Single Cell Expression Systems for the Production of Recombinant Proteins for Immunodiagnosis and Immunoprophylaxis of Toxoplasmosis
Abstract
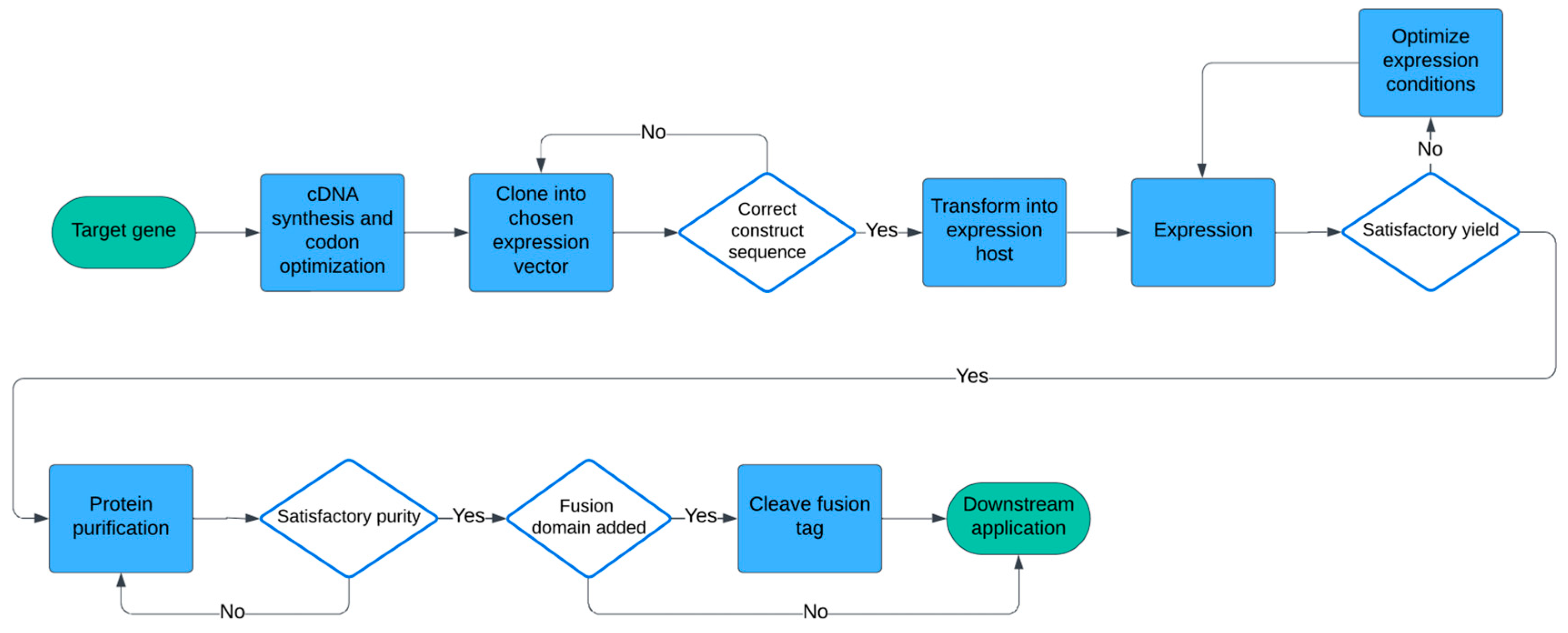
:1. Introduction
2. Materials and Methods
3. Prokaryotic Expression Systems
3.1. E. coli Expression Systems
T7 System
| Plasmid Vector | E. coli Host Strain | Protein | Expression Conditions | Yield | Application | Results | Reference, Year | |
|---|---|---|---|---|---|---|---|---|
| Diagnostic | Vaccine | |||||||
| pET29 | BL21 (DE3) pLysS | S-B10a-6xHis | 37 °C, 3 h | − | + | − | Strong immunoreactivity with human sera from both chronic and acute infections. | 1998, [49] |
| pUET1 | Rosetta (DE3) pLysS | 6xHis-GRA630–231-6xHis 6xHis-P35b26–170-6xHis 6xHis-SAG230–170-6xHis | LB, 30 °C, 8 h LB, 30 °C, 8 h LB, 37 °C, 3 h | 60–80 mg/L induced bacterial culture | + | − | Both r-GRA6 and r-p35 antigens detected antibodies more frequently (p < 0.01) from acute (93.9 and 87.9%) rather than chronic (60.6 and 53.0%) infections. The r-SAG2 gave a similar sensitivity in both groups of patients (93.9 and 95.5%) Both r-GRA6 and r-p35 antigens detected antibodies more frequently (p < 0.01) from acute (93.9 and 87.9%) rather than chronic (60.6 and 53.0%) infections. The r-SAG2 gave a similar sensitivity in both groups of patients (93.9 and 95.5%) GRA6 and p35 detected antibodies more frequently in acute infections (93.9% and 87.9%) compared to chronic infections (60.6% and 53.0%). SAG2 showed similar sensitivity in both acute and chronic cases (93.9% and 95.5%). | 2005, [50] |
| pET32a(+) | BL21 (DE3) | TRX-(Hisx6)-GRA2 | LB, 37 °C, 3 h | 12 mg/L induced bacterial culture | + | − | Specificity of IgG ELISA was 96.4%. Sensitivity was 95.8% to 100% for acute infection sera and 65.7% to 71.4% for chronic infection sera. | 2007, [51] |
| pUET1 | Rosetta (DE3) pLysS | 6xHis-MAG130–222-6xHis | LB, 37 °C, 16 h | 90 mg/L induced bacterial culture | + | − | Can distinguish between acute (97.3% sensitivity) and chronic (7.5% sensitivity) phases of toxoplasmosis. | 2007, [52] |
| pUET1 | Rosetta (DE3) pLysS | 6xHis-MIC125–182-6xHis 6xHis-MIC1183–456-6xHis 6xHis-MIC125–456-6xHis | LB, 37 °C | 16–24 mg/L induced bacterial culture | + | − | The three recombinant MIC1 proteins showed similar antigenicity for acute toxoplasmosis sera, but chronic infection sera had significantly lower sensitivity. | 2008, [53] |
| pET-28b(+) | Rosetta (DE3) | 6xHis-GRA718–236-6xHis | LB, 37 °C, 5 h | − | + | − | Western blot results indicated strong recognition of GRA7 by acute sera, weak detection by chronic sera, and no specific bands in negative sera. | 2009, [54] |
| pUET1 | Rosetta (DE3) pLysS | 6xHis-ROP185–396-6xHis 6xHis-GRA224–185-6xHis | LB, 37 °C, 16 h | 16 mg/L 28 mg/L induced bacterial culture | + | − | GRA2 and ROP1 showed higher sensitivity in acute infection sera (100% and 94.6%) than in chronic infection sera (22.5% and 15.5%). | 2009, [55] |
| pUET1 | BL21 (DE3) pLysS | 6xHis-GRA526–120-6xHis | LB, 30 °C, 8 h | 15 mg/L induced bacterial culture | + | − | Anti-GRA5 IgG antibodies were found in 70.9% of seropositive samples, similar to TLA-ELISA results. | 2010, [56] |
| pET-28b(+) | Rosetta (DE3) | 6xHis-S-GRA823–169-6xHis | LB, 30 °C, 5 h | 68 mg/L induced bacterial culture | + | − | IgM GRA8-ELISA had 97.1% specificity and 60.6% sensitivity. | 2011, [57] |
| pET-32c | BL21 (DE3) | Trx-(Hisx6)-SAG1309–318-SAG2109–118-SAG3347–356-6xHis | LB, 37 °C, 4 h | − | + | − | IgG ELISA showed 94.4% sensitivity and 100% specificity. IgM ELISA showed 96.9% sensitivity and 100% specificity | 2012, [58] |
| pUET1 | Rosetta (DE3) pLacI | 6xHis-MIC125–182-MAG130–222-6xHis | LB, 37 °C, 16 h | 43 mg/L induced bacterial culture | + | − | The IgG MIC1-MAG1-ELISA showed 90.8% sensitivity, similar to TLA (91.8%) and higher than MIC1, MAG1, or their mixture. | 2012, [59] |
| pUET1 | - | 6xHis-MIC125–182-MAG130–222-SAG149–198-6xHis | − | 20 mg/L induced bacterial culture | + | − | The MIC1-MAG1-SAG1-ELISA had 100% specificity and nearly 100% sensitivity, outperforming assays with just MIC1-MAG1 protein. | 2012, [60] |
| pET-30a(+) | Rosetta (DE3) | 6xHis-PDIc-6xHis | 25 °C, 8 h | − | − | + | Nasal immunization with PDI induced a protective immune response in mice, increasing the 30-day survival rate by about 31% compared to the control group. | 2013, [61] |
| pET-32a | BL21 (DE3) pLysS | 6xHis-GRA4-6xHis | LB, 6–8 h | − | − | + | Subcutaneous immunization induced high IgG levels, which declined after one week, and elevated IFN-γ, interleukin (IL) 10, and IL-4. However, it did not confer protection against T. gondii in mice. | 2013, [62] |
| pET-30 Ek/LIC | Rosetta (DE3) pLacI | 6xHis-SAG2-GRA1-ROP185–396-6xHis 6xHis-SAG2-GRA1-ROP185–250-6xHis | LB, 30 °C, 18 h | 31 mg/L 33 mg/L induced bacterial culture | + | − | IgG ELISA using SAG2-GRA1-ROP185–396—100%, sensitivity. IgG ELISA using SAG2-GRA1-ROP185–250—88.4% sensitivity. | 2015, [63] |
| pUET1 | Rosetta (DE3) pLacI | 6xHis-P3526–170-MAG130–222-6xHis 6xHis-MIC125–182-ROP1113–295-6xHis 6xHis-MAG130–222-ROP1113–295-6xHis | LB, 37 °C, 16 h | 43 mg/L 25 mg/L 36 mg/L induced bacterial culture | + | − | The reactivity of IgG ELISA for acute toxoplasmosis sera was 100% for P35-MAG1, 77.3% for MIC1-ROP1, and 86.4% for MAG1-ROP1, significantly higher than for chronic infection sera (26.2%, 36.1%, and 32.8%, respectively). IgM ELISA using P35-MAG1, MIC1-ROP1, and MAG1-ROP1 had a sensitivity of 81.8%, 72.7%, and 59.1%, respectively | 2015, [64] |
