Polysaccharides from Trametes versicolor as a Potential Prebiotic to Improve the Gut Microbiota in High-Fat Diet Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Polysaccharides
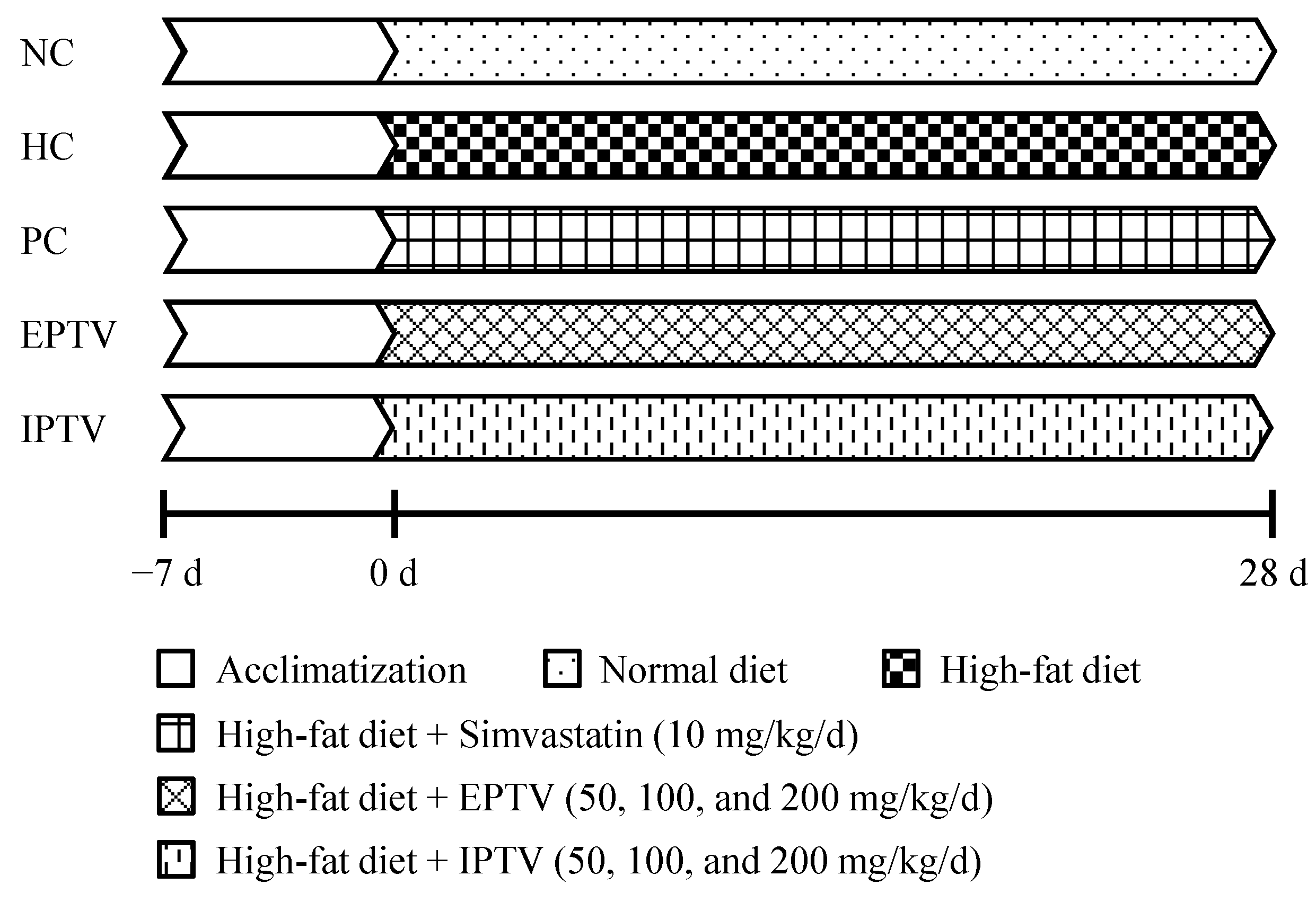
2.3. Animal Experiment
2.4. Biochemical Analyses
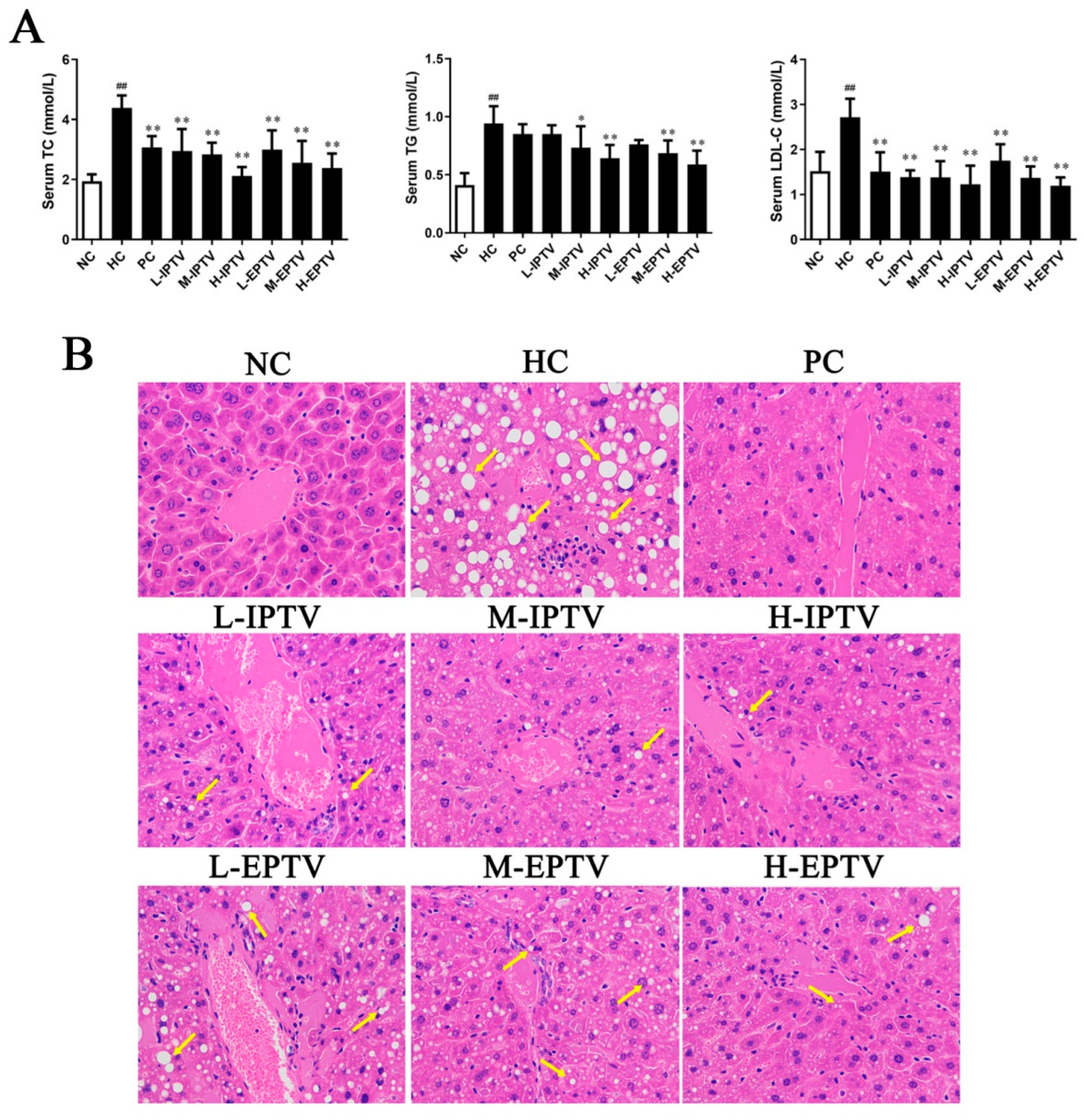
2.5. Histological Evaluation
2.6. Stool Sampling, DNA Extraction and Sequencing
2.7. Bioinformatics Analysis
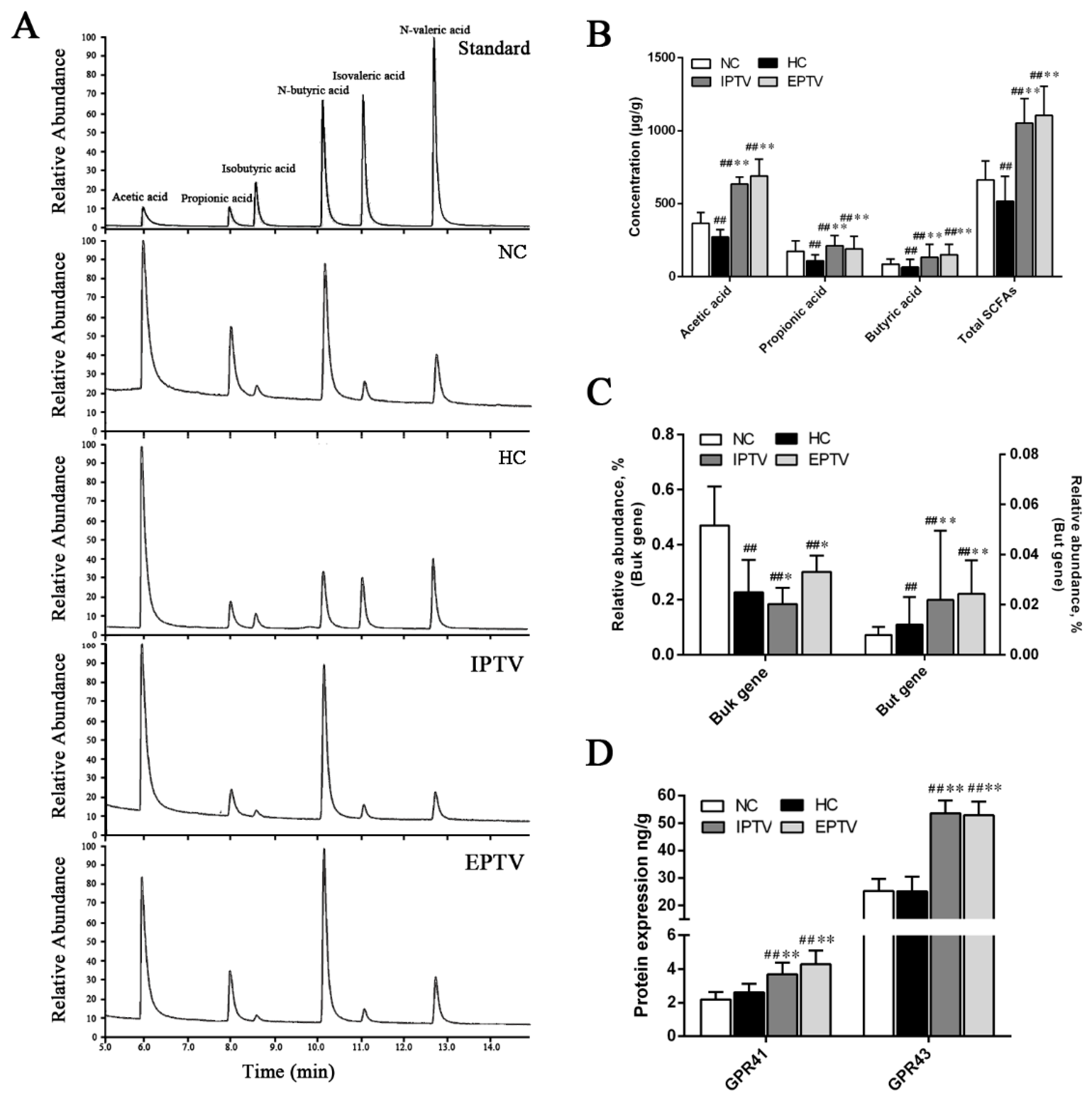
2.8. Short-Chain Fatty Acid (SCFA) Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. PTV Administration Normalized Body Weight Gain and Organ Indexes in HFD-Fed Mice
3.2. PTVs Alleviated Dyslipidemia in HFD-Fed Mice
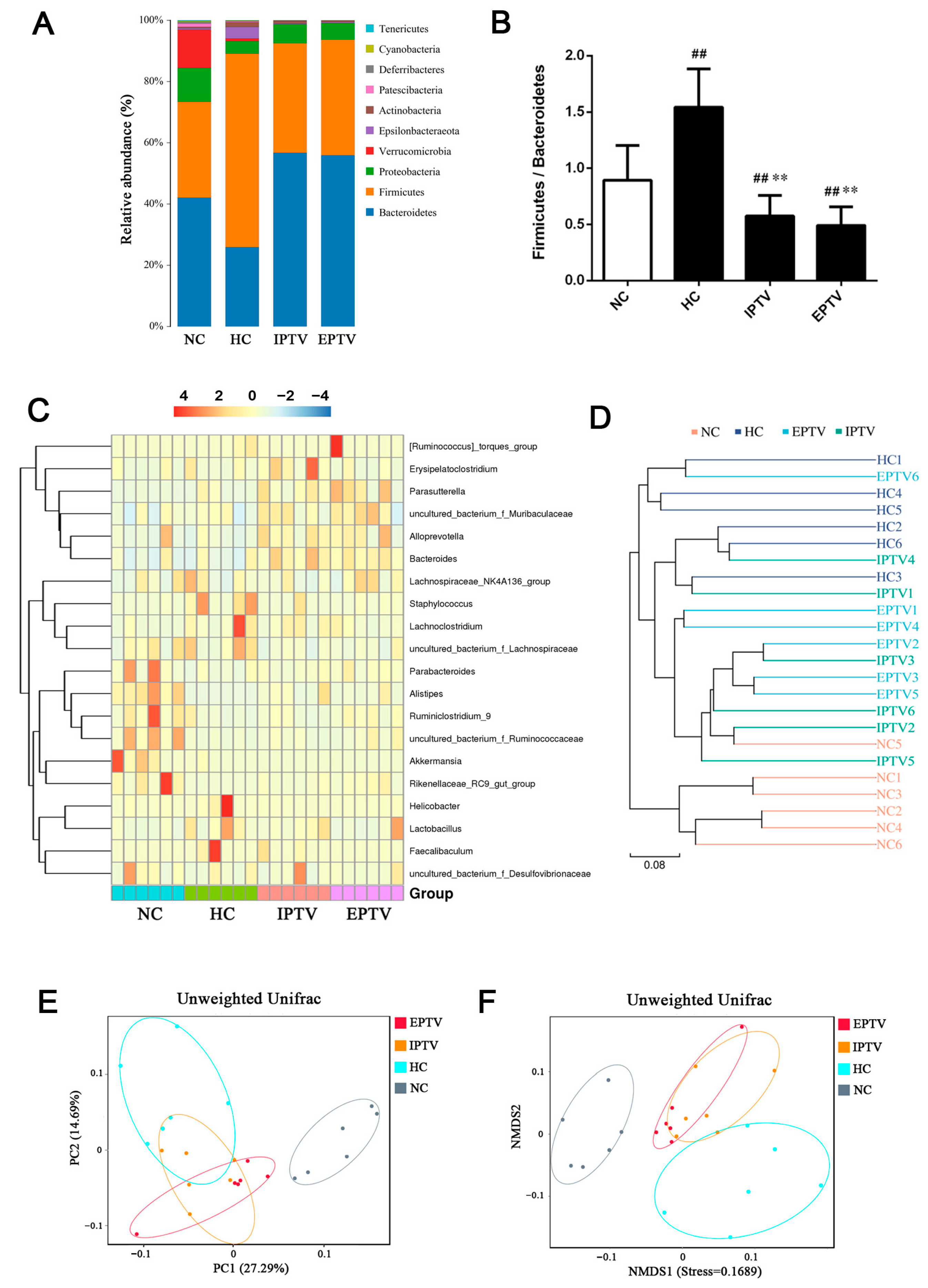
3.3. PTVs Restore the High-Fat-Diet-Induced Gut Bacterial Dysbiosis at Different Taxonomic Levels in HFD-Fed Mice
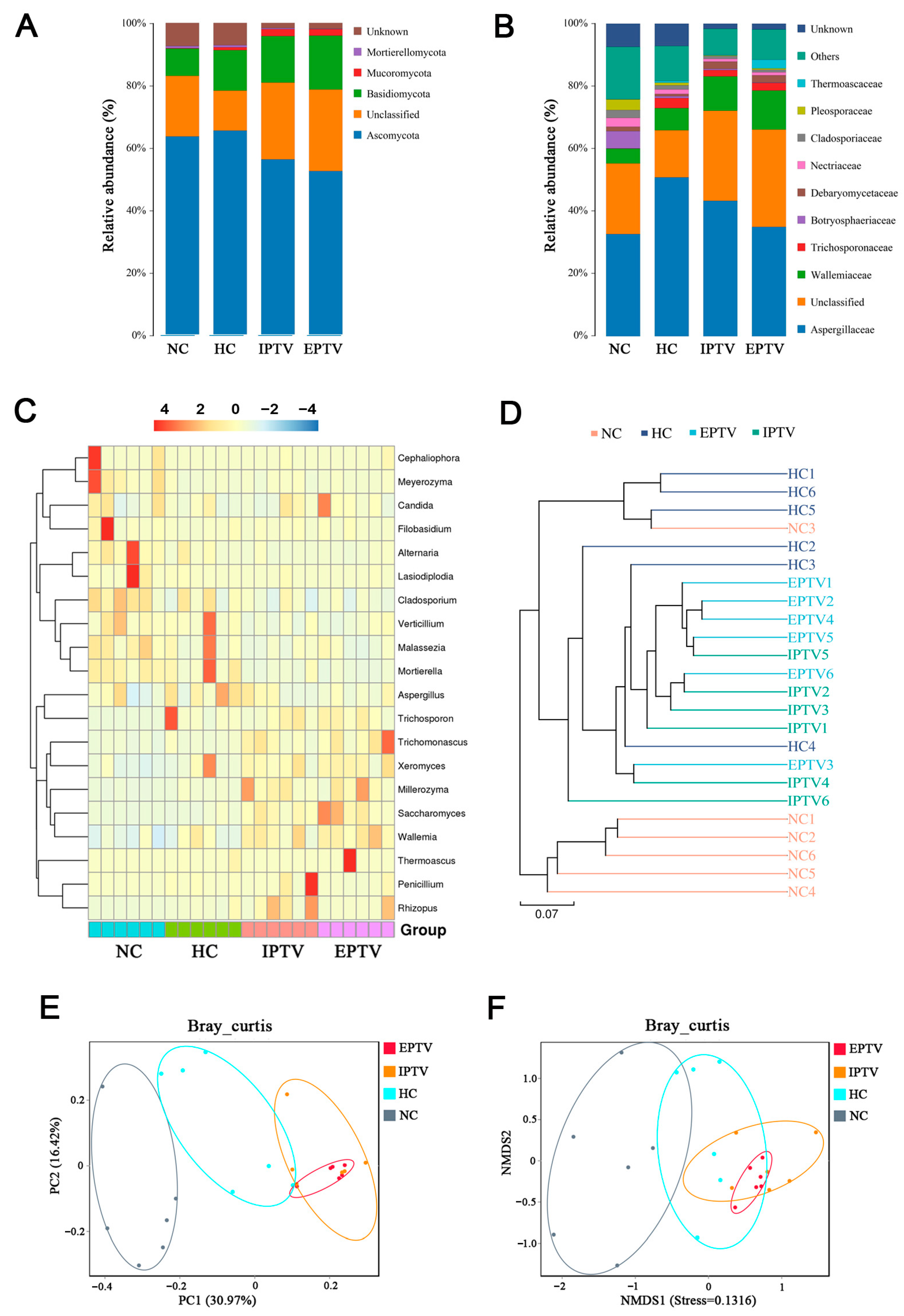
3.4. PTVs Regulated the Gut Fungal Microbes in HFD-Fed Mice
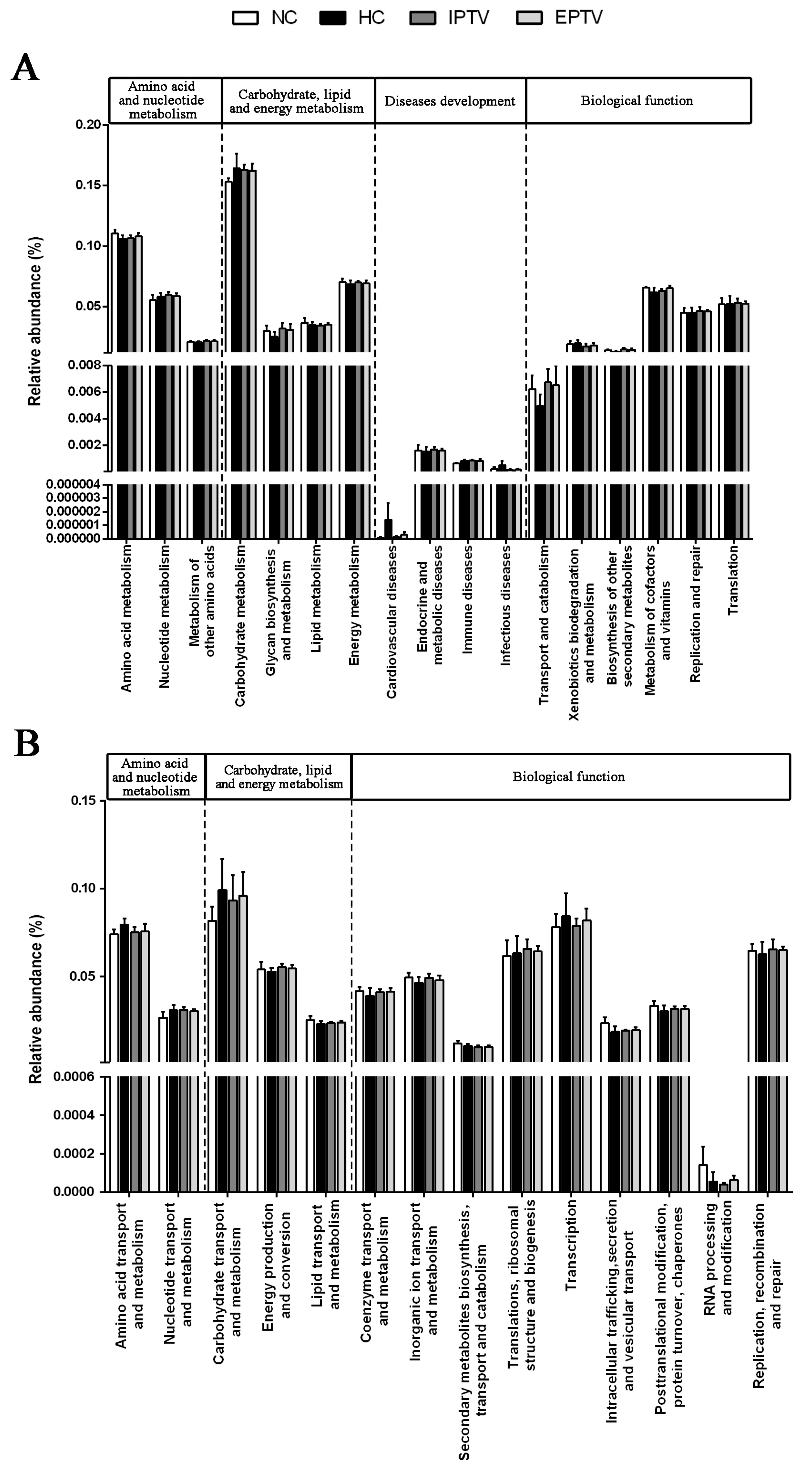
3.5. Effect of PTVs on Functional Genes in HFD-Fed Mice
3.6. PTVs Upregulate Butyrate Production by Buk and Butyryl-CoA Enzymes Accompanied by an Increase in SCFA Receptors
3.7. Intestinal Flora Correlate with Metabolic Parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mainieri, F.; La Bella, S.; Chiarelli, F. Hyperlipidemia and cardiovascular risk in children and adolescents. Biomedicines 2023, 11, 809. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lei, J.; Wang, R.; Li, K. Non-traditional risk factors as contributors to cardiovascular disease. Rev. Cardiovasc. Med. 2023, 24, 134. [Google Scholar] [CrossRef] [PubMed]
- Salazar, I.M.C.; Tibuakuu, M.; Blumenthal, R.S.; Sarkar, S. Cardiovascular disease in patients with diabetes: A comparison of professional society guidelines. Curr. Diabetes Rev. 2022, 18, e200821195733. [Google Scholar] [PubMed]
