Decitabine Increases the Transcription of RIG-I Gene to Suppress the Replication of Feline Calicivirus and Canine Influenza Virus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell, Virus, Natural Products, Antibodies, and Virus Titration
2.2. Primary Screening of Antiviral Natural Products by FCV-Induced CPE Inhibition
2.3. Evaluation of Cytotoxicity and Antiviral Efficacy of Natural Products
2.4. RNA Extraction, cDNA Synthesis, and Quantitative Real-Time PCR (qRT-PCR)
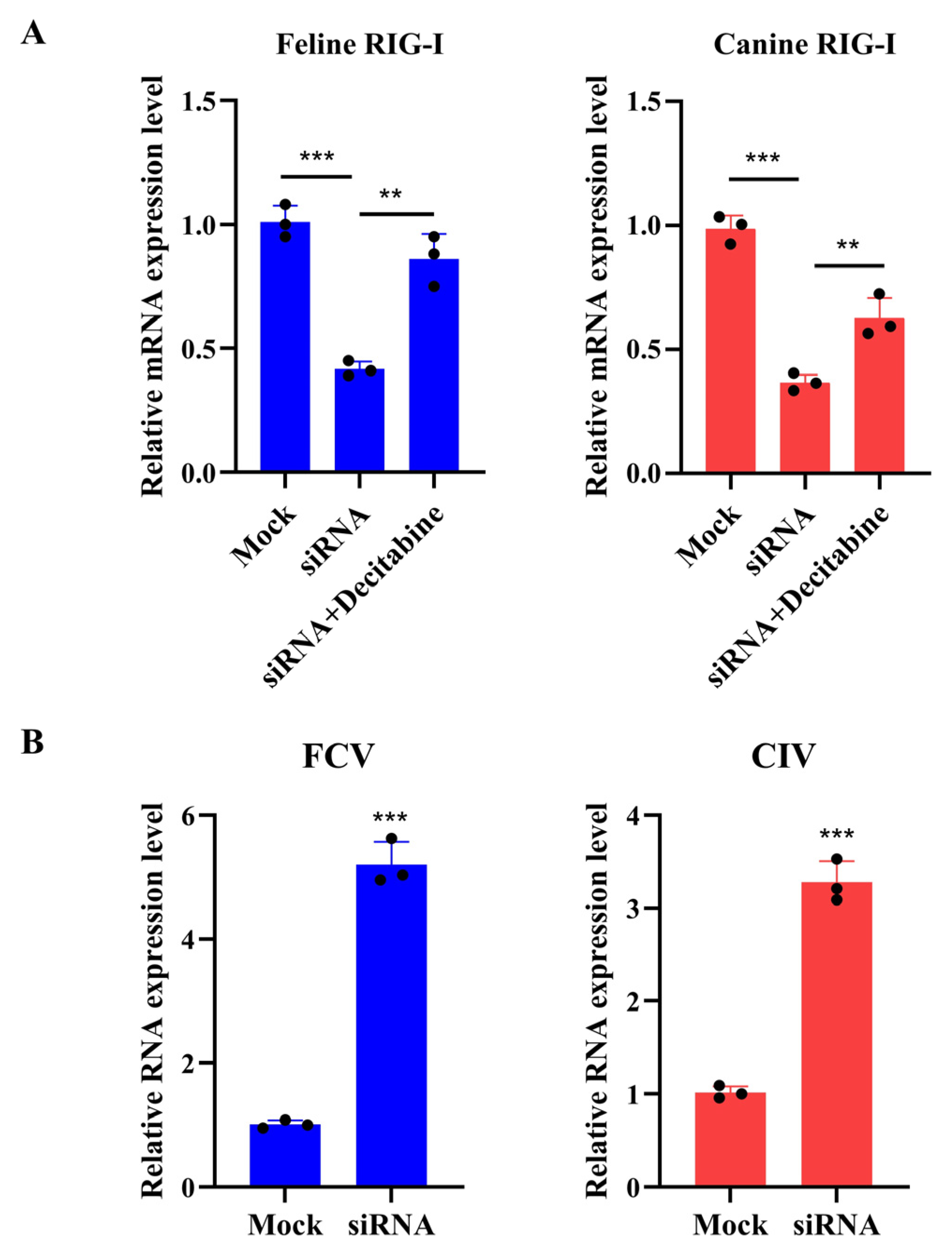
2.5. RNA Interference
2.6. Western Blotting
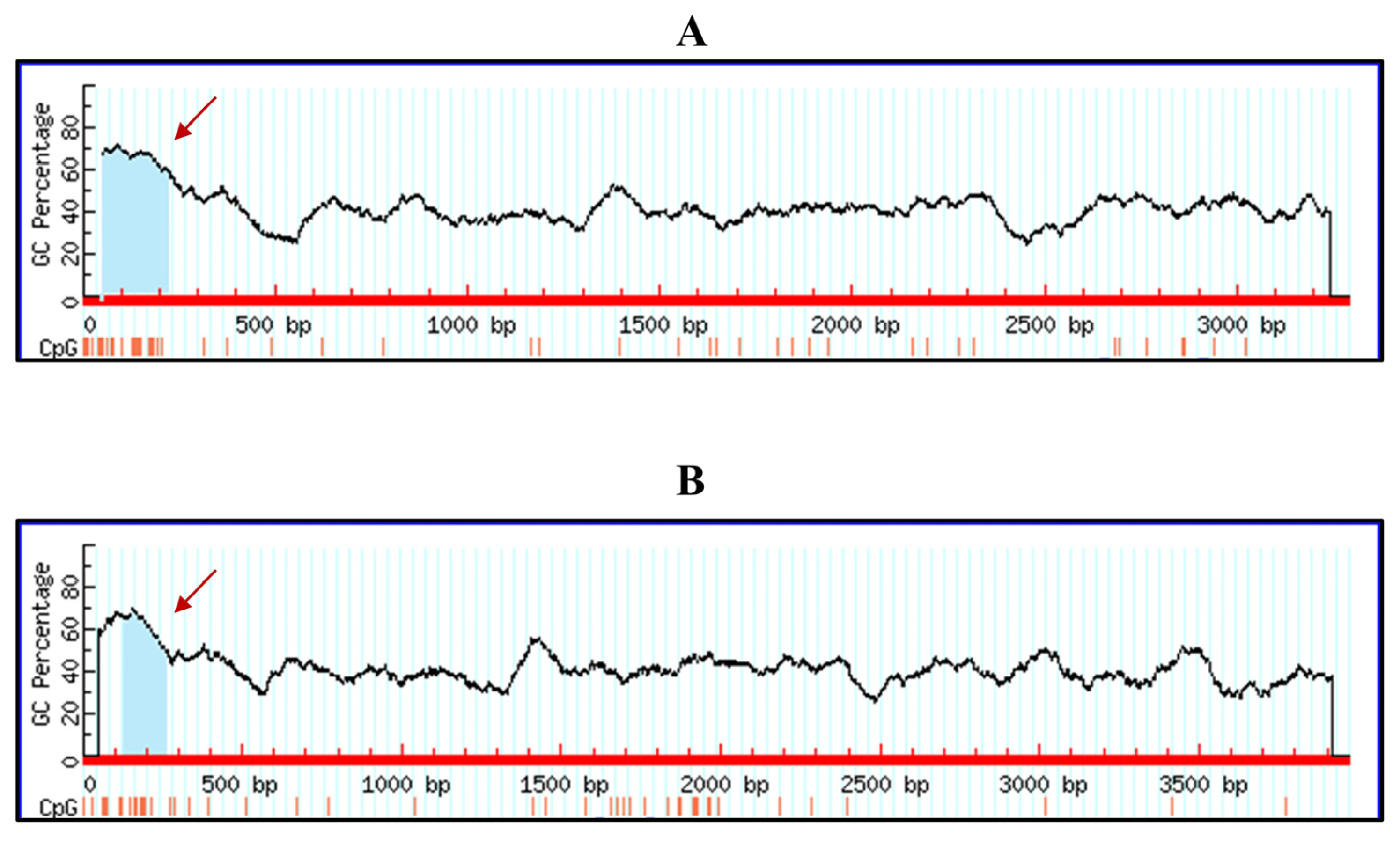
2.7. Bioinformatic Analysis of the CpG Island of Canine and Feline RIG-I Gene
2.8. Statistical Analysis
3. Results
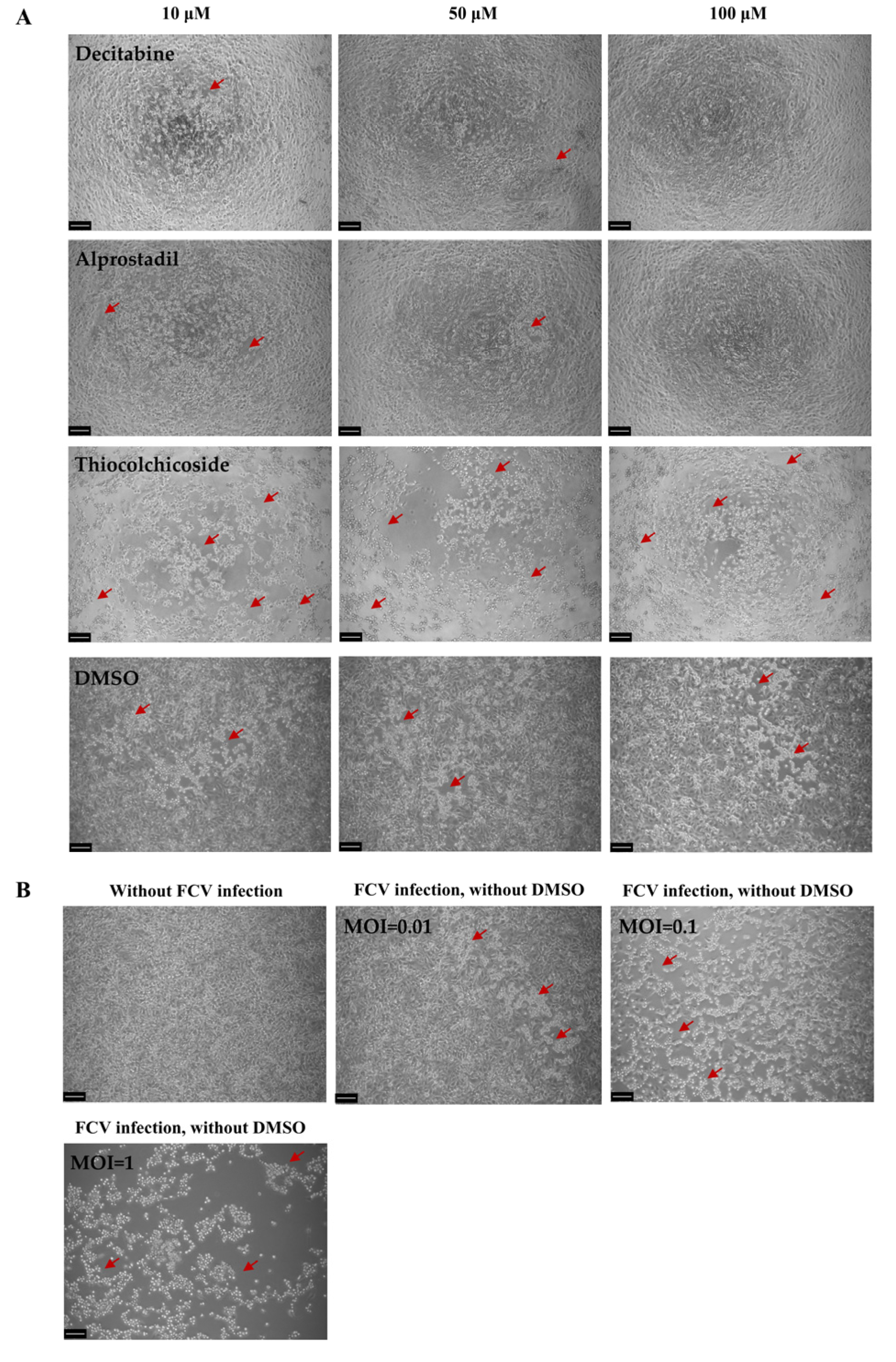
3.1. Primary Screening of Antiviral Natural Products Against Feline Calicivirus
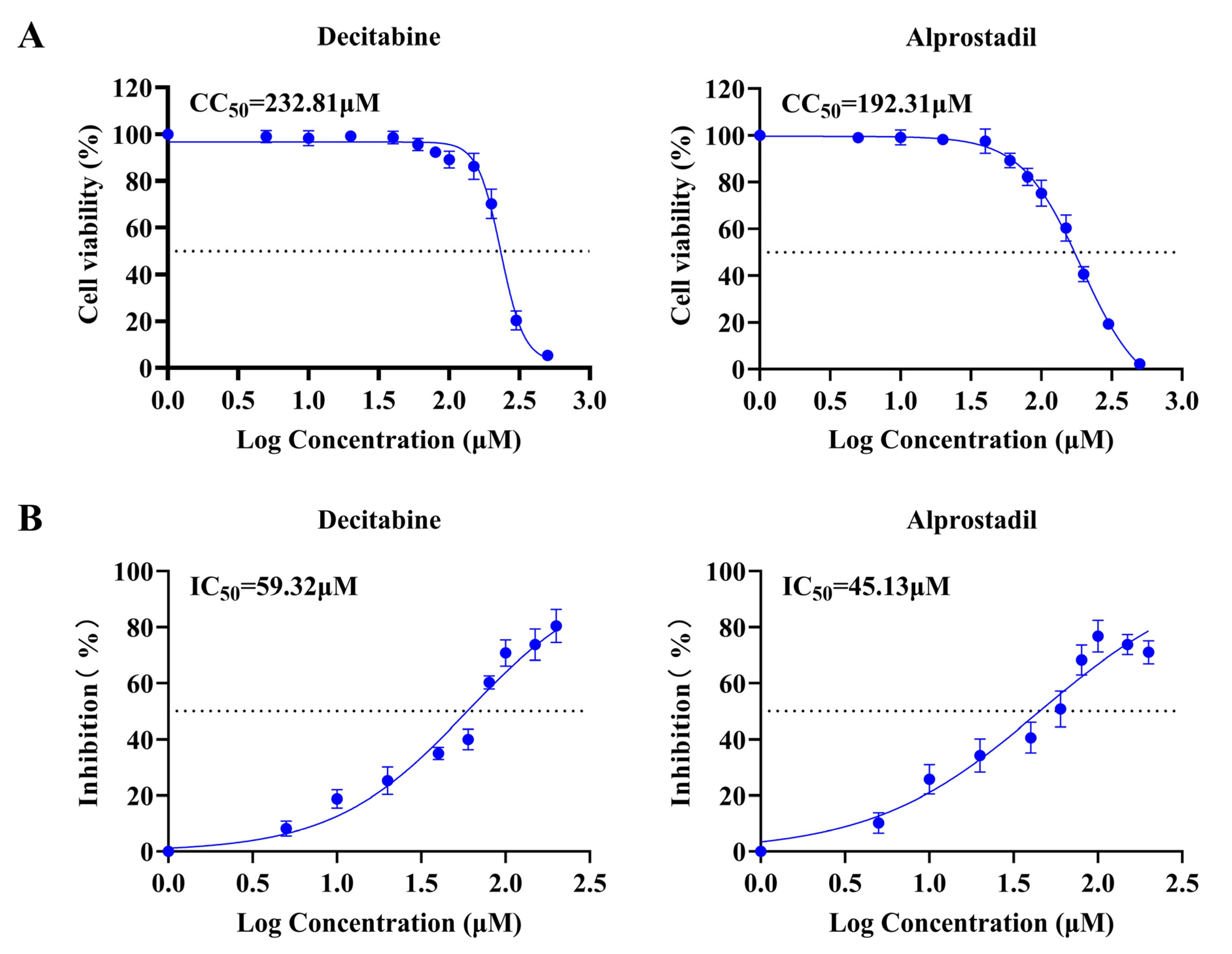
3.2. Evaluation of Cytotoxicity and Antiviral Efficacy of Natural Products
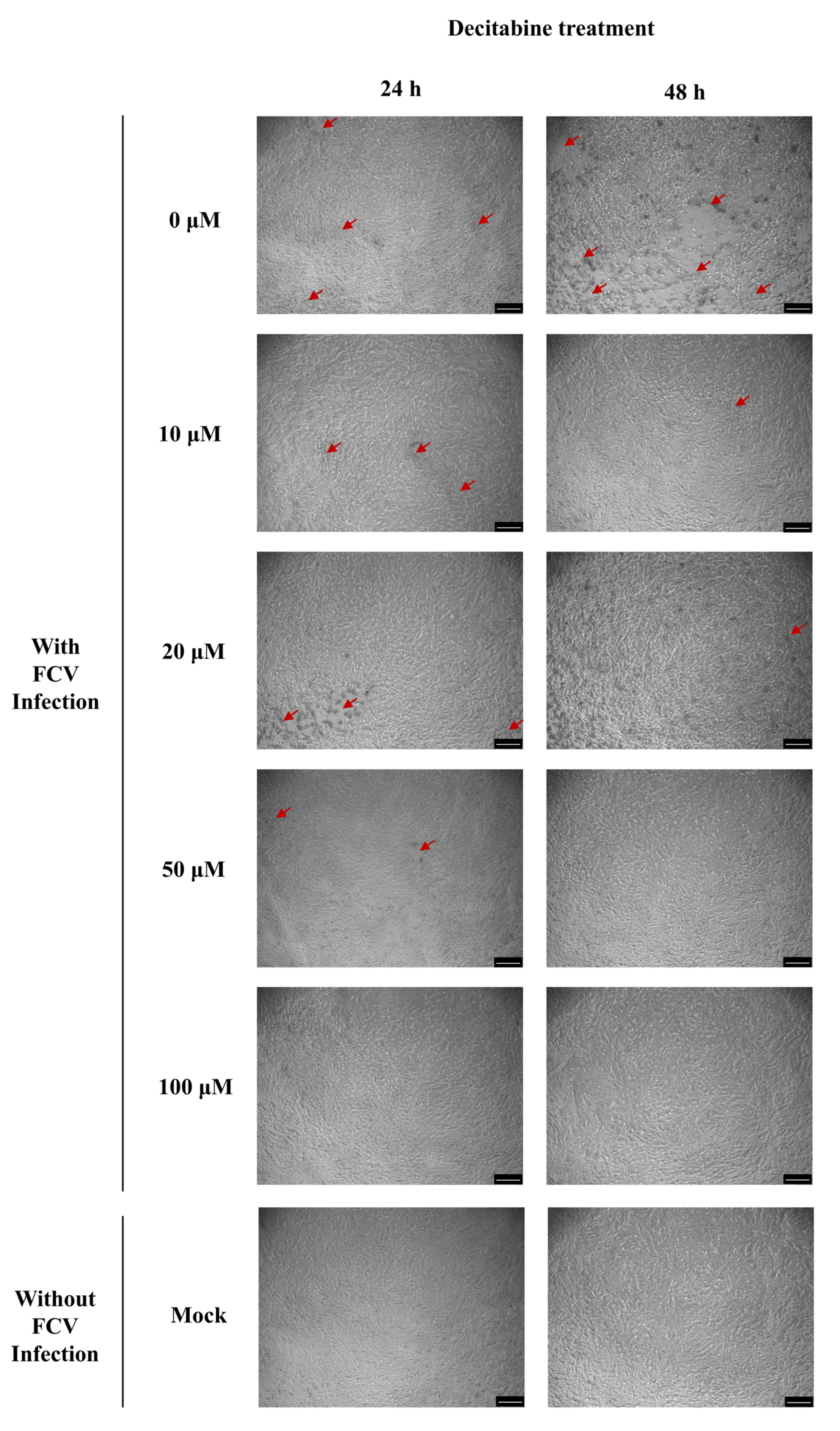
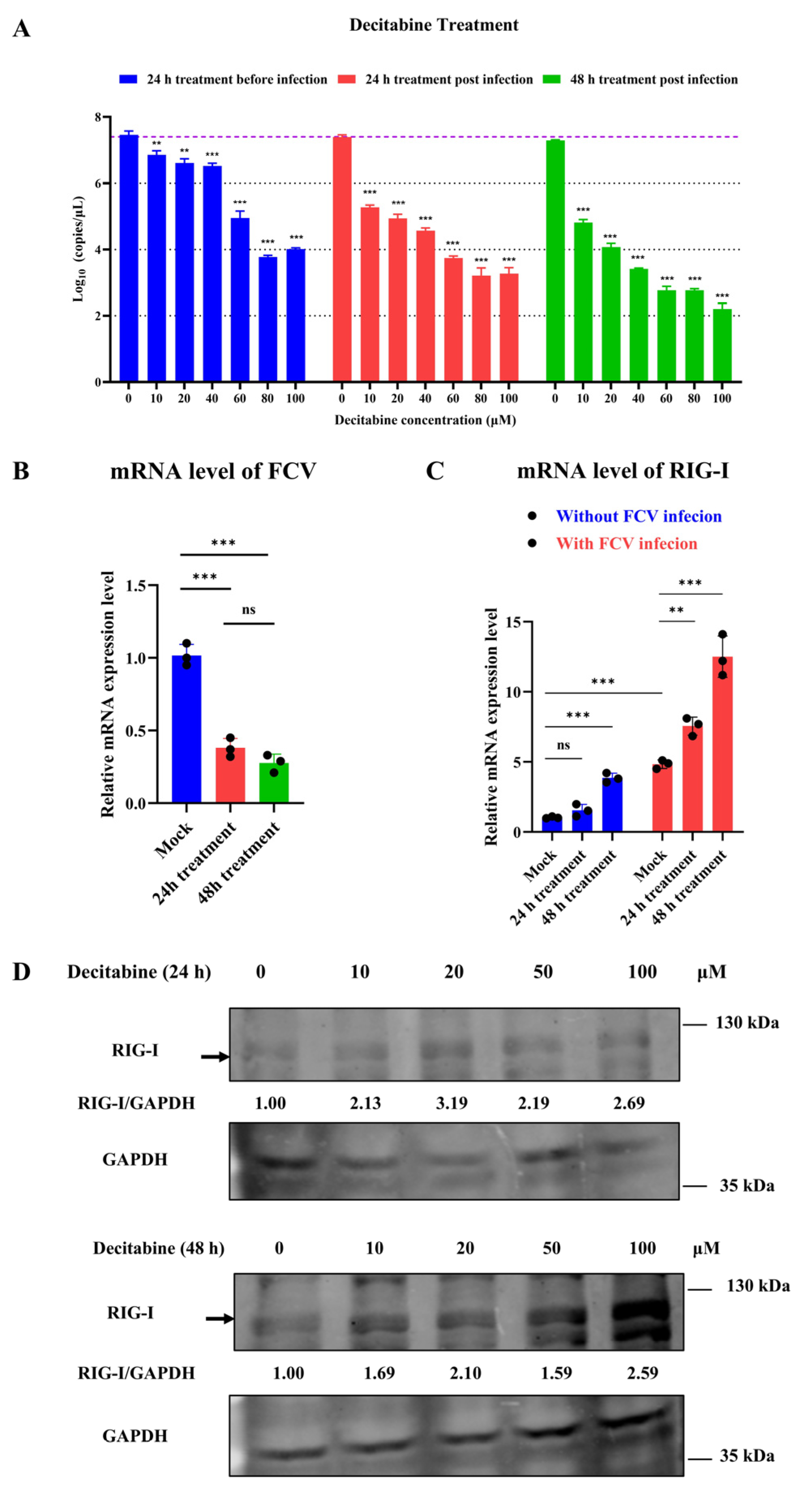
3.3. Potential Role of Decitabine Against Feline Calicivirus
3.4. Decitabine Enhances the Transcription of Canine RIG-I to Inhibit the Replication of Canine Influenza Virus
3.5. Bioinformatic Analysis of the Potential Methylation on Canine and Feline RIG-I Gene
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martinez, J.P.; Sasse, F.; Brönstrup, M.; Diez, J.; Meyerhans, A. Antiviral Drug Discovery: Broad-Spectrum Drugs from Nature. Nat. Prod. Rep. 2015, 32, 29–48. [Google Scholar] [CrossRef] [PubMed]
