Epidemiology, Virulence and Antimicrobial Resistance of Escherichia coli Isolated from Small Brazilian Farms Producers of Raw Milk Fresh Cheese
Abstract
1. Introduction
2. Materials and Methods
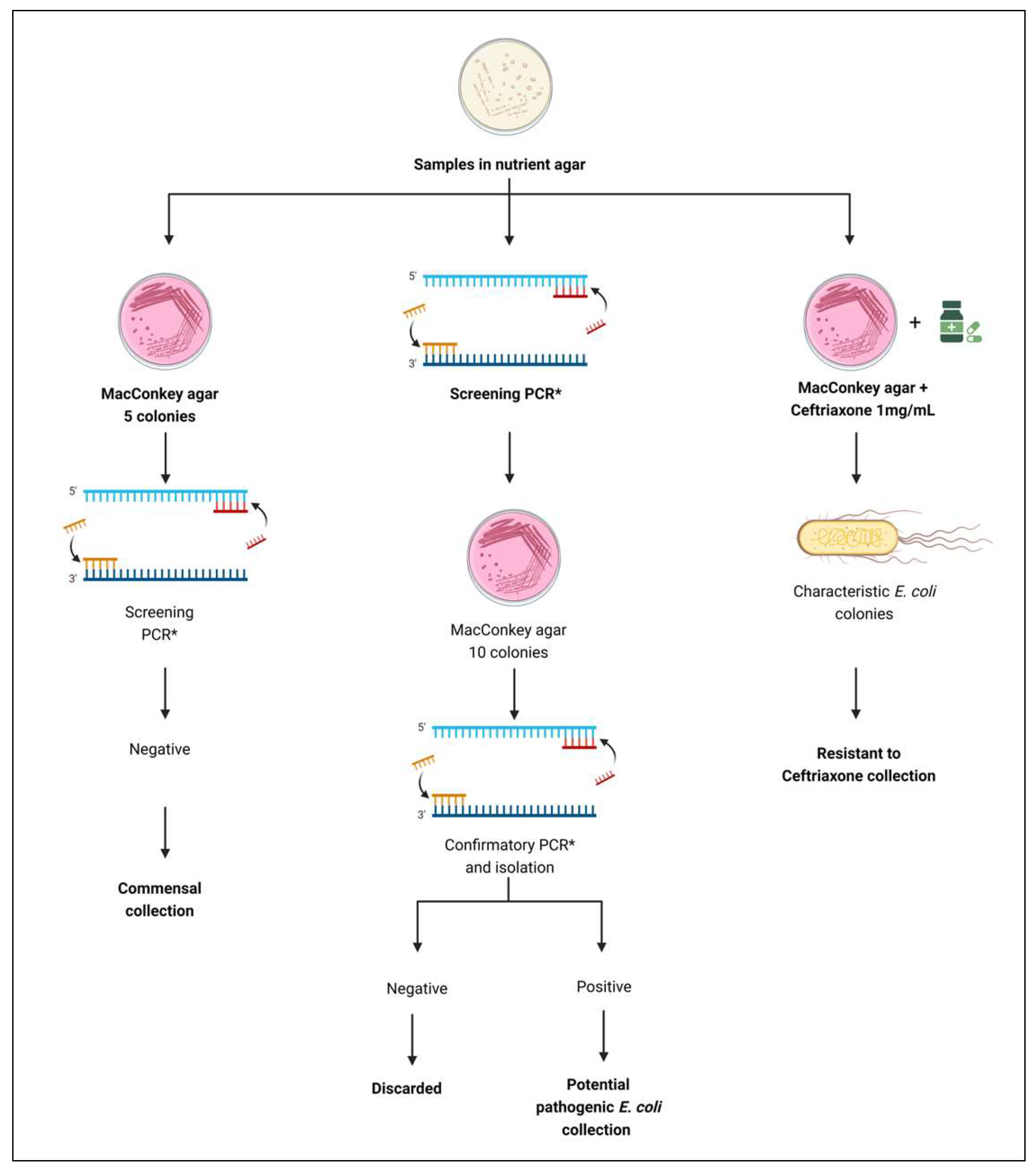
2.1. Sampling
2.2. Sample Preparation
2.3. E. coli Isolation
2.3.1. Collection of Commensal E. coli Isolates
2.3.2. Collection of Potentially Pathogenic E. coli Isolates
2.3.3. Collection of Ceftriaxone-Resistant E. coli Isolates
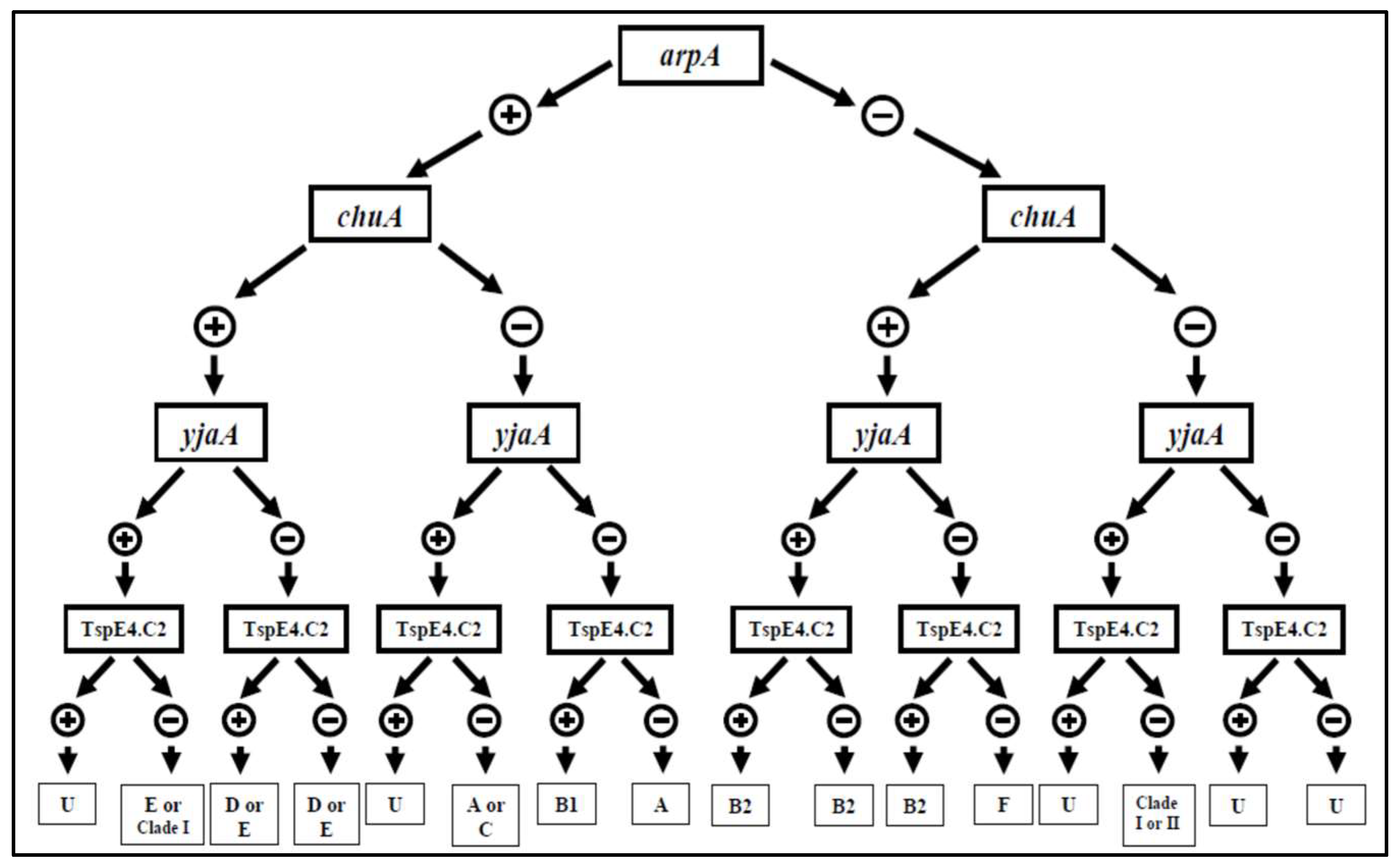
2.4. Phylogroup
2.5. Antimicrobial Susceptibility Test
2.6. Epidemiological Analysis of E. coli Isolates Using Pulsed-Field Gel Electrophoresis (PFGE)
2.7. Molecular Analysis for Identification of Resistance Genes by PCR
2.8. Serotyping for Detection of Somatic Antigen (O)
2.9. Multilocus Sequence Typing (MLST)
3. Results and Discussion
3.1. Collections of E. coli Isolates
3.1.1. Commensal
3.1.2. Potentially Pathogenic
3.1.3. Presence of ESBL/AmpC-Producing Isolates
3.2. Phylogrouping
3.3. Antimicrobial Sensitivity Test
3.4. Epidemiological Analysis of E. coli Isolates Using Pulsed-Field Electrophoresis Gel (PFGE)
3.5. PCR for Detecting Genes Related to Antimicrobial Resistance
3.6. Serological Testing for the Detection of Somatic Antigen (O)
3.7. Multilocus Sequence Typing (MLST)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grace, D. Burden of foodborne disease in low-income and middle-income countries and opportunities for scaling food safety interventions. Food Sec. 2023, 15, 1475–1488. [Google Scholar] [CrossRef]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L. Pathogenic E. coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef]
- Massella, E.; Giacometti, F.; Bonilauri, P.; Reid, C.J.; Djordjevic, S.P.; Merialdi, G.; Bacci, C.; Fiorentini, L.; Massi, P.; Bardasi, L.; et al. Antimicrobial Resistance Profile and ExPEC Virulence Potential in Commensal Escherichia coli of Multiple Sources. Antibiotics 2021, 10, 351. [Google Scholar] [CrossRef]
- Jenkins, C.; Bird, P.K.; Wensley, A.; Wilkinson, J.; Aird, H.; Mackintosh, A.; Greig, D.R.; Simpson, A.; Byrne, L.; Hughes, G.J. Outbreak of STEC O157:H7 linked to a milk pasteurisation failure at a dairy farm in England, 2019. Epidemiol. Infect. 2022, 150, e114. [Google Scholar] [CrossRef]
- Rosario, I.L.S.; Pia, A.K.R.; Rekowsky, B.S.S.; Elias, S.O.; Noronha, T.B.; Cuello, R.E.G.; Vieira, C.P.; Costa, M.P.; Conte-Junior, C.A. Predictive model for the growth of Shiga toxin-producing Escherichia coli in Minas Frescal cheese. Microb. Risk Anal. 2024, 27–28, 100308. [Google Scholar] [CrossRef]
- Haley, B.J.; Kim, S.W.; Salaheen, S.; Hovingh, E.; Van Kessel, J.A.S. Genome-Wide Analysis of Escherichia coli Isolated from Dairy Animals Identifies Virulence Factors and Genes Enriched in Multidrug-Resistant Strains. Antibiotics 2023, 12, 1559. [Google Scholar] [CrossRef]