- Li, M.; Wang, A.; Ren, S.H.; Wang, Z.Y.; Wang, Q.; Gou, C.Y.; Zhao, W.C.; Zhang, L.; Li, N. Factors associated with acute pancreatitis in patients with impacted duodenal papillary stones: A retrospective cohort study. Scand. J. Gastroenterol. 2022, 57, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Ezkurdia, A.; Ramírez, M.J.; Solas, M. Metabolic syndrome as a risk factor for Alzheimer’s disease: A focus on insulin resistance. Int. J. Mol. Sci. 2023, 24, 4354. [Google Scholar] [CrossRef] [PubMed]
- Cheeley, M.K.; Clegg, K.; Lockridge, C.; Schubert, T.J.; Jones, L.K. Statin intolerance: An overview of US and international guidance. Curr. Atheroscleros. Rep. 2023, 25, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Newman, C.B.; Preiss, D.; Tobert, J.A.; Jacobson, T.A.; Page, R.L.; Goldstein, L.B.; Chin, C.; Tannock, L.R.; Miller, M.; Raghuveer, G.; et al. Statin safety and associated adverse events: A scientific statement from the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 38–81. [Google Scholar] [CrossRef]
- Habtemariam, S. Trametes versicolor (Synn. Coriolus versicolor) polysaccharides in cancer therapy: Targets and efficacy. Biomedicines 2020, 8, 135. [Google Scholar]
- Lysakowska, P.; Sobota, A.; Wirkijowska, A. Medicinal mushrooms: Their bioactive components, nutritional value and application in functional food production—A review. Molecules 2023, 28, 5393. [Google Scholar] [CrossRef]
- Bains, A.; Chawla, P.; Kaur, S.; Najda, A.; Fogarasi, M.; Fogarasi, S. Bioactives from mushroom: Health attributes and food industry applications. Materials 2021, 14, 7640. [Google Scholar] [CrossRef]