- Vougogiannopoulou, K.; Corona, A.; Tramontano, E.; Alexis, M.N.; Skaltsounis, A.-L. Natural and Nature-Derived Products Targeting Human Coronaviruses. Molecules 2021, 26, 448. [Google Scholar] [CrossRef]
- Li, G.; Hilgenfeld, R.; Whitley, R.; De Clercq, E. Therapeutic Strategies for COVID-19: Progress and Lessons Learned. Nat. Rev. Drug Discov. 2023, 22, 449–475. [Google Scholar] [CrossRef] [PubMed]
- Pesavento, P.A.; Chang, K.-O.; Parker, J.S.L. Molecular Virology of Feline Calicivirus. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Hofmann-Lehmann, R.; Hosie, M.J.; Hartmann, K.; Egberink, H.; Truyen, U.; Tasker, S.; Belák, S.; Boucraut-Baralon, C.; Frymus, T.; Lloret, A.; et al. Calicivirus Infection in Cats. Viruses 2022, 14, 937. [Google Scholar] [CrossRef]
- Guo, H.; Miao, Q.; Zhu, J.; Yang, Z.; Liu, G. Isolation and Molecular Characterization of a Virulent Systemic Feline Calicivirus Isolated in China. Infect. Genet. Evol. 2018, 65, 425–429. [Google Scholar] [CrossRef]
- Pesavento, P.A.; Maclachlan, N.J.; Dillard-Telm, L.; Grant, C.K.; Hurley, K.F. Pathologic, Immunohistochemical, and Electron Microscopic Findings in Naturally Occurring Virulent Systemic Feline Calicivirus Infection in Cats. Vet. Pathol. 2004, 41, 257–263. [Google Scholar] [CrossRef]
- McDonagh, P.; Sheehy, P.A.; Fawcett, A.; Norris, J.M. Antiviral Effect of Mefloquine on Feline Calicivirus in Vitro. Vet. Microbiol. 2015, 176, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Cui, Z.; Li, G.; Zhang, L.; Zhang, Y.; Zhao, H.; Zhang, S.; Guo, Y.; Zhao, Y.; Men, F.; et al. Antiviral Effect of Copper Chloride on Feline Calicivirus and Synergy with Ribavirin in Vitro. BMC Vet. Res. 2020, 16, 231. [Google Scholar] [CrossRef]
