Abstract
As the mobile cassette carrier of the methicillin resistance gene mecA that is transported across staphylococci species, the evolution and origin of Staphylococcal Cassette Chromosome mec (SCCmec)—and in particular, the composition of mecA and SCCmec—have been extensively discussed in the scientific literature; however, information regarding its dissemination across geographical limits and evolution over decades remains limited. In addition, whole-genome sequencing-based macro-analysis was unable to provide sufficiently detailed evolutionary information on SCCmec. Herein, the cassette chromosome recombinase genes ccrAB/C, as essential components of SCCmec, were employed to explore the evolution of SCCmec. This work established the basic taxonomy of 33 staphylococci species. The CUB of mecA, ccrAB/C of 12 SCCmec types and core genome of 33 staphylococci species were subsequently compared; the phylogenetic relationship of ccrAB/C was observed via SCCmec typing on a temporal and geographical scale; and the duplicate appearance of ccrAB/C was illustrated by comparing SCCmec compositions. The results highlighted a deviation in the CUB of mecA and ccrAB/C, which evidenced their exogenous characteristics to staphylococci, and provided theological support for the phylogenetic analysis of ccrAB/C as representative of SCCmec. Importantly, the phylogenetic relationship of ccrAB/C did not exhibit centralization over time; instead, similarly to mecA, ccrAB/C with similar identities had close clades across decades and geographical limits and different SCCmec types, which enabled us to discriminate SCCmec based on the sequence identity of ccrAB/C. In addition, the duplicate appearance of ccrAB/C and fixed composition of the ccrAB/C complex among different strains were indicative of more complicated transmission mechanisms than targeting direct repeats of SCCmec.
1. Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) has long been recognized as a serious nosocomial pathogen due to its broad-spectrum resistance to β-lactam antimicrobial agents [1]. MRSA has been the subject of constant study over the last few decades; as such, Staphylococcal Cassette Chromosome mec (SCCmec) has been identified as a well-known carrier of the methicillin resistance gene mec, which has been transferred within the staphylococcal genus [2], causing ubiquitous dissemination in staphylococci. The increasing appearance of SCCmec in S. aureus can be attributed to the methicillin-resistant non-aureus staphylococci involved in the exploration of the evolution and origin of SCCmec [1,2,3,4]. It has been established that, due to the more common dissemination of coagulase-negative staphylococci (CoNS) in natural environments [5], methicillin-resistant coagulase-negative staphylococci (MR-CoNS) serve as reservoirs of SCCmec for S. aureus [6], facilitating the increasing emergence of new types of MRSA.
The evolution and origin of methicillin resistance genetic determinants should be considered based on the following factors:
Firstly, as methicillin resistance dominates gene-encoding penicillin-binding protein 2a (PBP2a), mecA was primarily analyzed to explore its dissemination and evolution within staphylococci. Incipient investigations demonstrated a ubiquitous presence and high identity of mecA in Mammaliicoccus sciuri (reassigned from Staphylococcus species to Mammaliicoccus species [7]) compared with MRSA [8,9]. Subsequently, modern Bayesian informatics and selective pressure analysis were utilized to evaluate the evolution and adaption of mecA in staphylococci, and it was proposed that Mammaliicoccus vitulinus, Mammaliicoccus fleurettii and M. sciuri constitute the phased evolution of mecA; more importantly, M. sciuri was inferred to be a precursor of SCCmec [10].
Secondly, since the initial designation of SCCmec composed of mecA and cassette chromosome recombinase ccrAB [11], the evolutionary analysis of methicillin resistance genetic determinants has been largely dependent on SCCmec structural comparison. For instance, based on the common presence of M. vitulinus, M. fleurettii and M. sciuri originating from mammals, through a sequence identity comparison of multiple serial Open Reading Frames (ORFs) within SCCmec, M. fleurettii was proposed as the evolutionary origin of SCCmec [3]. In addition, the evolutionary steps of SCCmec were hypothesized among M. vitulinus, M. fleurettii and M. sciuri; it was found that M. vitulinus and M. fleurettii constitute the mec complex, and M. sciuri is the precursor of SCCmec [4].
