Enhancing Antibiotic Efficacy and Combating Biofilm Formation: Evaluating the Synergistic Potential of Origanum vulgare Essential Oil against Multidrug-Resistant Gram-Negative Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Collection
2.2. Essential Oil Extraction
2.3. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
2.4. Tested Bacterial Isolates
2.5. Antibiotic Susceptibility Test
2.5.1. Disk Diffusion Method
2.5.2. Determination of MICs of Antibiotics
2.6. Antibacterial Activity of Essential Oil
2.6.1. Disk Diffusion Method
2.6.2. Determination of MICs and MBCs of Essential Oils
2.7. Checkerboard Assay
2.8. Biofilm Formation Test
2.9. Effect of OEO on Biofilm Adhesion and Preformed Biofilm
2.10. Statistical Analysis
3. Results
3.1. Extraction and Chemical Composition of Essential Oil
3.2. Antibiotic Susceptibility Testing of Clinical Strains
3.3. Activity of Oreganum Essential Oil
3.4. Combination of O. vulgare EO with Antibiotics
3.5. Biofilm Formation Test for Bacterial Strains
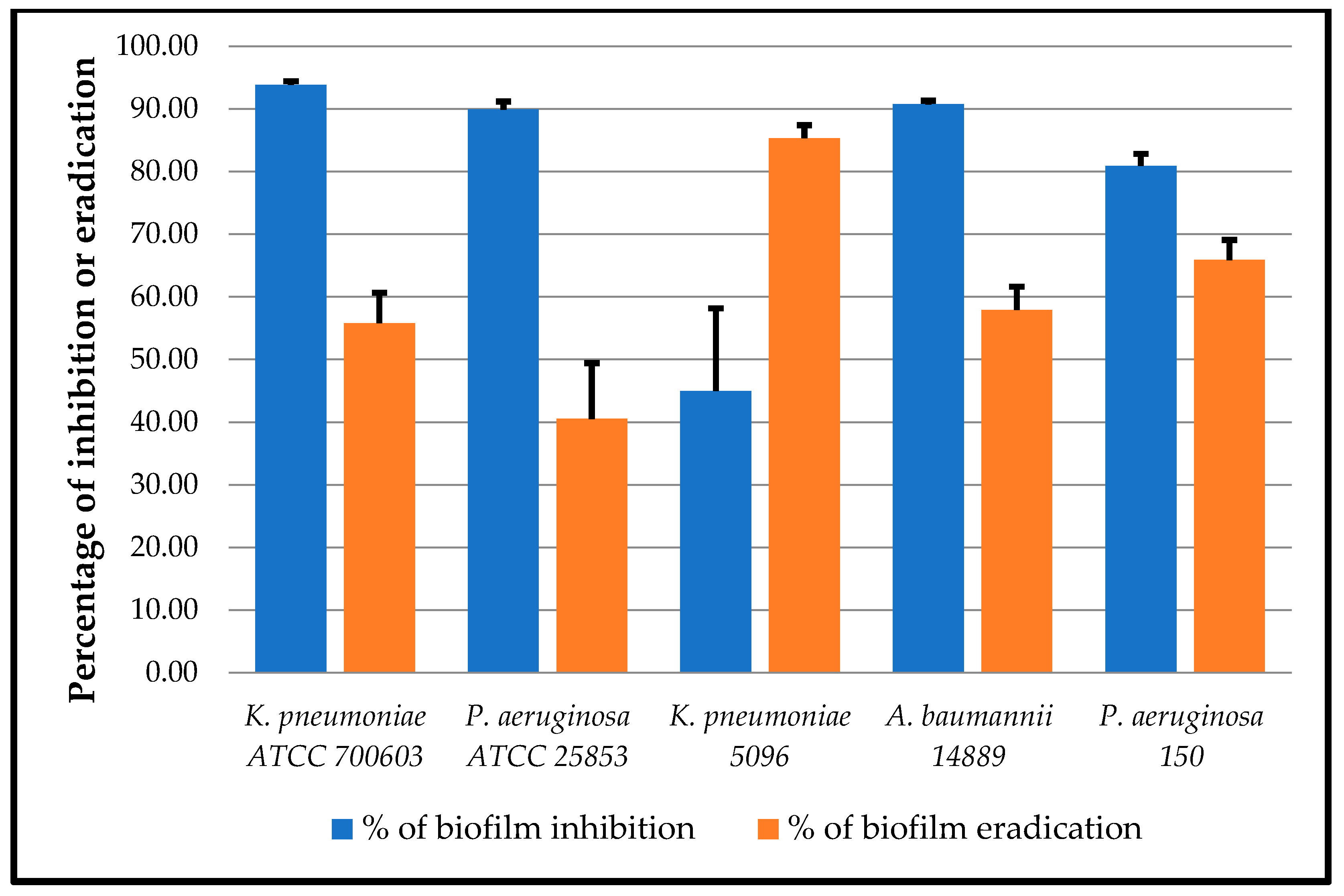
3.6. Antibiofilm Activity of Origanum Essential Oil
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ranjbar, R.; Alam, M.; Antimicrobial Resistance Collaborators (2022). Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Evid. Based Nurs. 2023, 27, 16. [Google Scholar] [CrossRef]
- Jonas, O.B.; Irwin, A.; Berthe, F.C.J.; Le Gall, F.G.; Marquez, P.V. Drug-Resistant Infections: A Threat to Our Economic Future. In World Bank Report; The World Bank: Washington, DC, USA, 2017; Volume 2. [Google Scholar]
- Walsh, T.R.; Gales, A.C.; Laxminarayan, R.; Dodd, P.C. Antimicrobial Resistance: Addressing a Global Threat to Humanity. PLoS Med. 2023, 20, e1004264. [Google Scholar] [CrossRef] [PubMed]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.R.M.; Mitra, S.; Emran, T.B.; Dhama, K.; Ripon, M.K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic Resistance in Microbes: History, Mechanisms, Therapeutic Strategies and Future Prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef] [PubMed]
- Wesseling, C.M.J.; Martin, N.I. Synergy by Perturbing the Gram-Negative Outer Membrane: Opening the Door for Gram-Positive Specific Antibiotics. ACS Infect. Dis. 2022, 8, 1731–1757. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Wang, Q.; Wang, Y.; Wen, X.; Peng, H.; Peng, R.; Shi, Q.; Xie, X.; Li, L. Outer Membrane Porins Contribute to Antimicrobial Resistance in Gram-Negative Bacteria. Microorganisms 2023, 11, 1690. [Google Scholar] [CrossRef] [PubMed]
- Hendrik, T.C.; Voorintholt, A.F.; Vos, M.C. Clinical and Molecular Epidemiology of Extended-Spectrum Beta-Lactamase-Producing Klebsiella Spp: A Systematic Review and Meta-Analyses. PLoS ONE 2015, 10, e0140754. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Carnelutti, A.; Peghin, M. Patient Specific Risk Stratification for Antimicrobial Resistance and Possible Treatment Strategies in Gram-Negative Bacterial Infections. Expert Rev. Anti. Infect. Ther. 2017, 15, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K. Persister Cells. Annu. Rev. Microbiol. 2010, 64, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.R. Nosocomial Infections and Infection Control. Medicine 2021, 49, 638–642. [Google Scholar] [CrossRef]