- Joshi, S.S.; Su, X.; D’Souza, D.H. Antiviral Effects of Grape Seed Extract against Feline Calicivirus, Murine Norovirus, and Hepatitis A Virus in Model Food Systems and under Gastric Conditions. Food Microbiol. 2015, 52, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Li, D.; Xie, Y.; Wang, K.; Zhang, Y.; Li, G.; Zhang, Q.; Chen, X.; Teng, Y.; Zhao, S.; et al. Nitazoxanide Protects Cats from Feline Calicivirus Infection and Acts Synergistically with Mizoribine In Vitro. Antivir. Res. 2020, 182, 104827. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ye, S.; Yao, C.; Wang, J.; Mao, J.; Xu, L.; Liu, Y.; Fu, C.; Lu, G.; Li, S. Antiviral Activity of Canine RIG-I against Canine Influenza Virus and Interactions between Canine RIG-I and CIV. Viruses 2021, 13, 2048. [Google Scholar] [CrossRef] [PubMed]
- Greve, G.; Schüler, J.; Grüning, B.A.; Berberich, B.; Stomper, J.; Zimmer, D.; Gutenkunst, L.; Bönisch, U.; Meier, R.; Blagitko-Dorfs, N.; et al. Decitabine Induces Gene Derepression on Monosomic Chromosomes: In Vitro and In Vivo Effects in Adverse-Risk Cytogenetics AML. Cancer Res. 2021, 81, 834–846. [Google Scholar] [CrossRef]
- Li, L.; Liu, W.; Sun, Q.; Zhu, H.; Hong, M.; Qian, S. Decitabine Downregulates TIGAR to Induce Apoptosis and Autophagy in Myeloid Leukemia Cells. Oxidative Med. Cell. Longev. 2021, 2021, 8877460. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Liu, P.; Wang, Y.; Zhu, Y.; Zeng, Q.; Hu, X.; Ren, Z.; Wang, Y. A Novel Cognition of Decitabine: Insights into Immunomodulation and Antiviral Effects. Molecules 2022, 27, 1973. [Google Scholar] [CrossRef] [PubMed]
