Lippia origanoides and Thymus vulgaris Essential Oils Synergize with Ampicillin against Extended-Spectrum Beta-Lactamase-Producing Escherichia coli
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Essential Oil Extraction (EO)
2.2. Gas Chromatography (GC) Analysis
2.3. Bacterial Strains
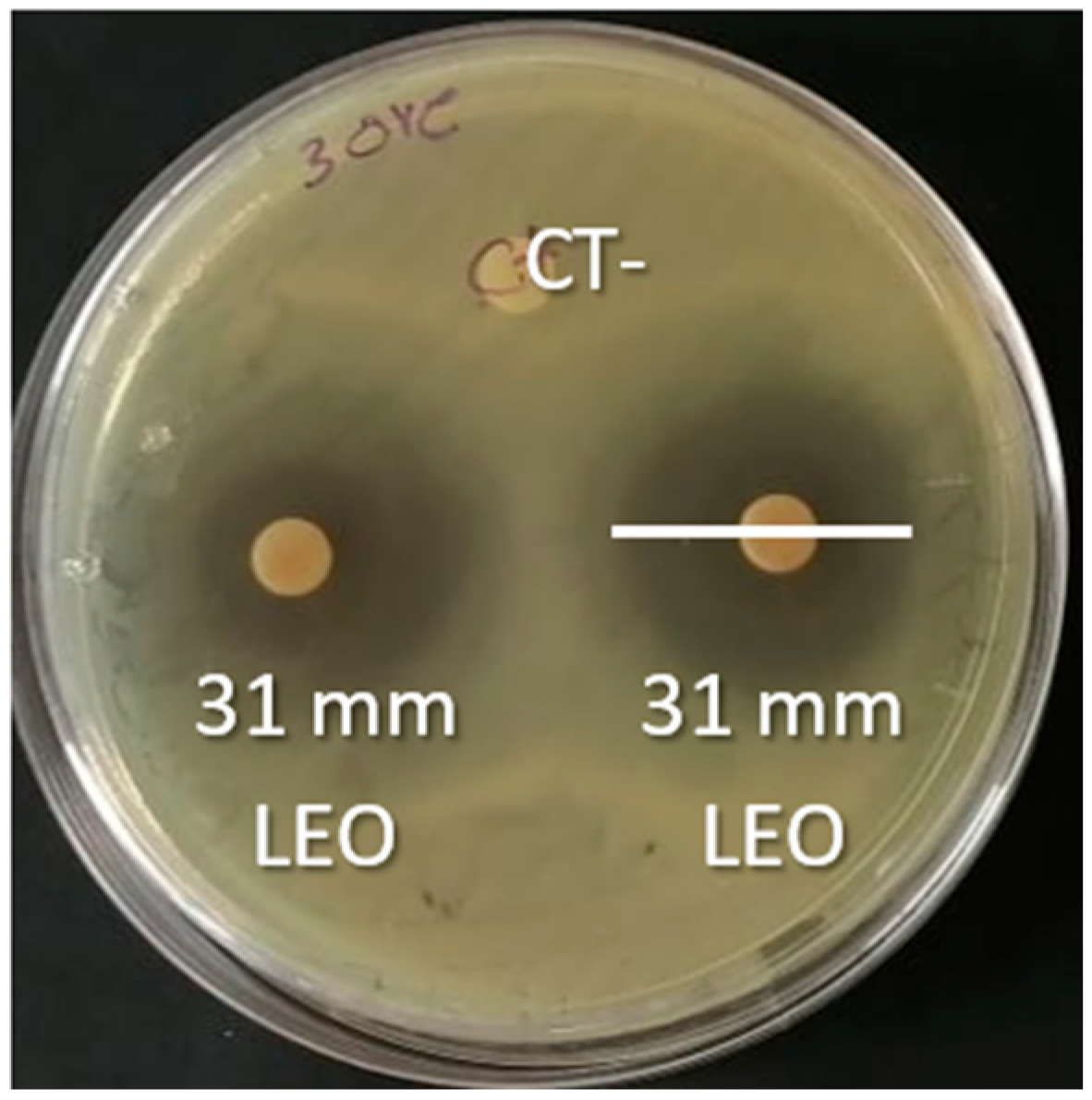
2.4. Antibacterial Activity by the Disk Diffusion Method
2.5. Minimal Inhibitory Concentration (MIC) and Minimal Bactericidal Concentration (MBC) of Essential Oils
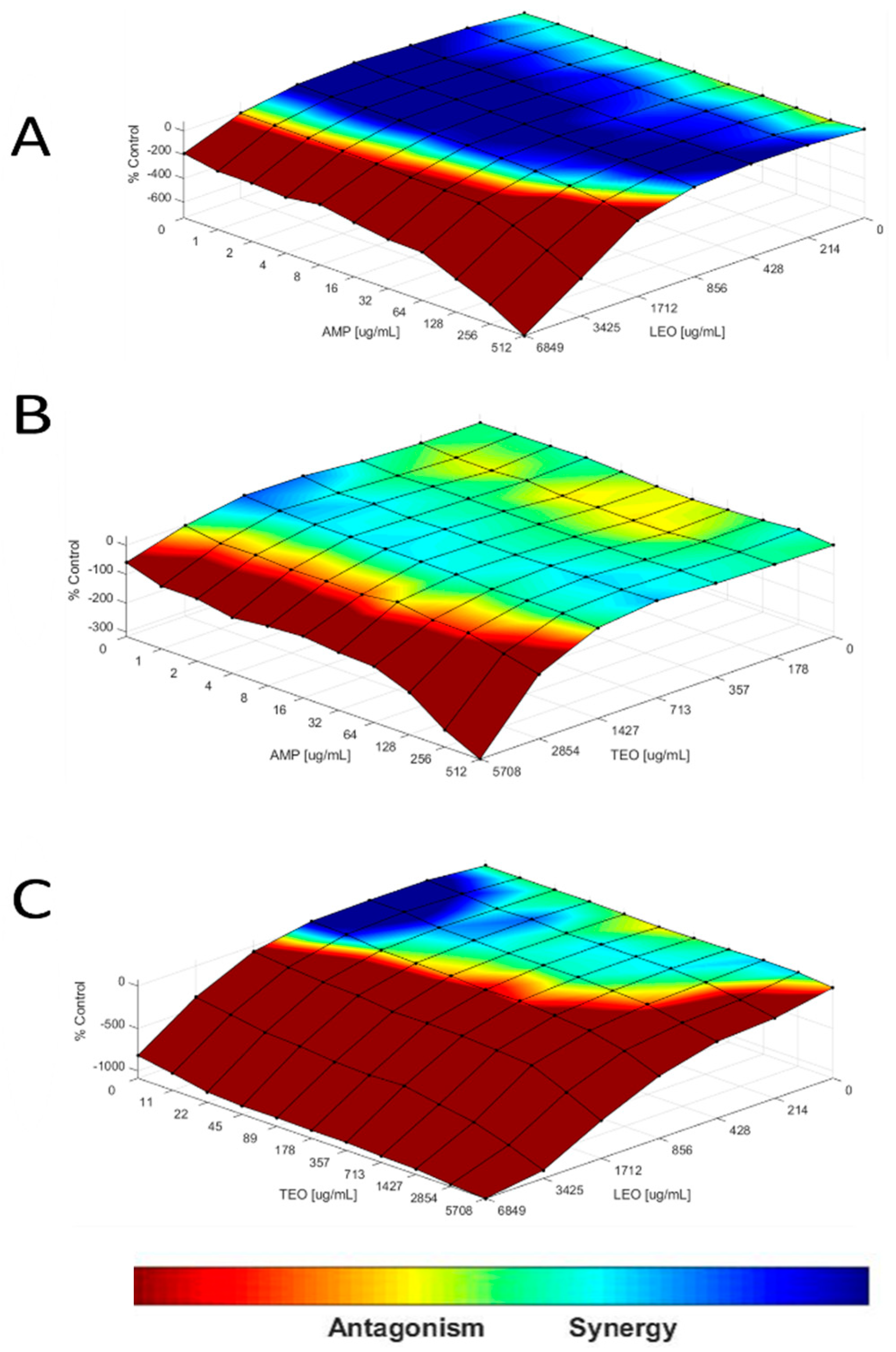
2.6. Interaction Assay
2.7. Loewe Additivity Model
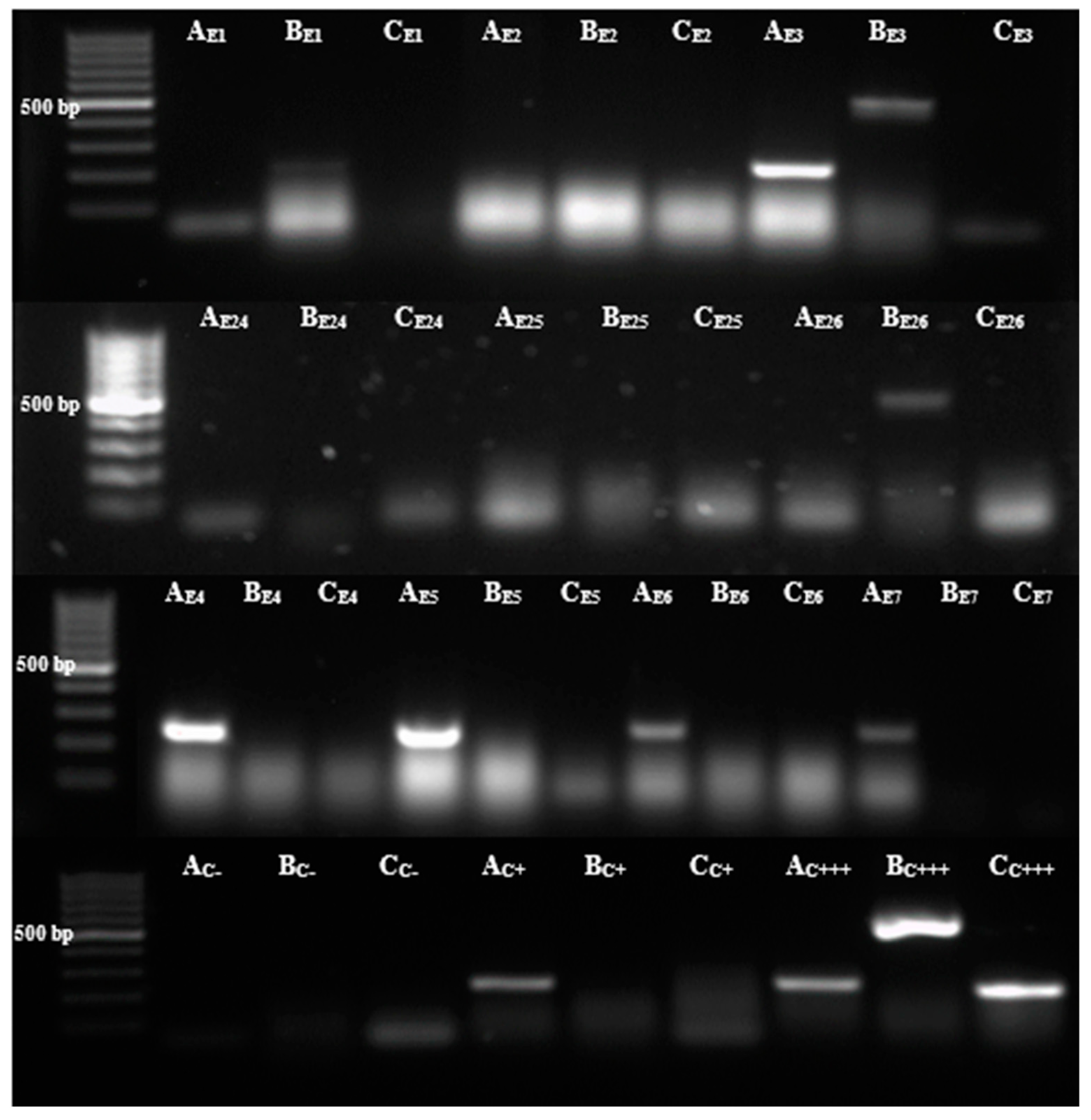
2.8. Detection of Antibiotic Resistance Genes β-Lactamics
2.9. Statistical Analysis
3. Results and Discussion
3.1. Carvacrol and Thymol Are the Major Components in LEO and TEO, Respectively
3.2. Bla Genes Are, in the Tested Clinical Isolates, Resistant to β-Lactam Antibiotics
3.3. Inhibitory Activity of TEO and LEO on ESBL Strains
3.4. ESBL-Producing E. coli Could Be Susceptible to T. vulgaris L. and L. origanoides EOs in Drug Interaction and In Vitro Susceptibility Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Madigan, M.T.; Guerrero, R.; Barrachina, C.; Ruiz Berraquero, F. Brock Biología de Los Microorganismos, 12th ed.; Pearson Educación: Madrid, Spain, 2009. [Google Scholar]
- del Arco, J. Antibióticos: Situación actual. Farm. Prof. 2014, 28, 29–33. [Google Scholar]
- Andersson, D.I.; Hughes, D. Selection and Transmission of Antibiotic-Resistant Bacteria. Microbiol Spectr. 2017, 5, 10–1128. [Google Scholar] [CrossRef]