- Tacconelli, E.; Magrini, N. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; World Health Organization: Geneva, Switzerland, 2017; Volume 43. [Google Scholar]
- Soyingbe, O.S.; Myeni, C.B.; Osunsanmi; Lawal, O.A.; Opoku, A.R. Antimicrobial and Efflux Pumps Inhibitory Activities of Eucalyptus Grandis Essential Oil against Respiratory Tract Infectious Bacteria. J. Med. Plants Res. 2015, 9, 343–348. [Google Scholar] [CrossRef][Green Version]
- Man, A.; Santacroce, L.; Jacob, R.; Mare, A.; Man, L. Antimicrobial Activity of Six Essential Oils against a Group of Human Pathogens: A Comparative Study. Pathogens 2019, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Kerekes, E.B.; Vidács, A.; Takó, M.; Petkovits, T.; Vágvölgyi, C.; Horváth, G.; Balázs, V.L.; Krisch, J. Anti-Biofilm Effect of Selected Essential Oils and Main Components on Mono-and Polymicrobic Bacterial Cultures. Microorganisms 2019, 7, 345. [Google Scholar] [CrossRef]
- Kachkoul, R.; Benjelloun Touimi, G.; Bennani, B.; El Habbani, R.; El Mouhri, G.; Mohim, M.; Sqalli Houssaini, T.; Chebaibi, M.; Koulou, A.; Lahrichi, A. The Synergistic Effect of Three Essential Oils against Bacteria Responsible for the Development of Lithiasis Infection: An Optimization by the Mixture Design. Evid.-Based Complement. Altern. Med. 2021, 2021, 1305264. [Google Scholar] [CrossRef]
- Bekka-Hadji, F.; Bombarda, I.; Djoudi, F.; Bakour, S.; Touati, A. Chemical Composition and Synergistic Potential of Mentha pulegium L. and Artemisia herba alba Asso. Essential Oils and Antibiotic against Multi-Drug Resistant Bacteria. Molecules 2022, 27, 1095. [Google Scholar] [CrossRef]
- Ejim, L.; Farha, M.A.; Falconer, S.B.; Wildenhain, J.; Coombes, B.K.; Tyers, M.; Brown, E.D.; Wright, G.D. Combinations of Antibiotics and Non antibiotic Drugs Enhance Antimicrobial Efficacy. Nat. Chem. Biol. 2011, 7, 348–350. [Google Scholar] [CrossRef] [PubMed]
- Yap, P.S.X.; Yiap, B.C.; Ping, H.C.; Lim, S.H.E. Essential Oils, A New Horizon in Combating Bacterial Antibiotic Resistance. Open Microbiol. J. 2014, 8, 6–14. [Google Scholar] [CrossRef]
- Meddour, R.; Meddour, O.; Derridj, A. Medicinal Plants and Their Traditional Uses in Kabylia (Algeria): An EthnobotanicalSurvey. Planta Med. 2011, 77, PF29. [Google Scholar] [CrossRef]
- Zinno, P.; Guantario, B.; Lombardi, G.; Ranaldi, G.; Finamore, A.; Allegra, S.; Mammano, M.M.; Fascella, G.; Raffo, A.; Roselli, M. Chemical Composition and Biological Activities of Essential Oils from Origanum vulgare Genotypes Belonging to the Carvacrol and Thymol Chemotypes. Plants 2023, 12, 1344. [Google Scholar] [CrossRef]
- Amaral, S.C.; Pruski, B.B.; de Freitas, S.B.; Allend, S.O.; Ferreira, M.R.A.; Moreira, C.; Pereira, D.I.B.; Junior, A.S.V.; Hartwig, D.D. Origanum vulgare Essential Oil: Antibacterial Activities and Synergistic Effect with Polymyxin B against Multidrug-Resistant Acinetobacter baumannii. Mol. Biol. Rep. 2020, 47, 9615–9625. [Google Scholar] [CrossRef]
- Silva, S.L.; Araújo, F.S.M.; Silva, P.O.A.; Silva, E.V.A.; Bezerra, M.M.S.L.; Diniz, A.F.; Oliveira, D.M.; Jesus, H.O.; Nascimento-Junior, B.B.; Medeiros, L.A.D.M.; et al. Evaluation of the Antimicrobial Effect of the Origanum vulgare L. Essential Oil onStrains of Klebsiella Pneumoniae. Braz. J. Biol. 2023, 83, e269317. [Google Scholar] [CrossRef]
