Differences in Lactation Performance, Rumen Microbiome, and Metabolome between Montbéliarde × Holstein and Holstein Cows under Heat Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Sample Collection
2.3. 16S rRNA Sequencing
2.4. LC-MS Analysis
2.5. Statistical Analysis
3. Results
3.1. THI and Physiological Parameters
3.2. Lactation Performance
3.3. Rumen Microbiome
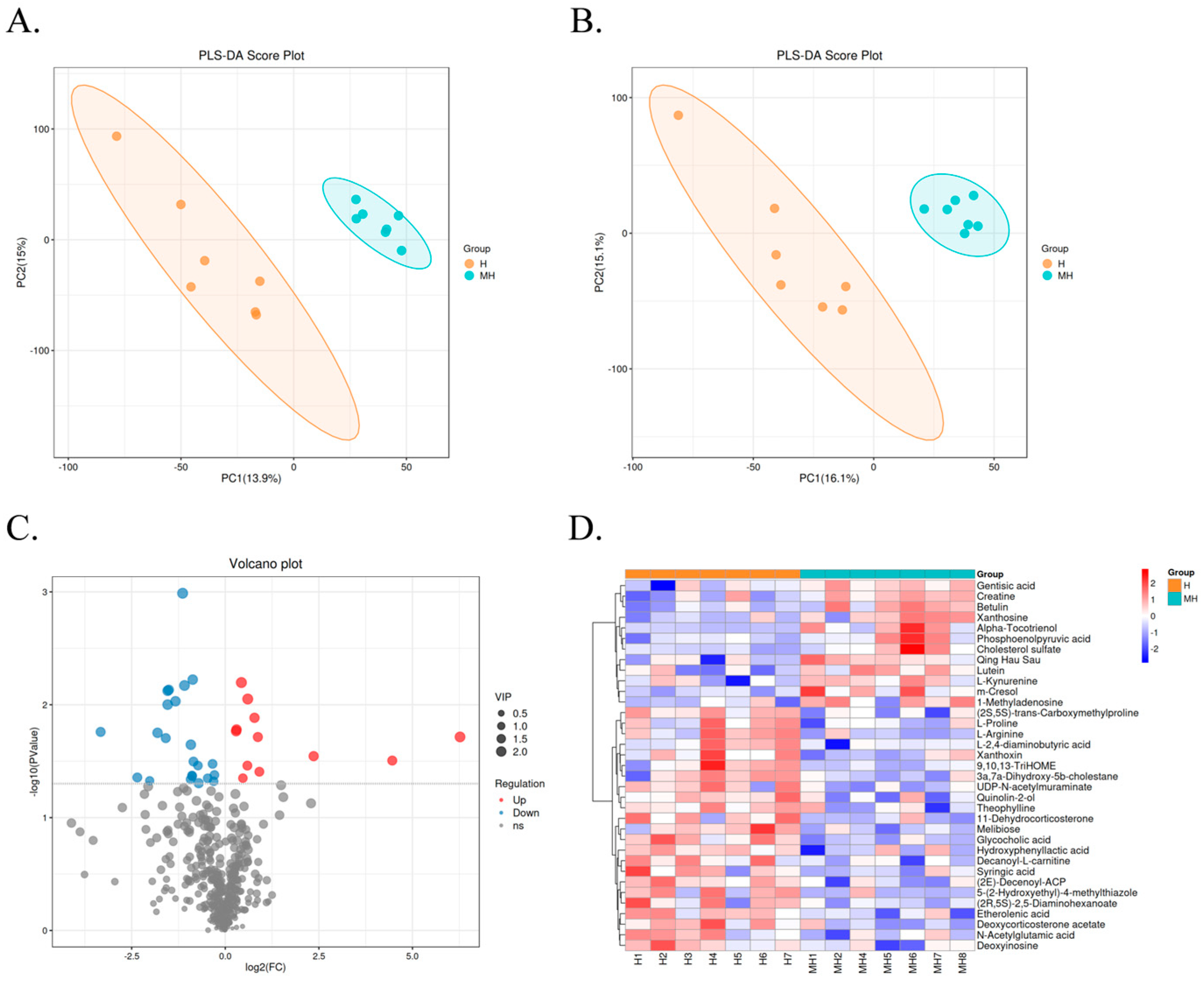
3.4. Rumen Metabolome
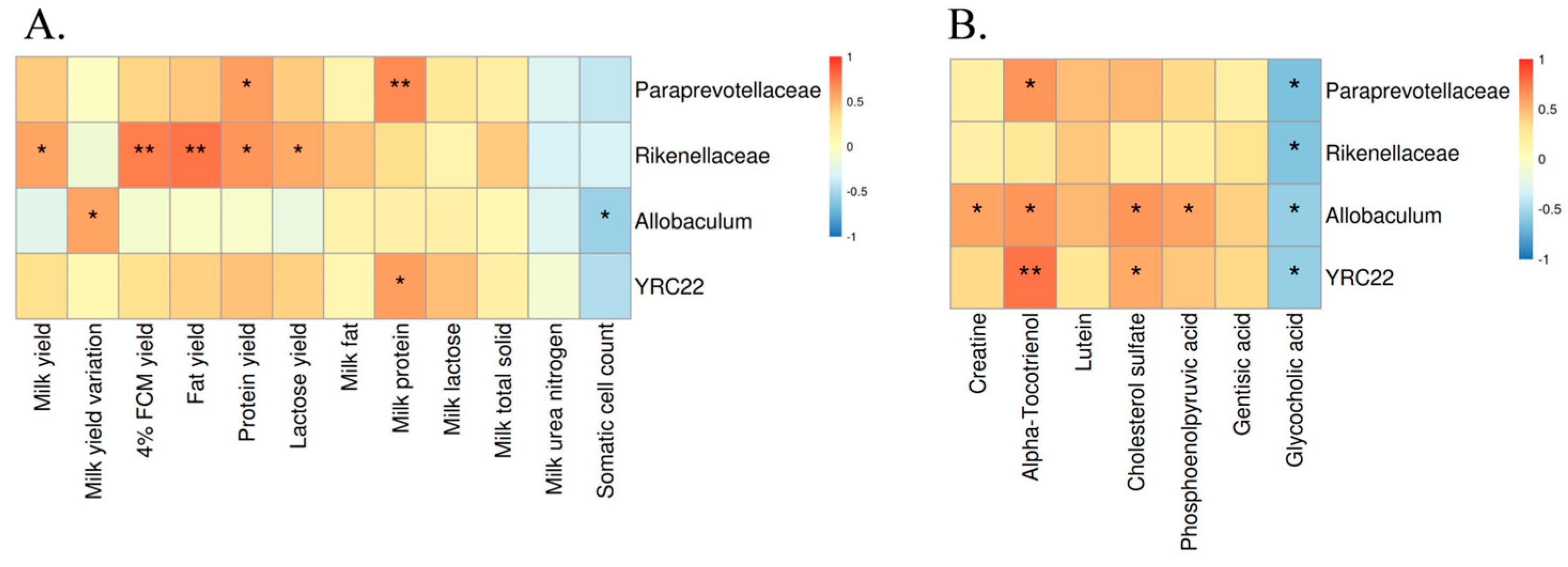
3.5. Correlation Analysis between Rumen Bacteria, Lactation Performance, and Metabolites
4. Discussion
4.1. Differences in Lactation Performance between Montbéliarde × Holstein and Holstein Cows under Heat Stress
4.2. Differences in Rumen Microbiome and Metabolome between Montbéliarde × Holstein and Holstein Cows under Heat Stress
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baile, C.A.; Forbes, J.M. Control of feed intake and regulation of energy balance in ruminants. Physiol. Rev. 1974, 54, 160–214. [Google Scholar] [CrossRef] [PubMed]
- Hammami, H.; Bormann, J.; M’hamdi, N.; Montaldo, H.H.; Gengler, N. Evaluation of heat stress effects on production traits and somatic cell score of Holsteins in a temperate environment. J. Dairy Sci. 2013, 96, 1844–1855. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Gao, S.; Ouyang, J.; Ma, L.; Bu, D. Impacts of Heat Stress-Induced Oxidative Stress on the Milk Protein Biosynthesis of Dairy Cows. Animals 2021, 11, 726. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, L.; Pang, F.; Zheng, Z.; Teng, Z.; Miao, T.; Fu, T.; Rushdi, H.E.; Yang, L.; Gao, T.; et al. Novel insights into heat tolerance using metabolomic and high-throughput sequencing analysis in dairy cows rumen fluid. Animal 2022, 16, 100478. [Google Scholar] [CrossRef] [PubMed]
- Gujar, G.; Tiwari, M.; Yadav, N.; Monika, D. Heat stress adaptation in cows—Physiological responses and underlying molecular mechanisms. J. Therm. Biol. 2023, 118, 103740. [Google Scholar] [CrossRef]
- Sørensen, M.K.; Norberg, E.; Pedersen, J.; Christensen, L.G. Invited Review: Crossbreeding in Dairy Cattle: A Danish Perspective. J. Dairy Sci. 2008, 91, 4116–4128. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, S.L.; Schmied, J.; Karrow, N.; Mallard, B.A. Impact of heat stress on dairy cattle and selection strategies for thermotolerance: A review. Front. Veter- Sci. 2023, 10, 1198697. [Google Scholar] [CrossRef]
