Nisin, a Probiotic Bacteriocin, Modulates the Inflammatory and Microbiome Changes in Female Reproductive Organs Mediated by Polymicrobial Periodontal Infection
Abstract
:1. Introduction
2. Material and Methods
2.1. Ethics Approval
2.2. Preparation of Periodontal Bacteria
2.3. Nisin Preparation
2.4. Infection of Mice
2.5. DNA Isolation from Samples/Tissues (Oral Swabs, Uterus, Ovary, Vagina, Heart and Aorta) to Evaluate Presence of Periodontal Pathogens via Reverse Transcription–Quantitative Polymerase Chain Reaction (RT-qPCR)
2.6. RNA Isolation from Samples/Tissues (Oral Swabs, Uterus, Ovary, Vagina, Heart, and Aorta) to Evaluate Immune Cytokine Levels via RT-qPCR
2.7. 16S Sequencing of the DNA Isolated from Tissues (Uterus, Ovary, Vagina and Heart)
2.8. Alpha Diversity
2.9. Beta Diversity
2.10. Statistical Analysis
3. Results
3.1. Oral Periodontal Pathogens Are Present in Female Upper Reproductive Organs, Plus Heart, and Aorta, Indicating Potential Hematogenous Transmission; Nisin Mitigates Changes in the Oral Cavity
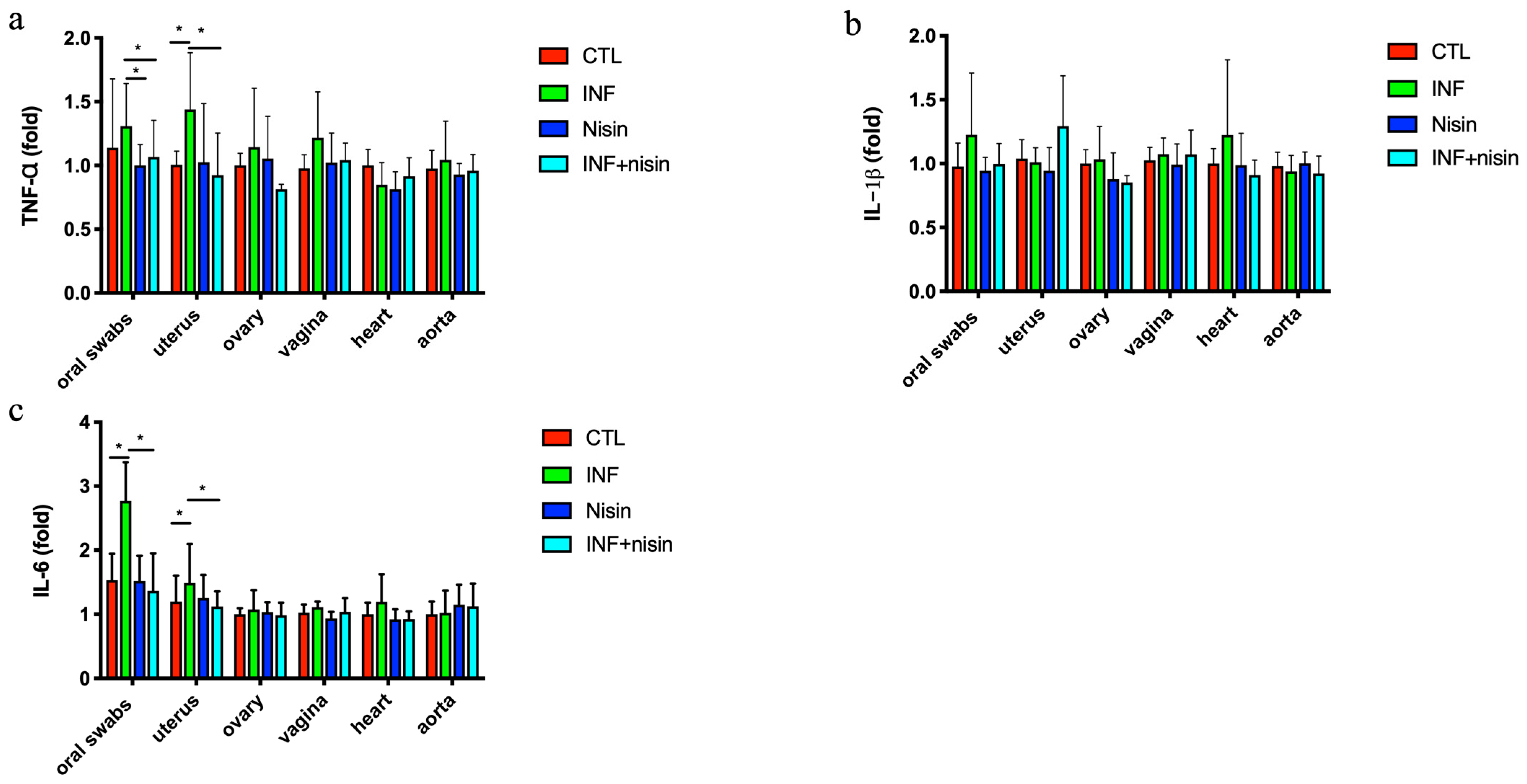
3.2. Polymicrobial Periodontal Infection Triggers an Increase in TNF-α and IL-6 Expression in the Oral Cavity and Uterus; Nisin Mitigates These Changes
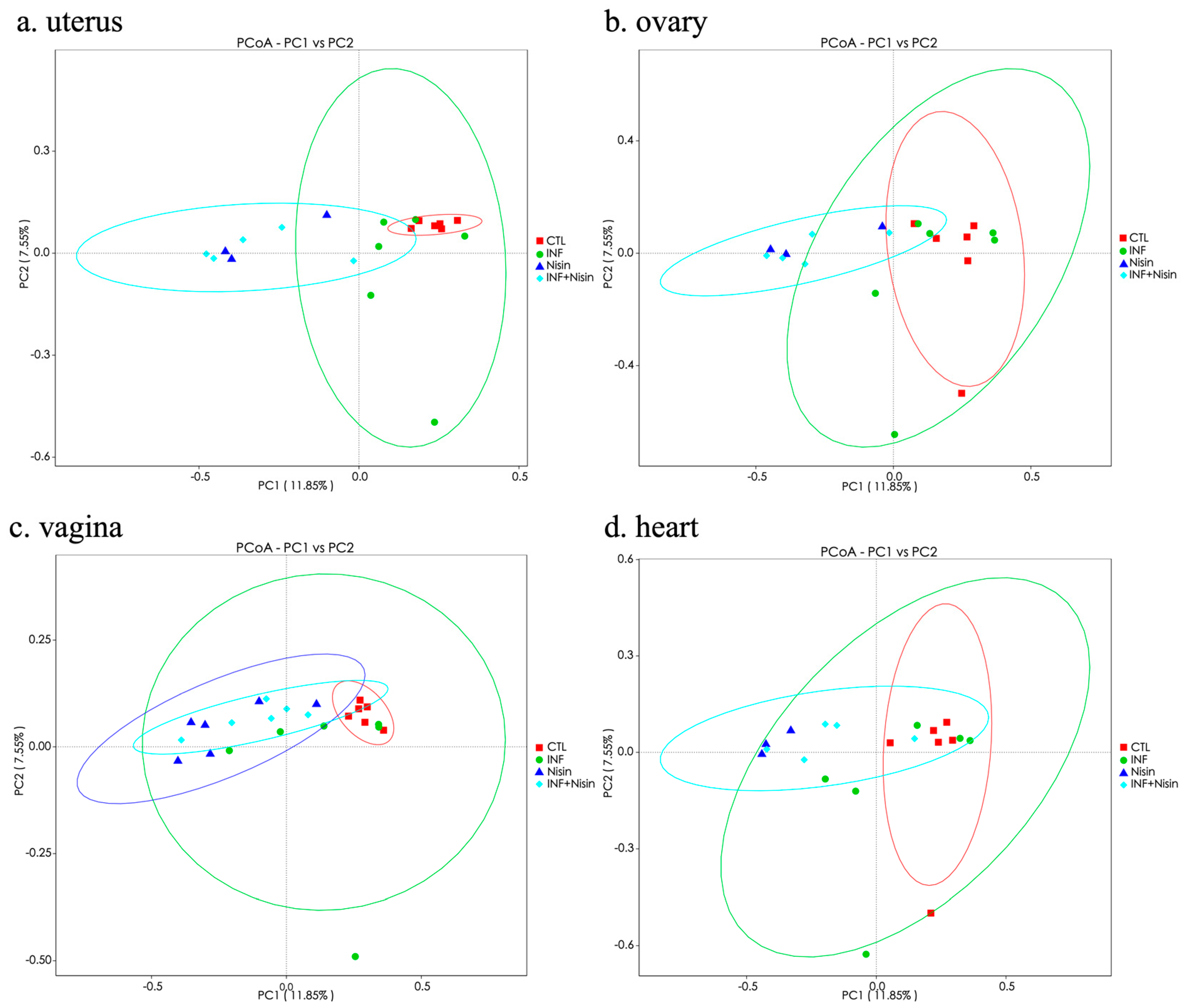
3.3. Polymicrobial Periodontal Infection Alters the Microbiome Alpha and Beta Diversity of the Female Reproductive System and Nisin Mediates Changes
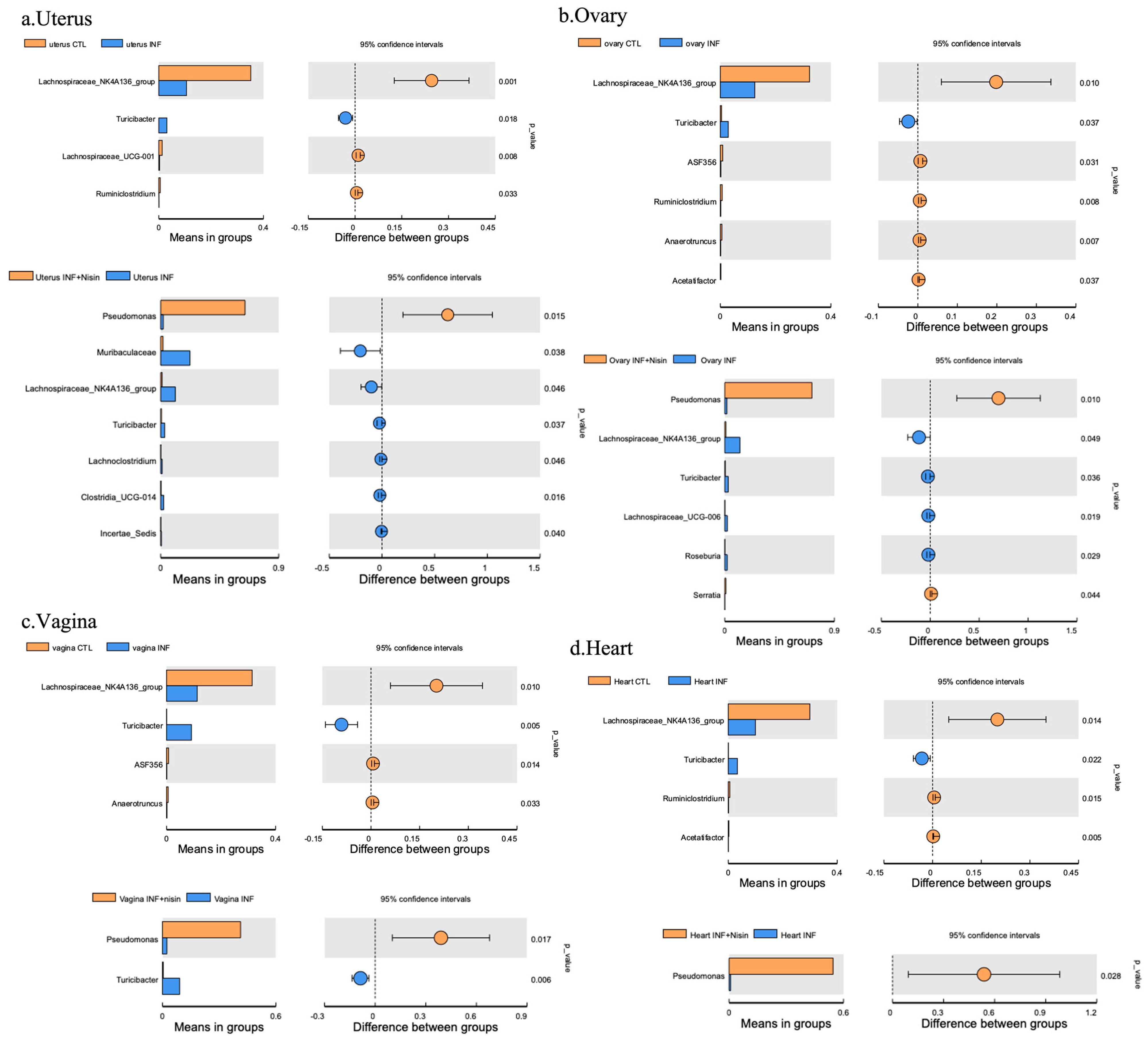
3.4. Periodontal Infection Shifts the Relative Abundance and Microbial Composition in the Uterus, Ovary and Vagina; Nisin Mitigates Some Changes and Establishes a New State
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, H.; Tal, R.; Clark, N.A.; Segars, J.H. Microbiota and pelvic inflammatory disease. Semin. Reprod. Med. 2014, 32, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Wassenaar, T.M.; Panigrahi, P. Is a foetus developing in a sterile environment? Lett. Appl. Microbiol. 