- Bariz, K.; Trabelsi, L.; Lahcene, S.; Salem-Bekhit, M.; Elossaily, G.M.; Alzahrani, H.A.; Alharbi, O.O.; Abbaci, M.; Abbaci, H.; Benguerba, Y.; et al. Evaluating the Synergistic Potency of Essential Oil sand Antibiotics Against Klebsiella Pneumoniae BLSEStrains. Cell. Mol. Biol. 2023, 69, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Sparkman, O.D. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy Robert P. Adams. J. Am. Soc. Mass Spectrom. 1997, 8, 671–672. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 31st ed.; M100. CLSI Suppl.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2021; Volume 41. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, Approved Standard, 9th ed.; CLSI: Wayne, PA, USA, 2012; Volume 32, ISBN 1562387839. [Google Scholar]
- Lahmar, A.; Bedoui, A.; Mokdad-Bzeouich, I.; Dhaouifi, Z.; Kalboussi, Z.; Cheraif, I.; Ghedira, K.; Chekir-Ghedira, L. Reversal of Resistance in Bacteria Underlies Synergistic Effect of Essential Oils with Conventional Antibiotics. Microb. Pathog. 2017, 106, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Timurkaynak, F.; Can, F.; Azap, Ö.K.; Demirbilek, M.; Arslan, H.; Karaman, S.Ö. In Vitro Activities ofNon-Traditional Antimicrobials Alone or in Combination against Multidrug-Resistant Strains of Pseudomonas aeruginosa and Acinetobacter baumannii Isolated from Intensive Care Units. Int. J. Antimicrob. Agents 2006, 27, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.D.; Shah, S.; Tammela, P. Defining Conditions for Biofilm Inhibition and Eradication Assays forGram-Positive Clinical Reference Strains. BMC Microbiol. 2018, 18, 173. [Google Scholar] [CrossRef] [PubMed]
- Jardak, M.; Mnif, S.; BenAyed, R.; Rezgui, F.; Aifa, S. Chemical Composition, Antibiofilm Activities of Tunisian Spices Essential Oils and Combinatorial Effect against Staphylococcus Epidermidis Biofilm. LWT 2021, 140, 110691. [Google Scholar] [CrossRef]
- Stepanovic, S.; Vukovic, D.; Hola, V.; Bonaventura, G.D.; Djukic, S.; Ruzicka, F.; Bonaventura, D.G. The Authors Printed inDenmark. All Rights Reserv. J. Compil. C 2007, 115, 891–900. [Google Scholar]
- Lagha, R.; Abdallah, F.B.; AL-Sarhan, B.O.; Al-Sodany, Y. Antibacterial and Biofilm Inhibitory Activity of Medicinal Plant Essential Oils Against Escherichia Coli Isolated from UTI Patients. Molecules 2019, 24, 1161. [Google Scholar] [CrossRef] [PubMed]
- Kavanaugh, N.L.; Ribbeck, K. Selected Antimicrobial Essential Oils Eradicate Pseudomonas Spp. And Staphylococcus Aureus Biofilms. Appl. Environ. Microbiol. 2012, 78, 4057–4061. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef]
- Amrouni, S.; Touati, M.; Hadef, Y.; Djahoudi, A. Effect of Essential Oil of Origanum vulgare and Thymus Ciliatus against Pseudomonas aeruginosa Producing VIM-2 Carbapenemase. Phytotherapie 2014, 12, 309–313. [Google Scholar] [CrossRef]
- Giamperi, L.; Fraternale, D.; Ricci, D. The in Vitro Action of Essential Oils on Different Organisms. J. Essent. Oil Res. 2002, 14, 312–318. [Google Scholar] [CrossRef]
