Microbial C and N Metabolism Alterations Based on Soil Metagenome and Different Shrub Invasion Stages in Sanjiang Plain Wetlands
Abstract
:1. Introduction
2. Materials and Methods
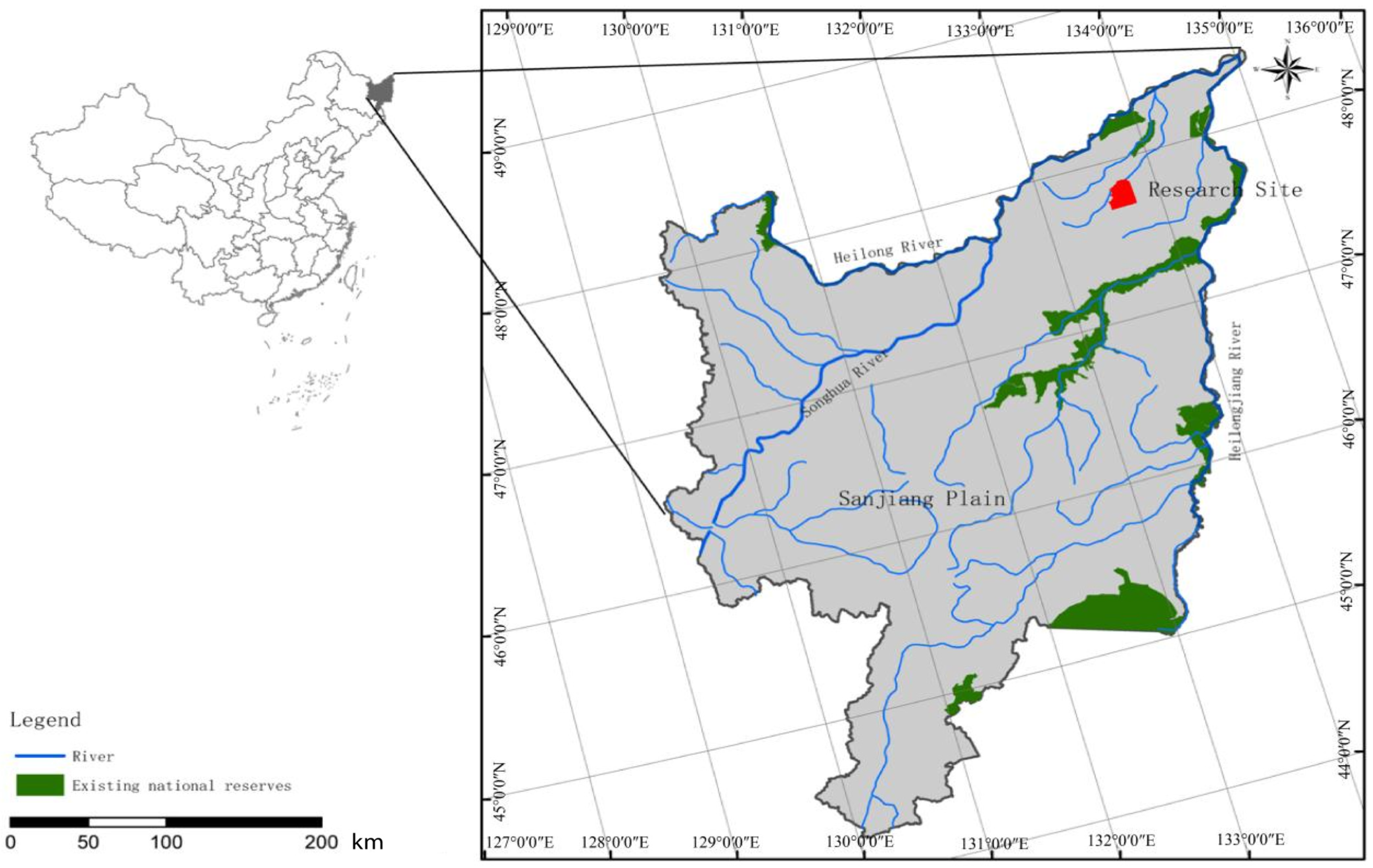
2.1. Research Area Description
2.2. Experimental Design
2.3. Soil Sample Collection and Analysis
2.4. Determination of Soil Physicochemical Properties
2.5. DNA Extraction, Metagenome Sequencing, and Data Processing
2.6. Statistical Analysis
3. Results
3.1. Soil Physicochemical Properties Changed with Shrub Expansion
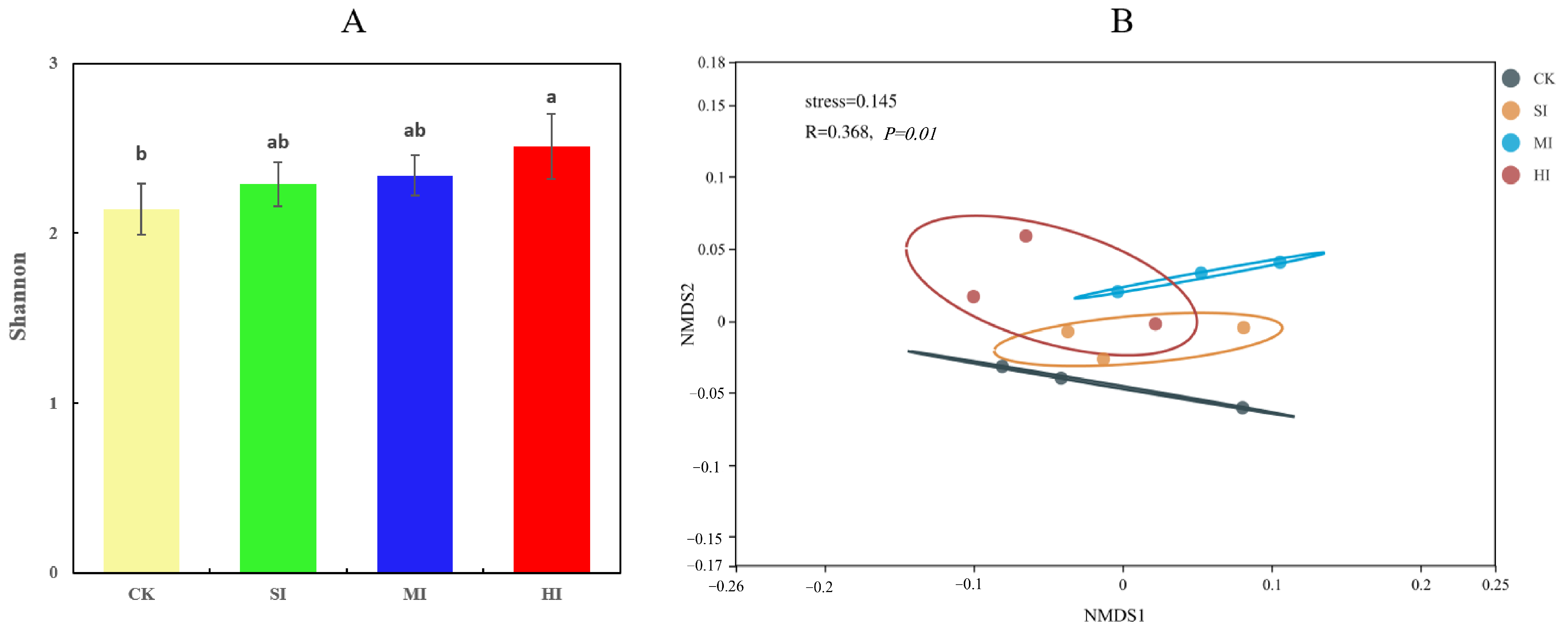
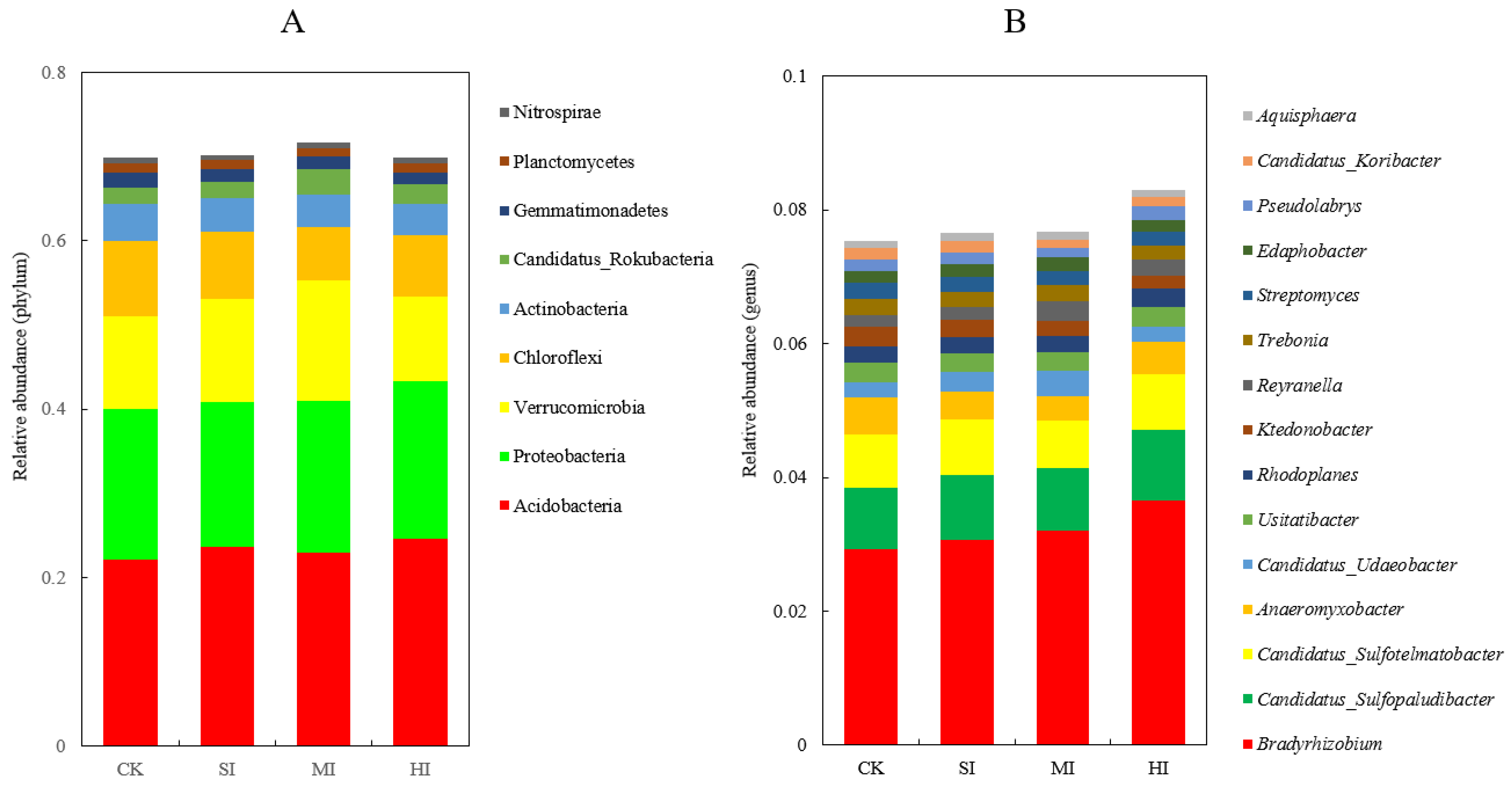
3.2. Comprehensive Characterization of Microbial Community Composition
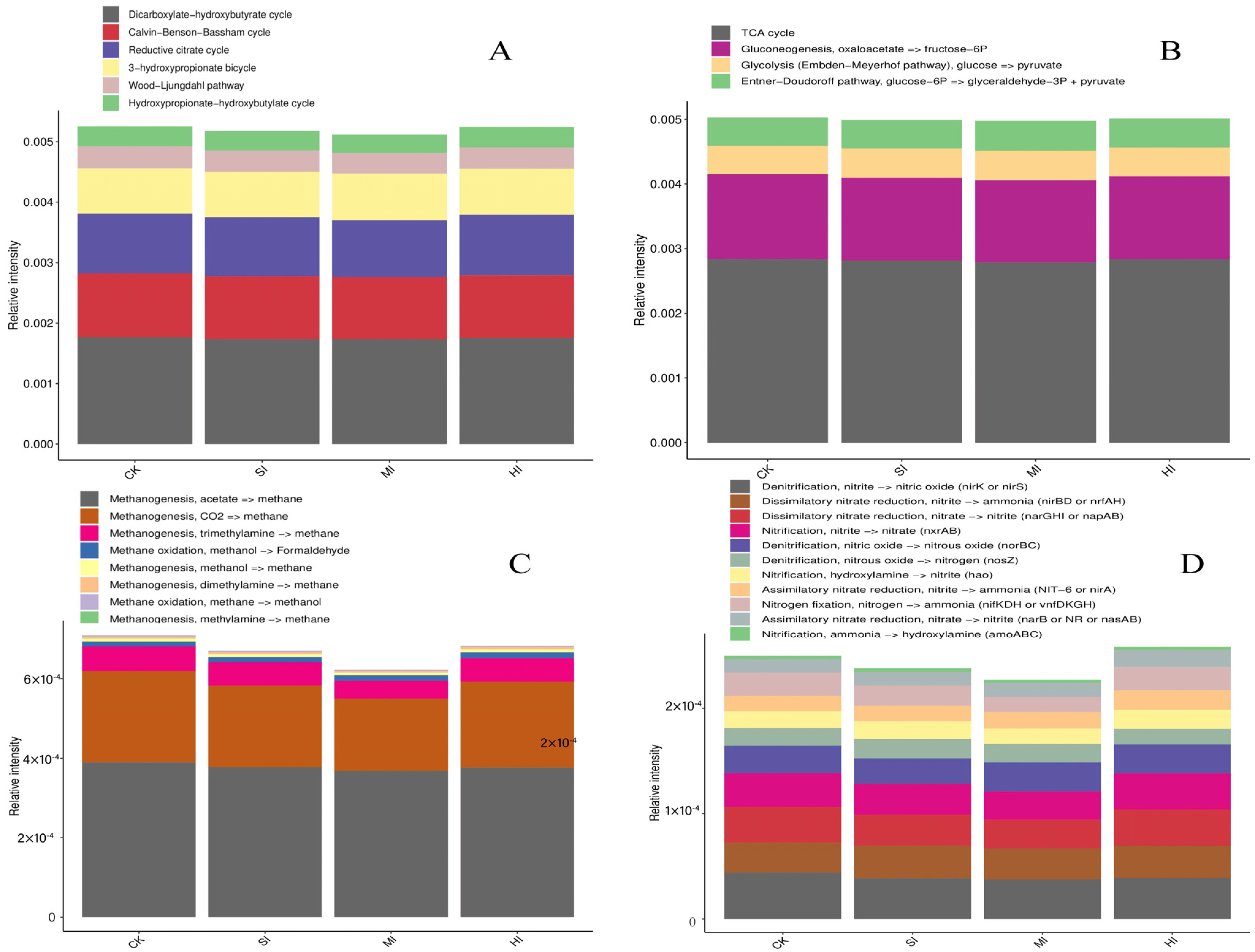
3.3. C and N Cycles under Different Shrub Expansion Intensities
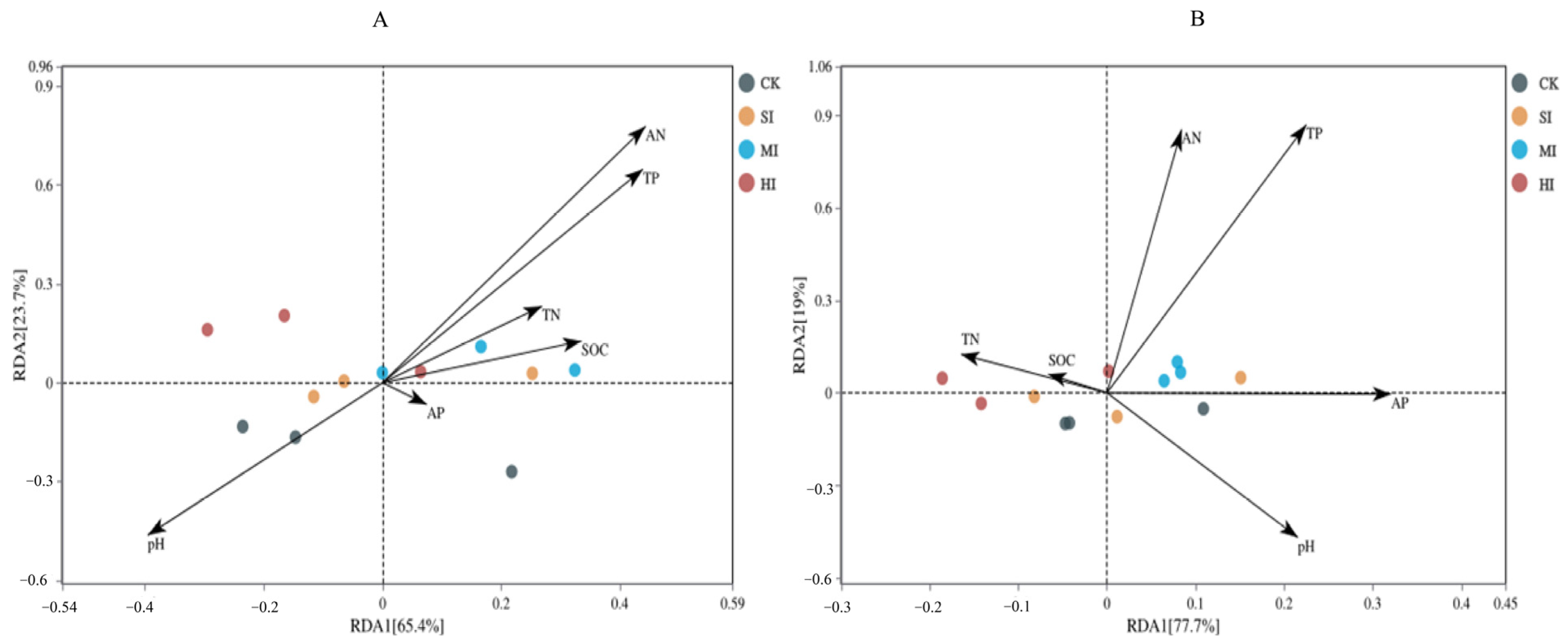
3.4. Correlation Analysis of Microbial and Environmental Factors
4. Discussion
4.1. Shrub Expansion Significantly Changed Soil Properties
4.2. Shrub Expansion Effects on Soil Microbial Community Structure
4.3. Shrub Expansion Effects on C and N Cycles
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, Z.; Zhang, C.; Liu, Z.; Song, C.; Xin, S. Effects of Long-Term (17 Years) Nitrogen Input on Soil Bacterial Community in Sanjiang Plain: The Largest Marsh Wetland in China. Microorganisms 2023, 11, 1552. [Google Scholar] [CrossRef]
- Liao, Q.; Lu, C.; Yuan, F.; Fan, Q.; Chen, H.; Yang, L.; Qiu, P.; Feng, Z.; Wang, C.; Zou, X. Soil carbon-fixing bacterial communities respond to plant community change in coastal salt marsh wetlands. Appl. Soil Ecol. 2023, 189, 104918. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, L.; Zhang, Y.; Wang, Z.; Guo, R. Seasonal fluctuations of marsh wetlands in the headwaters of the Brahmaputra, Ganges, and Indus Rivers, Tibetan Plateau based on the adapted LandTrendr model. Ecol. Indic. 2023, 152, 110360. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Zhao, W.; Li, J.; Xie, S.; Zhang, C.; He, X.; Yan, D.; Wang, M. Impact of Hydrological Changes on Wetland Landscape Dynamics and Implications for Ecohydrological Restoration in Honghe National Nature Reserve, Northeast China. Water 2023, 15, 3350. [Google Scholar] [CrossRef]