2014, 59, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.M.; Haick, A.; Nkwopara, E.; Garcia, R.; Rendi, M.; Agnew, K.; Fredricks, D.N.; Eschenbach, D. Colonization of the upper genital tract by vaginal bacterial species in nonpregnant women. Am. J. Obstet. Gynecol. 2015, 212, 611.e1–611.e9. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lu, Y.; Chen, T.; Li, R. The Female Vaginal Microbiome in Health and Bacterial Vaginosis. Front. Cell. Infect. Microbiol. 2021, 11, 631972. [Google Scholar] [CrossRef] [PubMed]
- Cassini, M.A.; Pilloni, A.; Condo, S.G.; Vitali, L.A.; Pasquantonio, G.; Cerroni, L. Periodontal bacteria in the genital tract: Are they related to adverse pregnancy outcome? Int. J. Immunopathol. Pharmacol. 2013, 26, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Song, X.; Wei, W.; Zhong, H.; Dai, J.; Lan, Z.; Li, F.; Yu, X.; Feng, Q.; Wang, Z.; et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat. Commun. 2017, 8, 875. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, A.; Black, A.Y.; Lortie, K.; Fleming, N.A. A case of adolescent pelvic inflammatory disease caused by a rare bacterium: Fusobacterium nucleatum. J. Pediatr. Adolesc. Gynecol. 2013, 26, e113–e115. [Google Scholar] [CrossRef] [PubMed]
- Chaparro, A.; Blanlot, C.; Ramirez, V.; Sanz, A.; Quintero, A.; Inostroza, C.; Bittner, M.; Navarro, M.; Illanes, S.E. Porphyromonas gingivalis, Treponema denticola and toll-like receptor 2 are associated with hypertensive disorders in placental tissue: A case-control study. J. Periodontal. Res. 2013, 48, 802–809. [Google Scholar] [CrossRef]
- Han, Y.W.; Fardini, Y.; Chen, C.; Iacampo, K.G.; Peraino, V.A.; Shamonki, J.M.; Redline, R.W. Term stillbirth caused by oral Fusobacterium nucleatum. Obstet. Gynecol. 2010, 115 Pt 2, 442–445. [Google Scholar] [CrossRef]
- Ye, C.; Katagiri, S.; Miyasaka, N.; Kobayashi, H.; Khemwong, T.; Nagasawa, T.; Izumi, Y. The periodontopathic bacteria in placenta, saliva and subgingival plaque of threatened preterm labor and preterm low birth weight cases: A longitudinal study in Japanese pregnant women. Clin. Oral Investig. 2020, 24, 4261–4270. [Google Scholar] [CrossRef]