- Nikolic, M.; Lazarevic, N.; Novakovic, J.; Jeremic, N.; Jakovljevic, V.; Zivkovic, V.; Bradic, J.; Pecarski, D.; Tel-Çayan, G.; Glamocija, J.; et al. Characterization, in vitro biological activity and in vivo cardioprotective properties of Trametes versicolor (L.: Fr.) Quél. heteropolysaccharides in a rat model of metabolic syndrome. Pharmaceuticals 2023, 16, 787. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Li, H.; Li, Y.; Zhao, Y.H.; Xiong, F.F.; Liu, Y.N.; Xue, H.R.; Yang, Z.Y.; Ni, S.; Sahil, A.; et al. Coriolus versicolor alleviates diabetic cardiomyopathy by inhibiting cardiac fibrosis and NLRP3 inflammasome activation. Phytother. Res. 2019, 33, 2737–2748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Aschenbrenner, D.; Yoo, J.Y.; Zuo, T. The gut mycobiome in health, disease, and clinical applications in association with the gut bacterial microbiome assembly. Lancet Microbe 2022, 3, e969–e983. [Google Scholar] [CrossRef] [PubMed]
- Perler, B.K.; Friedman, E.S.; Wu, G.D. The role of the gut microbiota in the relationship between diet and human health. Annu. Rev. Physiol. 2023, 85, 449–468. [Google Scholar] [CrossRef] [PubMed]
- Gowen, R.; Gamal, A.; Di Martino, L.; McCormick, T.S.; Ghannoum, M.A. Modulating the microbiome for Crohn’s disease treatment. Gastroenterology 2023, 164, 828–840. [Google Scholar] [CrossRef]
- Zhu, G.S.; Zhao, J.X.; Zhang, H.; Wang, G.; Chen, W. Gut microbiota and its metabolites: Bridge of dietary nutrients and Alzheimer’s disease. Adv. Nutr. 2023, 14, 819–839. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.W.; Arrieta, M.C. The intestinal mycobiome as a determinant of host immune and metabolic health. Curr. Opin. Microbiol. 2021, 62, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Shen, X.; Shi, X.; Sakandar, H.A.; Quan, K.Y.; Li, Y.L.; Jin, H.; Kwok, L.Y.; Zhang, H.P.; Sun, Z.H. Targeting gut microbiota and metabolism as the major probiotic mechanism-An evidence-based review. Trends Food Sci. Technol. 