- Jamrozik, E.; Selgelid, M.J. COVID-19 Human Challenge Studies: Ethical Issues. Lancet Infect. Dis. 2020, 20, e198–e203. [Google Scholar] [CrossRef]
- Li, Y.-T.; Linster, M.; Mendenhall, I.H.; Su, Y.C.F.; Smith, G.J.D. Avian Influenza Viruses in Humans: Lessons from Past Outbreaks. Br. Med. Bull. 2019, 132, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Ye, S.; Li, Q.; Bai, Y.; Wu, J.; Xu, L.; Wang, Z.; Wang, J.; Zhou, P.; Li, S. Molecular Characterization and Phylogenetic Analysis of Feline Calicivirus Isolated in Guangdong Province, China from 2018 to 2022. Viruses 2022, 14, 2421. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-Y.; Chuang, J.-H.; Wang, P.-W.; Lin, T.-K.; Wu, M.-T.; Hsu, W.-M.; Chuang, H.-C. 5-Aza-2′-Deoxycytidine Induces a RIG-I-Related Innate Immune Response by Modulating Mitochondria Stress in Neuroblastoma. Cells 2020, 9, 1920. [Google Scholar] [CrossRef]
- Mao, J.; Ye, S.; Deng, J.; Song, J.; Wang, Z.; Chen, A.; Zhou, P.; Li, S. Feline Calicivirus P39 Inhibits Innate Immune Responses by Autophagic Degradation of Retinoic Acid Inducible Gene I. Int. J. Mol. Sci. 2023, 24, 5254. [Google Scholar] [CrossRef] [PubMed]
- Dang, W.; Xu, L.; Yin, Y.; Chen, S.; Wang, W.; Hakim, M.S.; Chang, K.