- Daltro, D.D.; Fischer, V.; Alfonzo, E.P.M.; Dalcin, V.C.; Stumpf, M.T.; Kolling, G.J.; da Silva, M.; McManus, C. Infrared thermography as a method for evaluating the heat tolerance in dairy cows. Rev. Bras. De Zootec.-Braz. J. Anim. Sci. 2017, 46, 374–383. [Google Scholar] [CrossRef]
- Gu, Z.; Li, L.; Tang, S.; Liu, C.; Fu, X.; Shi, Z.; Mao, H. Metabolomics Reveals that Crossbred Dairy Buffaloes Are More Thermotolerant than Holstein Cows under Chronic Heat Stress. J. Agric. Food Chem. 2018, 66, 12889–12897. [Google Scholar] [CrossRef]
- Bang, N.N.; Gaughan, J.B.; Hayes, B.J.; Lyons, R.E.; McNeill, D.M. Application of infrared thermal technology to assess the level of heat stress and milk yield reduction of cows in tropical smallholder dairy farms. J. Dairy Sci. 2022, 105, 8454–8469. [Google Scholar] [CrossRef]
- Cuellar, C.J.; Saleem, M.; Jensen, L.M.; Hansen, P.J. Differences in body temperature regulation during heat stress and seasonal depression in milk yield between Holstein, Brown Swiss, and crossbred cows. J Dairy Sci 2023, 106, 3625–3632. [Google Scholar] [CrossRef]
- Kuczynska, B.; Puppel, K.; Golebiewski, M.; Kordyasz, M.; Grodzki, H.; Brzozowski, P. Comparison of fat and protein fractions of milk constituents in Montbeliarde and Polish Holstein-Friesian cows from one farm in Poland. Acta Vet. Brno 2012, 81, 139–144. [Google Scholar] [CrossRef]
- Dezetter, C.; Leclerc, H.; Mattalia, S.; Barbat, A.; Boichard, D.; Ducrocq, V. Inbreeding and crossbreeding parameters for production and fertility traits in Holstein, Montbéliarde, and Normande cows. J. Dairy Sci. 2015, 98, 4904–4913. [Google Scholar] [CrossRef]
- McCartney, C.A.; Bull, I.D.; Dewhurst, R.J. Chemical markers for rumen methanogens and methanogenesis. Animal 2013, 7, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Ramos, S.C.; Valencia, R.A.; Cho, Y.I.; Lee, S.S. Heat Stress: Effects on Rumen Microbes and Host Physiology, and Strategies to Alleviate the Negative Impacts on Lactating Dairy Cows. Front. Microbiol. 2022, 13, 804562. [Google Scholar] [CrossRef]