2023, 138, 178–198. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, M.; Wang, Y.; Zhang, S.; Jiang, X. Extracellular and intracellular polysaccharide extracts of Trametes versicolor improve lipid profiles via serum regulation of lipid-regulating enzymes in hyperlipidemic mice. Curr. Microbiol. 2020, 77, 3526–3537. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, X.; Jiang, W.; Tan, J.; Chen, G. Preparation, structural characterization and in vitro activity of ginger polysaccharide. Chem. Biol. Technol. Agric. 2023, 10, 126. [Google Scholar] [CrossRef]
- Zhao, B.; Cui, Y.; Fan, X.; Qi, P.; Liu, C.; Zhou, X.; Zhang, X. Anti-obesity effects of Spirulina platensis protein hydrolysate by modulating brain-liver axis in high-fat diet fed mice. PLoS ONE 2019, 14, e0218543. [Google Scholar] [CrossRef]
- Sheng, Y.; Zhao, C.; Zheng, S.; Mei, X.; Huang, K.; Wang, G.; He, X. Anti-obesity and hypolipidemic effect of water extract from Pleurotus citrinopileatus in C57BL/6J mice. Food Sci. Nutr. 2019, 7, 1295–1301. [Google Scholar] [CrossRef]
- Vetter, J. The mushroom glucans: Molecules of high biological and medicinal importance. Foods 2023, 12, 1009. [Google Scholar] [CrossRef]
- Michels, N.; Zouiouich, S.; Vanderbauwhede, B.; Vanacker, J.; Indave Ruiz, B.I.; Huybrechts, I. Human microbiome and metabolic health: An overview of systematic reviews. Obes. Rev. 2022, 23, e13409. [Google Scholar] [CrossRef]
- Wang, L.J.; Gou, X.L.; Ding, Y.; Liu, J.Y.; Wang, Y.; Wang, Y.Q.; Zhang, J.; Du, L.L.; Peng, W.; Fan, G. The interplay between herbal medicines and gut microbiota in metabolic diseases. Front. Pharmacol. 2023, 14, 1105405. [Google Scholar] [CrossRef]