- Switzman, H.; Coulibaly, P.; Adeel, Z. Modeling the impacts of dryland agricultural reclamation on groundwater resources in Northern Egypt using sparse data. J. Hydrol. 2015, 520, 420–438. [Google Scholar] [CrossRef]
- Li, T.; Peng, C.; Bu, Z.; Zhu, Q.; Song, H.; Guo, X.; Wang, M. Woody plants reduce the sensitivity of soil extracellular enzyme activity to nutrient enrichment in wetlands: A meta-analysis. Soil Biol. Biochem. 2021, 159, 108280. [Google Scholar] [CrossRef]
- Boone Wesley, W.; Albrecht Audrey, A.; Conrad Jeremy, R.; Lechowicz Chris, J.; Hellgren Eric, C.; McCleery Robert, A. Shrub encroachment threatens persistence of an endemic insular wetland rodent. J. Mammal. 2022, 103, 1182–1193. [Google Scholar] [CrossRef]
- Phillips, C.A.; Wurzburger, N. Elevated rates of heterotrophic respiration in shrub-conditioned arctic tundra soils. Pedobiol.-J. Soil Ecol. 2019, 72, 8–15. [Google Scholar] [CrossRef]
- Broadbent Arthur, A.D.; Michael, B.; Pritchard William, J.; Newbold Lindsay, K.; Tim, G.; Andrew, G.; Snell Helen, S.K.; Irene, C.; Antonios, M.; Grant Helen, K.; et al. Shrub expansion modulates belowground impacts of changing snow conditions in alpine grasslands. Ecol. Lett. 2021, 25, 52–64. [Google Scholar] [CrossRef]
- Oriol, G.; Karita, S.; Ninot Josep, M.; József, G.; Annamari, M.; Ahonen Saija, H.k.; Josep, P. Encroachment of shrubs into subalpine grasslands in the Pyrenees modifies the structure of soil fungal communities and soil properties. FEMS Microbiol. Ecol. 2019, 95, 28. [Google Scholar]
- Bai, X.; Zhai, G.; Yan, Z.; An, S.; Liu, J.; Huo, L.; Dippold, M.A.; Kuzyakov, Y. Effects of microbial groups on soil organic carbon accrual and mineralization during high- and low-quality litter decomposition. Catena 2024, 241, 108051. [Google Scholar] [CrossRef]
