Identification and Functional Analysis of ncRNAs Regulating Intrinsic Polymyxin Resistance in Foodborne Proteus vulgaris
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Cultural Conditions
2.2. Construction of ncRNA34 Mutant Strain
2.3. Construction of ncRNA34 Complemented Strain
2.4. Determination of the Minimum Inhibitory Concentration (MIC)
2.5. Spot Growth Assays
2.6. Quantitative Real-Time PCR (qRT-PCR)
2.7. RNA Secondary Structure Prediction
2.8. ncRNA Target Prediction
3. Results
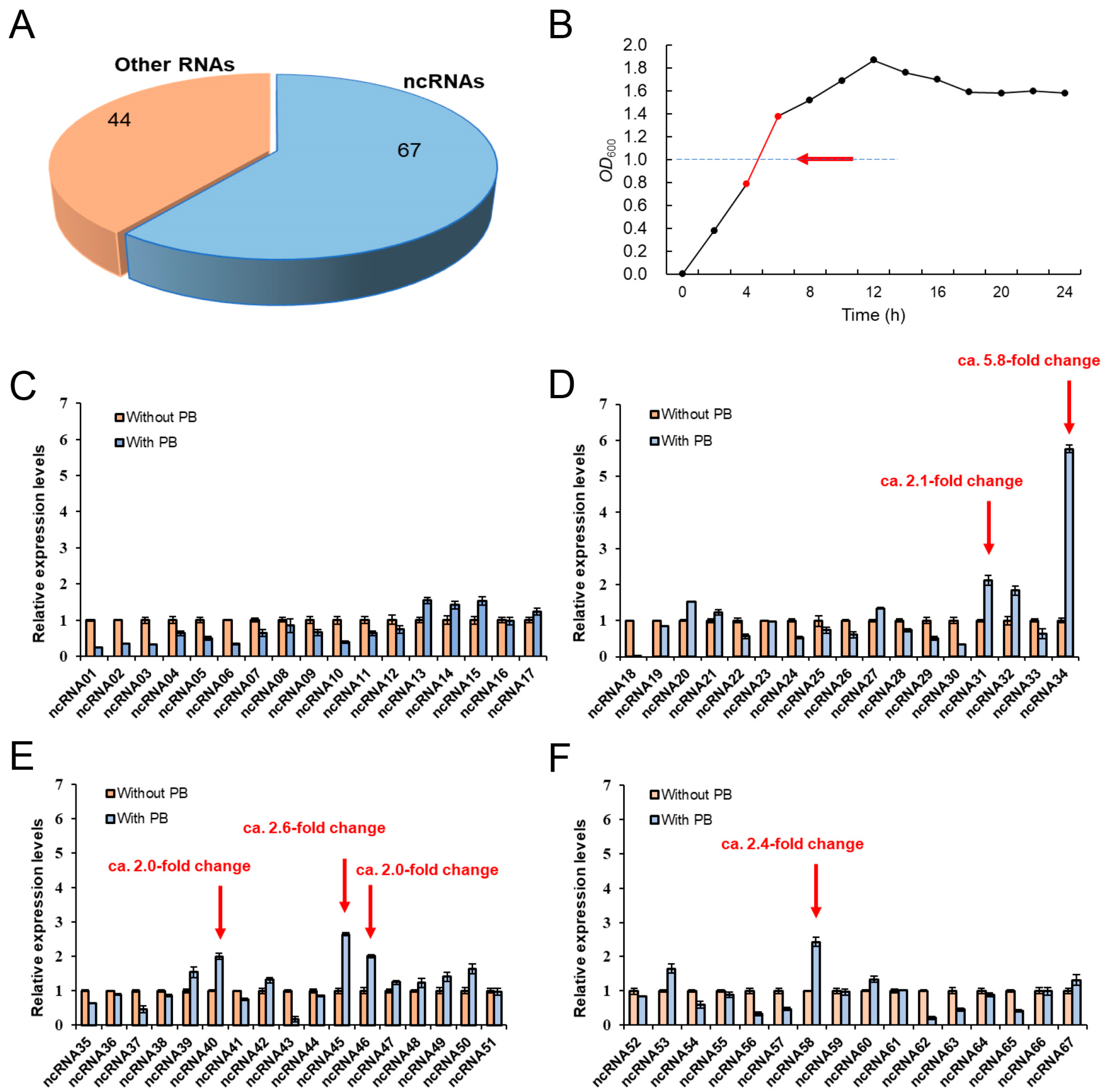
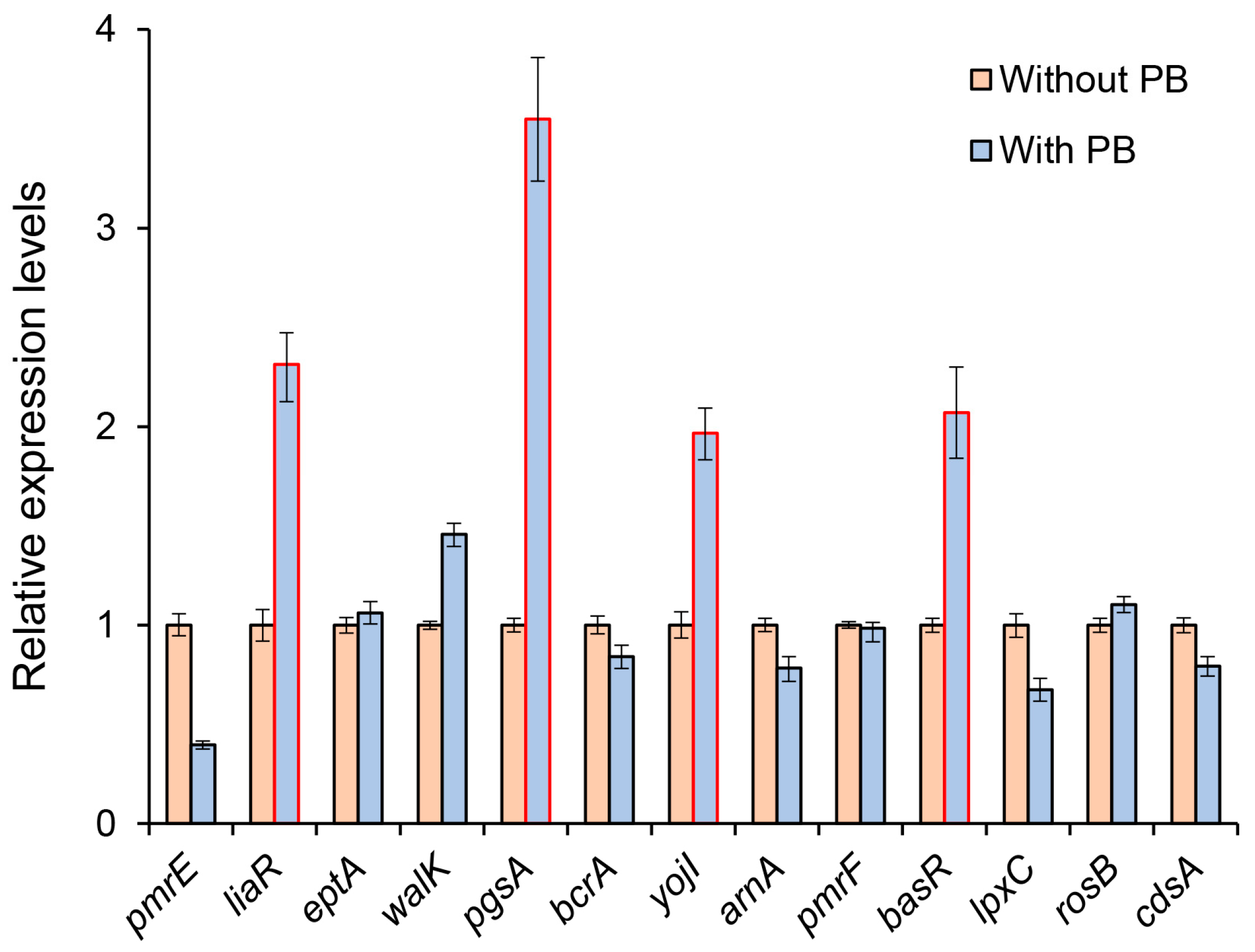
3.1. The Potential Role of ncRNAs in the Regulation of Polymyxin Resistance in Proteus vulgaris
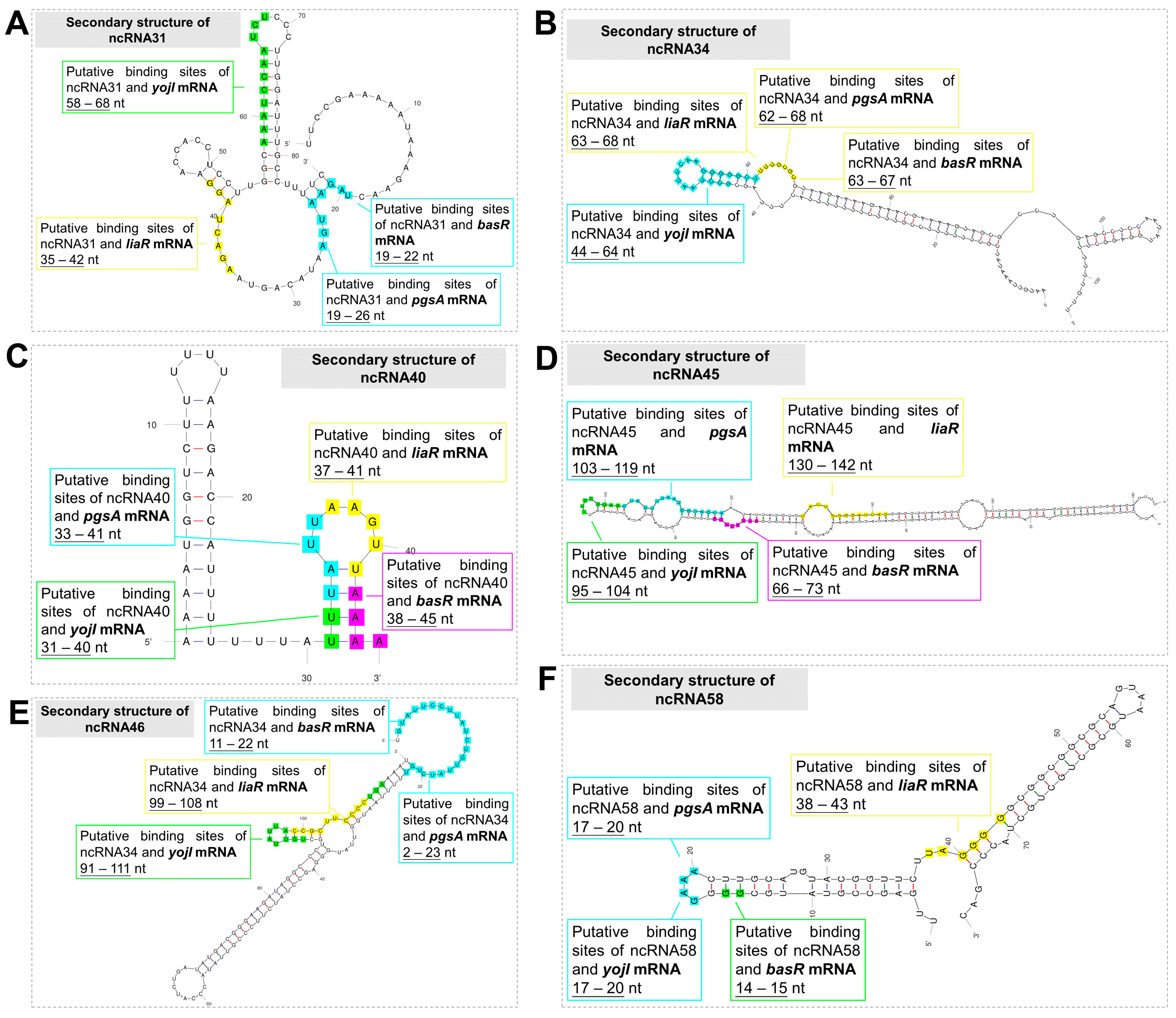
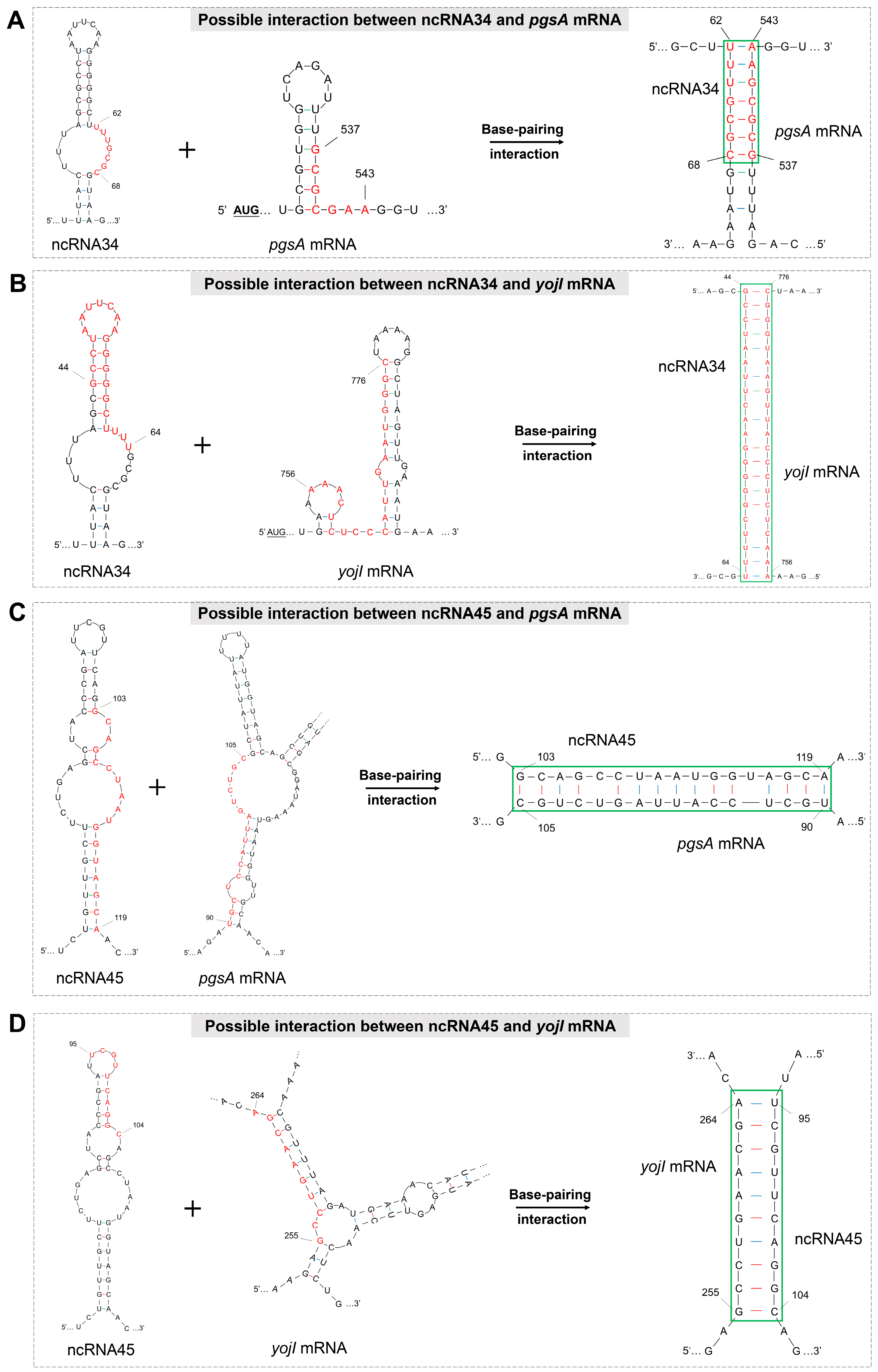
3.2. Prediction of ncRNA Secondary Structure and Potential Target Genes
3.3. Function Determination of Candidate ncRNAs
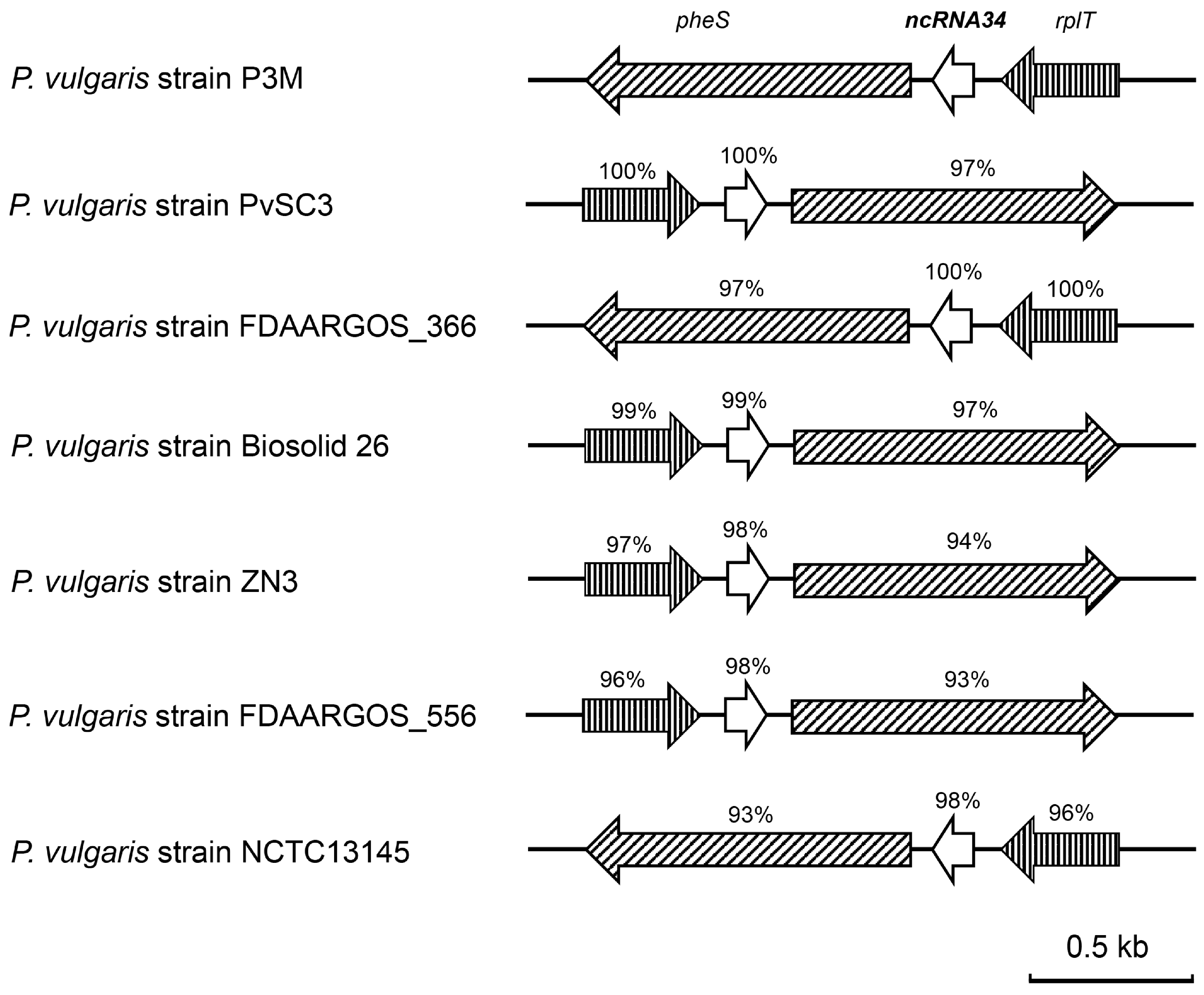
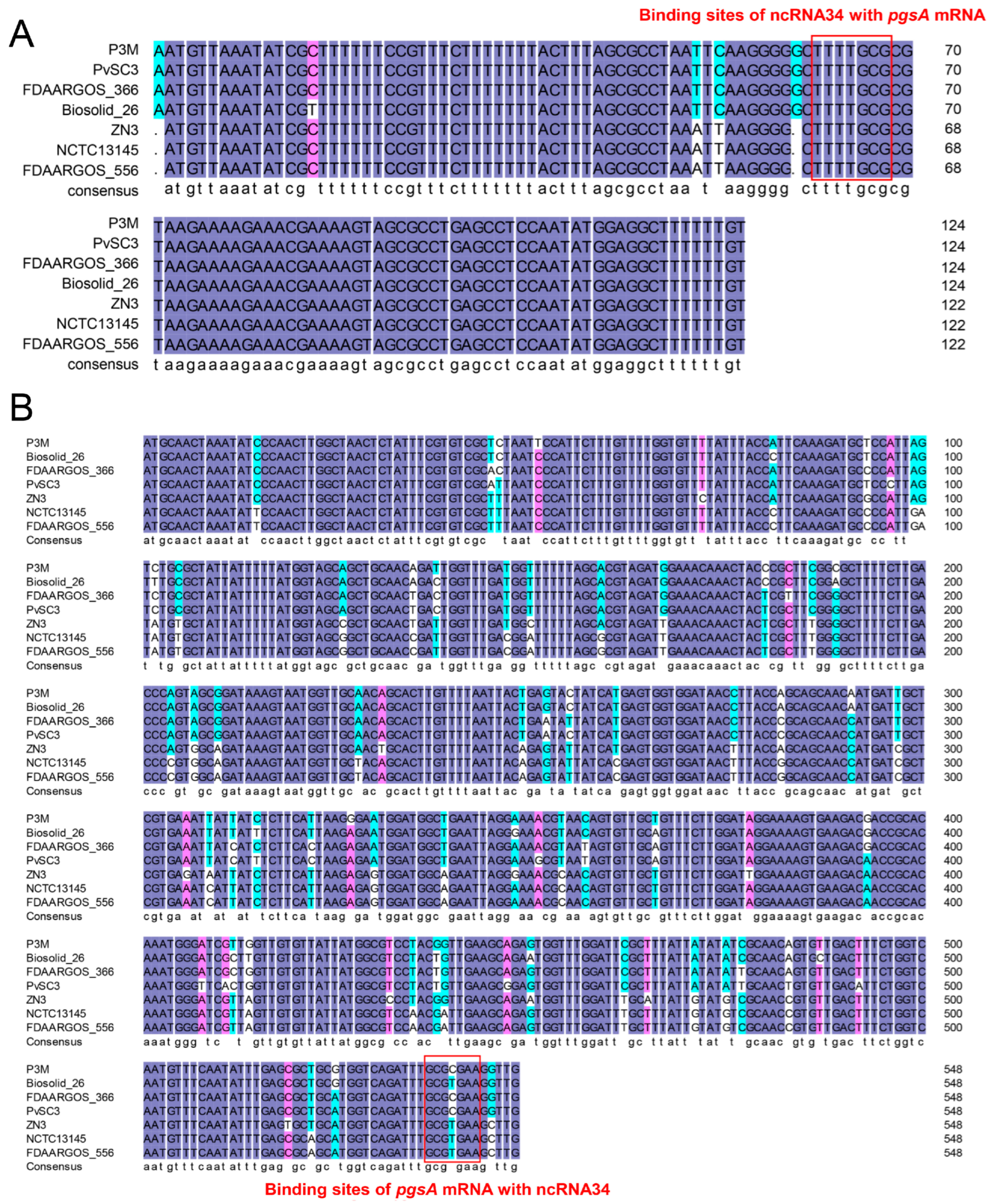
3.4. Evolutionary Conservation Analysis of ncRNA34-pgsA mRNA Interaction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hirsch, E.B.; Tam, V.H. Impact of multidrug-resistant Pseudomonas aeruginosa infection on patient outcomes. Expert. Rev. Pharmacoecon. Outcomes Res. 2010, 10, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, J.; Das, S.; Fatima, Z.; Hameed, S. Multidrug resistance: An emerging crisis. Interdiscip. Perspect. Infect. Dis. 2014, 2014, 541340. [Google Scholar] [CrossRef] [PubMed]
- Willems, R.P.J.; van Dijk, K.; Vehreschild, M.J.G.T.; Vehreschild, M.J.G.T.; Biehl, L.M.; Ket, J.C.F.; Remmelzwaal, S.; Vandenbroucke-Grauls, C.M.J.E. Incidence of infection with multidrug-resistant Gram-negative bacteria and vancomycin-resistant enterococci in carriers: A systematic review and meta-regression analysis. Lancet. Infect. Dis. 2023, 23, 719–731. [Google Scholar] [CrossRef]
