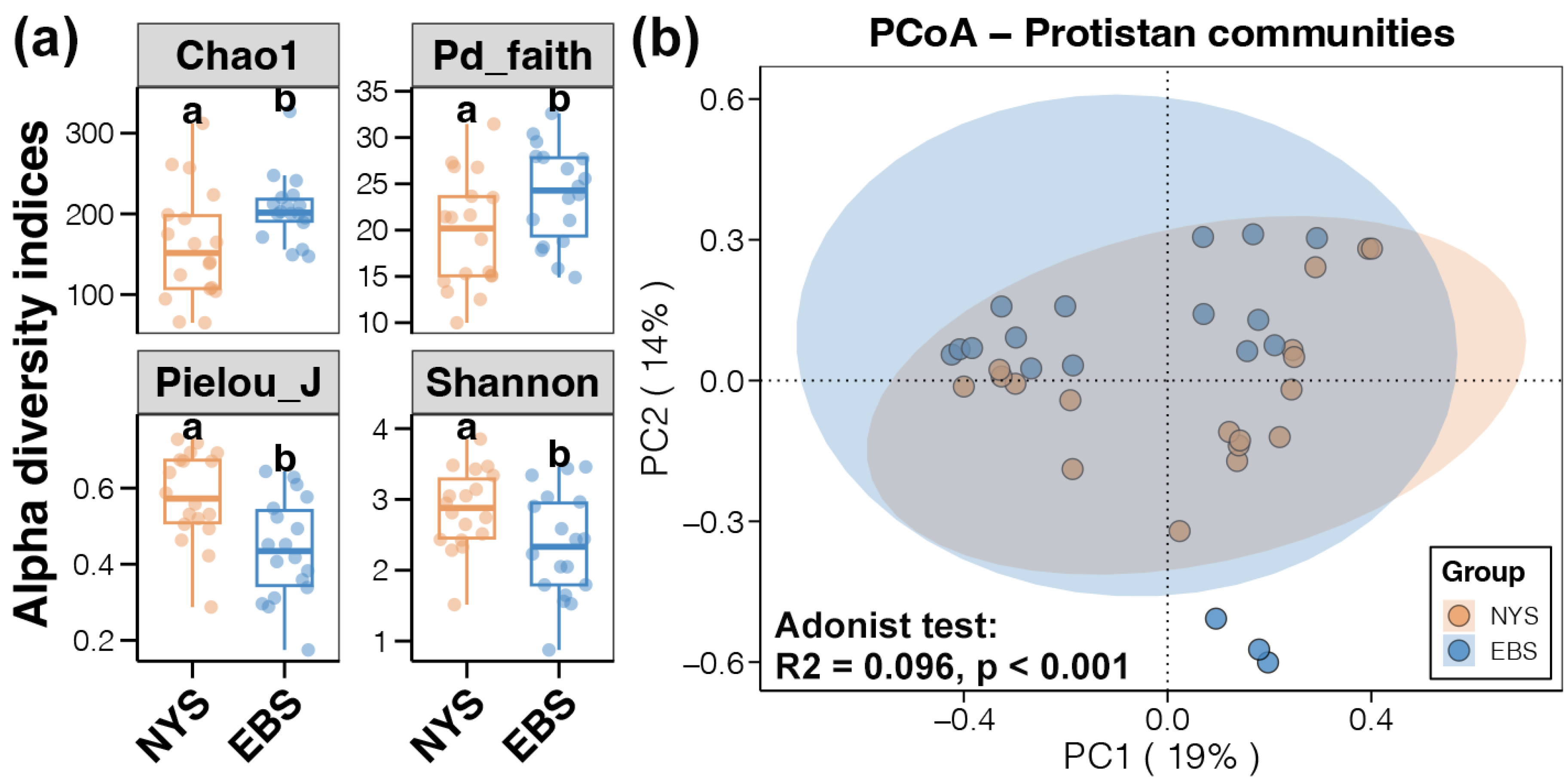
3.1. Composition and Diversity of Protist Communities
3.2. Unique and Shared Species in the Protist Communities
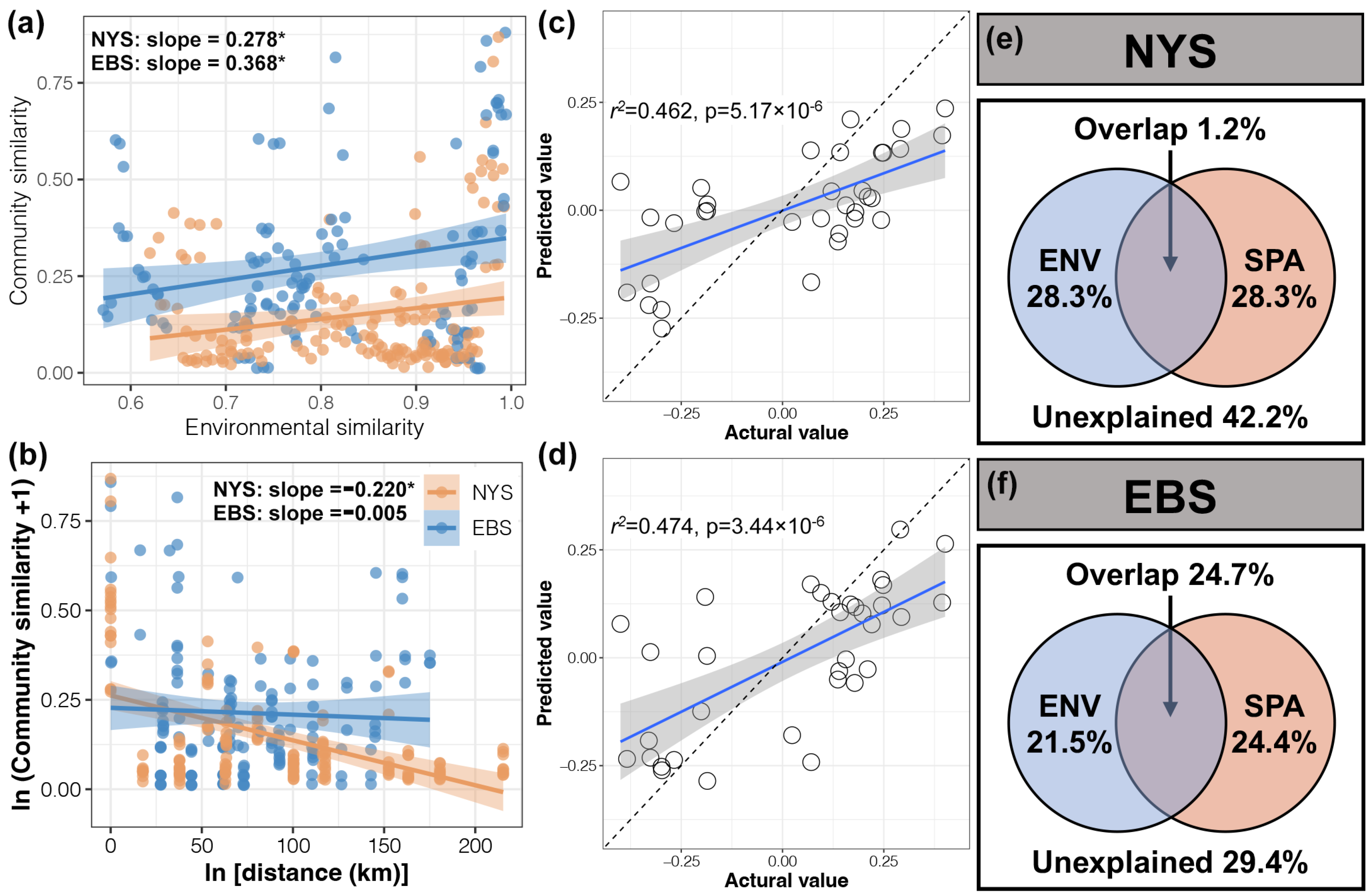
3.3. Factors Affecting the Composition of Protist Communities
3.4. Mechanisms Shaping Protist Community Assembly Processes
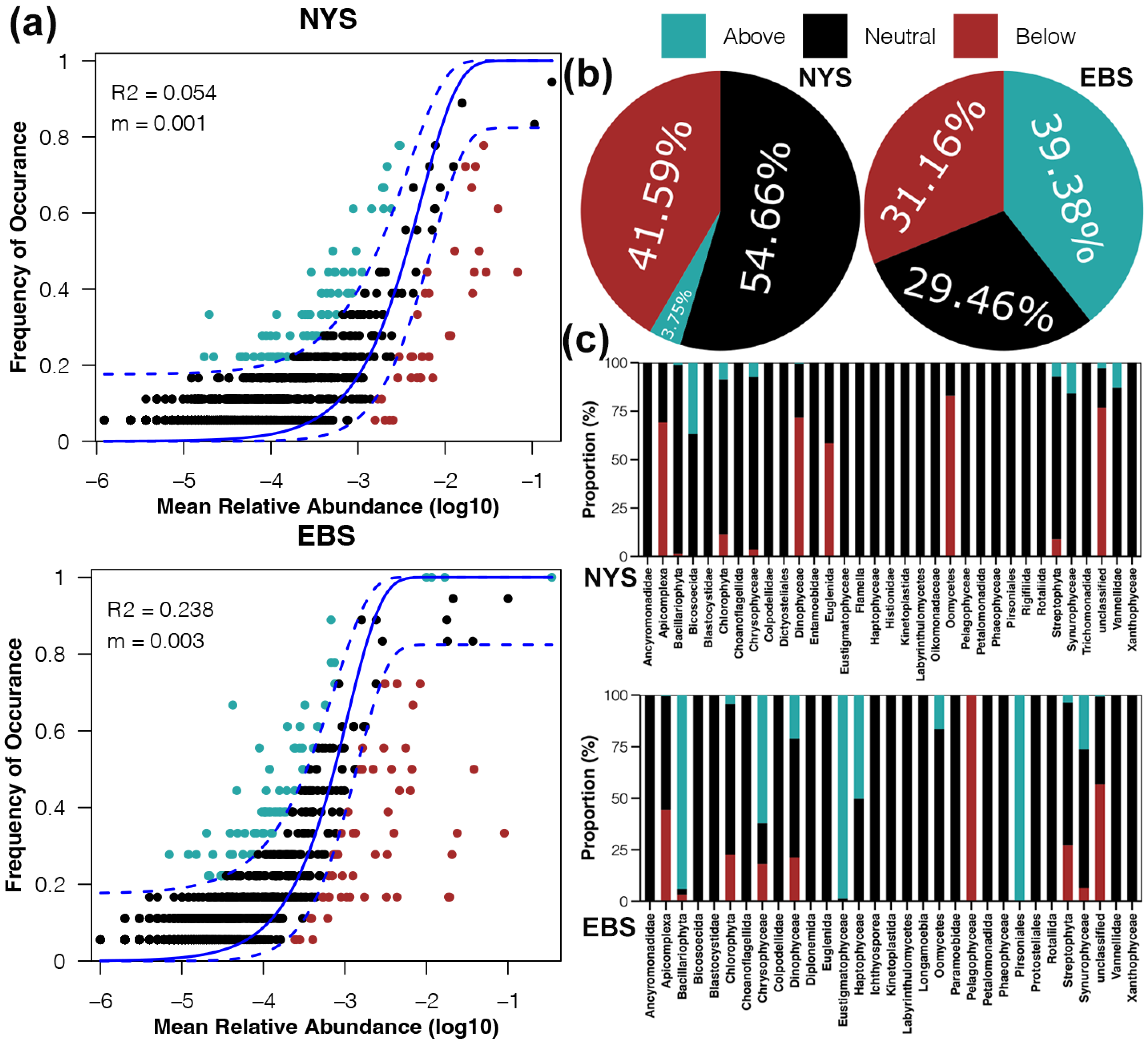
3.5. Differentiation of Protist Functional Diversity between Regions
4. Discussion
4.1. Comparison of Protist Community Composition between Regions
4.2. Environmental Factors Had a Greater Influence on the Composition of Protist Communities in the Areas with Higher Nutrient Salt Concentrations
4.3. Deterministic Processes Governed the Assembly of the Protist Communities in the NYS and EBS Estuaries
4.4. Consumers and Phototrophs Exhibited Biogeographic Patterns
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barbier, E.B.; Hacker, S.D.; Kennedy, C.; Koch, E.W.; Stier, A.C.; Silliman, B.R. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 2011, 81, 169–193. [Google Scholar] [CrossRef]
- Jeffries, T.C.; Schmitz Fontes, M.L.; Harrison, D.P.; Van-Dongen-Vogels, V.; Eyre, B.D.; Ralph, P.J.; Seymour, J.R. Bacterioplankton dynamics within a large anthropogenically impacted urban estuary. Front. Microbiol. 2015, 6, 1438. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-González, C.; Rodríguez-Pie, L.; Maister, O.; Rodellas, V.; Alorda-Keinglass, A.; Diego-Feliu, M.; Folch, A.; Garcia-Orellana, J.; Gasol, J.M. High spatial heterogeneity and low connectivity of bacterial communities along a Mediterranean subterranean estuary. Mol. Ecol. 2022, 31, 5745–5764. [Google Scholar] [CrossRef] [PubMed]
- Song, H.J.; Ji, R.B.; Xin, M.; Liu, P.; Zhang, Z.H.; Wang, Z.L. Spatial heterogeneity of seasonal phytoplankton blooms in a marginal sea: Physical drivers and biological responses. ICES J. Mar. Sci. 2019, 77, 408–418. [Google Scholar] [CrossRef]
- Clark, G.F.; Kelaher, B.P.; Dafforn, K.A.; Coleman, M.A.; Knott, N.A.; Marzinelli, E.M.; Johnston, E.L. What does impacted look like? High diversity and abundance of epibiota in modified estuaries. Environ. Pollut. 2015, 196, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, P.G.; Morais, H.; Crespo, D.; Tavares, D.; Pereira, E.; Pardal, M.Â. Seasonal characterization of mercury contamination along the Portuguese coast: Human health and environmental risk assessment. Environ. Sci. Pollut. Res. 2023, 30, 101121–101132. [Google Scholar] [CrossRef]