- Ao, M.; Miyauchi, M.; Furusho, H.; Inubushi, T.; Kitagawa, M.; Nagasaki, A.; Sakamoto, S.; Kozai, K.; Takata, T. Dental Infection of Porphyromonas gingivalis Induces Preterm Birth in Mice. PLoS ONE 2015, 10, e0137249. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Ren, H.; Guo, H.; Xing, W.; Liu, C.; Ji, Y.; Jiang, H.; Zhang, P.; Du, M. Periodontal infection with Porphyromonas gingivalis induces preterm birth and lower birth weight in rats. Mol. Oral Microbiol. 2018, 33, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Konishi, H.; Urabe, S.; Teraoka, Y.; Morishita, Y.; Koh, I.; Sugimoto, J.; Sakamoto, S.; Miyoshi, H.; Miyauchi, M.; Takata, T.; et al. Porphyromonas gingivalis, a cause of preterm birth in mice, induces an inflammatory response in human amnion mesenchymal cells but not epithelial cells. Placenta 2020, 99, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.M.; Gwak, J.W.; Kamarajan, P.; Fenno, J.C.; Rickard, A.H.; Kapila, Y.L. Biomedical applications of nisin. J. Appl. Microbiol. 2016, 120, 1449–1465. [Google Scholar] [CrossRef] [PubMed]
- Kajwadkar, R.; Shin, J.M.; Lin, G.H.; Fenno, J.C.; Rickard, A.H.; Kapila, Y.L. High-purity Nisin Alone or in Combination with Sodium Hypochlorite Is Effective against Planktonic and Biofilm Populations of Enterococcus faecalis. J. Endodont. 2017, 43, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.M.; Ateia, I.; Paulus, J.R.; Liu, H.R.; Fenno, J.C.; Rickard, A.H.; Kapila, Y.L. Antimicrobial nisin acts against saliva derived multi-species biofilms without cytotoxicity to human oral cells. Front. Microbiol. 2015, 6, 617. [Google Scholar] [CrossRef] [PubMed]
- Radaic, A.; Ye, C.C.; Parks, B.; Gao, L.; Kuraji, R.; Malone, E.; Kamarajan, P.; Zhan, L.; Kapila, Y.L. Modulation of pathogenic oral biofilms towards health with nisin probiotic. J. Oral Microbiol. 2020, 12, 1809302. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Kuraji, R.; Zhang, M.J.; Martinez, A.; Radaic, A.; Kamarajan, P.; Le, C.; Zhan, L.; Ye, C.C.; Range, H.; et al. Nisin probiotic prevents inflammatory bone loss while promoting reparative proliferation and a healthy microbiome. NPJ Biofilms Microbiomes 2022, 8, 45. [Google Scholar] [CrossRef]