- Xiaomin, S.; Yiming, L.; Yuying, Y.; Zhangqi, S.; Yongning, W.; Shaolin, W. Global impact of mcr-1-positive Enterobacteriaceae bacteria on “one health”. Crit. Rev. Microbiol. 2020, 46, 565–577. [Google Scholar] [CrossRef]
- Nang, S.C.; Azad, M.A.K.; Velkov, T.; Zhou, Q.T.; Li, J. Rescuing the Last-Line Polymyxins: Achievements and Challenges. Pharmacol. Rev. 2021, 73, 679–728. [Google Scholar] [CrossRef]
- Velkov, T.; Dai, C.; Ciccotosto, G.D.; Cappai, R.; Hoyer, D.; Li, J. Polymyxins for CNS infections: Pharmacology and neurotoxicity. Pharmacol. Ther. 2018, 181, 85–90. [Google Scholar] [CrossRef]
- Yang, Q.; Pogue, J.M.; Li, Z.; Nation, R.L.; Kaye, K.S.; Li, J. Agents of Last Resort: An Update on Polymyxin Resistance. Infect. Dis. Clin. N. Am. 2020, 34, 723–750. [Google Scholar] [CrossRef]
- Rigatto, M.H.; Falci, D.R.; Zavascki, A.P. Clinical Use of Polymyxin B. Adv. Exp. Med. Biol. 2019, 1145, 197–218. [Google Scholar] [PubMed]
- Nation, R.L.; Velkov, T.; Li, J. Colistin and polymyxin B: Peas in a pod, or chalk and cheese? Clin. Infect. Dis. 2014, 59, 88–94. [Google Scholar] [CrossRef]
- Wertheim, H.; Van Nguyen, K.; Hara, G.L.; Gelband, H.; Laxminarayan, R.; Mouton, J.; Cars, O. Global survey of polymyxin use: A call for international guidelines. J. Glob. Antimicrob. Resist. 2013, 1, 131–134. [Google Scholar] [CrossRef]