| pET-30a(+) | BL21 (DE3) | 6xHis-ADFd-6xHis | LB, 25 °C, 12 h | − | − | + | Intranasal immunization increased secretory IgA, IgG titers, splenocyte proliferation, and IL-2 and IFN-γ secretion. It improved survival by 36.36% and reduced liver and brain tachyzoite loads by 67.77% and 51.01%. | 2016, [65] |
| pET-28α | BL21 (DE3) | 6xHis-ROP18-6xHis | LB, 30 °C, 6 h | − | − | + | Intranasal immunizations with ROP18 in nanospheres induced higher levels of IgA and IgG2a as compared to groups inoculated intranasally with ROP18 alone or subcutaneously injected with ROP18 in montanide adjuvant. | 2017, [66] |
| pET-28α | BL21 (DE3) | 6xHis-SAG1-6xHis | LB, 30 °C | − | − | + | Intranasal immunization with SAG1 in nanospheres induced higher specific IgA and IgG2a responses compared to controls. | 2018, [67] |
| pET28a | BL21 | 6xHis-CDPK3e-6xHis | LB, 30 °C, 6 h | − | − | + | Intramuscular immunization induced spleen cell proliferation, IFN-γ release, and high IgG titers. It partially protected against acute toxoplasmosis, reducing brain cysts by 46.5%. | 2019, [68] |
| pET28a | BL21 (DE3) | 6xHis-GRA2-6xHis 6xHis-GRA7-6xHis 6xHis-TPIf-6xHis | LB, 37 °C, 4 h | − | + | − | The sensitivities of GRA2, GRA7, TPI, and their mixture were 85%, 83.3%, 88.3%, and 96.7%, respectively, with specificities of 85%, 90%, 100%, and 100%. GRA2 and GRA7 showed cross-reactivity. | 2019, [69] |
| pET-30 Ek/LIC | Rosetta (DE3) pLacI | 6xHis -SAG2-GRA1-ROP1-AMA167–287-6xHis 6xHis-AMA167–287-SAG2-GRA1-ROP1-6xHis 6xHis-AMA1287–569-SAG2-GRA1-ROP1-6xHis 6xHis-AMA167–569-SAG2-GRA1-ROP1-6xHis | TB, 23 °C, 18 h | 11–23 mg/L induced bacterial culture | + | − | All chimeric proteins demonstrated 100% sensitivity and specificity in IgG ELISA. Avidity results were comparable to commercial assays. | 2019, [70] |
| pET-30 Ek/LIC | Rosetta (DE3) pLysS | 6xHis-AMA167–287-6xHis 6xHis-AMA1287–569-6xHis 6xHis-AMA167–569-6xHis | LB, 30 °C, 4 h | 33 mg/L 31 mg/L 15 mg/L induced bacterial culture | + | − | The full-length AMA1 antigen outperforms its fragments in diagnostic assays. High reactivity with anti-T. gondii IgG (99.4%) and IgM (80.0%) antibodies. | 2020, [71] |
| pET-28a | BL21 (DE3) | 6xHis-MIC330–180-ROP885–185-SAG185–235-6xHis | LB, 37 °C, 6 h | − | − | + | Mice immunized with MIC3-ROP8-SAG1 showed stronger humoral and Th1 responses. Co-immunization with Freund and calcium phosphate nanoparticles impaired responses. Survival time increased by 15 days. | 2021, [72] |
| pET-28a (+) | BL21 (DE3) | 6xHis-ROP18377–546—MIC4302–471–SAG1130–299-6xHis | LB, 37 °C, 6 h | − | − | + | Vaccinated mice, particularly with ROP18-MIC4-SAG1-Freund, showed high levels of total IgG, IgG2a, and IFN-γ. Survival time increased by 15 days after the challenge. | 2023, [73] |
3.2. Protein Solubility
4. Yeast Expression Systems
5. Leishmania Tarentolae
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Molan, A.; Nosaka, K.; Hunter, M.; Wang, W. Global Status of Toxoplasma gondii Infection: Systematic Review and Prevalence Snapshots. Trop. Biomed. 2019, 36, 898–925. [Google Scholar] [PubMed]
- Attias, M.; Teixeira, D.E.; Benchimol, M.; Vommaro, R.C.; Crepaldi, P.H.; De Souza, W. The Life-Cycle of Toxoplasma gondii Reviewed Using Animations. Parasit. Vectors 2020, 13, 588. [Google Scholar] [CrossRef]
- Tenter, A.M.; Heckeroth, A.R.; Weiss, L.M. Toxoplasma gondii: From Animals to Humans. Int. J. Parasitol. 2000, 30, 1217–1258. [Google Scholar] [CrossRef]
- Deganich, M.; Boudreaux, C.; Benmerzouga, I. Toxoplasmosis Infection during Pregnancy. Trop. Med. Infect. Dis. 2022, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.; Dubey, J.P. Toxoplasma gondii: Transmission, Diagnosis and Prevention. Clin. Microbiol. Infect. 2002, 8, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lai, B.S.; Juhas, M.; Zhang, Y. Toxoplasma gondii Secretory Proteins and Their Role in Invasion and Pathogenesis. Microbiol. Res. 2019, 227, 126293. [Google Scholar] [CrossRef]
- Holec-Gasior, L. Toxoplasma gondii Recombinant Antigens as Tools for Serodiagnosis of Human Toxoplasmosis: Current Status of Studies. Clin. Vaccine Immunol. 2013, 20, 1343–1351. [Google Scholar] [CrossRef]
- Kotresha, D.; Noordin, R. Recombinant Proteins in the Diagnosis of Toxoplasmosis. APMIS 2010, 118, 529–542. [Google Scholar] [CrossRef]
- Buxton, D.; Thomson, K.M.; Maley, S.; Wright, S.; Bos, H.J. Experimental Challenge of Sheep 18 Months after Vaccination with a Live (S48) Toxoplasma gondii Vaccine. Vet. Rec. 1993, 133, 310–312. [Google Scholar] [CrossRef]
- Buxton, D. Toxoplasmosis: The First Commercial Vaccine. Parasitol. Today 1993, 9, 335–337. [Google Scholar] [CrossRef]
- Karakavuk, T.; Gül, C.; Karakavuk, M.; Gül, A.; Erkunt Alak, S.; Can, H.; Ün, C.; Döşkaya, M.; Gürüz, A.Y.; Değirmenci Döşkaya, A. Biotechnological Based Recombinant Protein Vaccines Developed Against Toxoplasmosis. Turk. Parazitolojii Derg. 2022, 46, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Mamaghani, A.J.; Fathollahi, A.; Arab-Mazar, Z.; Kohansal, K.; Fathollahi, M.; Spotin, A.; Bashiri, H.; Bozorgomid, A. Toxoplasma gondii Vaccine Candidates: A Concise Review. Ir. J. Med. Sci. 2023, 192, 231–261. [Google Scholar] [CrossRef]
- Kuriakose, A.; Chirmule, N.; Nair, P. Immunogenicity of Biotherapeutics: Causes and Association with Posttranslational Modifications. J. Immunol. Res. 2016, 2016, 1298473. [Google Scholar] [CrossRef]