- Li, Y.D.; Jing, H.M.; Kao, S.J.; Zhang, W.P.; Liu, H.B. Metabolic response of prokaryotic microbes to sporadic hypoxia in a eutrophic subtropical estuary. Mar. Pollut. Bull. 2020, 154, 111064. [Google Scholar] [CrossRef] [PubMed]
- Steidle, L.; Vennell, R. Phytoplankton retention mechanisms in estuaries: A case study of the Elbe estuary. Nonlin. Process. Geophys. 2024, 31, 151–164. [Google Scholar] [CrossRef]
- Zhao, Z.L.; Li, H.J.; Sun, Y.; Zhan, A.B.; Lan, W.L.; Woo, S.P.; Shau-Hwai, A.T.; Fan, J.F. Bacteria versus fungi for predicting anthropogenic pollution in subtropical coastal sediments: Assembly process and environmental response. Ecol. Indic. 2022, 134, 108484. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X.; Wang, X.; Wang, H.; Sun, Y.; Zhang, J.; Li, H. Contrasting benthic bacterial and fungal communities in two temperate coastal areas affected by different levels of anthropogenic pressure. Mar. Environ. Res. 2024, 198, 106501. [Google Scholar] [CrossRef]
- Leibold, M.; Holyoak, M.; Mouquet, N.; Amarasekare, P.; Chase, J.; Hoopes, M.; Holt, R.; Shurin, J.; Law, R.; Tilman, D.; et al. The metacommunity concept: A framework for multi-scale community ecology. Ecol. Lett. 2004, 7, 601–613. [Google Scholar] [CrossRef]
- Dini-Andreote, F.; Stegen, J.C.; van Elsas, J.D.; Salles, J.F. Disentangling mechanisms that mediate the balance between stochastic and deterministic processes in microbial succession. Proc. Natl. Acad. Sci. USA 2015, 112, 1326–1332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Deng, Y.; Feng, K.; Cai, W.W.; Li, S.Z.; Yin, H.Q.; Xu, M.Y.; Ning, D.L.; Qu, Y.Y. Deterministic assembly and diversity gradient altered the biofilm community performances of bioreactors. Environ. Sci. Technol. 2019, 53, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, Y.; Zhang, W.L.; Wang, C.; Wang, P.F.; Niu, L.; Wu, H.N. Homogeneous selection dominates the microbial community assembly in the sediment of the Three Gorges Reservoir. Sci. Total Environ. 2019, 690, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Vogt, J.C.; Olefeld, J.L.; Bock, C.; Boenigk, J.; Albach, D.C. Patterns of protist distribution and diversification in alpine lakes across Europe. Microbiol. Open 2021, 10, e1216. [Google Scholar] [CrossRef] [PubMed]
