Functional Abdominal Bloating and Gut Microbiota: An Update
Abstract
:1. Introduction
1.1. Overview of Abdominal Bloating and Distension
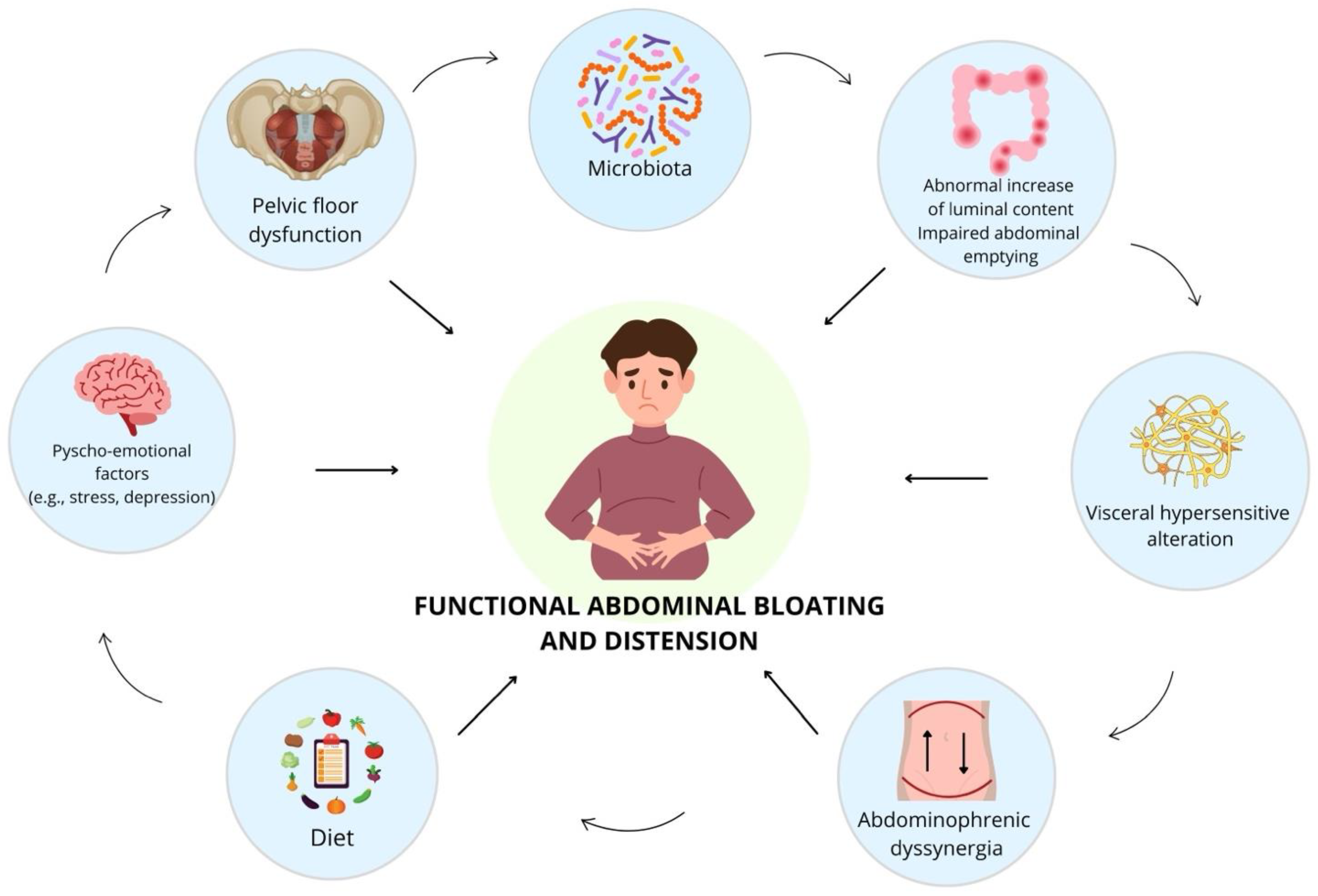
1.2. Pathophysiology and Contributing Factors
1.3. Diagnostic Challenges and Treatment Approaches
2. Methods
2.1. Literature Search
2.2. Study Selection and Data Extraction
2.3. Study Quality Assessment
2.4. Institutional Review Board Approval and Informed Consent
3. Results
3.1. The Multifactorial Pathophysiology of Functional Abdominal Bloating and Distension: The Contributing Factors
3.2. The Role of Microbiota in the Physiopathology of Bloating and Distension
3.3. Therapeutic Options for Bloating and Distension: Diet
3.4. Therapeutic Options for Bloating and Distension: Modulation of the Microbiota
3.5. Therapeutic Options for Bloating and Distension: Neuromodulators
3.6. Therapeutic Options for Bloating and Distension: Other Interventions
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lacy, B.E.; Cangemi, D.; Vazquez-Roque, M. Management of Chronic Abdominal Distension and Bloating. Clin. Gastroenterol. Hepatol. 2021, 19, 219–231.e1. Available online: http://www.cghjournal.org/article/S154235652030433X/fulltext (accessed on 24 September 2023). [CrossRef] [PubMed]
- Drossman, D.A. Functional gastrointestinal disorders: History, pathophysiology, clinical features, and Rome IV. Gastroenterology 2016, 150, 1262–1279.e2. [Google Scholar] [CrossRef] [PubMed]
- Schmulson, M.J.; Drossman, D.A. What Is New in Rome IV. J. Neurogastroenterol. Motil. 2017, 23, 151. Available online: https://pubmed.ncbi.nlm.nih.gov/28274109/ (accessed on 14 October 2023). [CrossRef] [PubMed]
- Mari, A.; Abu Backer, F.; Mahamid, M.; Amara, H.; Carter, D.; Boltin, D.; Dickman, R. Bloating and Abdominal Distension: Clinical Approach and Management. Adv. Ther. 2019, 36, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Lacy, B.E.; Mearin, F.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 150, 1393–1407.e5. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, A.K.; Talley, N.J.; Joos, S.K.; Tolman, K.G.; Hickam, D.H. Abdominal bloating in employed adults: Prevalence, risk factors, and association with other bowel disorders. Am. J. Gastroenterol. 2008, 103, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Sperber, A.D.; Bangdiwala, S.I.; Drossman, D.A.; Ghoshal, U.C.; Simren, M.; Tack, J.; Palsson, O.S. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology 2021, 160, 99–114.e3. [Google Scholar] [CrossRef] [PubMed]
- Farré, R.; Vanheel, H.; Vanuytsel, T.; Masaoka, T.; Törnblom, H.; Simrén, M.; Van Oudenhove, L.; Tack, J.F. In functional dyspepsia, hypersensitivity to postprandial distention correlates with meal-related symptom severity. Gastroenterology 2013, 145, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Houghton, L.A.; Lea, R.; Morris, J.; Reilly, B.; Whorwell, P.J. Bloating and Distention in Irritable Bowel Syndrome: The Role of Visceral Sensation. Gastroenterology 2008, 134, 1882–1889. [Google Scholar] [CrossRef]
- Wilder-Smith, C.H. The balancing act: Endogenous modulation of pain in functional gastrointestinal disorders. Gut 2011, 60, 1589–1599. [Google Scholar] [CrossRef]