- Uyeno, Y.; Sekiguchi, Y.; Tajima, K.; Takenaka, A.; Kurihara, M.; Kamagata, Y. An rRNA-based analysis for evaluating the effect of heat stress on the rumen microbial composition of Holstein heifers. Anaerobe 2010, 16, 27–33. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Y.; Liu, W.; Du, D.; Jiang, W.; Wang, Z.; Li, N.; Hu, Z. Altered rumen microbiome and correlations of the metabolome in heat-stressed dairy cows at different growth stages. Microbiol. Spectr. 2023, 11, e0331223. [Google Scholar] [CrossRef]
- Chang, H.; Wang, X.; Zeng, H.; Zhai, Y.; Huang, N.; Wang, C.; Han, Z. Comparison of ruminal microbiota, metabolomics, and milk performance between Montbéliarde×Holstein and Holstein cattle. Front. Vet. Sci. 2023, 10, 1178093. [Google Scholar] [CrossRef]
- Council, N.R. A Guide to Environmental Research on Animals; The National Academies Press: Washington, DC, USA, 1971; p. 382. [Google Scholar]
- Brog, R.A. Is Fat-Corrected Milk Sufficient? J. Dairy Sci. 1971, 54, 1137–1141. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2-Approximately Maximum-Likelihood Trees for Large Alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef] [PubMed]
- Demurtas, A.; Pescina, S.; Nicoli, S.; Santi, P.; Ribeiro de Araujo, D.; Padula, C. Validation of a HPLC-UV method for the quantification of budesonide in skin layers. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2021, 1164, 122512. [Google Scholar] [CrossRef] [PubMed]
- Zelena, E.; Dunn, W.B.; Broadhurst, D.; Francis-McIntyre, S.; Carroll, K.M.; Begley, P.; O’Hagan, S.; Knowles, J.D.; Halsall, A.; Wilson, I.D.; et al. Development of a robust and repeatable UPLC-MS method for the long-term metabolomic study of human serum. Anal. Chem. 2009, 81, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J.K. Global metabolic profiling of animal and human tissues via UPLC-MS. Nat. Protoc. 2013, 8, 17–32. [Google Scholar] [CrossRef]
- Rasmussen, J.A.; Villumsen, K.R.; Ernst, M.; Hansen, M.; Forberg, T.; Gopalakrishnan, S.; Gilbert, M.T.P.; Bojesen, A.M.; Kristiansen, K.; Limborg, M.T. A multi-omics approach unravels metagenomic and metabolic alterations of a probiotic and synbiotic additive in rainbow trout (Oncorhynchus mykiss). Microbiome 2022, 10, 21. [Google Scholar] [CrossRef]
- Navarro-Reig, M.; Jaumot, J.; García-Reiriz, A.; Tauler, R. Evaluation of changes induced in rice metabolome by Cd and Cu exposure using LC-MS with XCMS and MCR-ALS data analysis strategies. Anal. Bioanal. Chem. 2015, 407, 8835–8847. [Google Scholar] [CrossRef]