- Ray, M.; Manjunath, A.; Halami, P.M. Prevalence of polymyxin resistance through the food chain, the global crisis. J. Antibiot. 2022, 75, 185–198. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, J.; Cui, T.; Srinivas, S.; Feng, Y. Mechanistic insights into transferable polymyxin resistance among gut bacteria. J. Biol. Chem. 2018, 293, 4350–4365. [Google Scholar] [CrossRef]
- Moffatt, J.H.; Harper, M.; Boyce, J.D. Mechanisms of Polymyxin Resistance. Adv. Exp. Med. Biol. 2019, 1145, 55–71. [Google Scholar]
- Jiang, X.; Yang, K.; Han, M.L.; Yuan, B.; Li, J.; Gong, B.; Velkov, T.; Schreiber, F.; Wang, L.; Li, J. Outer Membranes of Polymyxin-Resistant Acinetobacter baumannii with Phosphoethanolamine-Modified Lipid A and Lipopolysaccharide Loss Display Different Atomic-Scale Interactions with Polymyxins. ACS Infect. Dis. 2020, 6, 2698–2708. [Google Scholar] [CrossRef]
- Loutet, S.A.; Valvano, M.A. Extreme antimicrobial Peptide and polymyxin B resistance in the genus burkholderia. Front. Microbiol. 2011, 2, 159. [Google Scholar] [CrossRef]
- Olaitan, A.O.; Morand, S.; Rolain, J.M. Mechanisms of polymyxin resistance: Acquired and intrinsic resistance in bacteria. Front. Microbiol. 2014, 5, 643. [Google Scholar] [CrossRef]
- Sud, I.J.; Feingold, D.S. Mechanism of polymyxin B resistance in Proteus mirabilis. J. Bacteriol. 1970, 104, 289–294. [Google Scholar] [CrossRef]
- Lin, Q.Y.; Tsai, Y.L.; Liu, M.C.; Lin, W.C.; Hsueh, P.R.; Liaw, S.J. Serratia marcescens arn, a PhoP-regulated locus necessary for polymyxin B resistance. Antimicrob. Agents Chemother. 2014, 58, 5181–5190. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, H.; Ma, Z.; Liu, Y.; Wu, Z.; Xu, H.; Qiao, M. Characterization of Proteus vulgaris Strain P3M, a Foodborne Multidrug-Resistant Bacterium Isolated from Penaeus vannamei in China. Microb. Drug. Resist. 2021, 27, 1360–1370. [Google Scholar] [CrossRef]
