Dynamics of Cellulose Degradation by Soil Microorganisms from Two Contrasting Soil Types
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Soil Properties
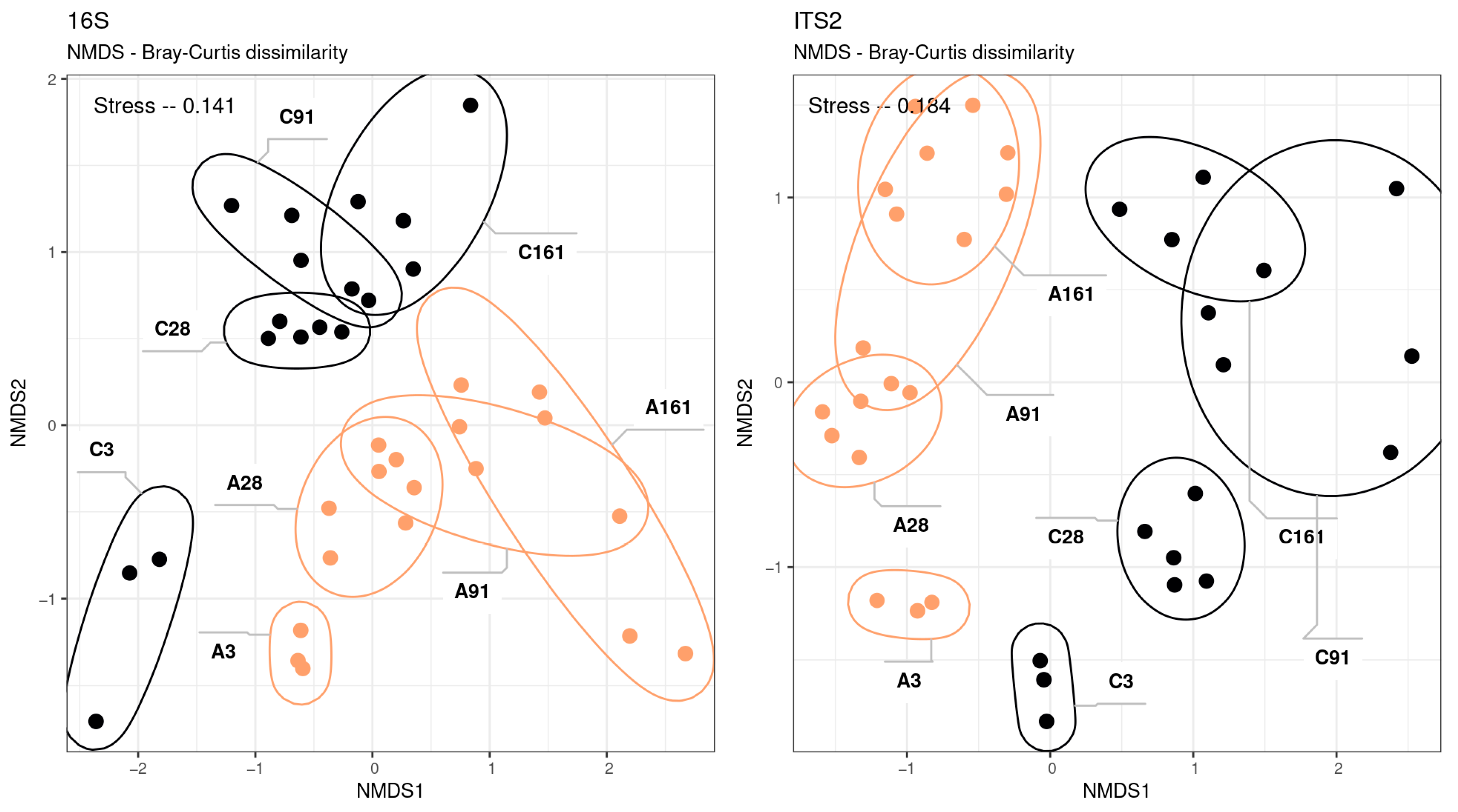
3.2. Amplicon Sequencing
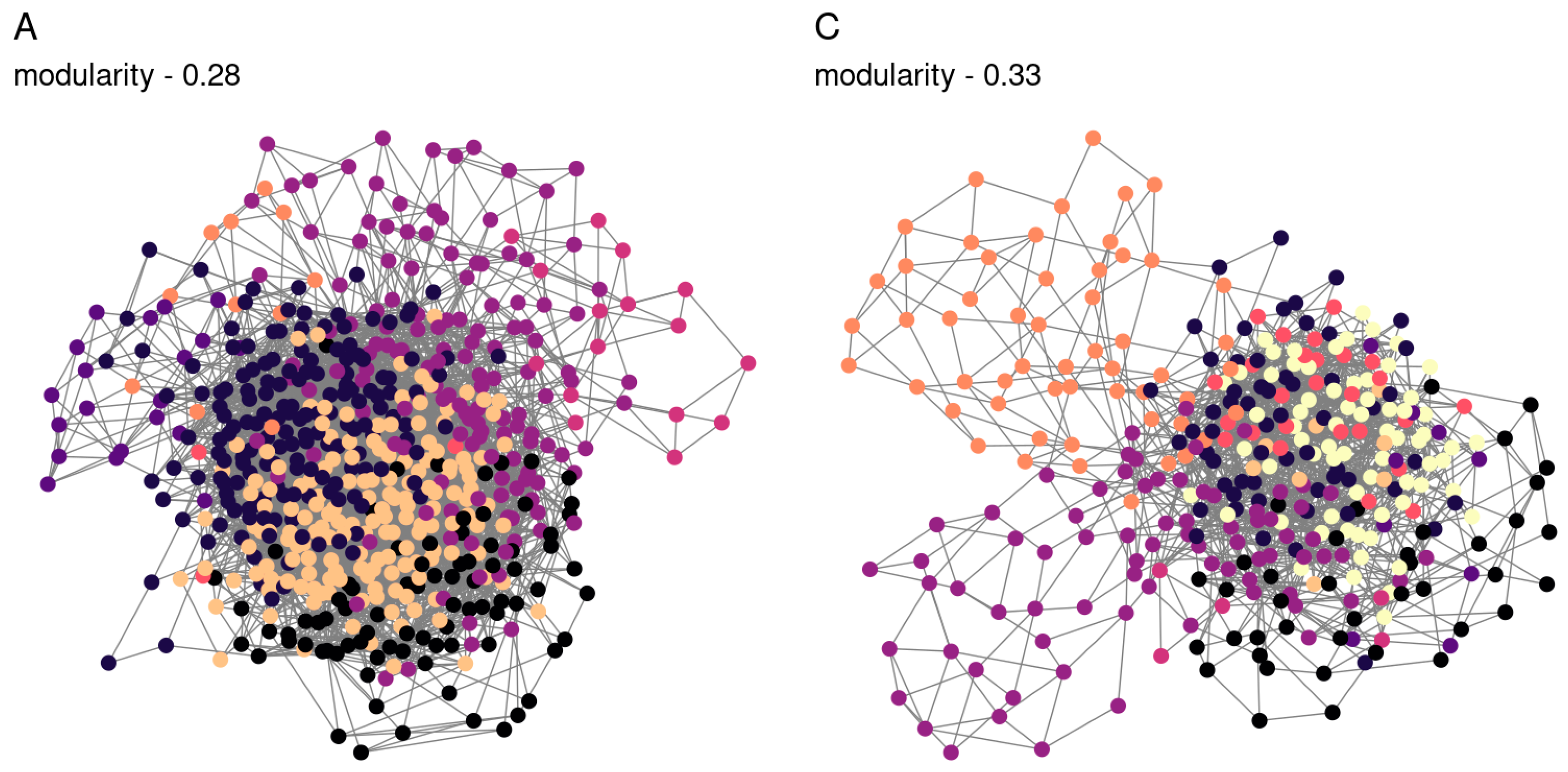
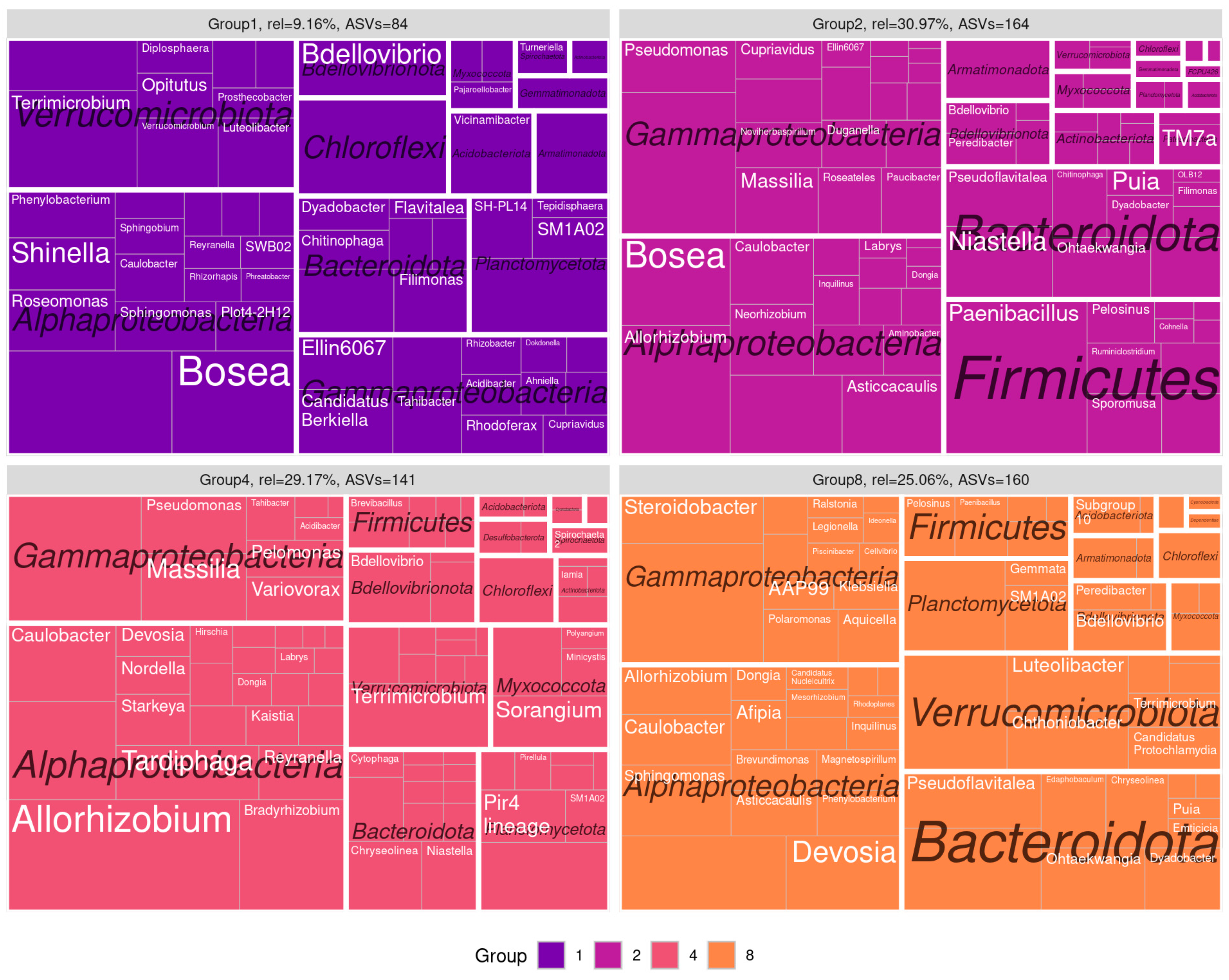
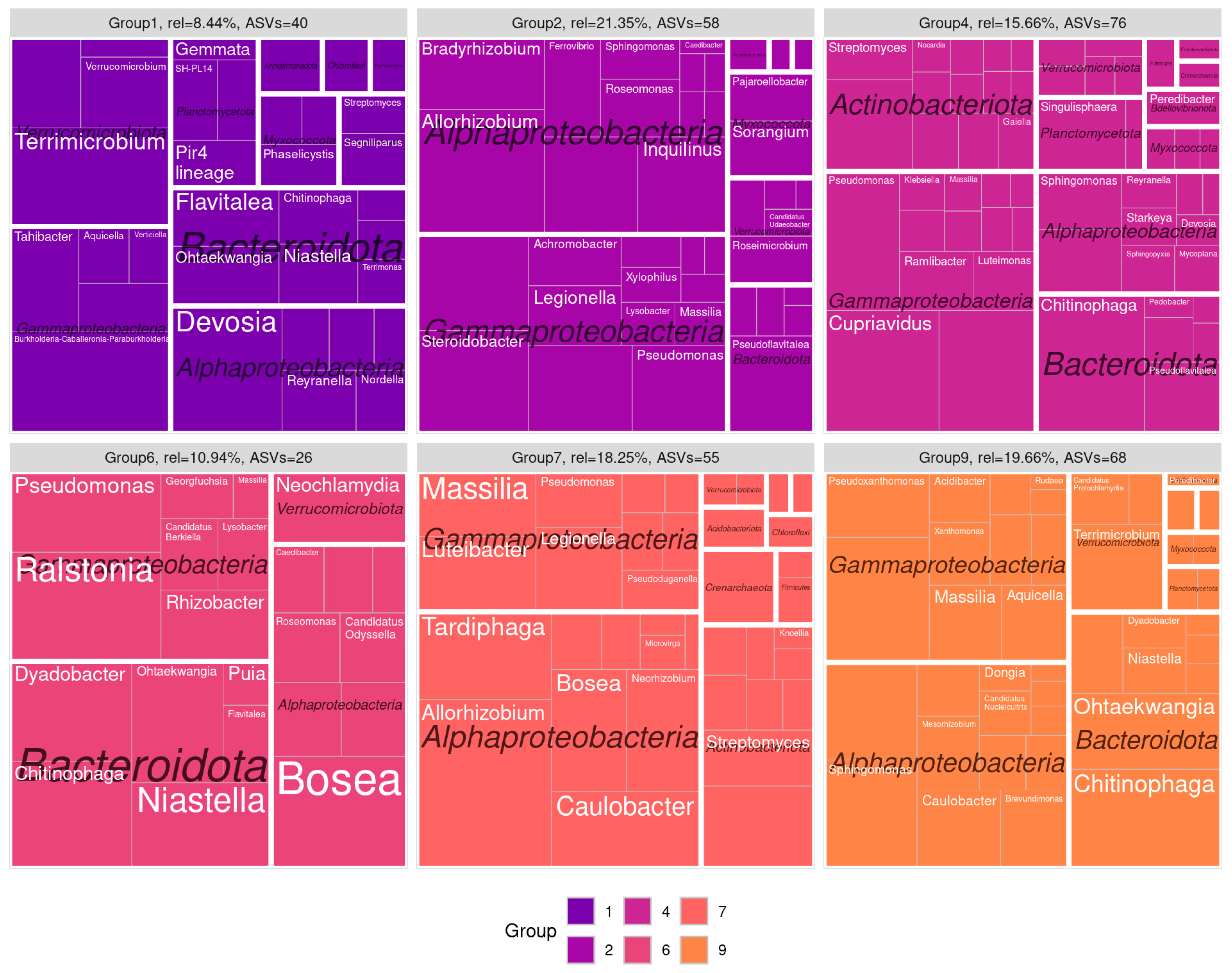
3.3. Co-Occurence Networks
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drula, E.; Garron, M.-L.; Dogan, S.; Lombard, V.; Henrissat, B.; Terrapon, N. The Carbohydrate-Active Enzyme Database: Functions and Literature. Nucleic Acids Res. 2022, 50, D571–D577. [Google Scholar] [CrossRef] [PubMed]
- Hayat, R.; Sheirdil, R.A.; Iftikhar-ul-Hassan, M.; Ahmed, I. Characterization and Identification of Compost Bacteria Based on 16S rRNA Gene Sequencing. Ann. Microbiol. 2013, 63, 905–912. [Google Scholar] [CrossRef]
- Soares, F.L.; Melo, I.S.; Dias, A.C.F.; Andreote, F.D. Cellulolytic Bacteria from Soils in Harsh Environments. World J. Microbiol. Biotechnol. 