- Ebani, V.V.; Nardoni, S.; Bertelloni, F.; Pistelli, L.; Mancianti, F. Antimicrobial Activity of Five Essential Oils against Bacteria and Fungi Responsible for Urinary Tract Infections. Molecules 2018, 23, 1668. [Google Scholar] [CrossRef] [PubMed]
- Moisa, C.; Copolovici, L.; Pop, G.; Lupitu, A.; Ciutina, V.; Copolovici, D. Essential Oil Composition, Total Phenolic Content, and Antioxidant Activity—Determined from Leaves, Flowers and Stems of Origanum vulgare L. Var. Aureum. Agric. Life Life Agric. Conf. Proc. 2018, 1, 555–561. [Google Scholar] [CrossRef]
- Goyal, S.; Tewari, G.; Pandey, H.K.; Kumari, A. Exploration of Productivity, Chemical Composition, and Antioxidant Potentialof Origanum vulgare L. Grown at Different Geographical Locations of Western Himalaya, India. J. Chem. 2021, 2021, 10–12. [Google Scholar] [CrossRef]
- Öner, E.K.; Yeşil, M. Effects of Altitudes on Secondary Metabolite Contents of Origanum majorana L. Sci. Rep. 2023, 13, 10765. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Khan, S.T.; Khan, N.A.; Mahmood, A.; Al-Kedhairy, A.A.; Alkhathlan, H.Z. The Composition of the Essential Oil and Aqueous Distillate of Origanum vulgare L. Growing in Saudi Arabia and Evaluation of Their Antibacterial Activity. Arab. J. Chem. 2018, 11, 1189–1200. [Google Scholar] [CrossRef]
- Magiorakos, A.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F. Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Microbiology 2011, 18, 268–281. [Google Scholar]
- Ait-Mimoune, N.; Hassaine, H.; Boulanoir, M. Bacteriological Profile of Urinary Tract Infections and Antibiotic Susceptibility of Escherichia Coli in Algeria. Iran. J. Microbiol. 2022, 14, 156–160. [Google Scholar] [CrossRef]
- Yahiaoui, M.; Robin, F.; Bakour, R.; Hamidi, M.; Bonnet, R.; Messai, Y. Antibiotic Resistance, Virulence, and Genetic Background of Community-Acquired Uropathogenic Escherichia Colifrom Algeria. Microb. Drug Resist. 2015, 21, 516–526. [Google Scholar] [CrossRef]
- Sabharwal, E.R.; Sharma, R. Fosfomycin: An Alternative Therapy for the Treatment of UTI a midst Escalating Antimicrobial Resistance. J. Clin. Diagnostic Res. 2015, 9, DC06–DC09. [Google Scholar] [CrossRef]
- Khorsi, K.; Messai, Y.; Hamidi, M.; Ammari, H.; Bakour, R. High Prevalence of Multidrug-Resistance in Acinetobacter baumannii and Dissemination of Carbapenemase-Encoding Genes Bla OXA-23-like, Bla OXA-24-like and Bla NDM-1in Algiers Hospitals. Asian Pac. J. Trop. Med. 2015, 8, 438–446. [Google Scholar] [CrossRef]
- Martins, H.S.I.; Bomfim, M.R.Q.; França, R.O.; Farias, L.M.; Carvalho, M.A.R.; Serufo, J.C.; Santos, S.G. Resistance Markers and Genetic Diversity in Acinetobacter baumannii Strains Recovered from Nosocomial Blood stream Infections. Int. J. Environ. Res. Public Health 2014, 11, 1465. [Google Scholar] [CrossRef] [PubMed]
- Nabti, L.Z.; Sahli, F.; Laouar, H.; Olowo-okere, A. Chemical Composition and Antibacterial Activity of Essential Oils from the Algerian Endemic Origanum. Antibiotics 2020, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, L.; Jaber, H.; Kamel, W.M. Antibacterial Activity of the Essential Oil Isolated from Origanum vulgare L. (Lamiaceae) Against Multi-Drug Resistant Bacteria. Int. J. Drug Deliv. Technol. 2022, 12, 81–84. [Google Scholar] [CrossRef]