- Oromí Durich, J. Resistencia bacteriana a los antibióticos. Med. Integral. 2000, 36, 367–370. [Google Scholar]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Gharavi, M.J.; Zarei, J.; Roshani-Asl, P.; Yazdanyar, Z.; Sharif, M.; Rashidi, N. Comprehensive study of antimicrobial susceptibility pattern and extended spectrum beta-lactamase (ESBL) prevalence in bacteria isolated from urine samples. Sci. Rep. 2021, 11, 578. [Google Scholar] [CrossRef]
- CDC. COVID-19: U.S. Impact on Antimicrobial Resistance, Special Report 2022; Department of Health and Human Services: Atlanta, GA, USA, 2022.
- Aouadhi, C.; Jouini, A.; Mechichi, D.; Boulares, M.; Hamrouni, S.; Maaroufi, A. Characterization of Primary Action Mode of Eight Essential Oils and Evaluation of Their Antibacterial Effect against Extended-Spectrum β-Lactamase (ESBL)-Producing Escherichia coli Inoculated in Turkey Meat. Molecules 2022, 27, 2588. [Google Scholar] [CrossRef]
- Yap, P.S.; Lim, S.H.; Hu, C.P.; Yiap, B.C. Combination of essential oils and antibiotics reduce antibiotic resistance in plasmid-conferred multidrug resistant bacteria. Phytomedicine 2013, 20, 710–713. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing. In CLSI Supplement M100, 28th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Bellio, P.; Fagnani, L.; Nazzicone, L.; Celenza, G. New and simplified method for drug combination studies by checkerboard assay. MethodsX 2021, 8, 101543. [Google Scholar] [CrossRef]