2012, 28, 2195–2203. [Google Scholar] [CrossRef]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The Carbohydrate-Active EnZymes Database (CAZy): An Expert Resource for Glycogenomics. Nucleic Acids Res. 2009, 37, D233–D238. [Google Scholar] [CrossRef]
- Terrapon, N.; Lombard, V.; Drula, É.; Lapébie, P.; Al-Masaudi, S.; Gilbert, H.J.; Henrissat, B. PULDB: The Expanded Database of Polysaccharide Utilization Loci. Nucleic Acids Res. 2018, 46, D677–D683. [Google Scholar] [CrossRef] [PubMed]
- Aakko, J.; Pietilä, S.; Toivonen, R.; Rokka, A.; Mokkala, K.; Laitinen, K.; Elo, L.; Hänninen, A. A Carbohydrate-Active Enzyme (CAZy) Profile Links Successful Metabolic Specialization of Prevotella to Its Abundance in Gut Microbiota. Sci. Rep. 2020, 10, 12411. [Google Scholar] [CrossRef]
- Štursová, M.; Žifčáková, L.; Leigh, M.B.; Burgess, R.; Baldrian, P. Cellulose Utilization in Forest Litter and Soil: Identification of Bacterial and Fungal Decomposers. FEMS Microbiol. Ecol. 2012, 80, 735–746. [Google Scholar] [CrossRef]