- Ghazal, T.S.A.; Schelz, Z.; Vidács, L.; Szemerédi, N.; Veres, K.; Spengler, G.; Hohmann, J. Antimicrobial, Multidrug Resistance Reversal and Biofilm Formation Inhibitory Effect of Origanum majorana Extracts, Essential Oil and Monoterpenes. Plants 2022, 11, 1432. [Google Scholar] [CrossRef] [PubMed]
- Zgurskaya, H.I.; Rybenkov, V.V. Permeability Barriers of Gram-Negative Pathogens. Ann. N. Y. Acad. Sci. 2020, 1459, 5–18. [Google Scholar] [CrossRef]
- Papadopoulos, C.J.; Carson, C.F.; Chang, B.J.; Riley, T.V. Role of the Mex AB-OprM Efflux Pump of Pseudomonas aeruginosa in Tolerance to Tea Tree (Melaleuca alternifolia) Oil and Its Monoterpene Components Terpinen-4-Ol,1,8-Cineole and α-Terpineol ∇. Appl. Environ. Microbiol. 2008, 74, 1932–1935. [Google Scholar] [CrossRef] [PubMed]
- Marotta, S.M.; Giarratana, F.; Parco, A.; Neri, D.; Ziino, G.; Giuffrida, A.; Panebianco, A. Evaluation of the Antibacterial Activity of Bergamot Essential Oils on Different Listeria Monocytogenes Strains. Ital. J. Food Saf. 2016, 5, 6176. [Google Scholar] [CrossRef]
- Sales, T.A.; Cardoso, M.D.G.; Guimarães, L.G.D.L.; Camargo, K.C.; Rezende, D.A.C.; Brandão, R.M.; Souza, R.V.; Ferreira, V.R.F.; Marques, A.E.; Magalhães, M.L.; et al. Essential Oils from the Leaves and Flowers of Callistemon viminalis ChemicalCharacterization and Evaluation of the Insecticide and Antifungal Activities. Am. J. Plant Sci. 2017, 8, 2516–2529. [Google Scholar] [CrossRef]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Rodriguez, M.R.; Cantu-Soto, E.U.; Vazquez-Armenta, F.J.; Bernal-Mercado, A.T.; Ayala-Zavala, J.F. Inhibition of Acinetobacter baumannii Biofilm Formation by Terpenes from Oregano (Lippia graveolens) Essential Oil. Antibiotics 2023, 12, 1539. [Google Scholar] [CrossRef] [PubMed]
- Ultee, A.; Bennik, M.H.J.; Moezelaar, R. The Phenolic Hydroxyl Group of Carvacrol Is Essential for Action against theFood-Borne Pathogen Bacillus Cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Iseppi, R.; Mariani, M.; Benvenuti, S.; Truzzi, E. Effects of Melaleuca alternifolia Chell (Tea Tree) and Eucalyptus globulus Labill. Essential Oils on Antibiotic-Resistant Bacterial Biofilms. Molecules 2023, 28, 1671. [Google Scholar] [CrossRef]
- Abdelatti, M.A.I.; AbdEl-Aziz, N.K.; El-Naenaeey, E.; Sayed, Y.M.; Ammar, A.M.; Alharbi, N.K.; Alharthi, A.; Zakai, S.A.; Abdelkhalek, A. Antibacterial and Anti-Efflux Activities of Cinnamon Essential Oil against Pan and Extensive Drug-Resistant Pseudomonas aeruginosa Isolated from Human and Animal Sources. Antibiotics 2023, 12, 1514. [Google Scholar] [CrossRef] [PubMed]
- Aelenei, P.; Miron, A.; Trifan, A.; Bujor, A.; Gille, E.; Aprotosoaie, A. Essential Oils and Their Components as Modulators of Antibiotic Activity against Gram-Negative Bacteria. Medicines 2016, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Akhmouch, A.A.; Hriouech, S.; Mzabi, A.; Tanghort, M.; Chefchaou, H.; Remmal, A.; Chami, N. Synergistic Action of AMX Associated with 1,8-Cineole and Its Effect on the ESBL Enzymatic Resistance Mechanism. Antibiotics 2022, 11, 1002. [Google Scholar] [CrossRef]
- Yang, S.K.; Yusoff, K.; Mai, C.W.; Lim, W.M.; Yap, W.S.; Lim, S.H.E.; Lai, K.S. Additivityvs. Synergism: Investigation of the Additive Interaction of Cinnamon Bark Oil and Meropenem in Combinatory Therapy. Molecules 2017, 22, 1733. [Google Scholar] [CrossRef]