- Stern, R.; Kraberg, A.; Bresnan, E.; Kooistra, W.; Lovejoy, C.; Montresor, M.; Morán, X.A.; Not, F.; Salas, R.; Siano, R.; et al. Molecular analyses of protists in long-term observation programmes—Current status and future perspectives. J. Plankton Res. 2018, 40, 519–536. [Google Scholar] [CrossRef]
- Zou, K.; Wang, R.L.; Xu, S.N.; Li, Z.Y.; Liu, L.; Li, M.; Zhou, L. Changes in protist communities in drainages across the Pearl River Delta under anthropogenic influence. Water Res. 2021, 200, 117294. [Google Scholar] [CrossRef] [PubMed]
- Fournier, B.; Steiner, M.; Brochet, X.; Degrune, F.; Mammeri, J.; Carvalho, D.L.; Siliceo, S.L.; Bacher, S.; Peña-Reyes, C.A.; Heger, T.J. Toward the use of protists as bioindicators of multiple stresses in agricultural soils: A case study in vineyard ecosystems. Ecol. Indic. 2022, 139, 108955. [Google Scholar] [CrossRef]
- Zhao, Z.B.; He, J.Z.; Geisen, S.; Han, L.L.; Wang, J.T.; Shen, J.P.; Wei, W.X.; Fang, Y.T.; Li, P.P.; Zhang, L.M. Protist communities are more sensitive to nitrogen fertilization than other microorganisms in diverse agricultural soils. Microbiome 2019, 7, 33. [Google Scholar] [CrossRef]
- Chen, B.Z.; Smith, S.L. Optimality-based approach for computationally efficient modeling of phytoplankton growth, chlorophyll-to-carbon, and nitrogen-to-carbon ratios. Ecol. Model. 2018, 385, 197–212. [Google Scholar] [CrossRef]
- Mo, Y.; Zhang, W.J.; Yang, J.; Lin, Y.S.; Yu, Z.; Lin, S.J. Biogeographic patterns of abundant and rare bacterioplankton in three subtropical bays resulting from selective and neutral processes. ISME J. 2018, 12, 2198–2210. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.S.; Li, H.J.; Yang, X.L.; Xu, Z.Z.; Tong, Y.D.; Yu, X.G. Comprehensive investigation and assessment of nutrient and heavy metal contamination in the surface water of coastal Bohai Sea in China. J. Ocean Univ. China 2020, 19, 843–852. [Google Scholar] [CrossRef]
- Berg, J.; Brandt Kristian, K.; Al-Soud Waleed, A.; Holm Peter, E.; Hansen Lars, H.; Sørensen Søren, J.; Nybroe, O. Selection for cu-tolerant bacterial communities with altered composition, but unaltered richness, via long-term cu exposure. Appl. Environ. Microbiol. 2012, 78, 7438–7446. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Guillou, L.; Bachar, D.; Audic, S.; Bass, D.; Berney, C.; Bittner, L.; Boutte, C.; Burgaud, G.; de Vargas, C.; Decelle, J.; et al. The Protist Ribosomal Reference database (PR2): A catalog of unicellular eukaryote small sub-unit rRNA sequences with curated taxonomy. Nucleic Acids Res. 2013, 41, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.B.; Boutros, P.C. VennDiagram: A package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinf. 2011, 12, 35. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Li, H.J.; Wang, L.L.; Zhou, Y.Q.; Li, S.Z.; Zhang, Z.J.; Feng, K.; Deng, Y. Environmental DNA metabarcoding reveals the influence of human activities on microeukaryotic plankton along the Chinese coastline. Water Res. 2023, 233, 119730. [Google Scholar] [CrossRef]
- Singer, D.; Seppey, C.V.W.; Lentendu, G.; Dunthorn, M.; Bass, D.; Belbahri, L.; Blandenier, Q.; Debroas, D.; de Groot, G.A.; de Vargas, C.; et al. Protist taxonomic and functional diversity in soil, freshwater and marine ecosystems. Environ. Int. 2021, 146, 106262. [Google Scholar] [CrossRef]
- Kulaš, A.; Žutinić, P.; Gulin Beljak, V.; Kepčija, R.M.; Perić, M.S.; Orlić, S.; Petrić, I.S.; Marković, T.; Gligora Udovič, M. Diversity of protist genera in periphyton of tufa-depositing karstic river. Ann. Microbiol. 2023, 73, 10. [Google Scholar] [CrossRef]
- Zhao, Z.L.; Li, H.J.; Sun, Y.; Shao, K.S.; Wang, X.C.; Ma, X.D.; Hu, A.y.; Zhang, H.K.; Fan, J.F. How habitat heterogeneity shapes bacterial and protistan communities in temperate coastal areas near estuaries. Environ. Microbiol. 2022, 24, 1775–1789. [Google Scholar] [CrossRef] [PubMed]
- Weisse, T. Functional diversity of aquatic ciliates. Eur. J. Protistol. 2017, 61, 331–358. [Google Scholar] [CrossRef] [PubMed]
- Kanso, E.A.; Lopes, R.M.; Strickler, J.R.; Dabiri, J.O.; Costello, J.H. Teamwork in the viscous oceanic microscale. Proc. Natl. Acad. Sci. USA 2021, 118, e2018193118. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Hu, Z.X.; Deng, Y.Y.; Shang, L.X.; Gobler, C.J.; Tang, Y.Z. Dependence of genome size and copy number of rRNA gene on cell volume in dinoflagellates. Harmful Algae 2021, 109, 102108. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Singh, S.P. Identification and profiling of microbial community from industrial sludge. Arch. Microbiol. 2022, 204, 234. [Google Scholar] [CrossRef] [PubMed]