- Hamilton, A.L.; Kamm, M.A.; Ng, S.C.; Morrison, M. Proteus spp. as Putative Gastrointestinal Pathogens. Clin. Microbiol. Rev. 2018, 31, e00085-17. [Google Scholar] [CrossRef]
- Yu, X.; Torzewska, A.; Zhang, X.; Yin, Z.; Drzewiecka, D.; Cao, H.; Liu, B.; Knirel, Y.A.; Rozalski, A.; Wang, L. Genetic diversity of the O antigens of Proteus species and the development of a suspension array for molecular serotyping. PLoS ONE 2017, 12, e0183267. [Google Scholar] [CrossRef] [PubMed]
- Kwiecinska-Pirog, J.; Skowron, K.; Bartczak, W.; Gospodarek-Komkowska, E. The Ciprofloxacin Impact on Biofilm Formation by Proteus mirabilis and P. vulgaris Strains. Jundishapur J. Microbiol. 2016, 9, e32656. [Google Scholar] [CrossRef]
- Baron, S.; Leulmi, Z.; Villard, C.; Olaitan, A.O.; Telke, A.A.; Rolain, J.M. Inactivation of the arn operon and loss of aminoarabinose on lipopolysaccharide as the cause of susceptibility to colistin in an atypical clinical isolate of Proteus vulgaris. Int. J. Antimicrob. Agents 2018, 51, 450–457. [Google Scholar] [CrossRef]
- Bakthavatchalam, Y.D.; Pragasam, A.K.; Biswas, I.; Veeraraghavan, B. Polymyxin susceptibility testing, interpretative breakpoints and resistance mechanisms: An update. J. Glob. Antimicrob. Resist. 2018, 12, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Aquilini, E.; Merino, S.; Knirel, Y.A.; Regué, M.; Tomás, J.M. Functional identification of Proteus mirabilis eptC gene encoding a core lipopolysaccharide phosphoethanolamine transferase. Int. J. Mol. Sci. 2014, 15, 6689–6702. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.S.; Liu, M.C.; Teng, L.J.; Wang, W.B.; Hsueh, P.R.; Liaw, S.J. Proteus mirabilis pmrI, an RppA-regulated gene necessary for polymyxin B resistance, biofilm formation, and urothelial cell invasion. Antimicrob. Agents Chemother. 2010, 54, 1564–1571. [Google Scholar] [CrossRef] [PubMed]
- Hinrichs, W.; Kisker, C.; Düvel, M.; Müller, A.; Tovar, K.; Hillen, W.; Saenger, W. Structure of the Tet repressor-tetracycline complex and regulation of antibiotic resistance. Science 1994, 264, 418–420. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.X.; Yi, X.X.; Cho, A.; O’Gara, F.; Wang, Y.P. CpxR Activates MexAB-OprM Efflux Pump Expression and Enhances Antibiotic Resistance in Both Laboratory and Clinical nalB-Type Isolates of Pseudomonas aeruginosa. PLoS Pathog. 2016, 12, e1005932. [Google Scholar] [CrossRef]
- Cho, H.; Kim, K.S. Escherichia coli OxyS RNA triggers cephalothin resistance by modulating the expression of CRP-associated genes. Biochem. Biophys. Res. Commun. 2018, 506, 66–72. [Google Scholar] [CrossRef]
- Liu, P.; Yue, C.; Liu, L.; Gao, C.; Lyu, Y.; Deng, S.; Tian, H.; Jia, X. The function of small RNA in Pseudomonas aeruginosa. PeerJ 2022, 10, e13738. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, K.S.; Haneke, K.; Papenfort, K.; Vogel, J. The target spectrum of SdsR small RNA in Salmonella. Nucleic Acids Res. 2016, 44, 10406–10422. [Google Scholar] [PubMed]