- Kuraji, R.; Ye, C.; Zhao, C.; Gao, L.; Martinez, A.; Miyashita, Y.; Radaic, A.; Kamarajan, P.; Le, C.; Zhan, L.; et al. Nisin lantibiotic prevents NAFLD liver steatosis and mitochondrial oxidative stress following periodontal disease by abrogating oral, gut and liver dysbiosis. NPJ Biofilms Microbiomes 2024, 10, 3. [Google Scholar] [CrossRef]
- Zhao, C.; Kuraji, R.; Ye, C.; Gao, L.; Radaic, A.; Kamarajan, P.; Taketani, Y.; Kapila, Y.L. Nisin a probiotic bacteriocin mitigates. brain microbiome dysbiosis and Alzheimer’s disease-like neuroinflammation triggered by periodontal disease. J. Neuroinflammation 2023, 20, 228. [Google Scholar] [CrossRef]
- Radaic, A.; Brody, H.; Contreras, F.; Hajfathalian, M.; Lucido, L.; Kamarajan, P.; Kapila, Y.L. Nisin and Nisin Probiotic Disrupt Oral Pathogenic Biofilms and Restore Their Microbiome Composition towards Healthy Control Levels in a Peri-Implantitis Setting. Microorganisms 2022, 10, 1336. [Google Scholar] [CrossRef] [PubMed]
- Radaic, A.; Malone, E.; Kamarajan, P.; Kapila, Y.L. Solid Lipid Nanoparticles Loaded with Nisin (SLN-Nisin) are More Effective Than Free Nisin as Antimicrobial, Antibiofilm, and Anticancer Agents. J. Biomed. Nanotechnol. 2022, 18, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Madanchi, H.; Shoushtari, M.; Kashani, H.H.; Sardari, S. Antimicrobial peptides of the vaginal innate immunity and their role in the fi ght against sexually transmitted diseases. New Microbes New Infect. 2020, 34, 100627. [Google Scholar] [CrossRef] [PubMed]
- Noll, K.S.; Sinko, P.J.; Chikindas, M.L. Elucidation of the Molecular Mechanisms of Action of the Natural Antimicrobial Peptide Subtilosin Against the Bacterial Vaginosis-associated Pathogen Gardnerella vaginalis. Probiotics Antimicrob. Proteins 2011, 3, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Kang, M.; Zhang, M.J.; Reza