- Findenig, B.; Chatzinotas, A.; Boenigk, J. Taxonomic and ecological characterization of stomatocysts of Spumella-like flagellates (chrysophyceae). J. Phycol. 2010, 46, 868–881. [Google Scholar] [CrossRef]
- Grossmann, L.; Bock, C.; Schweikert, M.; Boenigk, J. Small but Manifold—Hidden Diversity in “Spumella-like Flagellates”. J. Eukaryotic Microbiol. 2016, 63, 419–439. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Chang, S.L.; Lin, I.T.; Yao, M.C. Abundant and diverse Tetrahymena species living in the bladder traps of aquatic carnivorous Utricularia plants. Sci. Rep. 2019, 9, 13669. [Google Scholar] [CrossRef]
- Haraguchi, L.; Jakobsen, H.H.; Lundholm, N.; Carstensen, J. Phytoplankton community dynamic: A driver for ciliate trophic strategies. Front. Mar. Sci. 2018, 5, 272. [Google Scholar] [CrossRef]
- Armeli Minicante, S.; Piredda, R.; Quero, G.M.; Finotto, S.; Bernardi Aubry, F.; Bastianini, M.; Pugnetti, A.; Zingone, A. Habitat heterogeneity and connectivity: Effects on the planktonic protist community structure at two adjacent coastal sites (the lagoon and the gulf of Venice, northern Adriatic Sea, Italy) revealed by metabarcoding. Front. Microbiol. 2019, 10, 2736. [Google Scholar] [CrossRef]
- Jia, J.J.; Gao, Y.; Zhou, F.; Shi, K.; Johnes, P.J.; Dungait, J.A.J.; Ma, M.Z.; Lu, Y. Identifying the main drivers of change of phytoplankton community structure and gross primary productivity in a river-lake system. J. Hydrol. 2020, 583, 124633. [Google Scholar] [CrossRef]
- Feng, J.G.; Chen, H.R.; Hailong, Z.; Li, Z.X.; Yu, Y.; Zhang, Y.Z.; Bilal, M.; Qiu, Z.F. Turbidity Estimation from GOCI satellite data in the turbid estuaries of China’s coast. Remote Sens. 2020, 12, 3770. [Google Scholar] [CrossRef]
- Gris, B.; Morosinotto, T.; Giacometti, G.M.; Bertucco, A.; Sforza, E. Cultivation of Scenedesmus obliquus in photobioreactors: Effects of light intensities and light-dark cycles on growth, productivity, and biochemical composition. Appl. Biochem. Biotechnol. 2014, 172, 2377–2389. [Google Scholar] [CrossRef]
- Liu, M.; Fan, D.J.; Liao, Y.J.; Chen, B.; Yang, Z.S. Heavy metals in surficial sediments of the central Bohai Sea: Their distribution, speciation and sources. Acta Oceanol. Sin. 2016, 35, 98–110. [Google Scholar] [CrossRef]
- Zhang, H.X.; Shen, Y.M.; Tang, J. Numerical investigation of successive land reclamation effects on hydrodynamics and water quality in Bohai Bay. Ocean Eng. 2023, 268, 113483. [Google Scholar] [CrossRef]