- Azpiroz, F.; Malagelada, J.R. Abdominal bloating. Gastroenterology 2005, 129, 1060–1078. [Google Scholar] [CrossRef]
- Cann, P.A.; Read, N.W.; Brown, C.; Hobson, N.; Holdsworth, C.D. Irritable bowel syndrome: Relationship of disorders in the transit of a single solid meal to symptom patterns. Gut 1983, 24, 405–411. [Google Scholar] [CrossRef]
- Törnblom, H.; Van Oudenhove, L.; Sadik, R.; Abrahamsson, H.; Tack, J.; Simrén, M. Colonic transit time and IBS symptoms: What’s the link? Am. J. Gastroenterol. 2012, 107, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Lasser, R.B.; Bond, J.H.; Levitt, M.D. The Role of Intestinal Gas in Functional Abdominal Pain. N. Engl. J. Med. 1975, 293, 524–526. [Google Scholar] [CrossRef]
- Serra, J.; Azpiroz, F.; Malagelada, J.R. Mechanisms of intestinal gas retention in humans: Impaired propulsion versus obstructed evacuation. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, 138–143. [Google Scholar] [CrossRef]
- Tremolaterra, F.; Villoria, A.; Azpiroz, F.; Serra, J.; Aguadé, S.; Malagelada, J.R. Impaired Viscerosomatic Reflexes and Abdominal-Wall Dystony Associated With Bloating. Gastroenterology 2006, 130, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Burri, E.; Barba, E.; Huaman, J.W.; Cisternas, D.; Accarino, A.; Soldevilla, A.; Malagelada, J.-R.; Azpiroz, F. Mechanisms of postprandial abdominal bloating and distension in functional dyspepsia. Gut 2014, 63, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Chiarioni, G.; Salandini, L.; Whitehead, W.E. Biofeedback benefits only patients with outlet dysfunction, not patients with isolated slow transit constipation. Gastroenterology 2005, 129, 86–97. [Google Scholar] [CrossRef]
- Accarino, A.; Perez, F.; Azpiroz, F.; Quiroga, S.; Malagelada, J.R. Abdominal Distention Results From Caudo-ventral Redistribution of Contents. Gastroenterology 2009, 136, 1544–1551. [Google Scholar] [CrossRef]
- Villoria, A.; Azpiroz, F.; Burri, E.; Cisternas, D.; Soldevilla, A.; Malagelada, J.R. Abdomino-phrenic dyssynergia in patients with abdominal bloating and distension. Am. J. Gastroenterol. 2011, 106, 815–819. [Google Scholar] [CrossRef]
- Seo, A.Y.; Kim, N.; Oh, D.H. Bloating and Distension in Irritable Bowel Syndrome: Studies on Mechanisms and Treatment. J. Neurogastroenterol. Motil. 2013, 19, 433–453. [Google Scholar] [CrossRef] [PubMed]
- Marasco, G.; Cremon, C.; Barbaro, M.R.; Stanghellini, V.; Barbara, G. Gut microbiota signatures and modulation in irritable bowel syndrome. Microbiome Res. Rep. 2022, 1, 11. [Google Scholar] [CrossRef] [PubMed]
- Barbara, G.; Barbaro, M.R.; Fuschi, D.; Palombo, M.; Falangone, F.; Cremon, C.; Marasco, G.; Stanghellini, V. Corrigendum: Inflammatory and Microbiota-Related Regulation of the Intestinal Epithelial Barrier. Front. Nutr. 2021, 8, 718356. [Google Scholar] [CrossRef] [PubMed]
- Gargari, G.; Mantegazza, G.; Taverniti, V.; Gardana, C.; Valenza, A.; Rossignoli, F.; Barbaro, M.R.; Marasco, G.; Cremon, C.; Barbara, G.; et al. Fecal short-chain fatty acids in non-constipated irritable bowel syndrome: A potential clinically relevant stratification factor based on catabotyping analysis. Gut Microbes 2023, 15, 2274128. [Google Scholar] [CrossRef] [PubMed]