- Vazquez-Laslop, N.; Thum, C.; Mankin, A.S. Molecular mechanism of drug-dependent ribosome stalling. Mol. Cell 2008, 30, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.H.; Choi, E.C.; Weisblum, B. Transcriptional attenuation control of ermK, a macrolide-lincosamide-streptogramin B resistance determinant from Bacillus licheniformis. J. Bacteriol. 1991, 173, 4725–4735. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wilks, A. A rapid seamless method for gene knockout in Pseudomonas aeruginosa. BMC Microbiol. 2017, 17, 199. [Google Scholar] [CrossRef] [PubMed]
- Simon, R.; Priefer, U.B.; Puhler, A. A broad host range mobilization system for in vivo genetic engineering: Transposon mutagenesis in gram negative bacteria. Nat. Biotechnol. 1983, 1, 784–791. [Google Scholar] [CrossRef]
- Nunn, D.; Bergman, S.; Lory, S. Products of three accessory genes, pilB, pilC, and pilD, are required for biogenesis of Pseudomonas aeruginosa pili. J. Bacteriol. 1990, 172, 2911–2919. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 29th ed.; CLSI Approved Standard M100-S15: Wayne, PA, USA, 2018. [Google Scholar]
- Zhang, H.; Zhan, Y.; Yan, Y.; Liu, Y.; Hu, G.; Wang, S.; Yang, H.; Qiu, X.; Liu, Y.; Li, J.; et al. The Pseudomonas stutzeri-Specific Regulatory Noncoding RNA NfiS Targets katB mRNA Encoding a Catalase Essential for Optimal Oxidative Resistance and Nitrogenase Activity. J. Bacteriol. 2019, 201, e00334-19. [Google Scholar] [CrossRef]
- Zhan, Y.; Deng, Z.; Yan, Y.; Zhang, H.; Lu, C.; Yang, Z.; Shang, L.; Huang, Y.; Lv, F.; Liu, Y.; et al. NfiR, a New Regulatory Noncoding RNA (ncRNA), Is Required in Concert with the NfiS ncRNA for Optimal Expression of Nitrogenase Genes in Pseudomonas stutzeri A1501. Appl. Environ. Microbiol. 2019, 85, e00762-19. [Google Scholar] [CrossRef]
- Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef]