- Segatore, B.; Bellio, P.; Setacci, D.; Brisdelli, F.; Piovano, M.; Garbarino, J.A.; Nicoletti, M.; Amicosante, G.; Perilli, M.; Celenza, G. In vitro interaction of usnic acid in combination with antimicrobial agents against methicillin-resistant Staphylococcus aureus clinical isolates determined by FICI and ΔE model methods. Phytomedicine 2012, 19, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Bellio, P.; Segatore, B.; Mancini, A.; Di Pietro, L.; Bottoni, C.; Sabatini, A.; Brisdelli, F.; Piovano, M.; Nicoletti, M.; Amicosante, G.; et al. Interaction between lichen secondary metabolites and antibiotics against clinical isolates methicillin-resistant Staphylococcus aureus strains. Phytomedicine 2015, 22, 223–230. [Google Scholar] [CrossRef]
- Mejía-Argueta, E.L.; Santillán-Benítez, J.G.; Canales-Martinez, M.M.; Mendoza-Medellín, A. Antimicrobial activity of Syzygium aromaticum L. essential oil on extended-spectrum beta-lactamases-producing Escherichia coli. Bull. Natl. Res. Cent. 2020, 44, 201. [Google Scholar] [CrossRef]
- Lukas, B.; Schmiderer, C.; Novak, J. Essential oil diversity of European Origanum vulgare L. (Lamiaceae). Phytochemistry 2015, 119, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Calvo, I.L.; Parrak, T.V.; Acosta, A.V.; Escalante, E.F.; Díaz, V.L.; Dzib, G.; Peña, R.L. Phytochemical diversity of the essential oils of Mexican Oregano (Lippia graveolens Kunth) populations along an Edapho-climatic gradient. Chem. Biodivers. 2014, 11, 1010. [Google Scholar]
- Galovičová, L.; Borotová, P.; Valková, V.; Vukovic, N.L.; Vukic, M.; Štefániková, J.; Ďúranová, H.; Kowalczewski, P.; Čmiková, N.; Kačániová, M. Thymus vulgaris Essential Oil and Its Biological Activity. Plants 2021, 10, 1959. [Google Scholar] [CrossRef] [PubMed]