- Pessarelli, T.; Sorge, A.; Elli, L.; Costantino, A. The low-FODMAP diet and the gluten-free diet in the management of functional abdominal bloating and distension. Front. Nutr. 2022, 9, 1007716. [Google Scholar] [CrossRef] [PubMed]
- Cangemi, D.J.; Lacy, B.E. A Practical Approach to the Diagnosis and Treatment of Abdominal Bloating and Distension. Gastroenterol. Hepatol. 2022, 18, 75–84. [Google Scholar]
- Maxton, D.G.; Martin, D.F.; Whorwell, P.J.; Godfrey, M. Abdominal distension in female patients with irritable bowel syndrome: Exploration of possible mechanisms. Gut 1991, 32, 662–664. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M.M.; Murray, J.A.; Pimentel, M. AGA Clinical Practice Update on Small Intestinal Bacterial Overgrowth: Expert Review. Gastroenterology 2020, 159, 1526–1532. [Google Scholar] [CrossRef] [PubMed]
- Mezzasalma, V.; Manfrini, E.; Ferri, E.; Sandionigi, A.; La Ferla, B.; Schiano, I.; Michelotti, A.; Nobile, V.; Labra, M.; Di Gennaro, P. A Randomized, Double-Blind, Placebo-Controlled Trial: The Efficacy of Multispecies Probiotic Supplementation in Alleviating Symptoms of Irritable Bowel Syndrome Associated with Constipation. Biomed. Res. Int. 2016, 2016, 4740907. [Google Scholar] [CrossRef]
- Ringel-Kulka, T.; Palsson, O.S.; Maier, D.; Carroll, I.; Galanko, J.A.; Leyer, G.; Ringel, Y. Probiotic bacteria Lactobacillus acidophilus NCFM and Bifidobacterium lactis Bi-07 versus placebo for the symptoms of bloating in patients with functional bowel disorders: A double-blind study. J. Clin. Gastroenterol. 2011, 45, 518–525. Available online: https://pubmed.ncbi.nlm.nih.gov/21436726/ (accessed on 30 October 2023). [CrossRef]
- O’Sullivan, M.A.; O’Morain, C.A. Bacterial supplementation in the irritable bowel syndrome. A randomised doubleblind placebo-controlled crossover study. Dig. Liver Dis. 2000, 32, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Sharara, A.I.; Aoun, E.; Abdul-Baki, H.; Mounzer, R.; Sidani, S.; Elhajj, I. A randomized double-blind placebo-controlled trial of rifaximin in patients with abdominal bloating and flatulence. Am. J. Gastroenterol. 2006, 101, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, M.; Lembo, A. Microbiome and Its Role in Irritable Bowel Syndrome. Dig. Dis. Sci. 2020, 65, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Choi, J.K.; Ryu, H.S.; Choi, C.H.; Kang, E.H.; Park, K.S.; Min, Y.W.; Hong, K.S. Therapeutic Modulation of Gut Microbiota in Functional Bowel Disorders. J. Neurogastroenterol. Motil. 2017, 23, 2093–2879. [Google Scholar] [CrossRef] [PubMed]
- Kulka, T.R.; Benson, A.K.; Carroll, I.M.; Kim, J.; Legge, R.M.; Ringel, Y. Molecular characterization of the intestinal microbiota in patients with and without abdominal bloating. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G417–G426. [Google Scholar] [CrossRef] [PubMed]
- Noh, C.K.; Lee, K.J. Fecal microbiota alterations and small intestinal bacterial overgrowth in functional abdominal bloating/distention. J. Neurogastroenterol. Motil. 2020, 26, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Ringel, Y.; Carroll, I.M. Alterations in the Intestinal Microbiota and Functional Bowel Symptoms. Gastrointest. Endosc. Clin. N. Am. 2009, 19, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Mandarino, F.V.; Sinagra, E.; Raimondo, D.; Danese, S. The Role of Microbiota in Upper and Lower Gastrointestinal Functional Disorders. Microorganisms 2023, 11, 980. [Google Scholar] [CrossRef] [PubMed]