-O.; Peppelenbosch, M.P.; Pan, Q. IRF-1, RIG-I and MDA5 Display Potent Antiviral Activities against Norovirus Coordinately Induced by Different Types of Interferons. Antivir. Res. 2018, 155, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Kang, H.; Huang, J.; Li, Z.; Pan, Y.; Li, Y.; Chen, S.; Zhang, J.; Yin, H.; Qu, L. Feline Calicivirus Strain 2280 P30 Antagonizes Type I Interferon-Mediated Antiviral Innate Immunity through Directly Degrading IFNAR1 mRNA. PLoS Pathog 2020, 16, e1008944. [Google Scholar] [CrossRef] [PubMed]
- Yumiketa, Y.; Narita, T.; Inoue, Y.; Sato, G.; Kamitani, W.; Oka, T.; Katayama, K.; Sakaguchi, T.; Tohya, Y. Nonstructural Protein P39 of Feline Calicivirus Suppresses Host Innate Immune Response by Preventing IRF-3 Activation. Vet. Microbiol. 2016, 185, 62–67. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E.; Li, G. Approved Antiviral Drugs over the Past 50 Years. Clin. Microbiol. Rev. 2016, 29, 695–747. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.J.; Campoli-Richards, D.M. Acyclovir: An Updated Review of Its Antiviral Activity, Pharmacokinetic Properties and Therapeutic Efficacy. Drugs 1989, 37, 233–309. [Google Scholar] [CrossRef] [PubMed]
- D’Antonio, F.; Marinceu, D.; Prasad, S.; Khalil, A. Effectiveness and Safety of Prenatal Valacyclovir for Congenital Cytomegalovirus Infection: Systematic Review and Meta-analysis. Ultrasound Obstet. Gynecol. 2023, 61, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhu, J.; Miao, Q.; Qi, R.; Tang, A.; Liu, C.; Yang, H.; Yuan, L.; Liu, G. RPS5 Interacts with the Rabbit Hemorrhagic Disease Virus 3′ Extremities Region and Plays a Role in Virus Replication. Vet. Microbiol. 2020, 249, 108858. [Google Scholar] [CrossRef] [PubMed]