- Soares, S.J.A.; Guimarães, F.d.F.; Rossi, G.A.M.; Guerra, S.T.; Dalanezi, F.M.; Lopes, B.C.; Ribeiro Mioni, M.d.S.; Yamakawa, A.C.; da Silva, E.C.; de Moraes, G.N.; et al. Virulence Potential, Biofilm Formation, and Disinfectants Control of Escherichia coli from Raw Milk Bulk Tanks in the Southeast of Brazil. Dairy 2023, 4, 541–553. [Google Scholar] [CrossRef]
- Massé, J.; Vanier, G.; Fairbrother, J.M.; de Lagarde, M.; Arsenault, J.; Francoz, D.; Dufour, S.; Archambault, M. Description of Antimicrobial-Resistant Escherichia coli and Their Dissemination Mechanisms on Dairy Farms. Vet. Sci. 2023, 10, 242. [Google Scholar] [CrossRef]
- Gohil, N.; Panchasara, H.; Patel, S.; Singh, V. Molecular Biology Techniques for the Identification and Genotyping of Microorganisms. In Microbial Genomics in Sustainable Agroecosystems; Tripathi, V., Kumar, P., Tripathi, P., Kishore, A., Eds.; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Gill, A.; Martinez-Perez, A.; McIlwham, S.; Blais, B. Development of a method for the detection of verotoxin-producing E. coli in food. J. Food Protec. 2012, 75, 827–837. [Google Scholar] [CrossRef]
- Keskimaki, M.; Eklund, M.; Personen, H.; Heiskanen, T.; Siitonen, A. EPEC, EAEC and STEC in stool specimens: Prevalence and molecular epidemiology of isolates. Diagn. Microbiol. Infect. Dis. 2001, 40, 151–156. [Google Scholar] [CrossRef]
- Woodward, M.J.; Carroll, P.J.; Wray, C. Detection of entero- and verocytotoxin genes in E. coli from diarrhoeal disease in animals using the polymerase chain reaction. Vet. Microbiol. 1992, 31, 251–261. [Google Scholar] [CrossRef]
- Beaudry, M.; Zhu, C.; Fairbrother, J.M.; Harel, J. Genotypic and phenotypic characterization of E. coli isolates from dogs manifesting attaching and effacing lesions. J. Clin. Microbiol. 1996, 34, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Vidal, R.; Vidal, M.; Lagos, R.; Levine, M.; Prado, V. Multiplex PCR for diagnosis of enteric infections associated with diarrheagenic E. coli. J. Clin. Microbiol. 2004, 42, 1787–1789. [Google Scholar] [CrossRef]
- Ngeleka, M.; Pritchard, J.; Appleyard, G.; Middleton, D.; Fairbrother, J.M. Isolation and association of E. coli AIDA-I/STb, rather than EAST1 pathotype, with diarrhea in piglets and antibiotic sensitivity of isolates. J. Vet. Diagn. Investig. 2003, 5, 242–252. [Google Scholar] [CrossRef]
- Lortie, L.A.; Dubreuil, J.D.; Harel, J. Characterization of Escherichia coli strains producing heat-stable enterotoxin b (STb) isolated from humans with diarrhea. J. Clin. Microbiol. 1991, 29, 656–659. [Google Scholar] [CrossRef]
- Furrer, B.; Candrian, U.; Luthy, J. Detection and identification of E. coli producing heat-labile enterotoxin type I by enzymatic amplification of a specific DNA fragment. Lett. Appl. Microbiol. 1990, 10, 31–34. [Google Scholar] [CrossRef]