- Escobara, A.; Pérez, M.; Romanelli, G.; Guillermo, B. Thymol bioactivity: A review focusing on practical applications. Arab. J. Chem. 2020, 13, 9243–9269. [Google Scholar] [CrossRef]
- Kiskó, G.; Roller, S. Carvacrol and p-cymene inactivate Escherichia coli O157:H7 in apple juice. BMC Microbiol. 2005, 5, 36. [Google Scholar] [CrossRef]
- Mejía-Argueta, E.L.; Santillán-Benítez, J.G.; Mejía-Juárez, J. Identificación de cepas de E. coli productoras de betalactamasas de espectro extendido (BLEE) aisladas en el Centro Médico ISSEMyM de Toluca. CIENCIA Ergo-Sum. 2022, 29, e160. [Google Scholar] [CrossRef]
- Ballesteros-Monrreal, M.G.; Mendez-Pfeiffer, P.; Barrios-Villa, E.; Arenas-Hernández, M.M.P.; Enciso-Martínez, Y.; Sepúlveda-Moreno, C.O.; Bolado-Martínez, E.; Valencia, D. Uropathogenic Escherichia coli in Mexico, an Overview of Virulence and Resistance Determinants: Systematic Review and Meta-analysis. Arch. Med. Res. 2023, 54, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Garza-González, E.; Bocanegra-Ibarias, P.; Bobadilla-Del-Valle, M.; Ponce-de-León-Garduño, L.A.; Esteban-Kenel, V.; Silva-Sánchez, J.; Garza-Ramos, U.; Barrios-Camacho, H.; López-Jácome, L.E.; Colin-Castro, C.A.; et al. Drug resistance phenotypes and genotypes in Mexico in representative gram-negative species: Results from the infivar network. PLoS ONE 2021, 16, e0248614. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi, R.S.; Yaghoubi, A.; Amirfakhrian, R.; Hashemy, S.I.; Ghazvini, K. The prevalence of genes encoding ESBL among clinical isolates of Escherichia coli in Iran: A systematic review and meta-analysis. Gene Rep. 2020, 18, 100562. [Google Scholar] [CrossRef]