- Ansari, H.R.; Raghava, G.P.S. Identification of Conformational B-Cell Epitopes in an Antigen from Its Primary Sequence. Immunome Res. 2010, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Terpe, K. Overview of Bacterial Expression Systems for Heterologous Protein Production: From Molecular and Biochemical Fundamentals to Commercial Systems. Appl. Microbiol. Biotechnol. 2006, 72, 211–222. [Google Scholar] [CrossRef]
- Chen, R. Bacterial Expression Systems for Recombinant Protein Production: E. coli and Beyond. Biotechnol. Adv. 2012, 30, 1102–1107. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.L.; Ceccarelli, E.A. Recombinant Protein Expression in Escherichia coli: Advances and Challenges. Front. Microbiol. 2014, 5, 172. [Google Scholar] [CrossRef]
- Samuelson, J.C. Recent Developments in Difficult Protein Expression: A Guide to E. coli Strains, Promoters, and Relevant Host Mutations. Methods Mol. Biol. 2011, 705, 195–209. [Google Scholar] [CrossRef]
- Sweet, C.R. Expression of Recombinant Proteins From Lac Promoters. In E. coli Plasmid Vectors: Methods and Applications; Casali, N., Preston, A., Eds.; Humana Press: Totowa, NJ, USA, 2003; pp. 277–288. ISBN 978-1-59259-409-2. [Google Scholar]
- de Boer, H.A.; Comstock, L.J.; Vasser, M. The Tac Promoter: A Functional Hybrid Derived from the Trp and Lac Promoters. Proc. Natl. Acad. Sci. USA 1983, 80, 21–25. [Google Scholar] [CrossRef]
- Hehl, A.B.; Lekutis, C.; Grigg, M.E.; Bradley, P.J.; Dubremetz, J.F.; Ortega-Barria, E.; Boothroyd, J.C. Toxoplasma gondii Homologue of Plasmodium Apical Membrane Antigen 1 Is Involved in Invasion of Host Cells. Infect. Immun. 2000, 68, 7078–7086. [Google Scholar] [CrossRef]
- Dando, C.; Schroeder, E.R.; Hunsaker, L.A.; Deck, L.M.; Royer, R.E.; Zhou, X.; Parmley, S.F.; Vander Jagt, D.L. The Kinetic Properties and Sensitivities to Inhibitors of Lactate Dehydrogenases (LDH1 and LDH2) from Toxoplasma gondii: Comparisons with PLDH from Plasmodium falciparum. Mol. Biochem. Parasitol. 2001, 118, 23–32. [Google Scholar] [CrossRef]
- Reiff, S.B.; Vaishnava, S.; Striepen, B. The HU Protein Is Important for Apicoplast Genome Maintenance and Inheritance in Toxoplasma gondii. Eukaryot. Cell 2012, 11, 905–915. [Google Scholar] [CrossRef]
- Heintzelman, M.B.; Schwartzman, J.D. Characterization of Myosin-A and Myosin-C: Two Class XIV Unconventional Myosins from Toxoplasma gondii. Cell Motil. Cytoskeleton 1999, 44, 58–67. [Google Scholar] [CrossRef]
- Dunn, J.D.; Ravindran, S.; Kim, S.-K.; Boothroyd, J.C. The Toxoplasma gondii Dense Granule Protein GRA7 Is Phosphorylated upon Invasion and Forms an Unexpected Association with the Rhoptry Proteins ROP2 and ROP4. Infect. Immun. 2008, 76, 5853–5861. [Google Scholar] [CrossRef] [PubMed]
- Henriquez, F.L.; Nickdel, M.B.; McLeod, R.; Lyons, R.E.; Lyons, K.; Dubremetz, J.F.; Grigg, M.E.; Samuel, B.U.; Roberts, C.W. Toxoplasma gondii Dense Granule Protein 3 (GRA3) Is a Type I Transmembrane Protein That Possesses a Cytoplasmic Dilysine (KKXX) Endoplasmic Reticulum (ER) Retrieval Motif. Parasitology 2005, 131, 169–179. [Google Scholar] [CrossRef]
- Hehl, A.; Krieger, T.; Boothroyd, J.C. Identification and Characterization of SRS1, a Toxoplasma gondii Surface Antigen Upstream of and Related to SAG1. Mol. Biochem. Parasitol. 1997, 89, 271–282. [Google Scholar] [CrossRef]
- Lekutis, C.; Ferguson, D.J.; Boothroyd, J.C. Toxoplasma gondii: Identification of a Developmentally Regulated Family of Genes Related to SAG2. Exp. Parasitol. 2000, 96, 89–96. [Google Scholar] [CrossRef]
- Manger, I.D.; Hehl, A.B.; Boothroyd, J.C. The Surface of Toxoplasma Tachyzoites Is Dominated by a Family of Glycosylphosphatidylinositol-Anchored Antigens Related to SAG1. Infect. Immun. 1998, 66, 2237–2244. [Google Scholar] [CrossRef]
- Kimple, M.E.; Brill, A.L.; Pasker, R.L. Overview of Affinity Tags for Protein Purification. Curr. Protoc. Protein Sci. 2013, 73, 9.9.1–9.9.23. [Google Scholar] [CrossRef]
- Pina, A.S.; Batalha, Í.L.; Dias, A.M.G.C.; Roque, A.C.A. Affinity Tags in Protein Purification and Peptide Enrichment: An Overview. Methods Mol. Biol. 2021, 2178, 107–132. [Google Scholar] [CrossRef]
- Tenter, A.M.; Johnson, A.M. Recognition of Recombinant Toxoplasma gondii Antigens by Human Sera in an ELISA. Parasitol. Res. 1991, 77, 197–203. [Google Scholar] [CrossRef]
- Parmley, S.F.; Sgarlato, G.D.; Mark, J.; Prince, J.B.; Remington, J.S. Expression, Characterization, and Serologic Reactivity of Recombinant Surface Antigen P22 of Toxoplasma gondii. J. Clin. Microbiol. 1992, 30, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Redlich, A.; Müller, W.A. Serodiagnosis of Acute Toxoplasmosis Using a Recombinant Form of the Dense Granule Antigen GRA6 in an Enzyme-Linked Immunosorbent Assay. Parasitol. Res. 1998, 84, 700–706. [Google Scholar] [CrossRef]
- Lecordier, L.; Fourmaux, M.P.; Mercier, C.; Dehecq, E.; Masy, E.; Cesbron-Delauw, M.F. Enzyme-Linked Immunosorbent Assays Using the Recombinant Dense Granule Antigens GRA6 and GRA1 of Toxoplasma gondii for Detection of Immunoglobulin G Antibodies. Clin. Diagn. Lab. Immunol. 2000, 7, 607–611. [Google Scholar] [CrossRef]
- Wang, H.-L.; Zhang, T.-E.; Yin, L.-T.; Pang, M.; Guan, L.; Liu, H.-L.; Zhang, J.-H.; Meng, X.-L.; Bai, J.-Z.; Zheng, G.-P.; et al. Partial Protective Effect of Intranasal Immunization with Recombinant Toxoplasma gondii Rhoptry Protein 17 against Toxoplasmosis in Mice. PLoS ONE 2014, 9, e108377. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-D.; Chang, G.-N.; Chao, D. Protective Immunity against Toxoplasma gondii in Mice Induced by a Chimeric Protein RSAG1/2. Parasitol. Res. 2004, 92, 58–64. [Google Scholar] [CrossRef]