- Ojeniyi, B.; Ahrens, P.; Meyling, A. Detection of fimbrial and toxin genes in E. coli and their prevalence in piglets with diarrhea. The applfication of colony hybridization assay, polymerase chain reaction and phenotypic assays. J. Vet. Med. 1994, 41, 49–59. [Google Scholar] [CrossRef]
- Ewers, C.; Li, G.; Wilking, H.; Kiessling, S.; Alt, K.; Antao, E.M.; Laturnus, C.; Diehl, I.; Glodde, S.; Homeier, T.; et al. Avian pathogenic, uropathogenic, and newborn meningitis-causing E. coli: How closely related are they? Int. J. Med. Microbiol. 2007, 297, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.; Lorenzo, V.; Neilands, J.B. Nucleotide sequence of the iucD gene of the pColV-K30 aerobactin operon and topology of its product studied with phoA and lacZ gene fusions. J. Bacteriol. 1988, 170, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Dozois, C.M.; Dho-Moulin, M.; Bree, A.; Fairbrother, J.M.; Desautels, C.; Curtiss, R. Relationship between the Tsh autotransporter and pathogenicity of avian E. coli and localization and analysis of the Tsh genetic region. Infect. Immun. 2000, 68, 4145–4154. [Google Scholar] [CrossRef]
- Johnson, J.R.; Kuskowski, M.A.; Owens, K.; Gajewski, A.; Winokur, P.L. Phylogenetic Origin and Virulence Genotype in Relation to Resistance to Fluoroquinolones and/or Extended-Spectrum Cephalosporins and Cephamycins among E. coli Isolates from Animals and Humans. J. Infect. Dis. 2003, 188, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Sethabutr, O.; Venkatesan, M.; Yam, S.; Pang, L.W.; Smoak, B.L.; Sang, W.K.; Echeverria, P.; Taylor, D.N.; Isenbarger, D.W. Detection of PCR products of the ipaH gene from Shigella and enteroinvasive E. coli by enzyme linked immunosorbent assay. Diagn. Microbiol. Infect. Dis. 2000, 37, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Frankel, G.; Riley, L.; Giron, J.A.; Valmassoi, J.; Friedmann, A.; Strockbine, N.; Falkow, S.; Schoolnik, G.K. Detection of Shigella in feces using DNA amplification. J. Infect. Dis. 1990, 161, 1252–1256. [Google Scholar] [CrossRef]
- Boisen, N.; Struve, C.; Scheutz, F.; Krogfelt, K.A.; Nataro, J.P. New adhesin of enteroaggregative E. coli related to the Afa/Dr/AAF family. Infect. Immun. 2008, 76, 3281–3292. [Google Scholar] [CrossRef]
- Schmidt, H.; Knop, C.; Franke, S.; Aleksic, S.; Heesemann, J.; Karch, H. Development of PCR for screening of enteroaggregative E. coli. J. Clin. Microbiol. 1995, 33, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Clermont, C.; Christenson, J.K.; Denamur, E.; Gordon, D.M. The Clermont E. coli phylo-typing method revisited: Improvement of specificity and detection of new phylo-groups. Environ. Microbiol. Rep. 2003, 5, 58–65. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1996, 45, 493–496. [Google Scholar] [CrossRef]