- Nagoor Meeran, M.F.; Javed, H.; Al Taee, H. Pharmacological Properties and Molecular Mechanisms of Thymol: Prospects for Its Therapeutic Potential and Pharmaceutical Development. Front. Pharmacol. 2017, 8, 380. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Rodríguez, S.E.; López-Rivera, R.J.; García-Márquez, E.; Estarrón-Espinosa, M.; Espinosa-Andrews, H. Mexican oregano (Lippia graveolens) essential oil-in-water emulsions: Impact of emulsifier type on the antifungal activity of Candida albicans. Food Sci. Biotechnol. 2019, 28, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Béjaoui, A.; Chaabane, H.; Jemli, M.; Boulila, A.; Boussaid, M. Essential oil composition and antibacterial activity of Origanum vulgare subsp. glandulosum Desf. at different phenological stages. J. Med. Food 2013, 16, 1115–1120. [Google Scholar] [CrossRef]
- Iseppi, R.; Mariani, M.; Condò, C.; Sabia, C.; Messi, P. Essential Oils: A Natural Weapon against Antibiotic-Resistant Bacteria Responsible for Nosocomial Infections. Antibiotics 2021, 10, 417. [Google Scholar] [CrossRef] [PubMed]
- The European Committee on Antimicrobial Susceptibility Testing. Antimicrobial Susceptibility Testing EUCAST Disk Diffusion Method. EUCAST.org, Version 12.0. 2024. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/2024_manuals/Slide_show_v_12.0_EUCAST_Disk_Test_2024.pdf (accessed on 8 August 2024).