- Elisa, B.; Andrea, S.; Luca, B.; Wilma, B.; Nicola, G. Chimeric Antigens of Toxoplasma gondii: Toward Standardization of Toxoplasmosis Serodiagnosis Using Recombinant Products. J. Clin. Microbiol. 2006, 44, 2133–2140. [Google Scholar] [CrossRef]
- Song, K.J.; Yang, Z.; Chong, C.-K.; Kim, J.-S.; Lee, K.C.; Kim, T.-S.; Nam, H.-W. A Rapid Diagnostic Test for Toxoplasmosis Using Recombinant Antigenic N-Terminal Half of SAG1 Linked with Intrinsically Unstructured Domain of Gra2 Protein. Korean J. Parasitol. 2013, 51, 503–510. [Google Scholar] [CrossRef]
- Yang, Z.; Ahn, H.-J.; Nam, H.-W. High Expression of Water-Soluble Recombinant Antigenic Domains of Toxoplasma gondii Secretory Organelles. Korean J. Parasitol. 2014, 52, 367–376. [Google Scholar] [CrossRef]
- Arab-Mazar, Z.; Fallahi, S.; Koochaki, A.; Haghighi, A.; Seyyed Tabaei, S.J. Immunodiagnosis and Molecular Validation of Toxoplasma gondii-Recombinant Dense Granular (GRA) 7 Protein for the Detection of Toxoplasmosis in Patients with Cancer. Microbiol. Res. 2016, 183, 53–59. [Google Scholar] [CrossRef]
- Arab-Mazar, Z.; Javadi Mamaghani, A.; Fallahi, S.; Rajaeian, S.; Koochaki, A.; Seyyed Tabaei, S.J.; Rezaee, H. Immunodiagnosis and Molecular Validation of Toxoplasma gondii-Recombinant Dense Granular (GRA) 5 Protein for the Detection of Toxoplasmosis in Hemodialysis Patients. Semin. Dial. 2021, 34, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Hasan, T.; Shimoda, N.; Nakamura, S.; Fox, B.A.; Bzik, D.J.; Ushio-Watanabe, N.; Nishikawa, Y. Protective Efficacy of Recombinant Toxoplasma gondii Dense Granule Protein 15 against Toxoplasmosis in C57BL/6 Mice. Vaccine 2024, 42, 2299–2309. [Google Scholar] [CrossRef]
- Carson, M.; Johnson, D.H.; McDonald, H.; Brouillette, C.; Delucas, L.J. His-Tag Impact on Structure. Acta Crystallogr. D Biol. Crystallogr. 2007, 63, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Tran, K.C.; Arnold, J.J.; Teng, M.N. Purification and Characterization of Recombinant Human Respiratory Syncytial Virus Nonstructural Protein NS1. Protein Expr. Purif. 2008, 57, 261–270. [Google Scholar] [CrossRef]
- Yap, W.B.; Tey, B.T.; Ng, M.Y.T.; Ong, S.T.; Tan, W.S. N-Terminally His-Tagged Hepatitis B Core Antigens: Construction, Expression, Purification and Antigenicity. J. Virol. Methods 2009, 160, 125–131. [Google Scholar] [CrossRef]
- Goel, A.; Colcher, D.; Koo, J.-S.; Booth, B.J.M.; Pavlinkova, G.; Batra, S.K. Relative Position of the Hexahistidine Tag Effects Binding Properties of a Tumor-Associated Single-Chain Fv Construct. Biochim. Biophys. Acta-Gen. Subj. 2000, 1523, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Legler, P.M.; Mease, R.M.; Duncan, E.H.; Bergmann-Leitner, E.S.; Angov, E. Histidine Affinity Tags Affect MSP1(42) Structural Stability and Immunodominance in Mice. Biotechnol. J. 2012, 7, 133–147. [Google Scholar] [CrossRef]
- Nockemann, S.; Dlugonska, H.; Henrich, B.; Kitzerow, A.; Däubener, W. Expression, Characterization and Serological Reactivity of a 41 KDa Excreted–Secreted Antigen (ESA) from Toxoplasma gondii. Mol. Biochem. Parasitol. 1998, 97, 109–121. [Google Scholar] [CrossRef]
- Hiszczyńska-Sawicka, E.; Kur, J.; Pietkiewicz, H.; Holec-Gasior, L.; G1sior, A.; Myjak, P. Efficient Production of the Toxoplasma gondii GRA6, P35 and SAG2 Recombinant Antigens and Their Applications in the Serodiagnosis of Toxoplasmosis. Acta Parasitol. 2005, 50, 249–254. [Google Scholar]
- Golkar, M.; Rafati, S.; Abdel-Latif, M.S.; Brenier-Pinchart, M.-P.; Fricker-Hidalgo, H.; Sima, B.K.; Babaie, J.; Pelloux, H.; Cesbron-Delauw, M.-F.; Mercier, C. The Dense Granule Protein GRA2, a New Marker for the Serodiagnosis of Acute Toxoplasma Infection: Comparison of Sera Collected in Both France and Iran from Pregnant Women. Diagn. Microbiol. Infect. Dis. 2007, 58, 419–426. [Google Scholar] [CrossRef]
- Holec, L.; Hiszczyńska-Sawicka, E.; Gasior, A.; Brillowska-Dabrowska, A.; Kur, J. Use of MAG1 Recombinant Antigen for Diagnosis of Toxoplasma gondii Infection in Humans. Clin. Vaccine Immunol. 2007, 14, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Holec, L.; Gasior, A.; Brillowska-Dabrowska, A.; Kur, J. Toxoplasma gondii: Enzyme-Linked Immunosorbent Assay Using Different Fragments of Recombinant Microneme Protein 1 (MIC1) for Detection of Immunoglobulin G Antibodies. Exp. Parasitol. 2008, 119, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sadeghiani, G.; Zare, M.; Babaie, J.; Shokrgozar, M.-A.; Azadmanesh, K.; Fard-Esfahani, P.; Golkar, M. Heterologous Production of Dense Granule GRA7 Antigen of Toxoplasma gondii in Escherichia coli. Southeast Asian J. Trop. Med. Public Health 2009, 40, 692–700. [Google Scholar] [PubMed]
- Holec-Gasior, L.; Kur, J.; Hiszczyńska-Sawicka, E. GRA2 and ROP1 Recombinant Antigens as Potential Markers for Detection of Toxoplasma gondii-Specific Immunoglobulin G in Humans with Acute Toxoplasmosis. Clin. Vaccine Immunol. 2009, 16, 510–514. [Google Scholar] [CrossRef]
- Holec-Gasior, L.; Kur, J. Toxoplasma gondii: Recombinant GRA5 Antigen for Detection of Immunoglobulin G Antibodies Using Enzyme-Linked Immunosorbent Assay. Exp. Parasitol. 2010, 124, 272–278. [Google Scholar] [CrossRef]
- Babaie, J.; Miri, M.; Sadeghiani, G.; Zare, M.; Khalili, G.; Golkar, M. Expression and Single-Step Purification of GRA8 Antigen of Toxoplasma gondii in Escherichia coli. Avicenna J. Med. Biotechnol. 2011, 3, 67–77. [Google Scholar]