- Gruber, A.R.; Lorenz, R.; Bernhart, S.H.; Neuböck, R.; Hofacker, I.L. The Vienna RNA websuite. Nucleic Acids Res. 2008, 36, W70–W74. [Google Scholar] [CrossRef]
- Andronescu, M.; Condon, A.; Hoos, H.H.; Mathews, D.H.; Murphy, K.P. Efficient parameter estimation for RNA secondary structure prediction. Bioinformatics 2007, 23, i19–i28. [Google Scholar] [CrossRef]
- Jagodnik, J.; Chiaruttini, C.; Guillier, M. Stem-Loop Structures within mRNA Coding Sequences Activate Translation Initiation and Mediate Control by Small Regulatory RNAs. Mol. Cell 2017, 68, 158–170.e3. [Google Scholar] [CrossRef]
- Chen, Y.; Xue, D.; Sun, W.; Han, J.; Li, J.; Gao, R.; Zhou, Z.; Zhang, W.; Chen, M.; Lin, M.; et al. sRNA OsiA Stabilizes Catalase mRNA during Oxidative Stress Response of Deincoccus radiodurans R1. Microorganisms 2019, 7, 422. [Google Scholar] [CrossRef]
- Lahiry, A.; Stimple, S.D.; Wood, D.W.; Lease, R.A. Retargeting a Dual-Acting sRNA for Multiple mRNA Transcript Regulation. ACS Synth. Biol. 2017, 6, 648–658. [Google Scholar] [CrossRef]
- Zhan, Y.; Yan, Y.; Deng, Z.; Chen, M.; Lu, W.; Lu, C.; Shang, L.; Yang, Z.; Zhang, W.; Wang, W.; et al. The novel regulatory ncRNA, NfiS, optimizes nitrogen fixation via base pairing with the nitrogenase gene nifK mRNA in Pseudomonas stutzeri A1501. Proc. Natl. Acad. Sci. USA 2016, 113, E4348–E4356. [Google Scholar] [CrossRef]
- Li, M.; Zhu, J.; Lv, Z.; Qin, H.; Wang, X.; Shi, H. Recent Advances in RNA-Targeted Cancer Therapy. Chembiochem 2024, 25, e202300633. [Google Scholar] [CrossRef]
- Principles of Chemical Biology: From Antibiotic Resistance to RNA-Targeted Therapy via Autoxidation and Lipid Droplets. Cell Chem. Biol. 2017, 24, 533–534.