- CLSI. Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk and Dilution Susceptibility Test for Bacteria Isolated from Animals, 6th ed.; CLSI standard VET01S; CLSI: Wayne, PA, USA, 2023. [Google Scholar]
- Ribot, E.M.; Fair, M.A.; Gautom, R.; Cameron, D.N.; Hunter, S.B.; Swaminathan, B.; Barrett, T.J. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of E. coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog. Dis. 2006, 3, 59–67. [Google Scholar] [CrossRef]
- Harel, J.; Lapointe, H.; Fallara, A.; Lortie, L.A.; Bigras-Poulin, M.; Lariviere, S.; Fairbrother, J.M. Detection of genes for fimbrial antigens and enterotoxins associated with E. coli serogroups isolated from pigs with diarrhea. J. Clin. Microbiol. 1991, 29, 745–752. [Google Scholar] [CrossRef]
- Maynard, C.; Franc, S.B.; Sanschagrin, O.; Levesque, R.C.; Brousseau, R.; Masson, L.; Lariviere, S.; Harel, J. Heterogeneity among Virulence and Antimicrobial Resistance Gene Profiles of Extraintestinal E. coli Isolates of Animal and Human Origin. J. Clin. Microbiol. 2004, 42, 5444–5452. [Google Scholar] [CrossRef]
- Boyd, D.A.; Tyler, S.; Christianson, S.; McGeer, A.; Muller, M.P.; Willey, B.M.; Bryce, E.; Gardam, M.; Nordmann, P.; Mulvey, M.R. Complete nucleotide sequence of a 92 kb plasmid harboring the CTX-M-15 extended-spectrum beta-lactamase involved in an outbreak in long-term-care facilities in Toronto, Canada. Antimicrob. Agents Chemother. 2004, 48, 3758–3764. [Google Scholar] [CrossRef]
- Nuesch-Inderbinen, M.T.; Hachler, H.; Kayser, F.H. Detection of genes coding for extended-spectrum SHV b-lactamases in clinical isolates by a molecular genetic method, and comparison with E test. Eur. J. Clin. Microbiol. Infect. Dis. 1996, 15, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Maynard, A.D.; Baron, P.A.; Shvedova, A.A.; Kisin, E.R.; Catranova, V. Exposure to carbon nanotube material 1: Aerosol release during the handling of unrefined single walled carbon nanotube material. J. Toxicol. Environ. Health 2003, 67, 87–107. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Jiang, H.X.; Liao, X.P.; Liu, J.H.; Li, S.J.; Chen, X.Y.; Chen, C.X.; Lü, D.H.; Liu, Y.H. Prevalence of plasmid-mediated quinolone resistance qnr genes in poultry and swine clinical isolates of E. coli. Vet. Microbiol. 2008, 10, 414–420. [Google Scholar] [CrossRef]
- Ewing, B.; Green, P. Basecalling of automated sequencer traces using PHRED. II. Error probabilities. Genome Res. 1998, 8, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.G.M.; Cerqueira, A.M.F. Shiga toxin-producing Escherichia coli in the animal reservoir and food in Brazil. J. Appl. Microbiol. 2020, 128, 1568–1582. [Google Scholar] [CrossRef]
- Manges, A.R.; Johnson, J.R. Reservoirs of extraintestinal pathogenic E. coli. Microbiol. Spectrum 2015, 3, 3. [Google Scholar] [CrossRef]