- Jianfang, D.; Min, J.; Yanyun, W.; Lili, Q.; Rujun, G.; Jin, S. Evaluation of a Recombinant Multiepitope Peptide for Serodiagnosis of Toxoplasma gondii Infection. Clin. Vaccine Immunol. 2012, 19, 338–342. [Google Scholar] [CrossRef]
- Holec-Gąsior, L.; Ferra, B.; Drapała, D.; Lautenbach, D.; Kur, J. A New MIC1-MAG1 Recombinant Chimeric Antigen Can Be Used Instead of the Toxoplasma gondii Lysate Antigen in Serodiagnosis of Human Toxoplasmosis. Clin. Vaccine Immunol. 2012, 19, 57–63. [Google Scholar] [CrossRef]
- Holec-Gasior, L.; Ferra, B.; Drapala, D. MIC1-MAG1-SAG1 Chimeric Protein, a Most Effective Antigen for Detection of Human Toxoplasmosis. Clin. Vaccine Immunol. 2012, 19, 1977–1979. [Google Scholar] [CrossRef]
- Wang, H.-L.; Li, Y.-Q.; Yin, L.-T.; Meng, X.-L.; Guo, M.; Zhang, J.-H.; Liu, H.-L.; Liu, J.-J.; Yin, G.-R. Toxoplasma gondii Protein Disulfide Isomerase (TgPDI) Is a Novel Vaccine Candidate against Toxoplasmosis. PLoS ONE 2013, 8, e70884. [Google Scholar] [CrossRef]
- Ram, H.; Rao, J.R.; Tewari, A.K.; Banerjee, P.S.; Sharma, A.K. Molecular Cloning, Sequencing, and Biological Characterization of GRA4 Gene of Toxoplasma gondii. Parasitol. Res. 2013, 112, 2487–2494. [Google Scholar] [CrossRef]
- Ferra, B.; Holec-Gąsior, L.; Kur, J. A New Toxoplasma gondii Chimeric Antigen Containing Fragments of SAG2, GRA1, and ROP1 Proteins—Impact of Immunodominant Sequences Size on Its Diagnostic Usefulness. Parasitol. Res. 2015, 114, 3291–3299. [Google Scholar] [CrossRef] [PubMed]
- Drapała, D.; Holec-Gasior, L.; Kur, J. New Recombinant Chimeric Antigens, P35-MAG1, MIC1-ROP1, and MAG1-ROP1, for the Serodiagnosis of Human Toxoplasmosis. Diagn. Microbiol. Infect. Dis. 2015, 82, 34–39. [Google Scholar] [CrossRef]
- Liu, Z.; Yin, L.; Li, Y.; Yuan, F.; Zhang, X.; Ma, J.; Liu, H.; Wang, Y.; Zheng, K.; Cao, J. Intranasal Immunization with Recombinant Toxoplasma gondii Actin Depolymerizing Factor Confers Protective Efficacy against Toxoplasmosis in Mice. BMC Immunol. 2016, 17, 37. [Google Scholar] [CrossRef] [PubMed]
- Nabi, H.; Rashid, I.; Ahmad, N.; Durrani, A.; Akbar, H.; Islam, S.; Bajwa, A.A.; Shehzad, W.; Ashraf, K.; Imran, N. Induction of Specific Humoral Immune Response in Mice Immunized with ROP18 Nanospheres from Toxoplasma gondii. Parasitol. Res. 2017, 116, 359–370. [Google Scholar] [CrossRef]
- Naeem, H.; Sana, M.; Islam, S.; Khan, M.; Riaz, F.; Zafar, Z.; Akbar, H.; Shehzad, W.; Rashid, I. Induction of Th1 Type-Oriented Humoral Response through Intranasal Immunization of Mice with SAG1-Toxoplasma gondii Polymeric Nanospheres. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; An, R.; Chen, Y.; Chen, T.; Wen, H.; Yan, Q.; Shen, J.; Chen, L.; Du, J. Vaccination with Recombinant Toxoplasma gondii CDPK3 Induces Protective Immunity against Experimental Toxoplasmosis. Acta Trop. 2019, 199, 105148. [Google Scholar] [CrossRef]
- Luo, J.; Wan, J.; Tang, Z.; Shen, S. Identification of Novel Antigens for Serum IgG Diagnosis of Human Toxoplasmosis. Exp. Parasitol. 2019, 204, 107722. [Google Scholar] [CrossRef]
- Ferra, B.; Holec-Gąsior, L.; Gatkowska, J.; Dziadek, B.; Dzitko, K.; Grąźlewska, W.; Lautenbach, D. The First Study on the Usefulness of Recombinant Tetravalent Chimeric Proteins Containing Fragments of SAG2, GRA1, ROP1 and AMA1 Antigens in the Detection of Specific Anti-Toxoplasma gondii Antibodies in Mouse and Human Sera. PLoS ONE 2019, 14, e0217866. [Google Scholar] [CrossRef]
- Ferra, B.; Holec-Gąsior, L.; Gatkowska, J.; Dziadek, B.; Dzitko, K. Toxoplasma gondii Recombinant Antigen AMA1: Diagnostic Utility of Protein Fragments for the Detection of IgG and IgM Antibodies. Pathogens 2020, 9, 43. [Google Scholar] [CrossRef]
- Dodangeh, S.; Fasihi-Ramandi, M.; Daryani, A.; Valadan, R.; Asgarian-Omran, H.; Hosseininejad, Z.; Nayeri Chegeni, T.; Pagheh, A.S.; Javidnia, J.; Sarvi, S. Protective Efficacy by a Novel Multi-Epitope Vaccine, Including MIC3, ROP8, and SAG1, against Acute Toxoplasma gondii Infection in BALB/c Mice. Microb. Pathog. 2021, 153, 104764. [Google Scholar] [CrossRef] [PubMed]
- Nayeri, T.; Sarvi, S.; Fasihi-Ramandi, M.; Valadan, R.; Asgarian-Omran, H.; Ajami, A.; Khalilian, A.; Hosseininejad, Z.; Dodangeh, S.; Javidnia, J.; et al. Enhancement of Immune Responses by Vaccine Potential of Three Antigens, Including ROP18, MIC4, and SAG1 against Acute Toxoplasmosis in Mice. Exp. Parasitol. 2023, 244, 108427. [Google Scholar] [CrossRef]
- Qing, G.; Ma, L.-C.; Khorchid, A.; Swapna, G.V.T.; Mal, T.K.; Takayama, M.M.; Xia, B.; Phadtare, S.; Ke, H.; Acton, T.; et al. Cold-Shock Induced High-Yield Protein Production in Escherichia coli. Nat. Biotechnol. 2004, 22, 877–882. [Google Scholar] [CrossRef]
- Francis, D.M.; Page, R. Strategies to Optimize Protein Expression in E. coli. Curr. Protoc. Protein Sci. 2010, 5, 5.24.1–5.24.29. [Google Scholar] [CrossRef]
- Sonaimuthu, P.; Fong, M.Y.; Kalyanasundaram, R.; Mahmud, R.; Lau, Y.L. Sero-Diagnostic Evaluation of Toxoplasma gondii Recombinant Rhoptry Antigen 8 Expressed in E. coli. Parasit. Vectors 2014, 7, 297. [Google Scholar] [CrossRef]
- Sonaimuthu, P.; Ching, X.T.; Fong, M.Y.; Kalyanasundaram, R.; Lau, Y.L. Induction of Protective Immunity against Toxoplasmosis in BALB/c Mice Vaccinated with Toxoplasma gondii Rhoptry-1. Front. Microbiol. 2016, 7, 808. [Google Scholar] [CrossRef]
- Atroshenko, D.L.; Sergeev, E.P.; Golovina, D.I.; Pometun, A.A. Additivities for Soluble Recombinant Protein Expression in Cytoplasm of Escherichia coli. Fermentation 2024, 10, 120. [Google Scholar] [CrossRef]