- Gallucci, N.; Casero, C.; Oliva, M.; Zygadlo, J.; Demo, M. Interaction between Terpenes and Penicillin on Bacterial Strains Resistant to Beta-Lactam Antibiotics. J. Appl. Microbiol. 2006, 10, 30–32. [Google Scholar]
- Alotaibi, G.F. Factors Influencing Bacterial Biofilm Formation and Development. Am. J. Biomed. Sci. Res. 2021, 12, 617–626. [Google Scholar] [CrossRef]
- Di Domenico, E.G.; Farulla, I.; Prignano, G.; Gallo, M.T.; Vespaziani, M.; Cavallo, I.; Sperduti, I.; Pontone, M.; Bordignon, V.; Cilli, L.; et al. Biofilm Is a Major Virulence Determinant in Bacterial Colonization of Chronic Skin Ulcers Independently from the Multidrug Resistant Phenotype. Int. J. Mol. Sci. 2017, 18, 1077. [Google Scholar] [CrossRef] [PubMed]
- Flannery, A.; LeBerre, M.; Pier, G.B.; O’gara, J.P.; Kilcoyne, M. Glycomics Microarrays Reveal Differential in Situ Presentation of the Biofilm Polysaccharide Poly-n-Acetylglucosamine on Acinetobacter baumannii and Staphylococcus Aureus Cell Surfaces. Int. J. Mol. Sci. 2020, 21, 2465. [Google Scholar] [CrossRef] [PubMed]
- Cepas, V.; López, Y.; Muñoz, E.; Rolo, D.; Ardanuy, C.; Martí, S.; Xercavins, M.; Horcajada, J.P.; Bosch, J.; Soto, S.M. Relationship between Biofilm Formation and Antimicrobial Resistance in Gram-Negative Bacteria. Microb. Drug Resist. 2019, 25, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Syaiful, I.; Widodo, A.D.W.; Endraswari, P.D.; Alimsardjono, L.; Utomo, B.; Arfijanto, M.V. The Association between BiofilmFormation Ability and Antibiotic Resistance Phenotype in Clinical Isolates of Gram-Negative Bacteria: A Cross-Sectional Study. Bali Med. J. 2023, 12, 1014–1020. [Google Scholar] [CrossRef]
- Dos Santos Rodrigues, J.B.; de Carvalho, R.J.; de Souza, N.T.; de Sousa Oliveira, K.; Franco, O.L.; Schaffner, D.; de Souza, E.L.; Magnani, M. Effects of Oregano Essential Oil and Carvacrol on Biofilms of Staphylococcus Aureus from Food-Contact Surfaces. Food Control 2017, 73, 1237–1246. [Google Scholar] [CrossRef]
- Merghni, A.; Haddaji, N.; Bouali, N.; Alabbosh, K.F.; Adnan, M.; Snoussi, M.; Noumi, E. Comparative Study of Antibacterial, Antibiofilm, Antiswarming and Antiquorum Sensing Activities of Origanum vulgare Essential Oil and Terpinene-4-Ol against Pathogenic Bacteria. Life 2022, 12, 1616. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Yuk, H.G. Effects of Sublethal Thymol, Carvacrol, and Trans-Cinnamaldehyde Adaptation on Virulence Properties of Escherichia coli O157:H7. Appl. Environ. Microbiol. 2019, 85, e00271-19. [Google Scholar] [CrossRef]
- Gutierrez-Pacheco, M.M.; Gonzalez-Aguilar, G.A.; Martinez-Tellez, M.A.; Lizardi-Mendoza, J.; Madera-Santana, T.J.; Bernal-Mercado, A.T.; Vazquez-Armenta, F.J.; Ayala-Zavala, J.F. Carvacrol Inhibits Biofilm Formation and Production of Extracellular Polymeric Substances of Pectobacterium carotovorum Subsp. Carotovorum. Food Control 2018, 89, 210–218. [Google Scholar] [CrossRef]
- Ben Abdallah, F.; Lagha, R.; Gaber, A. Biofilm Inhibition and Eradication Properties of Medicinal Plant Essential Oils against Methicillin-Resistant Staphylococcus Aureus Clinical Isolates. Pharmaceuticals 2020, 13, 369. [Google Scholar] [CrossRef]