- Buckley, D.; Dharmasena, M.; Fraser, A.; Pettigrew, C.; Anderson, J.; Jiang, X. Efficacy of Silver Dihydrogen Citrate and Steam Vapor against a Human Norovirus Surrogate, Feline Calicivirus, in Suspension, on Glass, and on Carpet. Appl. Environ. Microbiol. 2018, 84, e00233-18. [Google Scholar] [CrossRef]
- Zhu, J.; Miao, Q.; Tang, J.; Wang, X.; Dong, D.; Liu, T.; Qi, R.; Yang, Z.; Liu, G. Nucleolin Mediates the Internalization of Rabbit Hemorrhagic Disease Virus through Clathrin-Dependent Endocytosis. PLoS Pathog. 2018, 14, e1007383. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Dos Anjos Borges, L.G.; Stadlbauer, D.; Ramos, I.; Bermúdez González, M.C.; He, J.; Ding, Y.; Wei, Z.; Ouyang, K.; Huang, W.; et al. Characterization of Swine-Origin H1N1 Canine Influenza Viruses. Emerg. Microbes Infect. 2019, 8, 1017–1026. [Google Scholar] [CrossRef]
- Sun, H.; Blackmon, S.; Yang, G.; Waters, K.; Li, T.; Tangwangvivat, R.; Xu, Y.; Shyu, D.; Wen, F.; Cooley, J.; et al. Zoonotic Risk, Pathogenesis, and Transmission of Avian-Origin H3N2 Canine Influenza Virus. J. Virol. 2017, 91, e00637-17. [Google Scholar] [CrossRef] [PubMed]
- Falchieri, M.; Reid, S.M.; Dastderji, A.; Cracknell, J.; Warren, C.J.; Mollett, B.C.; Peers-Dent, J.; Schlachter, A.-L.D.; Mcginn, N.; Hepple, R.; et al. Rapid Mortality in Captive Bush Dogs (Speothos venaticus) Caused by Influenza A of Avian Origin (H5N1) at a Wildlife Collection in the United Kingdom. Emerg. Microbes Infect. 2024, 13, 2361792. [Google Scholar] [CrossRef]