- Scheiblhofer, S.; Laimer, J.; Machado, Y.; Weiss, R.; Thalhamer, J. Influence of Protein Fold Stability on Immunogenicity and Its Implications for Vaccine Design. Expert Rev. Vaccines 2017, 16, 479–489. [Google Scholar] [CrossRef]
- Nozach, H.; Fruchart-Gaillard, C.; Fenaille, F.; Beau, F.; Ramos, O.H.P.; Douzi, B.; Saez, N.J.; Moutiez, M.; Servent, D.; Gondry, M.; et al. High Throughput Screening Identifies Disulfide Isomerase DsbC as a Very Efficient Partner for Recombinant Expression of Small Disulfide-Rich Proteins in E. coli. Microb. Cell Fact. 2013, 12, 37. [Google Scholar] [CrossRef]
- Baneyx, F.; Mujacic, M. Recombinant Protein Folding and Misfolding in Escherichia coli. Nat. Biotechnol. 2004, 22, 1399–1408. [Google Scholar] [CrossRef]
- Kaur, J.; Kumar, A.; Kaur, J. Strategies for Optimization of Heterologous Protein Expression in E. coli: Roadblocks and Reinforcements. Int. J. Biol. Macromol. 2018, 106, 803–822. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.D.; Hatahet, F.; Salo, K.E.H.; Enlund, E.; Zhang, C.; Ruddock, L.W. Pre-Expression of a Sulfhydryl Oxidase Significantly Increases the Yields of Eukaryotic Disulfide Bond Containing Proteins Expressed in the Cytoplasm of E. coli. Microb. Cell Fact. 2011, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Stern, D.; Seeber, F. Expression of in Vivo Biotinylated Recombinant Antigens SAG1 and SAG2A from Toxoplasma gondii for Improved Seroepidemiological Bead-Based Multiplex Assays. BMC Biotechnol. 2020, 20, 53. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Grigg, M.E.; Boothroyd, J.C.; Garcia, K.C. Structure of the Immunodominant Surface Antigen from the Toxoplasma gondii SRS Superfamily. Nat. Struct. Biol. 2002, 9, 606–611. [Google Scholar] [CrossRef]
- Karyolaimos, A.; de Gier, J.-W. Strategies to Enhance Periplasmic Recombinant Protein Production Yields in Escherichia coli. Front. Bioeng. Biotechnol. 2021, 9, 797334. [Google Scholar] [CrossRef]
- Allahyari, M.; Mohabati, R.; Babaie, J.; Amiri, S.; Siavashani, Z.J.; Zare, M.; Sadeghiani, G.; Golkar, M. Production of In-Vitro Refolded and Highly Antigenic SAG1 for Development of a Sensitive and Specific Toxoplasma IgG ELISA. J. Immunol. Methods 2015, 416, 157–166. [Google Scholar] [CrossRef]
- Jacquet, A.; Daminet, V.; Haumont, M.; Garcia, L.; Chaudoir, S.; Bollen, A.; Biemans, R. Expression of a Recombinant Toxoplasma gondii ROP2 Fragment as a Fusion Protein in Bacteria Circumvents Insolubility and Proteolytic Degradation. Protein Expr. Purif. 1999, 17, 392–400. [Google Scholar] [CrossRef]
- Graille, M.; Stura, E.A.; Bossus, M.; Muller, B.H.; Letourneur, O.; Battail-Poirot, N.; Sibaï, G.; Gauthier, M.; Rolland, D.; Le Du, M.-H.; et al. Crystal Structure of the Complex between the Monomeric Form of Toxoplasma gondii Surface Antigen 1 (SAG1) and a Monoclonal Antibody That Mimics the Human Immune Response. J. Mol. Biol. 2005, 354, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Mirzadeh, A.; Saadatnia, G.; Golkar, M.; Babaie, J.; Noordin, R. Production of Refolded Toxoplasma gondii Recombinant SAG1-Related Sequence 3 (SRS3) and Its Use for Serodiagnosis of Human Toxoplasmosis. Protein Expr. Purif. 2017, 133, 66–74. [Google Scholar] [CrossRef]
- Nigro, M.; Martin, V.; Kaufer, F.; Carral, L.; Angel, S.O.; Pszenny, V. High Level of Expression of the Toxoplasma gondii-Recombinant Rop2 Protein in Escherichia coli as a Soluble Form for Optimal Use in Diagnosis. Mol. Biotechnol. 2001, 18, 269–273. [Google Scholar] [CrossRef]
- Petsch, D.; Anspach, F.B. Endotoxin Removal from Protein Solutions. J. Biotechnol. 2000, 76, 97–119. [Google Scholar] [CrossRef]
- Daly, R.; Hearn, M.T.W. Expression of Heterologous Proteins in Pichia pastoris: A Useful Experimental Tool in Protein Engineering and Production. J. Mol. Recognit. 2005, 18, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Baghban, R.; Farajnia, S.; Rajabibazl, M.; Ghasemi, Y.; Mafi, A.; Hoseinpoor, R.; Rahbarnia, L.; Aria, M. Yeast Expression Systems: Overview and Recent Advances. Mol. Biotechnol. 2019, 61, 365–384. [Google Scholar] [CrossRef]
- Karbalaei, M.; Rezaee, S.A.; Farsiani, H. Pichia pastoris: A Highly Successful Expression System for Optimal Synthesis of Heterologous Proteins. J. Cell Physiol. 2020, 235, 5867–5881. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiao, T.; Xu, C.; Li, J.; Liu, G.; Yin, K.; Cui, Y.; Wei, Q.; Huang, B.; Sun, H. Protective Immune Response against Toxoplasma gondii Elicited by a Novel Yeast-Based Vaccine with Microneme Protein 16. Vaccine 2018, 36, 3943–3948. [Google Scholar] [CrossRef] [PubMed]
- Darby, R.A.J.; Cartwright, S.P.; Dilworth, M.V.; Bill, R.M. Which Yeast Species Shall I Choose? Saccharomyces cerevisiae versus Pichia pastoris (Review). Methods Mol. Biol. 2012, 866, 11–23. [Google Scholar] [CrossRef]
- Legastelois, I.; Buffin, S.; Peubez, I.; Mignon, C.; Sodoyer, R.; Werle, B. Non-Conventional Expression Systems for the Production of Vaccine Proteins and Immunotherapeutic Molecules. Hum. Vaccin. Immunother. 2017, 13, 947–961. [Google Scholar] [CrossRef]
- Chahed Bel-Ochi, N.; Bouratbine, A.; Mousli, M. Design and Characterization of a Recombinant Colorimetric SAG1–Alkaline Phosphatase Conjugate to Detect Specific Antibody Responses against Toxoplasma gondii. J. Immunol. Methods 2013, 394, 107–114. [Google Scholar] [CrossRef]
- Odenthal-Schnittler, M.; Tomavo, S.; Becker, D.; Dubremetz, J.F.; Schwarz, R.T. Evidence for N-Linked Glycosylation in Toxoplasma gondii. Biochem. J. 1993, 291 Pt 3, 713–721. [Google Scholar] [CrossRef]