Thirdly, ccrAB/C (ccrC was later discovered [12]), as another determinant of SCCmec function and in the excision and reintegration of SCCmec [2], were employed to analyze the evolution and phylogenic relationship of SCCmec variants. A previous report identified sequence variations among different isolates of the same ccrAB type, and high sequence conservation of ccrAB was observed within same geographical region, which led the authors to hypothesize that SCCmec may be transmitted horizontally [13]. However, in light of the increasing emergence of methicillin-resistant staphylococci and novel types of SCCmec, the underlying homology of novel types of ccrAB/C compared with previously established types is in need of updating. Notably, the close relationship of ccrAB of the SCCmec-like element in Macrococcus caseolyticus with ccrAB of S. aureus was emphasized via phylogenetic analysis, and the potential transmission of SCCmec across M. caseolyticus and S. aureus was elucidated [14].
Currently, high-resolution sequence polymorphisms (e.g., multi-locus sequence typing and single-nucleotide polymorphisms) and evolutionary deduction (e.g., Bayesian analysis) on methicillin-resistant staphylococci provide more detailed information based on whole-genome sequencing (WGS) [10,15]. For instance, through the application of WGS and Bayesian analysis, the evolutionary route of MRSA (sequence type (ST) 225) was observed within central Europe on a temporal and geographical scale [16]; additionally, the evolution, expansion and transmission routes of pandemic EMRSA-16 were observed within the United Kingdom [17]. Inspiringly, the origin of USA300 was observed in terms of the evolutionary route of ST8 and acquisition history of pathogenic elements (e.g., SCCmec and Panton–Valentine Leukocidin (PVL)) on a global scale [18]. Subsequently, the genome evolution of MRSA (involving multiple features, e.g., SCCmec, ST, antibiotic resistance genes, PVL and arginine catabolic mobile element) was observed on a larger scale (i.e., transcontinental dissemination, diverged time point and acquisition of SCCmec) [19]. The above evidence and exploration shed light on the evolutionary route of MRSA across time and geographical scale. Nevertheless, SCCmec acts as an independent transmission cassette transferred within staphylococci [2], and is usually of ancestral lineage and is unaccompanied by other pathogenic elements [20]. Regarding the potential emergence of new methicillin-resistant staphylococci in native surroundings and transmission of SCCmec across geographical limits, in our opinion, these macro-analyses on the evolution of SCCmec remain incomplete.
Regarding the potential difference in Codon Usage Bias (CUB) between exogenous and endogenous genes, SCCmec, as a mobile chromosome cassette, likely exhibits distinctive CUB compared to the core genome of staphylococci, since it has been suggested that CUB is capable of revealing a horizontal gene transfer within closely related organisms [21]. A related study performed evolutionary analysis of horizontally transferred genes by examining their CUB and tRNA profiles, and suggested that horizontally transferred genes exhibited varied CUB adaption frequency and extended residence times, and several horizontally transferred genes maintained atypical CUB compared with the core genome [22]. These findings support our hypothesis that CUB could be employed to observe the homologous relationship between SCCmec and the core genome of staphylococci, and genes within SCCmec are likely to maintain stable CUB/high sequence conservation over long-term evolution (as demonstrated by the high sequence conservation of mecA). Generally speaking, with the exception of two essential complexes (including mecA and ccrAB/C complexes), SCCmec commonly harbors multiple regulatory factors (e.g., mecR1, mecI), accessory genes (e.g., metabolic genes, insertion sequences, transposons), and other antibiotic resistance genes (e.g., fusC, ant4′, tetK, spc, ermA) [23,24], especially for the varied composition of genes within J1, J2, and J3 regions (separated by mecA and ccrAB/C complexes) [25], which constituted a complex exogenous mobile cassette accompanied by the loss or addition of regulatory, accessory, and other antibiotic resistance genes. In contrast with the occasional absence of these additional genes, mecA and ccrAB/C were commonly identified in methicillin-resistant staphylococci [26], and thus were established as reliable references for evolutionary exploration. However, to the best of our knowledge, recent works have predominantly focused on the evolution of mecA [10,27]. The high sequence conservation of mecA (observed in this study) among different SCCmec types and methicillin-resistant staphylococci limited its capacity for discriminating SCCmec variants and evolutionary routes within staphylococcal genus. Comparatively, ccrAB/C was rarely identified; thus, when it comes to the increasing emergence of novel types of SCCmec accompanied by existing or new types of ccrAB/C [26], the evolution of ccrAB/C is likely better able to elucidate the emergence of novel types of SCCmec [28] and transmission of SCCmec.