- Bouchard, J.; Walker, M.C.; Leclerc, J.M.; Lapointe, N.; Beaulieu, R.; Thibodeau, L. 5-Azacytidine and 5-Azadeoxycytidine Inhibit Human Immunodeficiency Virus Type 1 Replication in Vitro. Antimicrob. Agents Chemother. 1990, 34, 206–209. [Google Scholar] [CrossRef]
- Greggs, W.M.; Clouser, C.L.; Patterson, S.E.; Mansky, L.M. Discovery of Drugs That Possess Activity against Feline Leukemia Virus. J. Gen. Virol. 2012, 93, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Bautista, L.; Sirimanotham, C.; Espinoza, J.; Cheng, D.; Tay, S.; Drayman, N. A Drug Repurposing Screen Identifies Decitabine as an HSV-1 Antiviral. Microbiol. Spectr. 2024, 12, e01754-24. [Google Scholar] [CrossRef] [PubMed]
- Normand, C.; Thieulent, C.J.; Fortier, C.; Sutton, G.; Senamaud-Beaufort, C.; Jourdren, L.; Blugeon, C.; Vidalain, P.-O.; Pronost, S.; Hue, E.S. A Screening Study Identified Decitabine as an Inhibitor of Equid Herpesvirus 4 That Enhances the Innate Antiviral Response. Viruses 2024, 16, 746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.-S.; Dai, Q.; Chen, P.; Lu, M.; Kairis, E.L.; Murugaiah, V.; Xu, J.; Shukla, R.K.; Liang, X.; et al. 5-Methylcytosine (m5C) RNA Modification Controls the Innate Immune Response to Virus Infection by Regulating Type I Interferons. Proc. Natl. Acad. Sci. USA 2022, 119, e2123338119. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Tu, S.; Luo, D.; Dai, C.; Jin, M.; Chen, H.; Zou, J.; Zhou, H. Protein Arginine Methyltransferase 5 Mediates Arginine Symmetric Dimethylation of Influenza A Virus PB2 and Supports Viral Replication. J. Med. Virol. 2023, 95, e29171. [Google Scholar] [CrossRef]