- Hadjivasilis, A.; Tsioutis, C.; Michalinos, A.; Ntourakis, D.; Christodoulou, D.K.; Agouridis, A.P. New insights into irritable bowel syndrome: From pathophysiology to treatment. Ann. Gastroenterol. 2019, 32, 554–564. [Google Scholar] [CrossRef]
- Bellini, M.; Gambaccini, D.; Stasi, C.; Urbano, M.T.; Marchi, S.; Usai-Satta, P. Irritable bowel syndrome: A disease still searching for pathogenesis, diagnosis and therapy. World J. Gastroenterol. 2014, 20, 8807–8820. [Google Scholar]
- Simrén, M.; Barbara, G.; Flint, H.J.; Spiegel, B.M.R.; Spiller, R.C.; Vanner, S.; Verdu, E.F.; Whorwell, P.J.; Zoetendal, E.G. Intestinal microbiota in functional bowel disorders: A Rome foundation report. Gut 2013, 62, 159. Available online: https://pmc/articles/PMC3551212/ (accessed on 30 October 2023). [CrossRef] [PubMed]
- Nobaek, S.; Johansson, M.L.; Molin, G.; Ahrné, S.; Jeppsson, B. Alteration of intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome. Am. J. Gastroenterol. 2000, 95, 1231–1238. Available online: https://pubmed.ncbi.nlm.nih.gov/10811333/ (accessed on 30 October 2023). [CrossRef] [PubMed]
- King, T.S.; Elia, M.; Hunter, J.O. Abnormal colonic fermentation in irritable bowel syndrome. Lancet 1998, 352, 1187–1189. Available online: https://pubmed.ncbi.nlm.nih.gov/9777836/ (accessed on 30 October 2023). [CrossRef] [PubMed]
- Kim, H.J.; Camilleri, M.; McKinzie, S.; Lempke, M.B.; Burton, D.D.; Thomforde, G.M.; Zinsmeister, A.R. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment. Pharmacol. Ther. 2003, 17, 895–904. Available online: https://pubmed.ncbi.nlm.nih.gov/12656692/ (accessed on 30 October 2023). [PubMed]
- Collins, S.M.; Denou, E.; Verdu, E.F.; Bercik, P. The putative role of the intestinal microbiota in the irritable bowel syndrome. Dig. Liver Dis. 2009, 41, 850–853. Available online: https://pubmed.ncbi.nlm.nih.gov/19740713/ (accessed on 30 October 2023). [CrossRef] [PubMed]
- Vernia, P.; Di Camillo, M.; Marinaro, V.; Caprilli, R. Effect of predominant methanogenic flora on the outcome of lactose breath test in irritable bowel syndrome patients. Eur. J. Clin. Nutr. 2003, 57, 1116–1119. Available online: https://pubmed.ncbi.nlm.nih.gov/12947430/ (accessed on 30 October 2023). [CrossRef] [PubMed]
- Altomare, A.; Del Chierico, F.; Rocchi, G.; Emerenziani, S.; Nuglio, C.; Putignani, L.; Angeletti, S.; Presti, A.L.; Ciccozzi, M.; Russo, A.; et al. Association between dietary habits and fecal microbiota composition in irritable bowel syndrome patients: A pilot study. Nutrients 2021, 13, 1479. [Google Scholar] [CrossRef] [PubMed]
- Barbara, G.; Cremon, C.; Bellini, M.; Corsetti, M.; Di Nardo, G.; Falangone, F.; Fuccio, L.; Galeazzi, F.; Iovino, P.; Sarnelli, G.; et al. Italian guidelines for the management of irritable bowel syndrome: Joint Consensus from the Italian Societies of: Gastroenterology and Endoscopy (SIGE), Neurogastroenterology and Motility (SINGEM), Hospital Gastroenterologists and Endoscopists (AIGO). Dig. Liver Dis. 2022, 55, 187–207. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Bhattarai, Y.; Muniz Pedrogo, D.A.; Kashyap, P.C. Irritable bowel syndrome: A gut microbiota-related disorder? Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 312, G52–G62. [Google Scholar] [CrossRef]