To date, of the 15 SCCmec types identified according to the organization diversity of mecA and ccrAB/C complexes [26,29], the ccrAB/C complex was generally categorized by ccrA1B1 (in SCCmec types I and IX), ccrA2B2 (II and IV), ccrA3B3 (III), ccrA4B4 (VI and VIII), ccrA1B6 (X and XV), ccrA1B3 (XI), ccrC1 (V, VII and XIV) and ccrC2 (XII and XIII), while mecA complexes were generally characterized by mecA, mecR1, and mecI. Compared with the common presence of ccrAB/C, the mecA complex usually exhibited an absence of or variation in mecR1 and mecI [30]. As such, our work exploited ccrAB/C as a reference for SCCmec. To establish a solid CUB for the core genome, a tandem sequence of 31 housekeeping genes of staphylococci was deemed to be representative of the core genome, instead of a single housekeeping gene. Subsequently, the CUB of mecA and ccrAB/C was compared with the CUB of the core genome, and the phylogenetic relationship of ccrAB/C was constructed in terms of SCCmec typing, time, and geographical scale.
2. Materials and Methods
2.1. Collection of mecA-Positive Staphylococci and SCCmec Screening
As shown in Table 1, 176 mecA-positive staphylococci were obtained via conserved domain sequence alignment of mecA, which was achieved using the Basic Local Alignment Search Tool (BLAST) provided by the National Center for Biological Information (NCBI). The presence of coagulase gene coa was confirmed only in S. aureus, with the exception of coagulase-variable Staphylococcus pseudintermedius [5]; therefore, 8 S. pseudintermedius strains were classified as CoNS. According to previous demonstrations [28,31], the SCCmec region was generally designated within upstream attR at the end of orfX (namely rlmH, encoding 23S rRNA methyltransferase [32]) to downstream attL at the end of SCCmec; therefore, the conserved sequence of attR/L, i.e., direct repeats (DRs) (5′-GARGCDTATCATAAVT-3′) was utilized to extract SCCmec. According to our extraction method, 61 strains exhibited incomplete SCCmec structures (e.g., missing orfX or DRs). The SCCmec-like elements of these strains were found, at the very least, to cover the ccrAB/C and mecA complex. Subsequently, the SCCmec sequence was submitted to SCCmecFinder (https://cge.food.dtu.dk (accessed on 12 April 2024)) and assigned a 90% threshold identity and 60% gene length [33]. ORFs of SCCmec were annotated by RAST (https://rast.nmpdr.org/ (accessed on 15 April 2024)) [34,35,36]. The SCCmec types and sequences of 176 strains are described in detail in the Supplementary Materials.

Table 1.
mecA-positive staphylococci used in this study.
2.2. Basic Taxonomy of Staphylococci Species
The basic taxonomy of 33 staphylococci species was constructed based on 16S rRNA phylogeny (the tandem sequence of 31 housekeeping genes failed this phylogenetic analysis due to excessive sequence variation) to provide a phylogenetic background for further analysis. Briefly, 16S rRNA sequences of 33 staphylococci species were aligned by Multiple Sequence Comparison by Log-Expression (MUSCLE), and a Neighbor-Joining (NJ) tree (1000 bootstrap) was constructed using Molecular Evolutionary Genetic Analysis (MEGA) 11 software. The 16S rRNA sequences of 33 staphylococci species are listed in the Supplementary Materials.