- Ouhayoun, J.P. Penetrating the Plaque Biofilm: Impact of Essential Oil Mouthwash. J. Clin. Periodontol. 2003, 30, 10–12. [Google Scholar] [CrossRef] [PubMed]

| No. | RI | RT | Compounds | Area (%) |
|---|---|---|---|---|
| 1 | 844 | 5.231 | Hexenal | 0.04 |
| 2 | 923 | 8.758 | Thujene | 1.64 |
| 3 | 928 | 9.055 | α-Pinene | 0.75 |
| 4 | 940 | 9.895 | Camphène | 0.10 |
| 5 | 969 | 11.770 | L-β-Pinene | 0.16 |
| 6 | 983 | 12.713 | Octen-3-ol | 0.26 |
| 7 | 988 | 13.039 | 3-Octanone | 0.09 |
| 8 | 992 | 13.330 | β-Pinene | 1.68 |
| 9 | 1000 | 13.879 | α-Phellandrène | 0.30 |
| 10 | 1005 | 14.245 | (R)-α-Pinene | 0.12 |
| 11 | 1013 | 14.845 | 4-Carene | 2.43 |
| 12 | 1024 | 15.594 | β-Cymene | 8.56 |
| 13 | 1049 | 17.503 | β-Ocymene | 0.11 |
| 14 | 1060 | 18.314 | γ-terpinene | 13.95 |
| 15 | 1066 | 18.703 | Sabinene hydrate | 0.36 |
| 16 | 1084 | 20.057 | Terpinolene | 0.17 |
| 17 | 1103 | 21.446 | Linalol | 1.08 |
| 18 | 1136 | 23.812 | o-Xylene | 0.04 |
| 19 | 1145 | 24.481 | Ethanone, 1-(1,4-dimethyl-3-cyclohexen-1-yl) | 0.07 |
| 20 | 1160 | 25.596 | Borneol | 0.15 |
| 21 | 1172 | 26.476 | 4-Terpineol | 0.52 |
| 22 | 1187 | 27.556 | Terpineol | 0.07 |
| 23 | 1207 | 28.951 | p-menth-1-en-8-ol | 0.35 |
| 24 | 1233 | 30.780 | Thymol methyl | 0.12 |
| 25 | 1242 | 31.397 | Isothymolmethylether | 1.27 |
| 26 | 1274 | 33.546 | Carvone | 0.10 |
| 27 | 1294 | 34.963 | Ethanone | 0.08 |
| 28 | 1326 | 37.038 | Carvacrol | 61.51 |
| 29 | 1360 | 39.210 | Eugenol | 0.84 |
| 30 | 1375 | 40.147 | Cuminol | 0.09 |
| 31 | 1409 | 42.308 | β-Caryophyllène | 1.66 |
| 32 | 1504 | 48.075 | β-Bisabolene | 0.21 |
| 33 | 1518 | 48.892 | β-Sesquiphellandrene | 0.13 |
| 34 | 1528 | 49.435 | Eugenolacetate | 0.14 |
| 35 | 1539 | 50.081 | Humulene | 0.07 |
| 36 | 1570 | 51.847 | Caryophyllene oxide | 0.33 |
| Monoterpenes hydrocarbons (MH) | 29.85 | |||
| Oxygenated monoterpenes (OM) | 65.43 | |||
| Sesquiterpenes hydrocarbons (SH) | 2.07 | |||
| Oxygenated sesquiterpenes (OS) | 0.33 | |||
| Others | 1.49 | |||
| Total | 99.17 |
| Antibiotic | E. coli 45 | K. pneumoniae 5096 | A. baumannii 14889 | P. aeruginosa 150 |
|---|---|---|---|---|
| AMX | R | R | N | N |
| AMC | R | I | N | N |
| TIC | N | N | R | R |
| TCC | N | N | R | R |
| CZ | R | R | N | N |
| FOX | S | S | N | N |
| CTX | R | R | R | N |
| CTR | R | R | R | R |
| CAZ | R | R | R | S |
| IMP | S | S | R | R |
| GEN | R | S | R | R |
| AK | S | S | R | R |
| CIP | R | R | R | R |
| OFX | R | N | R | R |
| TET | S | R | R | N |
| SXT | R | R | R | R |
| CHL | S | S | N | N |
| FOS | S | S | N | R |
| Bacterial Strains | IZD (mm) | MIC (mg mL−1) | MBC (mg mL−1) |
|---|---|---|---|
| K. pneumoniae ATCC 700603 | 16 ± 1.0 | 2.35 ± 1.0 | 4.6 ± 2.0 |
| P. aeruginosa ATCC 27853 | 12,3 ± 1.1 | 14.0 ± 0.0 | 56.2 ± 0.0 |
| E. coli 45 | 26.6 ± 1.1 | 1.76 ± 0.0 | 2.9 ± 1.0 |
| K. pneumoniae 5096 | 17.6 ± 0.5 | 1.2 ± 0.5 | 4.6 ± 2.0 |
| A. baumannii 14889 | 32.3 ± 1.5 | 0.88 ± 0.0 | >3.52 |
| P. aeruginosa 150 | 7.6 ± 0.5 | 7.03 ± 0.0 | >28.1 |
| Strains | Combination | Individual MIC | Combined MIC | Individual FIC | FICI | Effect | MIC Reduction (%) |
|---|---|---|---|---|---|---|---|