- Ombarak, R.A.; Hinenoya, A.; Awastshi, S.P.; Iguchi, A.; Shima, A.; Elbagory, A.R.M.; Yamasaki, S. Prevalence and pathogenic potential of E. coli isolates from raw milk and raw milk cheese in Egypt. Int. J. Food Microbiol. 2016, 221, 69–76. [Google Scholar] [CrossRef]
- Ribeiro, L.F.; Nespolo, N.M.; Rossi, G.A.M.; Fairbrother, J.M. Exploring Extended-Spectrum Beta-Lactamase (ESBL)-Producing Escherichia coli in Food-Producing Animals and Animal-Derived Foods. Pathogens 2024, 13, 346. [Google Scholar] [CrossRef]
- Pas, C.; Latka, A.; Fieseler, L.; Briers, Y. Phage tailspike modularity and horizontal gene transfer reveals specificity towards E. coli O-antigen serogroups. Virol. J. 2023, 20, 174. [Google Scholar] [CrossRef]
- Lan, N.P.H.; Hien, N.H.; Phuong, T.L.T.; Thanh, D.P.; Thieu, N.T.V.; Ngoc, D.T.T.; Tuyen, H.T.; Vinh, P.V.; Ellington, M.J.; Thwaites, G.E.; et al. Phenotypic and genotypic characteristics of ESBL and AmpC producing organisms associated with bacteraemia in Ho Chi Minh City, Vietnam. Antimicrob. Resist. Infect. Control 2017, 6, 105. [Google Scholar] [CrossRef] [PubMed]
- Verraes, C.; Van Boxstael, S.; Van Meervenne, E.; Van Coillie, E.; Butaye, P.; Catry, B.; De Schaetzen, M.A.; Van HuffeL, X.; Imberechts, H.; Dierick, K.; et al. Antimicrobial Resistance in the Food Chain: A Review. Int. J. Environ. Res. Public Health 2013, 10, 2643–2669. [Google Scholar] [CrossRef]
- Stoppe, N.C.; Silva, J.S.; Carlos, C.; Sato, M.I.Z.; Saraiva, A.M.; Ottoboni, L.M.M.; Torres, T.T. Worldwide phylogenetic group patterns of Escherichia coli from commensal human and wastewater treatment plant isolates. Front. Microbiol. 2017, 8, 2512. [Google Scholar] [CrossRef] [PubMed]
- Carlos, C.; Pires, M.M.; Stoppe, N.C.; Hachich, E.M.; Sato, M.I.Z.; Gomes, T.A.T.; Amaral, L.A.; Ottoboni, L.M.M. E. coli phylogenetic group determination and its application in the identification of the major animal source of fecal contamination. BMC Microbiol. 2010, 10, 161. [Google Scholar] [CrossRef] [PubMed]
- Ciesielczuk, H.; Jenkins, C.; Chattaway, M.; Doumith, M.; Hope, R.; Woodford, N.; Wareham, D.W. Trends in ExPEC serogroups in the UK and their significance. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1661–1666. [Google Scholar] [CrossRef]
- Aslam, M.; Toufeer, M.; NArvaez-Bravo, C.; Lai, V.; Rempel, H.; Manges, A.; Diarra, M.S. Characterization of Extraintestinal Pathogenic E. coli isolated from retail poultry meats from Alberta, Canada. Int. J. Food Microbiol. 2014, 177, 49–56. [Google Scholar] [CrossRef]
- Cyoia, P.S.; Rodrigues, G.R.; Nishio, E.K.; Medeiros, L.P.; Koga, V.L.; PEreira, A.P.; Vespero, E.C.; Houle, S.; Dozois, C.M.; Nakazato, G.; et al. Presence of virulence genes and pathogenicity islands in extraintestinal pathogenic E. coli isolates from Brazil. J. Infect. Dev. Ctries. 2015, 29, 1068–1075. [Google Scholar] [CrossRef]