- Wang, Y.J.; Liu, D.Y.; Xiao, W.P.; Zhou, P.; Tian, C.G.; Zhang, C.S.; Du, J.Z.; Guo, H.; Wang, B.D. Coastal eutrophication in China: Trend, sources, and ecological effects. Harmful Algae 2021, 107, 102058. [Google Scholar] [CrossRef] [PubMed]
- Rabalais, N.; Cai, W.J.; Carstensen, J.; Conley, D.; Fry, B.; Hu, X.P.; Quiñones-Rivera, Z.; Rosenberg, R.; Slomp, C.P.; Turner, R.; et al. Eutrophication-driven deoxygenation in the coastal ocean. Oceanography 2014, 27, 172–183. [Google Scholar] [CrossRef]
- Yuan, G.Q.; Chen, Y.; Wang, Y.L.; Zhang, H.W.; Wang, H.X.; Jiang, M.X.; Zhang, X.N.; Gong, Y.C.; Yuan, S.B. Responses of protozoan communities to multiple environmental etresses (warming, eutrophication, and pesticide pollution). Animals 2024, 14, 1293. [Google Scholar] [CrossRef]
- Soares, E.M.; Figueredo, C.C.; Gücker, B.; Boëchat, I.G. Effects of growth condition on succession patterns in tropical phytoplankton assemblages subjected to experimental eutrophication. J. Plankton Res. 2013, 35, 1141–1153. [Google Scholar] [CrossRef]
- Bock, C.; Jensen, M.; Forster, D.; Marks, S.; Nuy, J.; Psenner, R.; Beisser, D.; Boenigk, J. Factors shaping community patterns of protists and bacteria on a European scale. Environ. Microbiol. 2020, 22, 2243–2260. [Google Scholar] [CrossRef] [PubMed]
- Woodhouse, J.N.; Ziegler, J.; Grossart, H.P.; Neilan, B.A. Cyanobacterial community composition and bacteria-bacteria interactions promote the stable occurrence of particle-associated bacteria. Front. Microbiol. 2018, 9, 777. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, H.J.; Wang, X.; Jin, Y.; Nagai, S.; Lin, S. Phytoplankton and Microzooplankton Community Structure and Assembly Mechanisms in Northwestern Pacific Ocean Estuaries with Environmental Heterogeneity and Geographic Segregation. Microbiol. Spectr. 2023, 11, e0492622. [Google Scholar] [CrossRef]
- Wu, H.N.; Bertilsson, S.; Li, Y.; Zhang, W.L.; Niu, L.; Cai, W.; Cong, H.; Zhang, C. Influence of rapid vertical mixing on bacterial community assembly in stratified water columns. Environ. Res. 2024, 243, 117886. [Google Scholar] [CrossRef] [PubMed]
- Luan, L.; Liang, C.; Chen, L.; Wang, H.; Xu, Q.; Jiang, Y.; Sun, B. Coupling Bacterial Community Assembly to Microbial Metabolism across Soil Profiles. mSystems 2020, 5, e00298-20. [Google Scholar] [CrossRef] [PubMed]