- Zhu, M.; Nicolas, F.; Wang, Q.; Xu, Z.; Liang, S.; Ye, J.; Lin, F.; Yuan, Z.; Mao, Z.; Wang, X.; et al. High functional breadth of microbial communities decreases home-field advantage of litter decomposition. Soil Biol. Biochem. 2024, 188, 109232. [Google Scholar] [CrossRef]
- Wei, Y.; Wei, B.; Masahiro, R.; Bi, Y.; Sun, X.; Zhang, Y.; Liu, N. Grazing facilitates litter-derived soil organic carbon formation in grasslands by fostering microbial involvement through microenvironment modification. Catena 2023, 232, 107389. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Y.; Feng, W.; Qin, S.; Liu, Z.; Bai, Y.; Yan, R.; Fa, K. Effects of xeric shrubs on soil microbial communities in a desert in northern China. Plant Soil 2017, 414, 281–294. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, W.; Tian, Y.; Bai, S.; Zuoma, D.; Zhang, D.; Ma, X.; Mu, X. The mitigation of microbial carbon and nitrogen limitations by shrub encroachment: Extracellular enzyme stoichiometry of the alpine grassland on the Qinghai-Tibetan Plateau. Biogeochemistry 2023, 165, 205–225. [Google Scholar] [CrossRef]
- Li, H.; Zhang, J.; Hu, H.; Chen, L.; Zhu, Y.; Shen, H.; Fang, J. Shift in soil microbial communities with shrub encroachment in Inner Mongolia grasslands, China. Eur. J. Soil Biol. 2017, 79, 40–47. [Google Scholar] [CrossRef]
- Ding, L.; Chen, H.; Wang, M.; Wang, P. Shrub expansion raises both aboveground and underground multifunctionality on a subtropical plateau grassland: Coupling multitrophic community assembly to multifunctionality and functional trade-off. Front. Microbiol. 2024, 14, 1339125. [Google Scholar] [CrossRef] [PubMed]
- Collins Courtney, G.; Stajich Jason, E.; Weber Sören, E.; Nuttapon, P.; Diez Jeffrey, M. Shrub range expansion alters diversity and distribution of soil fungal communities across an alpine elevation gradient. Mol. Ecol. 2018, 27, 2461–2476. [Google Scholar] [CrossRef]
- Zhou, H.C.; Ma, A.Z.; Zhou, X.R.; Chen, X.K.; Zhang, J.J.; Zhang, Q.W.; Qi, X.N.; Liu, G.H.; Zhuang, G.Q. Phosphorus Shapes Soil Microbial Community Composition and Network Properties During Grassland Expansion Into Shrubs in Tibetan Dry Valleys. Front. Plant Sci. 2022, 13, 848691. [Google Scholar] [CrossRef]