2.3. CUB Assay
As mentioned above, CUB comparison of core genome and SCCmec was performed to determine the differences between endogenous and exogenous genes. According to our SCCmec typing result, 12 SCCmec types were observed and subsequently subjected to this CUB assay. Briefly, Relative Synonymous Codon Usage (RSCU) values of the tandem sequences of 31 housekeeping genes (rsmE, glpE, gmk, tpi, gyrA, gyrB, rpoB, dnaG, frr, infA, infB, infC, nusA, pgk, pyrG, rplA, rplB, rplC, rplD, rplE, rplF, rpmA, rpsA, rpsB, rpsC, rpsE, smpB, tsf, recA, ftsZ and pyk), mecA, and ccrAB/C of 12 SCCmec types were calculated using CodonW 1.3 software with default parameters applied. A cluster dendrogram of RSCU profiles of core genome, mecA and ccrAB/C was plotted using an online tool (www.omicshare.com (accessed on 25 April 2024)). Details of the sequences and RSCU values of 31 housekeeping genes, mecA and ccrAB/C of 12 SCCmec types, are provided in the Supplementary Materials.
2.4. Phylogenetic Tree Construction of ccrAB/C
According to the preliminary screening of the SCCmec structures of these 176 staphylococcal strains, occasional absences of accessory and regulatory genes were observed within SCCmec (e.g., glpQ, merA, walK, IS431, Tn554, maoC, mecI and mecR1). In contrast, the ccrAB/C complex functioned as essential recombinases, which were commonly present within SCCmec. Meanwhile, mecA (with less than a 3-point mutation) sustained high sequence conservation in these 176 strains; comparatively, ccrAB/C exhibited sequence variation even within the same SCCmec type. Therefore, the phylogenetic relationship of ccrAB/C was constructed to reflect the phylogenetic correlation of SCCmec among these strains. A complete ccrAB/C sequence was subjected to a CD search of NCBI (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi (accessed on 7 May 2024)), and conserved domain regions and sequences of ccrAB/C were extracted separately and combined in tandem. Subsequently, a phylogenetic tree of the tandem conserved sequence of ccrAB/C was constructed via MUSCLE alignment and an NJ (1000 bootstrap) algorithm. The collection year and locus were obtained from the source information of the corresponding strain in the NCBI. iTol (https://itol.embl.de/, accessed on 9 May 2024) and Adobe Illustrator (AI) 26.0 software were employed to visualize and optimize the phylogenetic tree. The collection years, locus, and conserved domain sequences of ccrAB/C of 176 staphylococcal strains are provided in the Supplementary Materials.
2.5. Comparison of SCCmec Structures
During our screening of ccrAB/C within SCCmec or SCCmec-like elements, 35 strains tested positive for multiple ccrAB/C genes. Specifically, 2 strains contained ccrAB, ccrC1 and ccrC2, 12 strains contained ccrAB and ccrC1, and 21 strains contained ccrC1 and ccrC2, simultaneously. To highlight the characteristics of multiple ccrAB/C within these staphylococcal strains, the mecA and ccrAB/C complexes of SCCmec with multiple ccrAB/C genes were graphed by SnapGene 8, and compared with the regular SCCmec structure by AI 26.0 software. The characteristics of multiple ccrAB/C genes identified within 35 staphylococcal strains are described in detail in the Supplementary Materials.