- Meena, P.R.; Priyanka, P.; Singh, A.P. Extraintestinal pathogenic Escherichia coli (ExPEC) reservoirs, and antibiotics resistance trends: A one-health surveillance for risk analysis from “farm-to-fork”. Lett. Appl. Microbiol. 2023, 76, ovac016. [Google Scholar] [CrossRef]
- Dadi, B.R.; Abebe, T.; Zhang, L.; Mihret, A.; Abebe, W.; Amogne, W. Distribution of virulence genes and phylogenetics of uropathogenic Escherichia coli among urinary tract infection patients in Addis Ababa, Ethiopia. BMC Infect. Dis. 2020, 20, 108. [Google Scholar] [CrossRef]
- Yin, X.; Dudley, E.G.; Pinto, C.N.; M’ikanatha, N.M. Fluoroquinolone sales in food animals and quinolone resistance in non-typhoidal Salmonella from retail meats: United States, 2009–2018. J. Glob. Antim. Resist. 2022, 29, 163–167. [Google Scholar] [CrossRef]
- Bonyadian, M.; Moshtaghi, H.; Taheri, M.A. Molecular characterization and antibiotic resistance of enterotoxigenic and entero-aggregative E. coli isolated from raw milk and unpasteurized cheeses. Vet. Res. Forum. 2014, 5, 29–34. [Google Scholar]
- Do, K.H.; Seo, K.; Lee, W.K. Antimicrobial resistance, virulence genes, and phylogenetic characteristics of pathogenic Escherichia coli isolated from patients and swine suffering from diarrhea. BMC Microbiol. 2022, 22, 199. [Google Scholar] [CrossRef]
- Er, D.K.; Dundar, D.; Uzuner, H.; Osmani, A. Relationship between phylogenetic groups, antibiotic resistance and patient characteristics in terms of adhesin genes in cystitis and pyelonephritis isolates of E. coli. Microb. Pathog. 2015, 89, 188–194. [Google Scholar] [CrossRef]
- Schmid, A.; Hörmansdorfer, S.; Messelhäusser, U.; Käsbohrer, A.; Sauter-Louis, C.; Mansfeld, R. Prevalence of Extended-Spectrum -Lactamase-Producing E. coli on Bavarian Dairy and Beef Cattle Farms. Appl. Environ. Microbiol. 2013, 79, 3027–3032. [Google Scholar] [CrossRef]
- Farrokh, C.; Jordan, K.; Auvray, F.; Glass, K.; Oppegaard, H.; Raynaud, S.; Thevenot, D.; Condron, R.; De Reu, K.; Govaris, A.; et al. Review of Shiga-toxin-producing E. coli (STEC) and their significance in dairy production. Int. J. Food Microbiol. 2013, 162, 190–212. [Google Scholar] [CrossRef]
- Castro, M.; Soares, K.; Ribeiro, C.; Esteves, A. Evaluation of the Effects of Food Safety Training on the Microbiological Load Present in Equipment, Surfaces, Utensils, and Food Manipulator’s Hands in Restaurants. Biol. Life Sci. Forum 2024, 31, 10. [Google Scholar] [CrossRef]
- Gargiulo, A.H.; Duarte, S.G.; Campos, G.Z.; Landgraf, M.; Franco, B.D.G.M.; Pinto, U.M. Food Safety Issues Related to Eating In and Eating Out. Microorganisms 2022, 10, 2118. [Google Scholar] [CrossRef]
- Vincent, C.; Boerlin, P.; Daignault, D.; Dozois, C.M.; Dutil, L.; Galanakis, C.; Reid-Smith, R.J.; Tellier, P.P.; Tellis, P.A.; Ziebell, K.; et al. Food Reservoir for E. coli Causing Urinary Tract Infections. Emerg. Infect. Dis. 2010, 16, 88–95. [Google Scholar] [CrossRef]