- Biemans, R.; Grégoire, D.; Haumont, M.; Bosseloir, A.; Garcia, L.; Jacquet, A.; Dubeaux, C.; Bollen, A. The Conformation of Purified Toxoplasma gondii SAG1 Antigen, Secreted from Engineered Pichia pastoris, Is Adequate for Serorecognition and Cell Proliferation. J. Biotechnol. 1998, 66, 137–146. [Google Scholar] [CrossRef]
- Letourneur, O.; Gervasi, G.; Gaïa, S.; Pagès, J.; Watelet, B.; Jolivet, M. Characterization of Toxoplasma gondii Surface Antigen 1 (SAG1) Secreted from Pichia pastoris: Evidence of Hyper O-Glycosylation. Biotechnol. Appl. Biochem. 2001, 33, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Fong, M.Y.; Lau, Y.L.; Zulqarnain, M. Characterization of Secreted Recombinant Toxoplasma gondii Surface Antigen 2 (SAG2) Heterologously Expressed by the Yeast Pichia pastoris. Biotechnol. Lett. 2008, 30, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.-L.; Fong, M.-Y.; Idris, M.M.; Ching, X.-T. Cloning and Expression of Toxoplasma gondii Dense Granule Antigen 2 (GRA2) Gene by Pichia pastoris. Southeast Asian J. Trop. Med. Public Health 2012, 43, 10–16. [Google Scholar] [PubMed]
- Lau, Y.L.; Hasan, M.T.; Thiruvengadam, G.; Idris, M.M.; Init, I. Cloning and Expression of Toxoplasma gondii Dense Granular Protein 4 (GRA4) in Pichia pastoris. Trop. Biomed. 2010, 27, 525–533. [Google Scholar]
- Lau, Y.L.; Thiruvengadam, G.; Lee, W.W.; Fong, M.Y. Immunogenic Characterization of the Chimeric Surface Antigen 1 and 2 (SAG1/2) of Toxoplasma gondii Expressed in the Yeast Pichia pastoris. Parasitol. Res. 2011, 109, 871–878. [Google Scholar] [CrossRef]
- Chang, P.Y.; Fong, M.Y.; Nissapatorn, V.; Lau, Y.L. Evaluation of Pichia pastoris-Expressed Recombinant Rhoptry Protein 2 of Toxoplasma gondii for Its Application in Diagnosis of Toxoplasmosis. Am. J. Trop. Med. Hyg. 2011, 85, 485–489. [Google Scholar] [CrossRef]
- Lau, Y.L.; Fong, M.Y. Toxoplasma gondii: Serological Characterization and Immunogenicity of Recombinant Surface Antigen 2 (SAG2) Expressed in the Yeast Pichia pastoris. Exp. Parasitol. 2008, 119, 373–378. [Google Scholar] [CrossRef]
- Thiruvengadam, G.; Init, I.; Fong, M.Y.; Lau, Y.L. Optimization of the Expression of Surface Antigen SAG1/2 of Toxoplasma gondii in the Yeast Pichia pastoris. Trop. Biomed. 2011, 28, 506–513. [Google Scholar]
- Ling, L.Y.; Ithoi, I.; Yik, F.M. Optimization for High-Level Expression in Pichia pastoris and Purification of Truncated and Full Length Recombinant SAG2 of Toxoplasma gondii for Diagnostic Use. Southeast Asian J. Trop. Med. Public Health 2010, 41, 507–513. [Google Scholar]
- Fournier, J. A Review of Glycan Analysis Requirements. Biopharm Int. 2015, 28, 32–37. [Google Scholar]
- Werner, R.G.; Kopp, K.; Schlueter, M. Glycosylation of Therapeutic Proteins in Different Production Systems. Acta Paediatr. 2007, 96, 17–22. [Google Scholar] [CrossRef]
- Zhang, P.; Woen, S.; Wang, T.; Liau, B.; Zhao, S.; Chen, C.; Yang, Y.; Song, Z.; Wormald, M.R.; Yu, C.; et al. Challenges of Glycosylation Analysis and Control: An Integrated Approach to Producing Optimal and Consistent Therapeutic Drugs. Drug Discov. Today 2016, 21, 740–765. [Google Scholar] [CrossRef]
- Shen, W.; Xue, Y.; Liu, Y.; Kong, C.; Wang, X.; Huang, M.; Cai, M.; Zhou, X.; Zhang, Y.; Zhou, M. A Novel Methanol-Free Pichia pastoris System for Recombinant Protein Expression. Microb. Cell Fact. 2016, 15, 178. [Google Scholar] [CrossRef]
- Cos, O.; Ramón, R.; Montesinos, J.L.; Valero, F. Operational Strategies, Monitoring and Control of Heterologous Protein Production in the Methylotrophic Yeast Pichia pastoris under Different Promoters: A Review. Microb. Cell Fact. 2006, 5, 17. [Google Scholar] [CrossRef]
- Macauley-Patrick, S.; Fazenda, M.L.; McNeil, B.; Harvey, L.M. Heterologous Protein Production Using the Pichia pastoris Expression System. Yeast 2005, 22, 249–270. [Google Scholar] [CrossRef]
- Mohammadzadeh, R.; Karbalaei, M.; Soleimanpour, S.; Mosavat, A.; Rezaee, S.A.; Ghazvini, K.; Farsiani, H. Practical Methods for Expression of Recombinant Protein in the Pichia pastoris System. Curr. Protoc. 2021, 1, e155. [Google Scholar] [CrossRef]
- Zhou, H.; Gu, Q.; Zhao, Q.; Zhang, J.; Cong, H.; Li, Y.; He, S. Toxoplasma gondii: Expression and Characterization of a Recombinant Protein Containing SAG1 and GRA2 in Pichia pastoris. Parasitol. Res. 2007, 100, 829–835. [Google Scholar] [CrossRef]
- Fernández-Robledo, J.A.; Vasta, G.R. Production of Recombinant Proteins from Protozoan Parasites. Trends Parasitol. 2010, 26, 244–254. [Google Scholar] [CrossRef]
- Niimi, T. Recombinant Protein Production in the Eukaryotic Protozoan Parasite Leishmania tarentolae: A Review. Methods Mol. Biol. 2012, 824, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Taheri, T.; Seyed, N.; Mizbani, A.; Rafati, S. Leishmania-Based Expression Systems. Appl. Microbiol. Biotechnol. 2016, 100, 7377–7385. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, C.; Sitz, M.; Wolf, M.; Pohl, H.-D. Development of a Defined Medium for Heterologous Expression in Leishmania tarentolae. J. Basic Microbiol. 2008, 48, 488–495. [Google Scholar] [CrossRef]
- Kalef, D.A. Leishmania mexicana Recombinant Filamentous Acid Phosphatase as Carrier for Toxoplasma gondii Surface Antigen 1 Expression in Leishmania tarentolae. J. Parasit. Dis. 2021, 45, 1135–1144. [Google Scholar] [CrossRef]