- Li, S.; Lian, W.-H.; Han, J.-R.; Ali, M.; Lin, Z.-L.; Liu, Y.-H.; Li, L.; Zhang, D.-Y.; Jiang, X.-Z.; Li, W.-J.; et al. Capturing the Microbial Dark Matter in Desert Soils Using Culturomics-Based Metagenomics and High-Resolution Analysis. Npj Biofilms Microbiomes 2023, 9, 67. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil Bacterial and Fungal Communities across a pH Gradient in an Arable Soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Kimeklis, A.K.; Gladkov, G.V.; Orlova, O.V.; Afonin, A.M.; Gribchenko, E.S.; Aksenova, T.S.; Kichko, A.A.; Pinaev, A.G.; Andronov, E.E. The Succession of the Cellulolytic Microbial Community from the Soil during Oat Straw Decomposition. Int. J. Mol. Sci. 2023, 24, 6342. [Google Scholar] [CrossRef]
- Ábrego-Gacía, A.; Poggi-Varaldo, H.M.; Mendoza-Vargas, A.; Mercado-Valle, F.G.; Ríos-Leal, E.; Ponce-Noyola, T.; Calva-Calva, G. Effects of Fermented Oat Straw as a Lovastatin Carrier on in Vitro Methane Production and Rumen Microbiota. Front. Energy Res. 2021, 9, 630701. [Google Scholar] [CrossRef]