- Subhi, A.; Saad, A.S.A.; Osman, K.; Hashad, M.E.; Deif, H.N. Prevalence and Antibiogram of Escherichia coli isolates recovered from bovine milk. J. Appl. Vet. Sci. 2023, 8, 82–90. [Google Scholar] [CrossRef]
- Byarugaba, D.K.; Erima, B.; Wokorach, G.; Alafi, S.; Kibuuka, H.; Mworozi, E.; Musinguzi, A.K.; Kiyengo, J.; Najjuka, F.; Wabwire-Mangen, F. Resistome and virulome of high-risk pandemic clones of multidrug-resistant extra-intestinal pathogenic Escherichia coli (ExPEC) isolated from tertiary healthcare settings in Uganda. PLoS ONE 2023, 18, e0294424. [Google Scholar] [CrossRef] [PubMed]
- Bok, E.; Mazurek, J.; Stosik, M.; Wojciech, M.; Baldy-Chudzik, K. Prevalence of virulence determinants and antimicrobial resistance among commensal E. coli derived from dairy and beef cattle. Int. J. Environ. Res. Public Health. 2015, 12, 970–985. [Google Scholar] [CrossRef]
- Mazurek, J.; Pusz, P.; Bok, E.; Stosik, M.; Baldy-Chudzik, K. The phenotypic and genotypic characteristics of antibiotic resistance in E. coli populations isolated from farm animals with different exposure to antimicrobial agents. Pol. J. Microbiol. 2013, 62, 173–179. [Google Scholar] [CrossRef]
- Ramírez-Bayard, I.E.; Mejía, F.; Medina-Sánchez, J.R.; Cornejo-Reyes, H.; Castillo, M.; Querol-Audi, J.; Martínez-Torres, A.O. Prevalence of Plasmid-Associated Tetracycline Resistance Genes in Multidrug-Resistant Escherichia coli Strains Isolated from Environmental, Animal and Human Samples in Panama. Antibiotics 2023, 12, 280. [Google Scholar] [CrossRef]
- Juraschek, K.; Malekzadah, J.; Malorny, B.; Kasbohrer, A.; Schwarz, S.; Meemken, D.; Hammerl, J.A. Characterization of qnrB-carrying plasmids from ESBL- and non-ESBL-producing Escherichia coli. BMC Genom. 2022, 23, 365. [Google Scholar] [CrossRef] [PubMed]
- Wijetunge, D.S.; Gongati, S.; Debroy, C.; Kim, K.S.; Couraud, P.O.; Romero, I.A.; Weksler, B.; Kariyawasam, S. Characterizing the pathotype of neonatal meningitis causing E. coli (NMEC). BMC Microbiol. 2015, 15, 211. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.K.; Phan, M.-D.; Hancock, S.J.; Peters, K.M.; Alvarez-Fraga, L.; Forde, B.M.; Andersen, S.B.; Miliya, T.; Harris, P.N.A.; Beatson, S.A.; et al. High-risk Escherichia coli clones that cause neonatal meningitis and association with recrudescent infection. Microbiol. Infect. Dis. 2023, 12, RP91853. [Google Scholar] [CrossRef]
- Nguyen, Q.V.; Hochstrasser, L.; Chuard, C.; Hächler, H.; Regamey, C.; Descombes, E. Adult hemolytic-uremic syndrome associated with urosepsis due to Shigatoxin-producing E. coli O138:H-. Ren. Fail. 2007, 29, 747–750. [Google Scholar] [CrossRef]
- Osman, K.M.; Mustafa, A.M.; Elhariri, M.; Abdelhamed, G.S. Identification of serotypes and virulence markers of E. coli isolated from human stool and urine samples in Egypt. Indian J. Med. Microbiol. 2012, 30, 308–313. [Google Scholar] [CrossRef]