- Fowler, S.J.; Torresi, E.; Dechesne, A.; Smets, B.F. Biofilm thickness controls the relative importance of stochastic and deterministic processes in microbial community assembly in moving bed biofilm reactors. Interface Focus 2023, 13, 20220069. [Google Scholar] [CrossRef]
- Suzzi, A.L.; Stat, M.; Gaston, T.F.; Huggett, M.J. Spatial patterns in host-associated and free-living bacterial communities across six temperate estuaries. FEMS Microbiol. Ecol. 2023, 99, fiad061. [Google Scholar] [CrossRef]
- Jiang, R.M.; Wang, D.P.; Jia, S.Y.; Li, Q.S.; Liu, S.N.; Zhang, X.X. Dynamics of bacterioplankton communities in the estuary areas of the Taihu Lake: Distinct ecological mechanisms of abundant and rare communities. Environ. Res. 2024, 242, 117782. [Google Scholar] [CrossRef]
- Zhao, R.Z.; Zhang, W.J.; Zhao, Z.F.; Qiu, X.C. Determinants and assembly mechanism of bacterial community structure in ningxia section of the yellow River. Microorganisms 2023, 11, 496. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Ye, J.Q.; Liu, X.H.; Zhang, Y.L.; Zhang, J.Y.; Shen, L.Y.; Jin, Y.; Zhang, J.H.; Li, H.J. Spatiotemporal dynamics of bacterioplankton communities in the estuaries of two differently contaminated coastal areas: Composition, driving factors and ecological process. Mar. Pollut. Bull. 2024, 201, 116263. [Google Scholar] [CrossRef]
- Wu, W.X.; Lu, H.P.; Sastri, A.; Yeh, Y.C.; Gong, G.C.; Chou, W.C.; Hsieh, C.H. Contrasting the relative importance of species sorting and dispersal limitation in shaping marine bacterial versus protist communities. ISME J. 2018, 12, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Farjalla, V.F.; Srivastava, D.S.; Marino, N.A.; Azevedo, F.D.; Dib, V.; Lopes, P.M.; Rosado, A.S.; Bozelli, R.L.; Esteves, F.A. Ecological determinism increases with organism size. Ecology 2012, 93, 1752–1759. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Z.C.; Liu, W.C.; Liu, H.Y.; Zhang, Q.; Zeng, J.; Ren, C.; Yang, G.H.; Zhong, Z.K.; Han, X.H. Abundant and rare fungal taxa exhibit different patterns of phylogenetic niche conservatism and community assembly across a geographical and environmental gradient. Soil Biol. Biochem. 2023, 186, 109167. [Google Scholar] [CrossRef]