- Zhang, Q.; Feng, K.; Chang, Z.H.; He, S.H.; Xu, W.Q. Effects of shrub encroachment on plant and soil microbial in the forest-grassland ecotone. Chin. J. Plant Ecol. 2023, 47, 770–781. [Google Scholar] [CrossRef]
- Aanderud, Z.T.; Shuldman, M.I.; Drenovsky, R.E.; Richards, J.H. Shrub-interspace dynamics alter relationships between microbial community composition and belowground ecosystem characteristics. Soil Biol. Biochem. 2008, 40, 2206–2216. [Google Scholar] [CrossRef]
- Bani, A.; Pioli, S.; Ventura, M.; Panzacchi, P.; Borruso, L.; Tognetti, R.; Tonon, G.; Brusetti, L. The role of microbial community in the decomposition of leaf litter and deadwood. Appl. Soil Ecol. 2018, 126, 75–84. [Google Scholar] [CrossRef]
- Zhang, R.; Fu, X.; Zhong, H.; Sui, X.; Liu, Y. Changes in Soil Bacterial Community and Function in Winter Following Long-Term Nitrogen (N) Deposition in Wetland Soil in Sanjiang Plain, China. Microorganisms 2023, 11, 2634. [Google Scholar] [CrossRef]
- Weng, X.H.; Sui, X.; Li, M.S.; Liu, Y.N.; Zhang, R.T.; Yang, L.B. Effects of Simulated Nitrogen Deposition on Soil Microbial CarbonC Metabolism in Calamagrostis angustifolia Wetland in Sanjiang Plain. Huan Jing Ke Xue 2022, 43, 4674–4683. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Frey, B.; Yang, L.B.; Liu, Y.N.; Zhang, R.T.; Ni, H.W.; Li, M.H. Soil Acidobacterial community composition changes sensitively with wetland degradation in northeastern of China. Front. Microbiol. 2022, 13, 1052161. [Google Scholar] [CrossRef]
- Jinlei, C.; Ling, F.; Xiang, K. Composition, structure and regional characteristics of two forest communities in the central subtropics. For. Sci. 2019, 55, 159–172. [Google Scholar]