- Mollea, C.; Bosco, F.; Fissore, D. Agar Plate Methods for Assessing the Antibacterial Activity of Thyme and Oregano Essential Oils against S. epidermidis and E. coli. Antibiotics 2022, 11, 1809. [Google Scholar] [CrossRef] [PubMed]
- Iseppi, R.; Di Cerbo, A.; Aloisi, P.; Man, M.; Pellesi, V.; Provenzano, C.; Sabia, C. In Vitro Activity of Essential Oils Against Planktonic and Biofilm Cells of Extended-Spectrum β-Lactamase (ESBL)/Carbapenamase-Producing Gram-Negative Bacteria Involved in Human Nosocomial Infections. Antibiotics 2020, 9, 272. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.J.; Livinghouse, T.; Goeres, D.M.; Mettler, M.; Stewart, P.S. Antimicrobial Activity of Naturally Occurring Phenols and Derivatives Against Biofilm and Planktonic Bacteria. Front. Chem. 2019, 7, 653. [Google Scholar] [CrossRef]
- Guarda, A.; Rubilar, J.F.; Miltz, J.; Galotto, M.J. The antimicrobial activity of microencapsulated thymol and carvacrol. Int. J. Food Microbiol. 2011, 146, 144–150. [Google Scholar] [CrossRef]
- Si, H.; Hu, J.; Liu, Z.; Zeng, Z.-l. Antibacterial e¡ect of oregano essential oil alone and in combination with antibiotics against extended-spectrum b-lactamase-producing Escherichia coli. Mmunol. Med. Microbiol. 2007, 53, 190–194. [Google Scholar]
- Yang, S.-K.; Yusoff, K.; Thomas, W.; Akseer, R.; Alhosani, M.S.; Abushelaibi, A.; Lim, S.-H.-E.; Lai, K.-S. Lavender essential oil induces oxidative stress which modifies the bacterial membrane permeability of carbapenemase producing Klebsiella pneumoniae. Sci. Rep. 2020, 10, 819. [Google Scholar] [CrossRef]
- Liu, M.; Pan, Y.; Feng, M.; Guo, W.; Fan, X.; Feng, L.; Huang, J.; Cao, Y. Garlic essential oil in water nanoemulsion prepared by high-power ultrasound: Properties, stability and its antibacterial mechanism against MRSA isolated from pork. Ultrason. Sonochem. 2022, 90, 106201. [Google Scholar] [CrossRef] [PubMed]