- Alonso, D.; Etienne, R.S.; McKane, A.J. The merits of neutral theory. Trends Ecol. Evol. 2006, 21, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, M.M.; Wagner, K.; Schwab, C.; Urich, T.; Battin, T.J. Light availability impacts structure and function of phototrophic stream biofilms across domains and trophic levels. Mol. Ecol. 2018, 27, 2913–2925. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Jiao, Z.X.; Yan, Z.G.; Yan, X.Y.; Deng, X.H.; Xiong, W.; Tao, C.Y.; Liu, H.J.; Li, R.; Shen, Q.R.; et al. Predatory protists reduce bacteria wilt disease incidence in tomato plants. Nat. Commun. 2024, 15, 829. [Google Scholar] [CrossRef] [PubMed]
- Li, F.F.; Sun, A.Q.; Jiao, X.Y.; Bi, L.; Zheng, Y.; He, J.Z.; Hu, H.W. Specific protistan consumers and parasites are responsive to inorganic fertilization in rhizosphere and bulk soils. J. Soils Sediments 2021, 21, 3801–3812. [Google Scholar] [CrossRef]
- Dobson, A.; Lafferty, K.D.; Kuris, A.M.; Hechinger, R.F.; Jetz, W. Homage to linnaeus: How many parasites? How many hosts? Proc. Natl. Acad. Sci. USA 2008, 105, 11482–11489. [Google Scholar] [CrossRef]
- Aguilar-Díaz, H.; Carrero, J.C.; Argüello-García, R.; Laclette, J.P.; Morales-Montor, J. Cyst and encystment in protozoan parasites: Optimal targets for new life-cycle interrupting strategies? Trends Parasitol. 2011, 27, 450–458. [Google Scholar] [CrossRef]
- Krishnan, A.; Kloehn, J.; Lunghi, M.; Soldati-Favre, D. Vitamin and cofactor acquisition in apicomplexans: Synthesis versus salvage. J. Biol. Chem. 2020, 295, 701–714. [Google Scholar] [CrossRef]
- Caron, D.A. Mixotrophy stirs up our understanding of marine food webs. Proc. Natl. Acad. Sci. USA 2016, 113, 2806–2808. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, W.; Li, H.; Zhang, J.; Liu, X.; Jin, R.; Li, H. Comparing the Environmental Influences and Community Assembly of Protist Communities in Two Anthropogenic Coastal Areas. Microorganisms 2024, 12, 1618. https://doi.org/10.3390/microorganisms12081618
Qiao W, Li H, Zhang J, Liu X, Jin R, Li H. Comparing the Environmental Influences and Community Assembly of Protist Communities in Two Anthropogenic Coastal Areas. Microorganisms. 2024; 12(8):1618. https://doi.org/10.3390/microorganisms12081618
Chicago/Turabian StyleQiao, Wenwen, Hongbo Li, Jinyong Zhang, Xiaohan Liu, Ruofei Jin, and Hongjun Li. 2024. "Comparing the Environmental Influences and Community Assembly of Protist Communities in Two Anthropogenic Coastal Areas" Microorganisms 12, no. 8: 1618. https://doi.org/10.3390/microorganisms12081618