- Jeffery, I.B.; O’Toole, P.W.; Öhman, L.; Claesson, M.J.; Deane, J.; Quigley, E.M.M.; Simrén, M. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut 2012, 61, 997–1006. [Google Scholar] [CrossRef]
- Böhn, L.; Störsrud, S.; Liljebo, T.; Collin, L.; Lindfors, P.; Törnblom, H.; Simrén, M. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: A randomized controlled trial. Gastroenterology 2015, 149, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Molina-Infante, J.; Serra, J.; Fernandez-Bañares, F.; Mearin, F. The low-FODMAP diet for irritable bowel syndrome: Lights and shadows. Gastroenterol. Hepatol. 2016, 39, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.; Tonarelli, S.; Nagy, A.G.; Pancetti, A.; Costa, F.; Ricchiuti, A.; de Bortoli, N.; Mosca, M.; Marchi, S.; Rossi, A. Low FODMAP diet: Evidence, doubts, and hopes. Nutrients 2020, 12, 148. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, K.; Reed, D.E.; Schneider, T.; Dang, F.; Keshteli, A.H.; De Palma, G.; Madsen, K.; Bercik, P.; Vanner, S. FODMAPs alter symptoms and the metabolome of patients with IBS: A randomised controlled trial. Gut 2016, 66, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, H.M.; Lomer, M.C.E.; Farquharson, F.M.; Louis, P.; Fava, F.; Franciosi, E.; Scholz, M.; Tuohy, K.M.; Lindsay, J.O.; Irving, P.M.; et al. A diet low in FODMAPs reduces symptoms in patients with irritable bowel syndrome and a probiotic restores bifidobacterium species: A randomized controlled trial. Gastroenterology 2017, 153, 936–947. [Google Scholar] [CrossRef] [PubMed]
- Chey, W.D.; Chey, W.D.; Hashash, J.G.; Hashash, J.G.; Manning, L.; Manning, L.; Chang, L.; Chang, L. AGA clinical practice update on the role of diet in irritable bowel syndrome: Expert review. Gastroenterology 2022, 162, 1737–1745. [Google Scholar] [CrossRef] [PubMed]
- Almario, C.V.; Ballal, M.L.; Chey, W.D.; Nordstrom, C.; Khanna, D.; Spiegel, B.M.R. Burden of gastrointestinal symptoms in the United States: Results of a nationally representative survey of over 71,000 Americans. Am. J. Gastroenterol. 2018, 113, 1701. [Google Scholar] [CrossRef] [PubMed]
- Serra, J. Management of bloating. Neurogastroenterol. Motil. 2022, 34, e14333. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Ringel-Kulka, T.; McRorie, J.; Ringel, Y. Multi-center, double-blind, randomized, placebo-controlled, parallel-group study to evaluate the benefit of the probiotic Bifidobacterium infantis 35624 in non-patients with symptoms of abdominal discomfort and bloating. Off. J. Am. Coll. Gastroenterol. 2017, 112, 145–151. [Google Scholar] [CrossRef]
- Kim, H.J.; Roque, M.I.V.; Camilleri, M.; Stephens, D.; Burton, D.D.; Baxter, K.; Thomforde, G.; Zinsmeister, A.R. A randomized controlled trial of a probiotic combination VSL# 3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol. Motil. 2005, 17, 687–696. [Google Scholar] [PubMed]