- Armstrong, D.V. Heat stress interaction with shade and cooling. J. Dairy Sci. 1994, 77, 2044–2050. [Google Scholar] [CrossRef]
- Garner, J.B.; Douglas, M.L.; Williams, S.R.O.; Wales, W.J.; Marett, L.C.; Nguyen, T.T.T.; Reich, C.M.; Hayes, B.J. Genomic Selection Improves Heat Tolerance in Dairy Cattle. Sci. Rep. 2017, 6, 34114. [Google Scholar] [CrossRef]
- Becker, C.A.; Collier, R.J.; Stone, A.E. Invited review: Physiological and behavioral effects of heat stress in dairy cows. J. Dairy Sci. 2020, 103, 6751–6770. [Google Scholar] [CrossRef]
- Vitali, A.; Felici, A.; Lees, A.M.; Giacinti, G.; Maresca, C.; Bernabucci, U.; Gaughan, J.B.; Nardone, A.; Lacetera, N. Heat load increases the risk of clinical mastitis in dairy cattle. J. Dairy Sci. 2020, 103, 8378–8387. [Google Scholar] [CrossRef]
- Morse, D.; DeLorenzo, M.A.; Wilcox, C.J.; Collier, R.J.; Natzke, R.P.; Bray, D.R. Climatic Effects on Occurrence of Clinical Mastitis. J. Dairy Sci. 1988, 71, 848–853. [Google Scholar] [CrossRef]
- Xue, M.Y.; Sun, H.Z.; Wu, X.H.; Liu, J.X.; Guan, L.L. Multi-omics reveals that the rumen microbiome and its metabolome together with the host metabolome contribute to individualized dairy cow performance. Microbiome 2020, 8, 64. [Google Scholar] [CrossRef]
- Zhao, S.; Min, L.; Zheng, N.; Wang, J. Effect of Heat Stress on Bacterial Composition and Metabolism in the Rumen of Lactating Dairy Cows. Animals 2019, 9, 925. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Hu, X.; Bao, L.; Wu, K.; Zhao, Y.; Xiang, K.; Li, S.; Wang, Y.; Qiu, M.; Feng, L.; et al. Gut dysbiosis induces the development of mastitis through a reduction in host anti-inflammatory enzyme activity by endotoxemia. Microbiome 2022, 10, 205. [Google Scholar] [CrossRef]
- Perea, K.; Perz, K.; Olivo, S.K.; Williams, A.; Lachman, M.; Ishaq, S.L.; Thomson, J.; Yeoman, C.J. Feed efficiency phenotypes in lambs involve changes in ruminal, colonic, and small-intestine-located microbiota. J. Anim. Sci. 2017, 95, 2585–2592. [Google Scholar] [CrossRef]
- Zened, A.; Combes, S.; Cauquil, L.; Mariette, J.; Klopp, C.; Bouchez, O.; Troegeler-Meynadier, A.; Enjalbert, F. Microbial ecology of the rumen evaluated by 454 GS FLX pyrosequencing is affected by starch and oil supplementation of diets. FEMS Microbiol Ecol 2013, 83, 504–514. [Google Scholar] [CrossRef]