- Cheng, M.F.; Chen, W.L.; Hung, W.Y.; Huang, I.F.; Chiou, Y.H.; Chen, Y.S.; Lee, S.S.; Hung, C.H.; Wang, J.L. Emergence of extended spectrum-β-lactamase-producing E. coli O25b-ST131: A major community-acquired uropathogen in infants. Pediatr. Infect. Dis. J. 2015, 34, 469–475. [Google Scholar] [CrossRef]
- Cho, S.Y.; Kang, C.I.; Cha, M.K.; Wi, Y.M.; Ha, Y.E.; Chung, D.R.; Lee, N.Y.; Peck, K.R.; Song, J.H.; Korean Network for Study on Infectious Diseases. Clinical Features and Treatment Outcomes of Bloodstream Infections Caused by Extended-Spectrum β-Lactamase-Producing E. coli Sequence Type 131. Microb. Drug Resist. 2015, 21, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Dautzenberg, M.J.; Haverkate, M.R.; Bonten, M.J.; Bootsma, M.C. Epidemic potential of E. coli ST131 and Klebsiella pneumoniae ST258: A systematic review and meta-analysis. BMJ Open 2016, 6, e009971. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Ranjan, A.; Nandanwar, N.; Babbar, A.; Jadhav, S.; Ahmed, N. Genotypic and phenotypic profiles of E. coli isolates belonging to clinical sequence type 131 (ST131), clinical non-ST131, and fecal non-ST131 lineages from India. Antimicrob. Agents Chemother. 2014, 58, 7240–7249. [Google Scholar] [CrossRef] [PubMed]
- Nicolas-Chanoine, M.H.; Bertrand, X.; Madec, J.Y. E. coli ST131, an intriguing clonal group. Clin. Microbiol. Rev. 2014, 27, 543–574. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, L.F.; Rossi, G.A.M.; Sato, R.A.; de Souza Pollo, A.; Cardozo, M.V.; Amaral, L.A.d.; Fairbrother, J.M. Epidemiology, Virulence and Antimicrobial Resistance of Escherichia coli Isolated from Small Brazilian Farms Producers of Raw Milk Fresh Cheese. Microorganisms 2024, 12, 1739. https://doi.org/10.3390/microorganisms12081739
Ribeiro LF, Rossi GAM, Sato RA, de Souza Pollo A, Cardozo MV, Amaral LAd, Fairbrother JM. Epidemiology, Virulence and Antimicrobial Resistance of Escherichia coli Isolated from Small Brazilian Farms Producers of Raw Milk Fresh Cheese. Microorganisms. 2024; 12(8):1739. https://doi.org/10.3390/microorganisms12081739
Chicago/Turabian StyleRibeiro, Laryssa Freitas, Gabriel Augusto Marques Rossi, Rafael Akira Sato, Andressa de Souza Pollo, Marita Vedovelli Cardozo, Luiz Augusto do Amaral, and John Morris Fairbrother. 2024. "Epidemiology, Virulence and Antimicrobial Resistance of Escherichia coli Isolated from Small Brazilian Farms Producers of Raw Milk Fresh Cheese" Microorganisms 12, no. 8: 1739. https://doi.org/10.3390/microorganisms12081739
APA StyleRibeiro, L. F., Rossi, G. A. M., Sato, R. A., de Souza Pollo, A., Cardozo, M. V., Amaral, L. A. d., & Fairbrother, J. M. (2024). Epidemiology, Virulence and Antimicrobial Resistance of Escherichia coli Isolated from Small Brazilian Farms Producers of Raw Milk Fresh Cheese. Microorganisms, 12(8), 1739. https://doi.org/10.3390/microorganisms12081739