- Majeed, M.; Nagabhushanam, K.; Paulose, S.; Arumugam, S.; Mundkur, L. The effects of Bacillus coagulans MTCC 5856 on functional gas and bloating in adults: A randomized, double-blind, placebo-controlled study. Medicine 2023, 102, e33109. [Google Scholar] [CrossRef]
- Moshiree, B.; Drossman, D.; Shaukat, A. AGA clinical practice update on evaluation and management of belching, abdominal bloating, and distention: Expert review. Gastroenterology 2023, 165, 791–800.e3. [Google Scholar] [CrossRef] [PubMed]
- Takakura, W.; Pimentel, M. Small intestinal bacterial overgrowth and irritable bowel syndrome–an update. Front. Psychiatry 2020, 11, 664. [Google Scholar] [CrossRef]
- Pimentel, M. Review of rifaximin as treatment for SIBO and IBS. Expert Opin. Investig. Drugs 2009, 18, 349–358. [Google Scholar] [CrossRef]
- Ponziani, F.R.; Scaldaferri, F.; Petito, V.; Paroni, S.F.; Pecere, S.; Lopetuso, L.R.; Palladini, A.; Gerardi, V.; Masucci, L.; Pompili, M.; et al. The role of antibiotics in gut microbiota modulation: The eubiotic effects of rifaximin. Dig. Dis. 2016, 34, 269–278. [Google Scholar] [CrossRef]
- Cuomo, R.; Barbara, G.; Annibale, B. Rifaximin and diverticular disease: Position paper of the Italian Society of Gastroenterology (SIGE). Dig. Liver Dis. 2017, 49, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Menees, S.B.; Maneerattannaporn, M.; Kim, H.M.; Chey, W.D. The efficacy and safety of rifaximin for the irritable bowel syndrome: A systematic review and meta-analysis. Off. J. Am. Coll. Gastroenterol. 2012, 107, 28–35. [Google Scholar] [CrossRef]
- Chey, W.D.; Shah, E.D.; DuPont, H.L. Mechanism of action and therapeutic benefit of rifaximin in patients with irritable bowel syndrome: A narrative review. Ther. Adv. Gastroenterol. 2020, 13, 1756284819897531. [Google Scholar] [CrossRef]
- Arora, U.; Sachdeva, K.; Garg, P.; Baitha, U.; Kedia, S.; Kalaivani, M.; Ahuja, V.; Kumar, A.; Ranjan, P.; Vikram, N.K.; et al. S573 Efficacy of Rifaximin in Patients with Abdominal Bloating or Distension: A Systematic Review and Meta-Analysis. Off. J. Am. Coll. Gastroenterol. 2022, 117, e406. [Google Scholar] [CrossRef]
- Pimentel, M.; Lembo, A.; Chey, W.D.; Zakko, S.; Ringel, Y.; Yu, J.; Mareya, S.M.; Shaw, A.L.; Bortey, E.; Forbes, W.P. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N. Engl. J. Med. 2011, 364, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Lacy, B.E.; Cangemi, D.J. A pragmatic approach to the evaluation and treatment of abdominal bloating. Off. J. Am. Coll. Gastroenterol. 2022, 117, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Drossman, D.A.; Tack, J.; Ford, A.C.; Szigethy, E.; Törnblom, H.; Van Oudenhove, L. Neuromodulators for functional gastrointestinal disorders (disorders of gut− brain interaction): A Rome foundation working team report. Gastroenterology 2018, 154, 1140–1171. [Google Scholar] [CrossRef]
- E Lacy, B.; A Saito, Y.; Camilleri, M.; Bouras, E.; DiBaise, J.K.; Herrick, L.M.; A Szarka, L.; Tilkes, K.; Zinsmeister, A.R.; Talley, N.J. Effects of antidepressants on gastric function in patients with functional dyspepsia. Off. J. Am. Coll. Gastroenterol. 2018, 113, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Sharbafchi, M.R.; Afshar, H.; Adhamian, P.; Feizi, A.; Daghaghzadeh, H.; Adibi, P. Effects of venlafaxine on gastrointestinal symptoms, depression, anxiety, stress, and quality of life in patients with the moderate-to-severe irritable bowel syndrome. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2020, 25, 115. [Google Scholar]