- Pujo, J.; Petitfils, C.; Le Faouder, P.; Eeckhaut, V.; Payros, G.; Maurel, S.; Perez-Berezo, T.; Van Hul, M.; Barreau, F.; Blanpied, C.; et al. Bacteria-derived long chain fatty acid exhibits anti-inflammatory properties in colitis. Gut 2021, 70, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Ni, J.; Zhang, M.; Xu, Y.; Li, Y.; Karim, N.; Chen, W. Mulberry Anthocyanins Ameliorate DSS-Induced Ulcerative Colitis by Improving Intestinal Barrier Function and Modulating Gut Microbiota. Antioxidants 2022, 11, 1674. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Luo, H.; Zhang, G.; Qiu, W.; Dong, Y.; Wang, Y.; Huang, X.; Wang, Y. Analysis of Differences in Hindgut Microbiota of Holstein Dairy Cows During Heat Stress. China Anim. Husb. Vet. Med. 2019, 46, 2273–2280. [Google Scholar] [CrossRef]
- Yi, X.; Cai, R.; Shaoyong, W.; Wang, G.; Yan, W.; He, Z.; Li, R.; Chao, M.; Zhao, T.; Deng, L.; et al. Melatonin promotes gut anti-oxidative status in perinatal rat by remodeling the gut microbiome. Redox Biol. 2023, 65, 102829. [Google Scholar] [CrossRef]
- Gaowa, N.; Panke-Buisse, K.; Wang, S.; Wang, H.; Cao, Z.; Wang, Y.; Yao, K.; Li, S. Brisket Disease Is Associated with Lower Volatile Fatty Acid Production and Altered Rumen Microbiome in Holstein Heifers. Animals 2020, 10, 1712. [Google Scholar] [CrossRef]
- Liu, H.; Bai, Y.; Yu, Y.; Qi, Z.; Zhang, G.; Li, G.; Yu, Y.; An, T. Maternal transfer of resorcinol-bis(diphenyl)-phosphate perturbs gut microbiota development and gut metabolism of offspring in rats. Environ. Int. 2023, 178, 108039. [Google Scholar] [CrossRef] [PubMed]
- Hua, C.; Tian, J.; Tian, P.; Cong, R.; Luo, Y.; Geng, Y.; Tao, S.; Ni, Y.; Zhao, R. Feeding a High Concentration Diet Induces Unhealthy Alterations in the Composition and Metabolism of Ruminal Microbiota and Host Response in a Goat Model. Front. Microbiol. 2017, 8, 138. [Google Scholar] [CrossRef]
- Gleason, C.B.; Settlage, R.E.; Beckett, L.M.; White, R.R. Characterizing Effects of Ingredients Differing in Ruminally Degradable Protein and Fiber Supplies on the Ovine Rumen Microbiome Using Next-Generation Sequencing. Front. Anim. Sci. 2021, 2, 745848. [Google Scholar] [CrossRef]