Sailani, M.; Kuraji, R.; Martinez, A.; Ye, C.; Kamarajan, P.; Le, C.; Zhan, L.; et al. Polymicrobial periodontal disease triggers a wide radius of effect and unique virome. NPJ Biofilms Microbiomes 2020, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Rivera, M.F.; Lee, J.Y.; Aneja, M.; Goswami, V.; Liu, L.; Velsko, I.M.; Chukkapalli, S.S.; Bhattacharyya, I.; Chen, H.; Lucas, A.R.; et al. Polymicrobial infection with major periodontal pathogens induced periodontal disease and aortic atherosclerosis in hyperlipidemic ApoE(null) mice. PLoS ONE 2013, 8, e57178. [Google Scholar] [CrossRef]
- Chukkapalli, S.S.; Velsko, I.M.; Rivera-Kweh, M.F.; Zheng, D.; Lucas, A.R.; Kesavalu, L. Polymicrobial oral infection with four periodontal bacteria orchestrates a distinct inflammatory response and atherosclerosis in apoe null mice. PLoS ONE 2015, 10, e0143291. [Google Scholar] [CrossRef]
- Nahid, M.A.; Rivera, M.; Lucas, A.; Chan, E.K.; Kesavalu, L. Polymicrobial infection with periodontal pathogens specifically enhances microRNA miR-146a in ApoE−/− mice during experimental periodontal disease. Infect. Immun. 2011, 79, 1597–1605. [Google Scholar] [CrossRef]
- Velsko, I.M.; Chukkapalli, S.S.; Rivera-Kweh, M.F.; Zheng, D.; Aukhil, I.; Lucas, A.R.; Larjava, H.; Kesavalu, L. Periodontal pathogens invade gingiva and aortic adventitia and elicit inflammasome activation in αvβ6 integrin-deficient mice. Infect. Immun. 2015, 83, 4582–4593. [Google Scholar] [CrossRef]
- Magoč, T.; Steven, L. Salzberg. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Edgar, R. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucl. Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucl. Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Kuczynski, J.; Stombaugh, J.; Walters, W.A.; González, A.; Caporaso, J.G.; Knight, R. Using QIIME to Analyze 16S RRNA Gene Sequences from Microbial Communities. Curr. Protoc. Microbiol. 2012, 27, 1E-5. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Greenbaum, S.; Greenbaum, G.; Moran-Gilad, J.; Weintraub, A.Y. Ecological dynamics of the vaginal microbiome in relation to health and disease. Am. J. Obstet. Gynecol. 