- Song, Q.Q.; Wang, Y.K.; Huang, L.X.; Shen, M.Y.; Yu, Y.; Yu, Q.; Chen, Y.; Xie, J.H. Review of the relationships among polysaccharides, gut microbiota, and human health. Food Res. Int. 2021, 140, 109858. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, Q.; Lin, F.D.; Zheng, B.D.; Huang, Y.Y.; Yang, Y.C.; Xue, C.H.; Xiao, M.T.; Ye, J. Neoagarotetraose alleviates high fat diet induced obesity via white adipocytes browning and regulation of gut microbiota. Carbohydr. Polym. 2022, 296, 119903. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L.; He, Y.; Zeng, L.; He, J.; Yang, Y.; Zhang, T. Effect of κ-carrageenan on glucolipid metabolism and gut microbiota in high-fat diet-fed mice. J. Funct. Foods 2021, 86, 104707. [Google Scholar] [CrossRef]
- Zhou, Y.M.; Lu, H.Y.; Zhao, S.; Yan, B.B.; Wang, H.; Zhou, X.L.; Xiao, Y. The beneficial effects of Tartary buckwheat (Fagopyrum tataricum Gaertn.) on diet-induced obesity in mice are related to the modulation of gut microbiota composition. Food Sci. Hum. Wellness 2023, 12, 1323–1330. [Google Scholar] [CrossRef]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Tkach, S.; et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017, 17, 120. [Google Scholar] [CrossRef]
- Lv, W.W.; Song, J.Y.; Raka, R.N.; Sun, J.L.; Shi, G.Z.; Wu, H.; Xiao, J.S.; Xu, D.X. Effects of food emulsifiers on high fat-diet-induced obesity, intestinal inflammation, changes in bile acid profile, and liver dysfunction. Food Res. Int. 2023, 173, 113302. [Google Scholar] [CrossRef]
- Ke, S.; Yu, Y.; Xu, Q.; Zhang, B.; Wang, S.; Jin, W.; Wei, B.; Wang, H. Composition-activity relationships of polysaccharides from Saccharina japonica in regulating gut microbiota in short-term high-fat diet-fed mice. J. Agric. Food Chem. 2021, 69, 11121–11130. [Google Scholar] [CrossRef]