- Hobbie, S.E. Interactions between Litter Lignin and Nitrogenitter Lignin and Soil Nitrogen Availability during Leaf Litter Decomposition in a Hawaiian Montane Forest. Ecosystems 2000, 3, 484–494. [Google Scholar] [CrossRef]
- Lebedeva, I.I.; Tonkonogov, V.D.; Gerasimova, M.I. A New Classification System of Soils of Russia: Preliminary Results of Discussion. Eurasian Soil Sci. 2008, 41, 93–99. [Google Scholar] [CrossRef]
- Semenov, M.V.; Chernov, T.I.; Tkhakakhova, A.K.; Zhelezova, A.D.; Ivanova, E.A.; Kolganova, T.V.; Kutovaya, O.V. Distribution of Prokaryotic Communities throughout the Chernozem Profiles under Different Land Uses for over a Century. Appl. Soil Ecol. 2018, 127, 8–18. [Google Scholar] [CrossRef]
- Du, Z.; Angers, D.A.; Ren, T.; Zhang, Q.; Li, G. The Effect of No-till on Organic C Storage in Chinese Soils Should Not Be Overemphasized: A Meta-Analysis. Agric. Ecosyst. Environ. 2017, 236, 1–11. [Google Scholar] [CrossRef]
- Orlova, O.V.; Kichko, A.A.; Pershina, E.V.; Pinaev, A.G.; Andronov, E.E. Succession of Bacterial Communities in the Decomposition of Oats Straw in Two Soils with Contrasting Properties. Eurasian Soil Sci. 2020, 53, 1620–1628. [Google Scholar] [CrossRef]
- Gladkov, G.; Kimeklis, A.; Zverev, A.; Pershina, E.; Ivanova, E.; Kichko, A.; Andronov, E.; Abakumov, E. Soil Microbiome of the Postmining Areas in Polar Ecosystems in Surroundings of Nadym, Western Siberia, Russia. Open Agric. 2019, 4, 684–696. [Google Scholar] [CrossRef]
- Kurtz, Z.D.; Müller, C.L.; Miraldi, E.R.; Littman, D.R.; Blaser, M.J.; Bonneau, R.A. Sparse and Compositionally Robust Inference of Microbial Ecological Networks. PLoS Comput. Biol. 2015, 11, e1004226. [Google Scholar] [CrossRef] [PubMed]
- Meinshausen, N.; Bühlmann, P. High-Dimensional Graphs and Variable Selection with the Lasso. Ann. Stat. 2006, 34, 1436–1462. [Google Scholar] [CrossRef]
- Csárdi, G.; Nepusz, T.; Müller, K.; Horvát, S.; Traag, V.; Zanini, F.; Noom, D. Igraph for R: R Interface of the Igraph Library for Graph Theory and Network Analysis. 2024. Available online: https://igraph.org (accessed on 15 July 2024).