2019, 220, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Baud, A.; Hillion, K.H.; Plainvert, C.; Tessier, V.; Tazi, A.; Mandelbrot, L.; Poyart, C.; Kennedy, S.P. Microbial diversity in the vaginal microbiota and its link to pregnancy outcomes. Sci. Rep. 2023, 4, 9061. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, C.; Mangogna, A.; Bossi, F.; Ricci, G.; Kishore, U.; Bulla, R. Uterine Immunity and Microbiota: A Shifting Paradigm. Front. Immunol. 2019, 10, 2387. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Smith, M.A.; Champagne, C.; Elter, J.; Beck, J.; Offenbacher, S. Porphyromonas gingivalis infection during pregnancy increases maternal tumor necrosis factor alpha, suppresses maternal interleukin-10, and enhances fetal growth restriction and resorption in mice. Infect. Immun. 2003, 71, 5156–5162. [Google Scholar] [CrossRef] [PubMed]
- Ksiazek, M.; Mizgalska, D.; Eick, S.; Thogersen, I.B.; Enghild, J.J.; Potempa, J. KLIKK proteases of Tannerella forsythia: Putative virulence factors with a unique domain structure. Front. Microbiol. 2015, 6, 312. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A. Virulence mechanisms of Tannerella forsythia. Periodontology 2010, 54, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Dong, G.; Xiao, W.; Xiao, E.; Miao, F.; Syverson, A.; Missaghian, N.; Vafa, R.; Cabrera-Ortega, A.A.; Rossa, C., Jr.; et al. Effect of Aging on Periodontal Inflammation, Microbial Colonization, and Disease Susceptibility. J. Dent. Res. 2016, 95, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, J.; Xu, Y.; Yang, H.; Wang, J.; Xue, C.; Yan, X.; Su, L. Anti-inflammation effects of fucosylated chondroitin sulphate from Acaudina molpadioides by altering gut microbiota in obese mice. Food Funct. 2019, 10, 1736–1746. [Google Scholar] [CrossRef] [PubMed]
- Manuel, C.R.; Latuga, M.S.; Ashby, C.R., Jr.; Reznik, S.E. Immune tolerance attenuates gut dysbiosis, dysregulated uterine gene expression and high-fat diet potentiated preterm birth in mice. Am. J. Obstet. Gynecol. 2019, 220, 596.e1–596.e28. [Google Scholar] [CrossRef]