- Rodrigues, M.; Bertoncini-Silva, C.; Joaquim, A.G.; Machado, C.D.; Zambelli Ramalho, L.N.; Carlos, D.; Fassini, P.G.; Miguel Suen, V.M. Beneficial effects of eugenol supplementation on gut microbiota and hepatic steatosis in high-fat-fed mice. Food Funct. 2022, 13, 3381–3390. [Google Scholar] [CrossRef] [PubMed]
- Ou-yang, J.; Li, X.P.; Liu, C.W.; Jie, O.Y.; Tang, J.Y.; Liu, Q.; Huang, J.A.; Liu, Z. Moringa-Fu brick tea extract attenuated high-fat diet-induced obesity via modulating bile acid metabolism and gut microbiota in rats. J. Funct. Foods 2023, 109, 105766. [Google Scholar] [CrossRef]
- Li, F.; Jiang, C.; Krausz, K.W.; Li, Y.; Albert, I.; Hao, H.; Fabre, K.M.; Mitchell, J.B.; Patterson, A.D.; Gonzalez, F.J. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat. Commun. 2013, 4, 2384. [Google Scholar] [CrossRef] [PubMed]
- Kan, J.; Chen, C.; Huo, T.; Xie, W.; Hui, Y.; Liu, J.; Jin, C. Polyphenolic-enriched peach peels extract regulates lipid metabolism and improves the gut microbiota composition in high fat diet-fed mice. J. Funct. Foods 2020, 72, 104082. [Google Scholar] [CrossRef]
- Leonardi, I.; Gao, I.H.; Lin, W.Y.; Allen, M.; Li, X.V.; Fiers, W.D.; De Celie, M.B.; Putzel, G.G.; Yantiss, R.K.; Johncilla, M.; et al. Mucosal fungi promote gut barrier function and social behavior via Type 17 immunity. Cell 2022, 185, 831–846. [Google Scholar] [CrossRef]
- Nagpal, R.; Neth, B.J.; Wang, S.; Mishra, S.P.; Craft, S.; Yadav, H. Gut mycobiome and its interaction with diet, gut bacteria and Alzheimer’s disease markers in subjects with mild cognitive impairment: A pilot study. EBioMedicine 2020, 59, 102950. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, K.; Zeng, Y.; Luo, Y.; Peng, J.; Zhang, J.; Kuang, T.; Fan, G. Gut mycobiome and metabolic diseases: The known, the unknown, and the future. Pharmacol. Res. 2023, 193, 106807. [Google Scholar] [CrossRef]