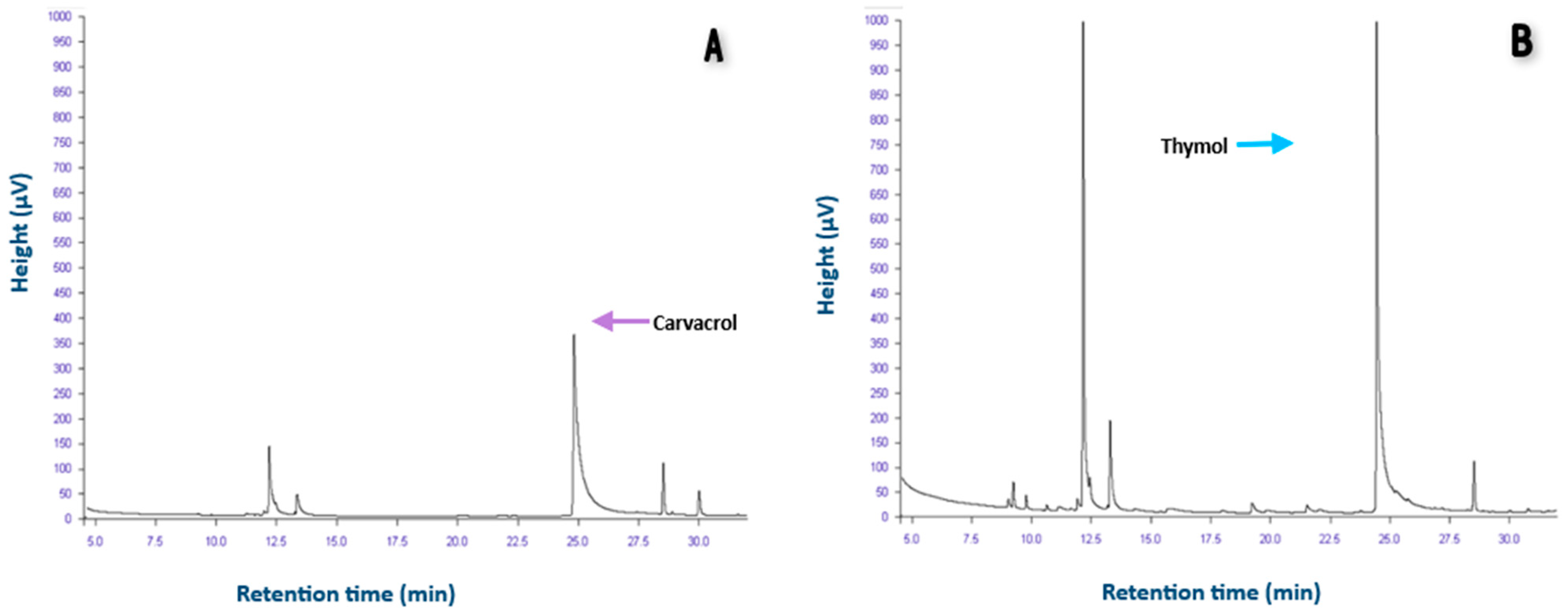
| Compound | LEO 1 (mg/L) | TEO 1 (mg/L) | LEO (%) | TEO (%) |
|---|---|---|---|---|
| Thymol | 62.9 ± 0.2 | 828.4 ± 0.4 | 0.005 | 39.9 |
| Carvacrol | 612.2 ± 0.2 | 112.4 ± 0.6 | 70.4 | 4.6 |
| p-cymene | -- | -- | 24.6 | 12.2 |
| Gene | LEO | TEO | ||
|---|---|---|---|---|
| MIC µg/mL | MBC µg/mL | MIC µg/mL | MBC µg/mL | |
| blaTEM | 632 | 1265 | 940 | 1275 |
| blaTEM + blaCTX-M | 892 | 1784 | 923 | 2215 |
| blaCTX-M | 713 | 1427 | 738 | 1477 |
| Unidentified | 815 | 1733 | 844 | 1582 |
| ATCC 25922 | 357 | 713 | 369 | 738 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bastida-Ramírez, L.J.; Buendía-González, L.; Mejía-Argueta, E.L.; Sandoval-Cabrera, A.; García-Fabila, M.M.; Pavón-Romero, S.H.; Padua-Ahumada, M.; Santillán-Benítez, J.G. Lippia origanoides and Thymus vulgaris Essential Oils Synergize with Ampicillin against Extended-Spectrum Beta-Lactamase-Producing Escherichia coli. Microorganisms 2024, 12, 1702. https://doi.org/10.3390/microorganisms12081702
Bastida-Ramírez LJ, Buendía-González L, Mejía-Argueta EL, Sandoval-Cabrera A, García-Fabila MM, Pavón-Romero SH, Padua-Ahumada M, Santillán-Benítez JG. Lippia origanoides and Thymus vulgaris Essential Oils Synergize with Ampicillin against Extended-Spectrum Beta-Lactamase-Producing Escherichia coli. Microorganisms. 2024; 12(8):1702. https://doi.org/10.3390/microorganisms12081702
Chicago/Turabian StyleBastida-Ramírez, Levi Jafet, Leticia Buendía-González, Euridice Ladisu Mejía-Argueta, Antonio Sandoval-Cabrera, María Magdalena García-Fabila, Sergio Humberto Pavón-Romero, Monica Padua-Ahumada, and Jonnathan Guadalupe Santillán-Benítez. 2024. "Lippia origanoides and Thymus vulgaris Essential Oils Synergize with Ampicillin against Extended-Spectrum Beta-Lactamase-Producing Escherichia coli" Microorganisms 12, no. 8: 1702. https://doi.org/10.3390/microorganisms12081702