- Narayan, P.; Ayers, D.F.; Rottman, F.M.; Maroney, P.A.; Nilsen, T.W. Unequal Distribution of N6-Methyladenosine in Influenza Virus mRNAs. Mol. Cell. Biol. 1987, 7, 1572–1575. [Google Scholar] [PubMed]
- Courtney, D.G.; Kennedy, E.M.; Dumm, R.E.; Bogerd, H.P.; Tsai, K.; Heaton, N.S.; Cullen, B.R. Epitranscriptomic Enhancement of Influenza A Virus Gene Expression and Replication. Cell Host Microbe 2017, 22, 377–386.e5. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, S.; Wang, Z.; Chen, A.; Chen, Y.; Lou, G.; Xie, Q.; Lu, G.; Li, S. Decitabine Increases the Transcription of RIG-I Gene to Suppress the Replication of Feline Calicivirus and Canine Influenza Virus. Microorganisms 2025, 13, 143. https://doi.org/10.3390/microorganisms13010143
Ye S, Wang Z, Chen A, Chen Y, Lou G, Xie Q, Lu G, Li S. Decitabine Increases the Transcription of RIG-I Gene to Suppress the Replication of Feline Calicivirus and Canine Influenza Virus. Microorganisms. 2025; 13(1):143. https://doi.org/10.3390/microorganisms13010143
Chicago/Turabian StyleYe, Shaotang, Zhen Wang, Aolei Chen, Ying Chen, Gaoming Lou, Qingmei Xie, Gang Lu, and Shoujun Li. 2025. "Decitabine Increases the Transcription of RIG-I Gene to Suppress the Replication of Feline Calicivirus and Canine Influenza Virus" Microorganisms 13, no. 1: 143. https://doi.org/10.3390/microorganisms13010143
APA StyleYe, S., Wang, Z., Chen, A., Chen, Y., Lou, G., Xie, Q., Lu, G., & Li, S. (2025). Decitabine Increases the Transcription of RIG-I Gene to Suppress the Replication of Feline Calicivirus and Canine Influenza Virus. Microorganisms, 13(1), 143. https://doi.org/10.3390/microorganisms13010143