- Lawler, J.M.; Barnes, W.S.; Wu, G.; Song, W.; Demaree, S. Direct Antioxidant Properties of Creatine. Biochem. Biophys. Res. Commun. 2002, 290, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cui, Z.; Jiang, Y.; Aisikaer, A.; Wu, Q.; Zhang, F.; Wang, W.; Bo, Y.; Yang, H. Dietary Guanidine Acetic Acid Improves Ruminal Antioxidant Capacity and Alters Rumen Fermentation and Microflora in Rapid-Growing Lambs. Antioxidants 2023, 12, 772. [Google Scholar] [CrossRef]
- Packer, L.; Weber, S.U.; Rimbach, G. Molecular Aspects of α-Tocotrienol Antioxidant Action and Cell Signalling. J. Nutr. 2001, 131, 369S–373S. [Google Scholar] [CrossRef]
- Kamat, J.P.; Devasagayam, T.P.A. Tocotrienols from palm oil as potent inhibitors of lipid peroxidation and protein oxidation in rat brain mitochondria. Neurosci. Lett. 1995, 195, 179–182. [Google Scholar] [CrossRef]
- Xu, C.Z.; Wang, H.F.; Yang, J.Y.; Wang, J.H.; Duan, Z.Y.; Wang, C.; Liu, J.X.; Lao, Y. Effects of feeding lutein on production performance, antioxidative status, and milk quality of high-yielding dairy cows. J. Dairy Sci. 2014, 97, 7144–7150. [Google Scholar] [CrossRef]
- Xu, D.; Ma, R.; Ju, Y.; Song, X.; Niu, B.; Hong, W.; Wang, R.; Yang, Q.; Zhao, Z.; Zhang, Y.; et al. Cholesterol sulfate alleviates ulcerative colitis by promoting cholesterol biosynthesis in colonic epithelial cells. Nat. Commun. 2022, 13, 4428. [Google Scholar] [CrossRef]
- Morino, K.; Kunimura, K.; Sugiura, Y.; Izumi, Y.; Matsubara, K.; Akiyoshi, S.; Maeda, R.; Hirotani, K.; Sakata, D.; Mizuno, S.; et al. Cholesterol sulfate limits neutrophil recruitment and gut inflammation during mucosal injury. Front Immunol 2023, 14, 1131146. [Google Scholar] [CrossRef]
- Li, Y.J.; Zang, Y.T.; Zhao, X.H.; Liu, L.; Qiu, Q.H.; Ouyang, K.H.; Qu, M.R. Dietary Supplementation With Creatine Pyruvate Alters Rumen Microbiota Protein Function in Heat-Stressed Beef Cattle. Front. Microbiol. 2021, 12, 715088. [Google Scholar] [CrossRef] [PubMed]
- Saeedavi, M.; Goudarzi, M.; Fatemi, I.; Basir, Z.; Noori, S.M.A.; Mehrzadi, S. Gentisic acid mitigates gentamicin-induced nephrotoxicity in rats. Tissue Cell 2023, 84, 102191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tong, J.; Zhang, Y.; Xiong, B.; Jiang, L. Metabolomics reveals potential biomarkers in the rumen fluid of dairy cows with different levels of milk production. Asian-Australas. J. Anim. Sci. 2020, 33, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, C.; Xia, C.; Zhang, H.; Sun, L.; Gao, Y. Plasma metabolic profiling of dairy cows affected with clinical ketosis using LC/MS technology. Vet. Q. 2014, 34, 152–158. [Google Scholar] [CrossRef]