- Lee, A.; Fujita, H.; Kobayashi, H. Effects of Drainage on Open-Water Mire Pools: Open Water Shrinkage and Vegetation Change of Pool Plant Communities. Wetlands 2017, 37, 741–751. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, B.; Shen, J.; Xu, F.; Li, N.; Jia, P.; Jia, Y.; An, S. Shifts in C-degradation genes and microbial metabolic activity with vegetation types affected the surface soil organic carbon pool. Soil Biol. Biochem. 2024, 192, 109371. [Google Scholar] [CrossRef]
- Li, Y.P.; Li, W.T.; Li, J.; Feng, Y.L. Temporal dynamics of plant−soil feedback and related mechanisms depend on environmental context during invasion processes of a subtropical invader. Plant Soil 2023, 496, 539–554. [Google Scholar] [CrossRef]
- Li, J.; Dong, L.; Fan, M.; Shangguan, Z. Long-term vegetation restoration promotes lignin phenol preservation and microbial anabolism in forest plantations: Implications for soil organic carbon dynamics. Sci. Total Environ. 2024, 928, 172635. [Google Scholar] [CrossRef]
- He, Y.; Jia, B.; Wei, C.; Fan, F.; Wilschut, R.A.; Lu, X. Leaf litter presence in the non-growing season prolongs plant legacy effects on soil fungal communities and succeeding plant growth. J. Ecol. 2023, 111, 1997–2009. [Google Scholar] [CrossRef]
- Mengjie, Y.; Xinrui, Y.; Ting, W.; Qunli, S.; Xianting, W.; Yuhuan, W. Different vegetation communities did not amplify spatial heterogeneity of soil microbial diversity and community in a subtropical Sphagnum-dominated peatland. Plant Soil 2023, 495, 271–285. [Google Scholar]
- Rango, A.; Tartowski, S.L.; Laliberte, A.; Wainwright, J.; Parsons, A. Islands of hydrologically enhanced biotic productivity in natural and managed arid ecosystems. J. Arid Environ. 2005, 65, 235–252. [Google Scholar]
- Cai, Y.; Yan, Y.; Xu, D.; Xu, X.; Wang, C.; Wang, X.; Chen, J.; Xin, X.; Eldridge, D.J. The fertile island effect collapses under extreme overgrazing: Evidence from a shrub-encroached grassland. Plant Soil 2020, 448, 201–212. [Google Scholar] [CrossRef]
- Štraus, D.; Redondo, M.Á.; Castaño, C.; Juhanson, J.; Clemmensen, K.E.; Hallin, S.; Oliva, J. Plant–soil feedbacks among boreal forest species. J. Ecol. 2023, 112, 138–151. [Google Scholar] [CrossRef]