| K. pneumoniae ATCC 700603 | CZ/OEO CTX/OEO GEN/OEO | 128/2.4 8/2.4 8/2.4 | 128/1.2 8/1.2 8/1.2 | 1/0.5 | 1.5 | I | 0/50 0/50 0/50 |
| P. aeroginosa ATCC 27853 | CTX/OEO | 16/14.06 | 0.25/3.52 | 0.016/0.25 | 0.27 | S | 98.44/93.75 |
| E. coli 45 | CZ/OEO CTX/OEO GEN/OEO CIP/OEO | 2048/1.76 2048/1.76 32/1.76 64/1.76 | 1024/0.88 1024/0.88 4/0.44 64/0.88 | 0.5/0.5 0.5/0.5 0.125/0.25 1/0.5 | 1.00 1.00 0.38 1.5 | A A S I | 50/50 50/50 87.5/75 0/50 |
| K. pneumoniae 5096 | CZ/OEO CTX/OEO GEN/OEO CIP/OEO | 2048/1.2 128/1.2 32/1.2 16/1.2 | 1024/0.6 64/0.6 1/0.6 16/1.2 | 0.5/0.5 0.5/0.5 0.031/0.5 1/1 | 1.00 1.00 0.53 2.00 | A A PS I | 50/50 50/50 96.88/50 0/0 |
| A. baumannii 14889 | CTX/OEO GEN/OEO CIP/OEO | 1024/0.88 4096/0.88 256/0.88 | 32/0.22 4096/0.22 16/0.055 | 0.031/0.25 1/0.25 0.062/0.062 | 0.28 1.25 0.12 | S I S | 96.88/75 0/75 93.75/93.75 |
| P. aeruginosa 150 | GEN/OEO CIP/OEO | 128/7.03 32/7.03 | 64/1.76 16/3.52 | 0.5/0.25 0.5/0.5 | 0.75 1.00 | PS A | 50/75 50/75 |
| Strains | OD630 ± SD | Biofilm Formation |
|---|---|---|
| K. pneumoniae ATCC 700603 | 0.944 ± 0.125 | Moderate biofilm producer |
| P. aeruginosa ATCC 27853 | 1.062 ± 0.054 | Moderate biofilm producer |
| E. coli 45 | 0.240 ± 0.011 | Non-biofilm producer |
| K. pneumoniae 5096 | 0.372 ± 0.101 | Weak biofilm producer |
| A. baumannii 14889 | 1.385 ± 0.162 | Strong biofilm producer |
| P. aeruginosa 150 | 1.080 ± 0.106 | Moderate biofilm producer |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saoudi, B.; Bariz, K.; Saci, S.; Belounis, Y.; Ait Issad, H.; Abbaci, M.; Mustapha, M.A.; Nabti, E.-H.; Alenazy, R.; Alhussaini, M.S.; et al. Enhancing Antibiotic Efficacy and Combating Biofilm Formation: Evaluating the Synergistic Potential of Origanum vulgare Essential Oil against Multidrug-Resistant Gram-Negative Bacteria. Microorganisms 2024, 12, 1651. https://doi.org/10.3390/microorganisms12081651
Saoudi B, Bariz K, Saci S, Belounis Y, Ait Issad H, Abbaci M, Mustapha MA, Nabti E-H, Alenazy R, Alhussaini MS, et al. Enhancing Antibiotic Efficacy and Combating Biofilm Formation: Evaluating the Synergistic Potential of Origanum vulgare Essential Oil against Multidrug-Resistant Gram-Negative Bacteria. Microorganisms. 2024; 12(8):1651. https://doi.org/10.3390/microorganisms12081651
Chicago/Turabian StyleSaoudi, Bilal, Karim Bariz, Sarah Saci, Yousra Belounis, Hakima Ait Issad, Mohamed Abbaci, Mohamed Abou Mustapha, El-Hafid Nabti, Rawaf Alenazy, Mohammed Sanad Alhussaini, and et al. 2024. "Enhancing Antibiotic Efficacy and Combating Biofilm Formation: Evaluating the Synergistic Potential of Origanum vulgare Essential Oil against Multidrug-Resistant Gram-Negative Bacteria" Microorganisms 12, no. 8: 1651. https://doi.org/10.3390/microorganisms12081651
APA StyleSaoudi, B., Bariz, K., Saci, S., Belounis, Y., Ait Issad, H., Abbaci, M., Mustapha, M. A., Nabti, E.-H., Alenazy, R., Alhussaini, M. S., Alyahya, A. A. I., Alqasmi, M., Alhumaidi, M. S., Almufarriji, F. M., & Houali, K. (2024). Enhancing Antibiotic Efficacy and Combating Biofilm Formation: Evaluating the Synergistic Potential of Origanum vulgare Essential Oil against Multidrug-Resistant Gram-Negative Bacteria. Microorganisms, 12(8), 1651. https://doi.org/10.3390/microorganisms12081651