| Items | H (n = 13) | MH (n = 13) | p-Value |
|---|---|---|---|
| Respiratory rate (bpm) | 82.35 ± 2.16 | 71.98 ± 1.54 | <0.001 |
| Rectal temperature (°C) | 39.34 ± 0.08 | 39.08 ± 0.05 | 0.011 |
| Items | H (n = 13) | MH (n = 13) | p-Value |
|---|---|---|---|
| Yield, kg/d | |||
| Milk | 39.55 ± 1.52 | 43.12 ± 1.44 | 0.101 |
| Milk yield variation 1 | −3.50 ± 1.35 | −0.01 ± 0.80 | 0.035 |
| 4% FCM | 36.46 ± 1.45 | 40.66 ± 1.33 | 0.044 |
| Fat | 1.38 ± 0.07 | 1.56 ± 0.06 | 0.048 |
| Protein | 1.25 ± 0.05 | 1.42 ± 0.05 | 0.021 |
| Lactose | 1.96 ± 0.08 | 2.17 ± 0.07 | 0.060 |
| Milk composition, % | |||
| Fat | 3.51 ± 0.16 | 3.63 ± 0.08 | 0.493 |
| Protein | 3.17 ± 0.05 | 3.29 ± 0.03 | 0.044 |
| Lactose | 4.96 ± 0.02 | 5.03 ± 0.03 | 0.049 |
| Total solid | 12.53 ± 0.19 | 12.85 ± 0.10 | 0.145 |
| Milk urea nitrogen, mg/dL | 12.78 ± 0.40 | 12.34 ± 0.28 | 0.384 |
| Somatic cell count, 104/mL 2 | 10.33 (65.48) | 4.39 (2.63) | 0.002 |
| Items | H (n = 7) | MH (n = 7) | p-Value |
|---|---|---|---|
| Goods_coverage | 0.99 (0.00) | 0.99 (0.01) | 0.482 |
| Simpson | 0.99 (0.01) | 0.99 (0.00) | 0.655 |
| Shannon | 8.91 (0.78) | 8.97 (0.33) | 0.406 |
| Chao1 | 2074.14 (525.58) | 1930.04 (270.25) | 0.949 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weng, H.; Zeng, H.; Wang, H.; Chang, H.; Zhai, Y.; Li, S.; Han, Z. Differences in Lactation Performance, Rumen Microbiome, and Metabolome between Montbéliarde × Holstein and Holstein Cows under Heat Stress. Microorganisms 2024, 12, 1729. https://doi.org/10.3390/microorganisms12081729
Weng H, Zeng H, Wang H, Chang H, Zhai Y, Li S, Han Z. Differences in Lactation Performance, Rumen Microbiome, and Metabolome between Montbéliarde × Holstein and Holstein Cows under Heat Stress. Microorganisms. 2024; 12(8):1729. https://doi.org/10.3390/microorganisms12081729
Chicago/Turabian StyleWeng, Hantong, Hanfang Zeng, Haihui Wang, Haomiao Chang, Yunfei Zhai, Shujie Li, and Zhaoyu Han. 2024. "Differences in Lactation Performance, Rumen Microbiome, and Metabolome between Montbéliarde × Holstein and Holstein Cows under Heat Stress" Microorganisms 12, no. 8: 1729. https://doi.org/10.3390/microorganisms12081729