- Ma, L.; Ni, Y.; Wang, Z.; Tu, W.; Ni, L.; Zhuge, F.; Zheng, A.; Hu, L.; Zhao, Y.; Zheng, L.; et al. Spermidine improves gut barrier integrity and gut microbiota function in diet-induced obese mice. Gut Microbes 2020, 12, 1832857. [Google Scholar] [CrossRef]
- Jung, M.J.; Lee, J.; Shin, N.R.; Kim, M.S.; Hyun, D.W.; Yun, J.H.; Kim, P.S.; Whon, T.W.; Bae, J.W. Chronic Repression of mTOR Complex 2 Induces Changes in the Gut Microbiota of Diet-induced Obese Mice. Sci. Rep. 2016, 6, 30887. [Google Scholar] [CrossRef] [PubMed]
- Horie, M.; Miura, T.; Hirakata, S.; Hosoyama, A.; Sugino, S.; Umeno, A.; Murotomi, K.; Yoshida, Y.; Koike, T. Comparative analysis of the intestinal flora in type 2 diabetes and nondiabetic mice. Exp. Anim. 2017, 66, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Barandouzi, Z.A.; Starkweather, A.R.; Henderson, W.A.; Gyamfi, A.; Cong, X.S. Altered Composition of Gut Microbiota in Depression: A Systematic Review. Front. Psychiatry 2020, 11, 541. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Li, J.; Liu, F.; Lyu, N.; Wang, K.; Wang, L.; Liang, S.; Tao, H.; Zhu, B.; Alkasir, R. Analysis of the Gut Microflora in Patients With Parkinson’s Disease. Front. Neurosci. 2019, 13, 1184. [Google Scholar] [CrossRef]

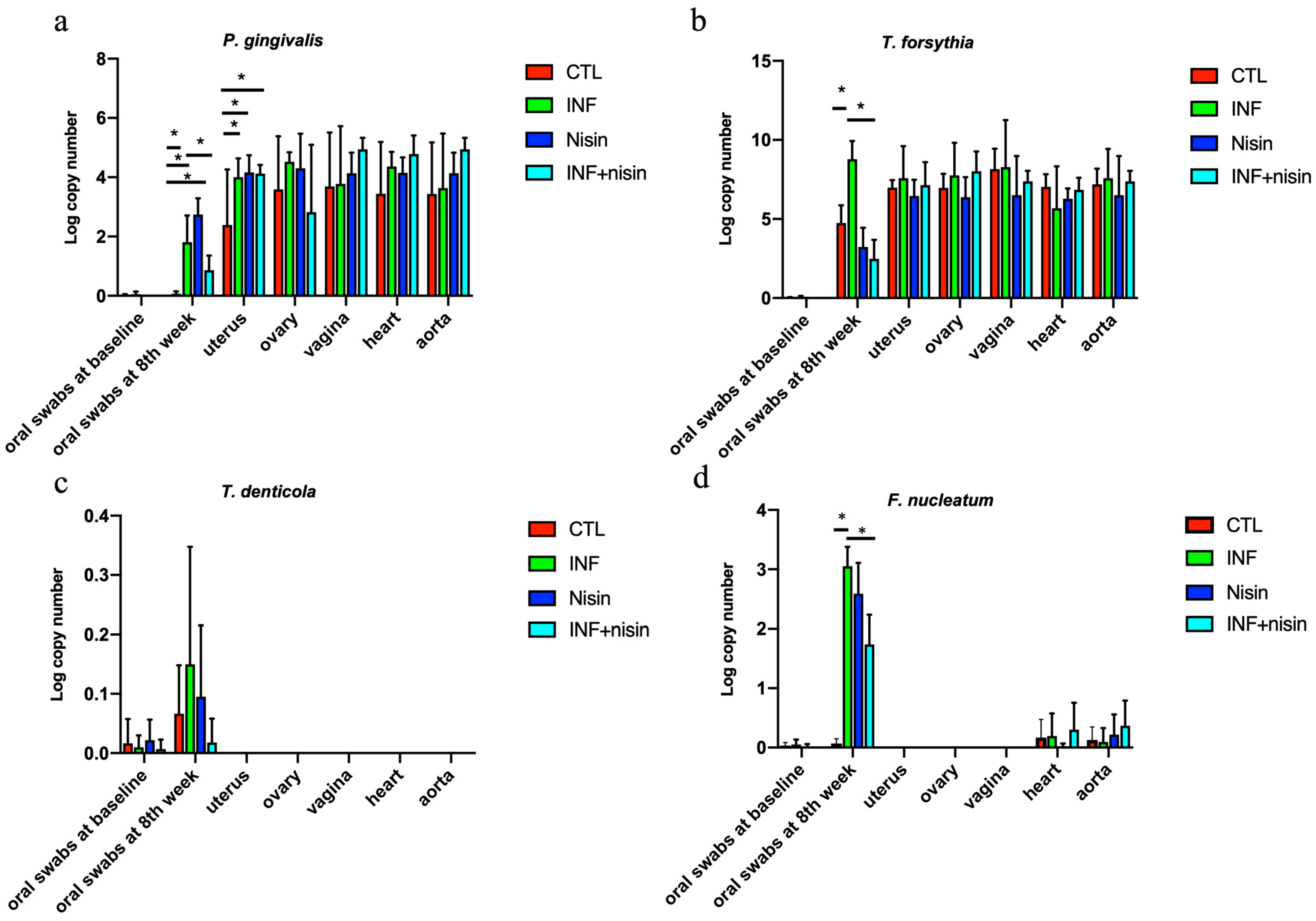



| Oral Swab | Uterus | Ovary | Vagina | Heart | Aorta | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTL | INF | Nisin | INF+Nisin | CTL | INF | Nisin | INF+Nisin | CTL | INF | Nisin | INF+Nisin | CTL | INF | Nisin | INF+Nisin | CTL | INF | Nisin | INF+Nisin | CTL | INF | Nisin | INF+Nisin | |
| P. gingivalis | 100.0 (6/6) | 100.0 (6/6) | 100.0 (6/6) | 100.0 (6/6) | 66.7 (4/6) | 100.0 (6/6) | 100.0 (6/6) | 100.0 (6/6) | 50.0 (3/6) | 100.0 (6/6) | 100.0 (6/6) | 66.7 (4/6) | 100.0 (6/6) | 100.0 (6/6) | 100.0 (6/6) | 100.0 (6/6) | 83.3 (5/6) | 100.0 (6/6) | 100.0 (6/6) | 100.0 (6/6) | 83.3 (5/6) | 83.3 (5/6) | 100.0 (6/6) | 100.0 (6/6) |