- Wilschut Rutger, A.; Hume Benjamin, C.C.; Ekaterina, M.; van Kleunen, M. Plant-soil feedback effects on conspecific and heterospecific successors of annual and perennial Central European grassland plants are correlated. Nat. Plants 2023, 9, 1057–1066. [Google Scholar] [CrossRef]
- Liang, D.S.; Mu, C.C.; Gao, X.; Lu, Y. Environmental gradient distribution patterns of wetland plant community diversity and controlling factors in Songnen Plain. Acta Ecol. Sin. 2023, 43, 339–351. [Google Scholar]
- Mou, X.M.; Li, F.-C.; Jia, B.; Chen, J.; Guan, Z.-H.; Li, Y.-Q.; Guggenberger, G.; Kuzyakov, Y.; Wang, L.; Li, X.G. Decreasing carbon allocation belowground in alpine meadow soils by shrubification. Geoderma 2024, 443, 116810. [Google Scholar]
- Xin, S.; Wang, M.; Beat, F.; Liu, Y.; Zhang, R.; Ni, H.; Yu, S.; Xin, H.; Li, M.H. Differential Responses of Soil Bacterial and Fungal Communities to Simulated Nitrogen Deposition in a Temperate Wetland of Northeastern China. J. Soil Sci. Plant Nutr. 2023, 24, 467–482. [Google Scholar] [CrossRef]
- Paolo, Z.; Dolores, A.; Jordi, S.; Romà, O.; Liu, L.; Josep, P. Effects of nitrogen 515 deposition on soil enzymatic activity and soil microbial community in a Mediterranean holm oak forest. 516 Geoderma 2023, 430, 116354. [Google Scholar]
- Bao, Y.; Dolfing, J.; Guo, Z.; Chen, R.; Wu, M.; Li, Z.; Lin, X.; Feng, Y. Important ecophysiological roles of non-dominant Actinobacteria in plant residue decomposition, especially in less fertile soils. Microbiome 2021, 9, 84. [Google Scholar] [CrossRef]
- Liu, J.; Sui, Y.; Yu, Z.; Yao, Q.; Shi, Y.; Chu, H.; Jin, J.; Liu, X.; Wang, G. Diversity and distribution patterns of acidobacterial communities in the black soil zone of northeast China. Soil Biol. Biochem. 2016, 95, 212–222. [Google Scholar] [CrossRef]
- Li, N.; Du, H.; Li, M.-H.; Na, R.; Dong, R.; He Hong, S.; Zong, S.; Huang, L.; Wu, Z. Deyeuxia angustifolia upward migration and nitrogen deposition change soil microbial community structure in an alpine tundra. Soil Biol. Biochem. 2023, 180, 109009. [Google Scholar] [CrossRef]