- Majidiani, H.; Dalimi, A.; Ghaffarifar, F.; Pirestani, M. Multi-Epitope Vaccine Expressed in Leishmania tarentolae Confers Protective Immunity to Toxoplasma gondii in BALB/c Mice. Microb. Pathog. 2021, 155, 104925. [Google Scholar] [CrossRef]
- Redondo, A.; Wood, D.; Amaral, S.; Ferré, J.; Goti, D.; Bertran, J. Production of Toxoplasma gondii Recombinant Antigens in Genome-Edited Escherichia coli. Appl. Biochem. Microbiol. 2021, 57, 152–160. [Google Scholar] [CrossRef]
- Feldman, S.F.; Temkin, E.; Wulffhart, L.; Nutman, A.; Schechner, V.; Shitrit, P.; Shvartz, R.; Schwaber, M.J.; Carmeli, Y. Effect of Temperature on Escherichia coli Bloodstream Infection in a Nationwide Population-Based Study of Incidence and Resistance. Antimicrob. Resist. Infect. Control 2022, 11, 144. [Google Scholar] [CrossRef]
- Shin, M.-K.; Yoo, H.S. Animal Vaccines Based on Orally Presented Yeast Recombinants. Vaccine 2013, 31, 4287–4292. [Google Scholar] [CrossRef]
- Kim, K.; Bülow, R.; Kampmeier, J.; Boothroyd, J.C. Conformationally Appropriate Expression of the Toxoplasma Antigen SAG1 (P30) in CHO Cells. Infect. Immun. 1994, 62, 203–209. [Google Scholar] [CrossRef]
- Aghdasi, M.; Ghaffarifar, F.; Forooghi, F.; Dalimi Asl, A.H.; Sharifi, Z.; Maspi, N. Expression of Plasmid Encoded GRA4 Gene of Toxoplasma gondii RH Strain in CHO Eukaryotic Cells. Iran. J. Parasitol. 2018, 13, 392–398. [Google Scholar]
| Plasmid Vector | E. coli Host Strain | Protein | Expression Conditions | Yield | Application | Results | Reference, Year | |
|---|---|---|---|---|---|---|---|---|
| Diagnostic | Vaccine | |||||||
| pGEX-1N | JM105 | GST-H4 GST-H11 | − | − | + | − | Both antigens were highly specific for T. gondii antibodies. H4- and H11-ELISA can distinguish between acute and chronic phases of toxoplasmosis. | 1991, [32] |
| pGEX-2T | JM101 | P22a27–172 | LB, 37 °C, 3 h | − | + | − | Acute infection sera showed stronger IgG reaction with P22 protein (immunoblots and ELISA). IgA and IgM P22-ELISA showed no reactivity. | 1992, [33] |
| pGEX-3X | SURE | GST-GRA6 | − | − | + | − | GRA6-IgG EIA can distinguish between acute and chronic phases of toxoplasmosis. | 1998, [34] |
| pGEX-3X pGEX2T pGEX-3X | JM101 | GST-GRA1 GST-GRA6-Nt GST-GRA6-Ct | LB, 37 °C | − | + | − | GRA6-Ct IgG ELISA—10% sensitivity. GRA6-Nt IgG ELISA—96% sensitivity. GRA1 IgG ELISA—68% sensitivity | 2000, [35] |
| pGEX-6p-1 | BL21 Star (DE3) | SAG1/2, SAG1 SAG2 | 37 °C, 4 h | − | − | + | Vaccination with SAG1/2 protected 73% (11/15) of mice from a lethal challenge. SAG1 immunization—60% survival rate. SAG2 immunization—53% survival rate. | 2004, [37] |
| pGEX-SN | − | GST-EC2 (MIC2157–235-MIC3234–307-SAG1182–312) GST-EC3 (GRA336–134-GRA724–102-M2AP37–263) | − | GST-EC2 8 mg/L GST-EC3 5 mg/L induced bacterial culture | + | − | IgG and IgM ELISAs using EC2 and EC3 perform similarly to commercial assays. IgM-capture assays with chimeric antigens enhance postnatal congenital toxoplasmosis diagnosis. | 2006, [38] |
| pGEX-4T-1 | BL21 (DE3) | GST-GRA2-SAG1A | − | − | + | − | GRA2-SAG1A rapid diagnostic test showed 100% specificity 100% and 97.1% sensitivity. | 2013, [39] |
| pGEX-6P-1 | Rosetta (DE3) | GST-ROP17 | − | − | − | + | Intranasal ROP17 immunization in mice induces systemic and local immune responses. Provides protection against lethal T. gondii infections. Reduces tachyzoite burdens in host tissues. Increases animal survival rates. | 2014, [36] |
| pGEX-4T-1 | BL21 (DE3) pLysS | GST-GRA2 (aa 25–185, 25–135, 25–105, 75–185, 106–185, 25–105) GST-GRA3 (aa 39–138, 39–222) GST-ROP2 (aa 29–561, 29–323 324–561, 29–197, 324–483, 403–561, 324–430, 431–561) GST-MIC2 (aa 1–723, 1–651, 1–425, 219–651, 1–284, 142–425, 421–651, 1–215, 216–425, 142–284) GST-GRA231–71-MIC21–284 | 30 °C | − | + | − | GRA231–71-MIC21–284 shows a high diagnostic potential and may be used for developing a serological assay. | 2014, [40] |
| pGEX-6p-1 | BL21 | GST-GRA7 | LB, 37 °C, 6–8 h | − | + | − | GRA7-ELISA showed 92% sensitivity and 94% specificity. Results align closely with LAMP technique results. | 2016, [41] |
| pGEX-6p-1 | BL21 | GST-GRA5 | LB, 37 °C, 4 h | − | + | − | GRA5-ELISA with sera from hemodialysis patients showed 96% sensitivity and 93% specificity. | 2021, [42] |
| pGEX-4T1 | BL21 (DE3) | GRA15 | LB, 37 °C, 4 h | − | − | + | GRA15 immunization in mice induced IgG1 and IgG2c, boosted spleen cell proliferation and interferon γ (IFN-γ) production, improved survival rates, and reduced parasite burden against the Pru strain. | 2024, [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sołowińska, K.; Holec-Gąsior, L. Single Cell Expression Systems for the Production of Recombinant Proteins for Immunodiagnosis and Immunoprophylaxis of Toxoplasmosis. Microorganisms 2024, 12, 1731. https://doi.org/10.3390/microorganisms12081731
Sołowińska K, Holec-Gąsior L. Single Cell Expression Systems for the Production of Recombinant Proteins for Immunodiagnosis and Immunoprophylaxis of Toxoplasmosis. Microorganisms. 2024; 12(8):1731. https://doi.org/10.3390/microorganisms12081731
Chicago/Turabian StyleSołowińska, Karolina, and Lucyna Holec-Gąsior. 2024. "Single Cell Expression Systems for the Production of Recombinant Proteins for Immunodiagnosis and Immunoprophylaxis of Toxoplasmosis" Microorganisms 12, no. 8: 1731. https://doi.org/10.3390/microorganisms12081731
APA StyleSołowińska, K., & Holec-Gąsior, L. (2024). Single Cell Expression Systems for the Production of Recombinant Proteins for Immunodiagnosis and Immunoprophylaxis of Toxoplasmosis. Microorganisms, 12(8), 1731. https://doi.org/10.3390/microorganisms12081731