- Lin, H.; Peddada, S.D. Analysis of Compositions of Microbiomes with Bias Correction. Nat. Commun. 2020, 11, 3514. [Google Scholar] [CrossRef]
- Xia, Q.; Rufty, T.; Shi, W. Soil Microbial Diversity and Composition: Links to Soil Texture and Associated Properties. Soil Biol. Biochem. 2020, 149, 107953. [Google Scholar] [CrossRef]
- Wolińska, A.; Kuźniar, A.; Zielenkiewicz, U.; Izak, D.; Szafranek-Nakonieczna, A.; Banach, A.; Błaszczyk, M. Bacteroidetes as a Sensitive Biological Indicator of Agricultural Soil Usage Revealed by a Culture-Independent Approach. Appl. Soil Ecol. 2017, 119, 128–137. [Google Scholar] [CrossRef]
- Moo-Young, M.; Chahal, D.S.; Swan, J.E.; Robinson, C.W. SCP Production by Chaetomium cellulolyticum, a New Thermotolerant Cellulolytic Fungus. Biotechnol. Bioeng. 1977, 19, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Mueller, G.M.; Foster, M.S.; Bills, G.F. Biodiversity of Fungi; Elsevier: Amsterdam, The Netherlands, 2004; ISBN 978-0-12-509551-8. [Google Scholar]
- Andersen, B.; Poulsen, R.; Hansen, G.H. Cellulolytic and Xylanolytic Activities of Common Indoor Fungi. Int. Biodeterior. Biodegrad. 2016, 107, 111–116. [Google Scholar] [CrossRef]
- Shokoohi, E.; Moyo, N.; Gouveia, F. Relationship of Nematodes in Natural and Disturbed Land with Physicochemical Properties in Magoebaskloof, Limpopo Province, South Africa. Biologia 2023, 78, 3223–3233. [Google Scholar] [CrossRef]
- Hidalgo, D.; Corona, F.; Martín-Marroquín, J.M. Manure Biostabilization by Effective Microorganisms as a Way to Improve Its Agronomic Value. Biomass Convers. Biorefinery 2022, 12, 4649–4664. [Google Scholar] [CrossRef]
- Pylak, M.; Oszust, K.; Frąc, M. Review Report on the Role of Bioproducts, Biopreparations, Biostimulants and Microbial Inoculants in Organic Production of Fruit. Rev. Environ. Sci. Biotechnol. 2019, 18, 597–616. [Google Scholar] [CrossRef]
- Kyrychenko, O.V. Market Analysis and Microbial Biopreparations Creation for Crop Growing in Ukraine. Biotechnol. Acta 2015, 8, 42–52. [Google Scholar] [CrossRef]
- Neher, D.A. Ecology of Plant and Free-Living Nematodes in Natural and Agricultural Soil. Annu. Rev. Phytopathol. 2010, 48, 371–394. [Google Scholar] [CrossRef]
- Balla, A.; Silini, A.; Cherif-Silini, H.; Bouket, A.C.; Boudechicha, A.; Luptakova, L.; Alenezi, F.N.; Belbahri, L. Screening of Cellulolytic Bacteria from Various Ecosystems and Their Cellulases Production under Multi-Stress Conditions. Catalysts 2022, 12, 769. [Google Scholar] [CrossRef]
- Vieira, S.; Sikorski, J.; Gebala, A.; Boeddinghaus, R.S.; Marhan, S.; Rennert, T.; Kandeler, E.; Overmann, J. Bacterial Colonization of Minerals in Grassland Soils Is Selective and Highly Dynamic. Environ. Microbiol. 2020, 22, 917–933. [Google Scholar] [CrossRef]
- Sanwal, K.C. A Key to the Species of the Nematode Genus Aphelenchoides Fischer, 1894. Can. J. Zool. 1961, 39, 143–148. [Google Scholar] [CrossRef]