- Ford, A.C.; Wright-Hughes, A.; Alderson, S.L.; Ow, P.L.; Ridd, M.J.; Foy, R.; Bianco, G.; Bishop, F.L.; Cook, M.C.H.; Everitt, H.A.; et al. Amitriptyline at low-dose and titrated for irritable bowel syndrome as second-line treatment in primary care (ATLANTIS): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023, 402, 1773–1785. [Google Scholar] [CrossRef] [PubMed]
- Black, C.J.; Thakur, E.R.; A Houghton, L.; Quigley, E.M.M.; Moayyedi, P.; Ford, A.C. Efficacy of psychological therapies for irritable bowel syndrome: Systematic review and network meta-analysis. Gut 2020, 69, 1441–1451. [Google Scholar] [CrossRef]
- Lacy, B.E.; Pimentel, M.; Brenner, D.M.; Chey, W.D.; Keefer, L.A.; Long, M.D.; Moshiree, B. ACG clinical guideline: Management of irritable bowel syndrome. Off. J. Am. Coll. Gastroenterol. 2021, 116, 17–44. [Google Scholar] [CrossRef]
- Wei, L.; Singh, R.; Ro, S.; Ghoshal, U.C. Gut microbiota dysbiosis in functional gastrointestinal disorders: Underpinning the symptoms and pathophysiology. JGH Open 2021, 5, 976–987. [Google Scholar] [CrossRef]
- Chassard, C.; Dapoigny, M.; Scott, K.P.; Crouzet, L.; Del’Homme, C.; Marquet, P.; Martin, J.C.; Pickering, G.; Ardid, D.; Eschalier, A.; et al. Functional dysbiosis within the gut microbiota of patients with constipated-irritable bowel syndrome. Aliment. Pharmacol. Ther. 2012, 35, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.; Gdanetz, K.; Schmidt, A.W.; Schmidt, T.M. H2 generated by fermentation in the human gut microbiome influences metabolism and competitive fitness of gut butyrate producers. Microbiome 2023, 11, 133. [Google Scholar] [CrossRef] [PubMed]
- Miquel, S.; Martín, R.; Lashermes, A.; Gillet, M.; Meleine, M.; Gelot, A.; Eschalier, A.; Ardid, D.; Bermúdez-Humarán, L.G.; Sokol, H.; et al. Anti-nociceptive effect of Faecalibacterium prausnitzii in non-inflammatory IBS-like models. Sci. Rep. 2016, 6, 19399. [Google Scholar] [CrossRef] [PubMed]
- Meynier, M.; Daugey, V.; Mallaret, G.; Gervason, S.; Meleine, M.; Barbier, J.; Aissouni, Y.; Lolignier, S.; Bonnet, M.; Ardid, D.; et al. Pasteurized akkermansia muciniphila improves irritable bowel syndrome-like symptoms and related behavioral disorders in mice. Gut Microbes 2024, 16, 2298026. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crucillà, S.; Caldart, F.; Michelon, M.; Marasco, G.; Costantino, A. Functional Abdominal Bloating and Gut Microbiota: An Update. Microorganisms 2024, 12, 1669. https://doi.org/10.3390/microorganisms12081669
Crucillà S, Caldart F, Michelon M, Marasco G, Costantino A. Functional Abdominal Bloating and Gut Microbiota: An Update. Microorganisms. 2024; 12(8):1669. https://doi.org/10.3390/microorganisms12081669
Chicago/Turabian StyleCrucillà, Salvatore, Federico Caldart, Marco Michelon, Giovanni Marasco, and Andrea Costantino. 2024. "Functional Abdominal Bloating and Gut Microbiota: An Update" Microorganisms 12, no. 8: 1669. https://doi.org/10.3390/microorganisms12081669