- Bozkurt, B.; Terlemez, G.; Sezgin, E. Basidiomycota species in Drosophila gut are associated with host fat metabolism. Sci. Rep. 2023, 13, 13807. [Google Scholar] [CrossRef]
- Mar Rodriguez, M.; Perez, D.; Javier Chaves, F.; Esteve, E.; Marin-Garcia, P.; Xifra, G.; Vendrell, J.; Jove, M.; Pamplona, R.; Ricart, W.; et al. Obesity changes the human gut mycobiome. Sci. Rep. 2015, 5, 14600. [Google Scholar] [CrossRef] [PubMed]
- Coppola, S.; Avagliano, C.; Calignano, A.; Berni Canani, R. The protective role of butyrate against obesity and obesity-related diseases. Molecules 2021, 26, 682. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.T.; Fujimura, Y.; Mimura, I.; Fujii, Y.; Nguyen, N.L.; Arakawa, K.; Morita, H. Cultivable butyrate-producing bacteria of elderly Japanese diagnosed with Alzheimer’s disease. J. Microbiol. 2018, 56, 760–771. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Everard, A.; Duparc, T. Gut microbiota, enteroendocrine functions and metabolism. Curr. Opin. Pharmacol. 2013, 13, 935–940. [Google Scholar] [CrossRef] [PubMed]
- De Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Peng, Y.; Huang, Y.; Xie, M.; Dai, Z.; Cai, H.; Dong, W.; Xu, W.; Xie, Z.; Chen, D.; et al. Fluoride induced leaky gut and bloom of Erysipelatoclostridium ramosum mediate the exacerbation of obesity in high-fat-diet fed mice. J. Adv. Res. 2023, 50, 35–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hao, J.; Miao, D.; Xiao, P.; Jiang, X.; E-Hu, L. Compound chenpi tea consumption reduces obesity-related metabolic disorders by modulating gut microbiota and serum metabolites in mice. J. Sci. Food Agric. 2024, 104, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.F.; Wang, S.W.; Wang, X.X.; Weng, Y.Y.; Fan, X.Y.; Sheng, H.; Zhu, X.T.; Lou, L.J.; Zhang, F. The flavonoid-rich Quzhou Fructus Aurantii extract modulates gut microbiota and prevents obesity in high-fat diet-fed mice. Nutr. Diabetes 2019, 9, 30. [Google Scholar] [CrossRef]