- Tran, T.L.Q.; Anani, H.; Trinh, H.T.; Pham, T.P.T.; Dang, V.K.; Ho, V.M.; Bui, N.H.L.; Nguyen, N.H.; Raoult, D.; Trinh, T.T.; et al. Chitinophaga Vietnamensis Sp. Nov., a Multi-Drug Resistant Bacterium Infecting Humans. Int. J. Syst. Evol. Microbiol. 2020, 70, 1758–1768. [Google Scholar] [CrossRef] [PubMed]
- Hovde, B.T.; Steichen, S.A.; Starkenburg, S.R.; Brown, J.K. Vampirovibrio chlorellavorus Draft Genome Sequence, Annotation, and Preliminary Characterization of Pathogenicity Determinants. Phycol. Res. 2020, 68, 23–29. [Google Scholar] [CrossRef]
- Trifonova, T.; Kosmacheva, A.; Sprygin, A.; Chesnokova, S.; Byadovskaya, O. Enzymatic Activity and Microbial Diversity of Sod-Podzolic Soil Microbiota Using 16S rRNA Amplicon Sequencing Following Antibiotic Exposure. Antibiotics 2021, 10, 970. [Google Scholar] [CrossRef]
- Evdokimova, E.V.; Gladkov, G.V.; Kuzina, N.I.; Ivanova, E.A.; Kimeklis, A.K.; Zverev, A.O.; Kichko, A.A.; Aksenova, T.S.; Pinaev, A.G.; Andronov, E.E. The Difference between Cellulolytic ‘Culturomes’ and Microbiomes Inhabiting Two Contrasting Soil Types. PLoS ONE 2020, 15, e0242060. [Google Scholar] [CrossRef]
- Bao, Z.; Ikunaga, Y.; Matsushita, Y.; Morimoto, S.; Takada-Hoshino, Y.; Okada, H.; Oba, H.; Takemoto, S.; Niwa, S.; Ohigashi, K.; et al. Combined Analyses of Bacterial, Fungal and Nematode Communities in Andosolic Agricultural Soils in Japan. Microbes Environ. 2012, 27, 72–79. [Google Scholar] [CrossRef]
- Karpinska, A.; Ryan, D.; Germaine, K.; Dowling, D.; Forrestal, P.; Kakouli-Duarte, T. Soil Microbial and Nematode Community Response to the Field Application of Recycled Bio-Based Fertilisers in Irish Grassland. Sustainability 2021, 13, 12342. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, H.; Chen, L.; Yuan, Y.; Fang, H.; Luan, L.; Chen, Y.; Wang, X.; Liu, M.; Li, H.; et al. Nematodes and Microorganisms Interactively Stimulate Soil Organic Carbon Turnover in the Macroaggregates. Front. Microbiol. 2018, 9, 2803. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gladkov, G.V.; Kimeklis, A.K.; Orlova, O.V.; Lisina, T.O.; Kichko, A.A.; Bezlepsky, A.D.; Andronov, E.E. Dynamics of Cellulose Degradation by Soil Microorganisms from Two Contrasting Soil Types. Microorganisms 2024, 12, 1728. https://doi.org/10.3390/microorganisms12081728
Gladkov GV, Kimeklis AK, Orlova OV, Lisina TO, Kichko AA, Bezlepsky AD, Andronov EE. Dynamics of Cellulose Degradation by Soil Microorganisms from Two Contrasting Soil Types. Microorganisms. 2024; 12(8):1728. https://doi.org/10.3390/microorganisms12081728
Chicago/Turabian StyleGladkov, Grigory V., Anastasiia K. Kimeklis, Olga V. Orlova, Tatiana O. Lisina, Arina A. Kichko, Alexander D. Bezlepsky, and Evgeny E. Andronov. 2024. "Dynamics of Cellulose Degradation by Soil Microorganisms from Two Contrasting Soil Types" Microorganisms 12, no. 8: 1728. https://doi.org/10.3390/microorganisms12081728