- Liu, Y.; Peng, Y.; Bai, Y.; Wang, Y.; Men, J.; Men, M.; Peng, Z. Responses of soil bacterial functional group diversity to nitrogen enrichment in global grasslands. Catena 2024, 237, 107807. [Google Scholar] [CrossRef]
- Mao, R.; Zhang, X.-H.; Li, S.-Y.; Song, C.-C. Long-term phosphorus addition enhances the biodegradability of dissolved organic carbon in a nitrogen-limited temperate freshwater wetland. Sci. Total Environ. 2017, 605–606, 332–336. [Google Scholar] [CrossRef]
- Andrews, H.M.; Krichels, A.H.; Homyak, P.M.; Piper, S.; Aronson, E.L.; Botthoff, J.; Greene, A.C.; Jenerette, G.D. Wetting-induced soil CO2 emission pulses are driven by interactions among soil temperature, carbon, and nitrogen limitation in the Colorado Desert. Glob. Change Biol. 2023, 29, 3205–3220. [Google Scholar] [CrossRef]
- Huang, J.; Yang, J.; Han, M.; Wang, B.; Sun, X.; Jiang, H. Microbial carbon fixation and its influencing factors in saline lake water. Sci. Total Environ. 2023, 877, 162922. [Google Scholar] [CrossRef]
- Huang, J.; Yang, J.; Han, M.; Wang, B.; Sun, X.; Jiang, H. Changes in soil microbial carbon fixation pathways along the oasification process in arid desert region: A confirmation based on metagenome analysis. Catena 2024, 239, 107955. [Google Scholar]
- Becker, J.C.; Rodibaugh, K.J.; Hahn, D.; Nowlin, W.H. Bacterial community composition and carbon metabolism in a subtropical riverscape. Hydrobiologia 2017, 792, 209–226. [Google Scholar] [CrossRef]
- Zhang, W.; Kang, X.; Kang, E.; Audet, J.; Davidson, T.A.; Zhang, X.; Yan, L.; Li, Y.; Yan, Z.; Zhang, K.; et al. Soil water content, carbon, and nitrogen determine the abundances of methanogens, methanotrophs, and methane emission in the Zoige alpine wetland. J. Soils Sediments 2021, 22, 470–481. [Google Scholar] [CrossRef]
- Petersen, D.G.; Blazewicz, S.J.; Firestone, M.; Herman, D.J.; Turetsky, M.; Waldrop, M. Abundance of microbial genes associated with nitrogen cycling as indices of biogeochemical process rates across a vegetation gradient in Alaska. Environ. Microbiol. 2012, 14, 993–1008. [Google Scholar] [CrossRef]