| T. denticola | 50.0 (3/6) | 50.0 (3/6) | 66.7 (4/6) | 16.7 (1/6) | 0.0 (0/6) | 0.0 (0/6) | 0.0 (0/6) | 0.0 (0/6) | 0.0 (0/6) | 0.0 (0/6) | 0.0 (0/6) | 0.0 (0/6) | 0.0 (0/6) | 0.0 (0/6) | 0.0 (0/6) | 0.0 (0/6) | 0.0 (0/6) | 0.0 (0/6) | 0.0 (0/6) | 0.0 (0/6) | 0.0 (0/6) | 0.0 (0/6) | 0.0 (0/6) | 0.0 (0/6) |
| T. forsythia | 100.0 (6/6) | 100.0 (6/6) | 100.0 (6/6) | 100.0 (6/6) | 100.0 (6/6) | 100.0 (6/6) | 100.0 (6/6) | 100.0 (6/6) | 100.0 (6/6) | 100.0 (6/6) | 100.0 (6/6) | 100.0 (6/6) | 100.0 (6/6) | 100.0 (6/6) | 100.0 (6/6) | 100.0 (6/6) | 100.0 (6/6) | 83.3 (5/6) | 100.0 (6/6) | 100.0 (6/6) | 100.0 (6/6) | 100.0 (6/6) | 100.0 (6/6) | 100.0 (6/6) |
| F. nucleatum | 100.0 (6/6) | 100.0 (6/6) | 100.0 (6/6) | 100.0 (6/6) | 0.0 (0/6) | 0.0 (0/6) | 0.0 (0/6) | 0.0 (0/6) | 0.0 (0/6) | 0.0 (0/6) | 0.0 (0/6) | 0.0 (0/6) | 0.0 (0/6) | 0.0 (0/6) | 0.0 (0/6) | 0.0 (0/6) | 33.3 (2/6) | 33.3 (2/6) | 0.0 (0/6) | 16.7 (1/6) | 33.3 (2/6) | 16.7 (1/6) | 33.3 (2/6) | 50.0 (3/6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, C.; Zhao, C.; Kuraji, R.; Gao, L.; Rangé, H.; Kamarajan, P.; Radaic, A.; Kapila, Y.L. Nisin, a Probiotic Bacteriocin, Modulates the Inflammatory and Microbiome Changes in Female Reproductive Organs Mediated by Polymicrobial Periodontal Infection. Microorganisms 2024, 12, 1647. https://doi.org/10.3390/microorganisms12081647
Ye C, Zhao C, Kuraji R, Gao L, Rangé H, Kamarajan P, Radaic A, Kapila YL. Nisin, a Probiotic Bacteriocin, Modulates the Inflammatory and Microbiome Changes in Female Reproductive Organs Mediated by Polymicrobial Periodontal Infection. Microorganisms. 2024; 12(8):1647. https://doi.org/10.3390/microorganisms12081647
Chicago/Turabian StyleYe, Changchang, Chuanjiang Zhao, Ryutaro Kuraji, Li Gao, Hélène Rangé, Pachiyappan Kamarajan, Allan Radaic, and Yvonne L. Kapila. 2024. "Nisin, a Probiotic Bacteriocin, Modulates the Inflammatory and Microbiome Changes in Female Reproductive Organs Mediated by Polymicrobial Periodontal Infection" Microorganisms 12, no. 8: 1647. https://doi.org/10.3390/microorganisms12081647