| Strains or Plasmids | Characteristics | Sources |
|---|---|---|
| P. vulgaris strains | ||
| P3M | Wild-type strain | [19] |
| ∆ncRNA34 | ncRNA34 deletion mutant strain | This study |
| com-ncRNA34 | ncRNA34 complemented strain, Tcr | This study |
| E. coli strains | ||
| E. coli DH5α | Competent cell for cloning | CWBIO Company |
| E. coli S17 | Mobilizing donor strain, Smr | [34] |
| Plasmids | ||
| pEX18Tc | Suicide plasmid used for constructing the deletion mutant strain, Tcr | [35] |
| pDN18 | Broad-spectrum clone plasmid used for the construction of functional complemented strain, Tcr | [36] |
| Antibiotic | MIC (mg/L), Interpretation |
|---|---|
| Colistin E (CT) | >1024, Resistant |
| Polymyxin B (PB) | >1024, Resistant |
| No. | Gene Name | Function | Location a | Strand b |
|---|---|---|---|---|
| 1 | pmrE | Nucleotide sugar dehydrogenase | 54448–55614 | − |
| 2 | liaR | Response regulator | 421750–422412 | − |
| 3 | eptA | Metal-dependent hydrolase | 435097–436788 | − |
| 4 | walK | Histidine kinase | 687783–689081 | + |
| 5 | pgsA | CDP-diacylglycerol-glycerol-3-phosphate-3-phosphatidyltransferase | 1627067–1627615 | + |
| 6 | bcrA | ATP-binding protein | 1674978–1675691 | − |
| 7 | yojI | ATPase component | 1739261–1740082 | − |
| 8 | arnA | Methionyl-tRNA formyltransferase | 2166583–2168565 | − |
| 9 | pmrF | Phosphotransferase | 2168565–2169545 | − |
| 10 | basR | Response regulator | 2491364–2492029 | + |
| 11 | lpxC | N-acetylglucosamine deacetylase | 2834499–2835419 | − |
| 12 | rosB | Kef family K (+) transporter | 3000038–3001798 | + |
| 13 | cdsA | Phosphatidate cytidylyltransferase | 3018284–3019219 | + |
| Candidate ncRNA | Possible Target mRNA | Binding Sites on ncRNA (5′–3′) | Stem–Loop Structure a | Binding Sites on Target mRNA (5′–3′) | Post-Transcriptional Translation Process b |
|---|---|---|---|---|---|
| ncRNA31 | liaR mRNA | 35 to 42 | T | 342 to 349 | N |
| pgsA mRNA | 19 to 26 | T | 39 to 46 | N | |
| yojI mRNA | 58 to 68 | T | 224 to 234 | N | |
| basR mRNA | 19 to 22 | F | 106 to 109 | N | |
| ncRNA34 | liaR mRNA | 63 to 68 | T | 31 to 37 | N |
| pgsA mRNA | 62 to 68 | T | 537 to 543 | P | |
| yojI mRNA | 44 to 64 | T | 756 to 776 | P | |
| basR mRNA | 63 to 67 | T | 316 to 320 | N | |
| ncRNA40 | liaR mRNA | 37 to 41 | T | 230 to 234 | N |
| pgsA mRNA | 33 to 41 | T | 5 to 13 | N | |
| yojI mRNA | 31 to 40 | T | 9 to 18 | N | |
| basR mRNA | 38 to 45 | T | 230 to 237 | N | |
| ncRNA45 | liaR mRNA | 130 to 142 | T | 131 to 145 | N |
| pgsA mRNA | 103 to 119 | T | 90 to 105 | P | |
| yojI mRNA | 95 to 104 | T | 255 to 264 | P | |
| basR mRNA | 66 to 73 | T | 90 to 97 | N | |
| ncRNA46 | liaR mRNA | 99 to 108 | T | 118 to 127 | N |
| pgsA mRNA | 2 to 23 | F | 330 to 352 | N | |
| yojI mRNA | 91 to 111 | T | 81 to 99 | N | |
| basR mRNA | 11 to 22 | F | 90 to 105 | N | |
| ncRNA58 | liaR mRNA | 38 to 43 | F | 416 to 421 | N |
| pgsA mRNA | 17 to 20 | T | 492 to 495 | N | |
| yojI mRNA | 17 to 20 | T | 695 to 698 | N | |
| basR mRNA | 14 to 15 | F | 567 to 568 | N |
| Strains | MIC (mg/L), Interpretation |
|---|---|
| P3M | >1024, Resistant |
| ∆ncRNA34 | 1024, Resistant |
| com-ncRNA34 | >1024, Resistant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Wu, T.; Ruan, H. Identification and Functional Analysis of ncRNAs Regulating Intrinsic Polymyxin Resistance in Foodborne Proteus vulgaris. Microorganisms 2024, 12, 1661. https://doi.org/10.3390/microorganisms12081661
Zhang H, Wu T, Ruan H. Identification and Functional Analysis of ncRNAs Regulating Intrinsic Polymyxin Resistance in Foodborne Proteus vulgaris. Microorganisms. 2024; 12(8):1661. https://doi.org/10.3390/microorganisms12081661
Chicago/Turabian StyleZhang, Hongyang, Tao Wu, and Haihua Ruan. 2024. "Identification and Functional Analysis of ncRNAs Regulating Intrinsic Polymyxin Resistance in Foodborne Proteus vulgaris" Microorganisms 12, no. 8: 1661. https://doi.org/10.3390/microorganisms12081661