- Pereira e Silva, M.C.; Semenov, A.V.; van Elsas, J.D.; Salles, J.F. Seasonal variations in the diversity and abundance of diazotrophic communities across soils. Fed. Eur. Microbiol. Soc. 2011, 77, 57–68. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Zhang, H.K.; Liang, J.H.; Zhang, B.G.; Jiang, W.T.; Tian, L.L.; Li, Y.; Cai, Y.J. Soil denitrifying enzyme activity and its influencing factors in a bamboo forest riparian zone in the upper reaches of the Taihu Lake Basin, China. Chin. J. Appl. Ecol. 2021, 32, 3070–3078. [Google Scholar]

| N Level | pH | SOC (g/kg) | TN (g/kg) | AN (g/kg) | TP (g/kg) | AP (mg/kg) |
|---|---|---|---|---|---|---|
| CK | 5.35 ± 0.11 a | 33.48 ± 3.87 a | 3.01 ± 0.24 a | 0.25 ± 0.02 b | 0.84 ± 0.05 b | 8.75 ± 0.61 b |
| SI | 5.22 ± 0.11 a | 39.85 ± 5.91 a | 3.75 ± 0.26 a | 0.31 ± 0.01 a | 1.03 ± 0.07 a | 8.25 ± 0.57 b |
| MI | 5.26 ± 0.14 a | 35.42 ± 4.41 a | 3.27 ± 0.15 a | 0.32 ± 0.02 a | 1.07 ± 0.08 a | 8.17 ± 0.92 b |
| HI | 5.23 ± 0.12 a | 34.44 ± 3.59 a | 3.31 ± 0.21 a | 0.34 ± 0.02 a | 1.09 ± 0.09 a | 10.12 ± 0.88 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Wang, S.; Zhong, H.; Fu, X.; Li, L.; Wang, L.; Liu, Y. Microbial C and N Metabolism Alterations Based on Soil Metagenome and Different Shrub Invasion Stages in Sanjiang Plain Wetlands. Microorganisms 2024, 12, 1648. https://doi.org/10.3390/microorganisms12081648
Zhang R, Wang S, Zhong H, Fu X, Li L, Wang L, Liu Y. Microbial C and N Metabolism Alterations Based on Soil Metagenome and Different Shrub Invasion Stages in Sanjiang Plain Wetlands. Microorganisms. 2024; 12(8):1648. https://doi.org/10.3390/microorganisms12081648
Chicago/Turabian StyleZhang, Rongtao, Shenzheng Wang, Haixiu Zhong, Xiaoyu Fu, Lin Li, Li Wang, and Yingnan Liu. 2024. "Microbial C and N Metabolism Alterations Based on Soil Metagenome and Different Shrub Invasion Stages in Sanjiang Plain Wetlands" Microorganisms 12, no. 8: 1648. https://doi.org/10.3390/microorganisms12081648