- Yin, C.M.; Noratto, G.D.; Fan, X.Z.; Chen, Z.Y.; Yao, F.; Shi, D.F.; Gao, H. The impact of mushroom polysaccharides on gut microbiota and its beneficial effects to host: A review. Carbohydr. Polym. 2020, 250, 116942. [Google Scholar] [CrossRef]
- Yu, H.N.; Guo, Z.Z.; Shen, S.R.; Shan, W.G. Effects of taurine on gut microbiota and metabolism in mice. Amino Acids 2016, 48, 1601–1617. [Google Scholar] [CrossRef]
- Heisel, T.; Montassier, E.; Johnson, A.; Al-Ghalith, G.; Lin, Y.W.; Wei, L.N.; Knights, D.; Gale, C.A. High-fat diet changes fungal microbiomes and interkingdom relationships in the murine gut. mSphere 2017, 2, 1128. [Google Scholar] [CrossRef] [PubMed]
- Chin, V.K.; Yong, V.C.; Chong, P.P.; Amin Nordin, S.; Basir, R.; Abdullah, M. Mycobiome in the gut: A multiperspective review. Mediat. Inflamm. 2020, 2020, 9560684. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, M.; Huang, Z.; Zheng, X.; Hou, M.; Zhang, S. Polysaccharides from Trametes versicolor as a Potential Prebiotic to Improve the Gut Microbiota in High-Fat Diet Mice. Microorganisms 2024, 12, 1654. https://doi.org/10.3390/microorganisms12081654
Bai M, Huang Z, Zheng X, Hou M, Zhang S. Polysaccharides from Trametes versicolor as a Potential Prebiotic to Improve the Gut Microbiota in High-Fat Diet Mice. Microorganisms. 2024; 12(8):1654. https://doi.org/10.3390/microorganisms12081654
Chicago/Turabian StyleBai, Ming, Zhenfeng Huang, Xiaoya Zheng, Mingyong Hou, and Song Zhang. 2024. "Polysaccharides from Trametes versicolor as a Potential Prebiotic to Improve the Gut Microbiota in High-Fat Diet Mice" Microorganisms 12, no. 8: 1654. https://doi.org/10.3390/microorganisms12081654