3. Results and Discussion
3.1. Basic Taxonomy of 33 Staphylococci Species
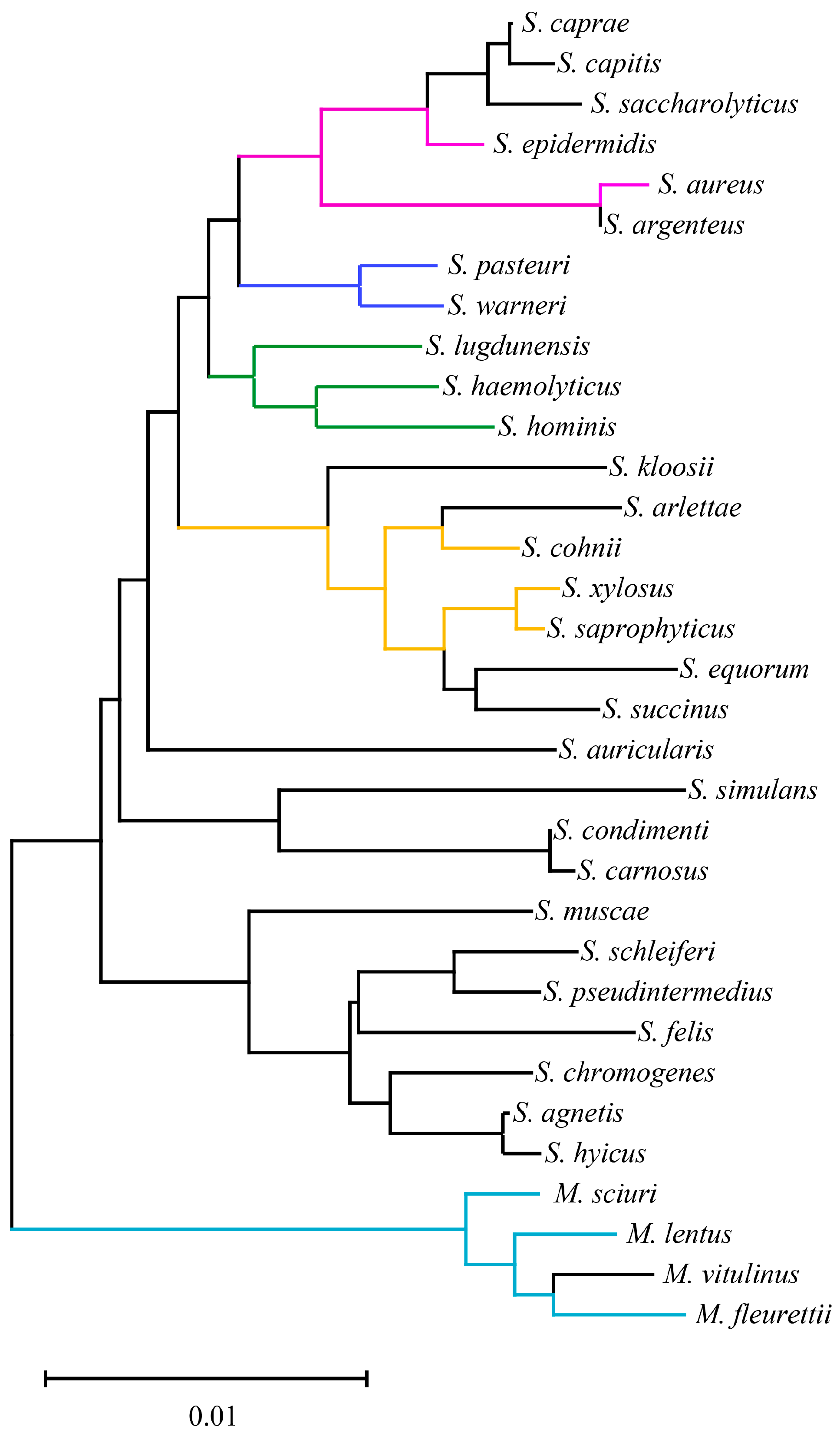
As shown in Figure 1, the clade length remained within the range of 0–0.02 for 33 staphylococci species. Notably, according to previous research [7], the reassigned M. sciuri, Mammaliicoccus lentus, M. vitulinus and M. fleurettii exhibited greater taxonomic distances from other staphylococci species. Interestingly, the current emerging methicillin-resistant staphylococci species generally showed close taxonomic relationships as opposed to random distribution within the staphylococci genus. For instance, according to previous reports involving MR-CoNS investigations in animals, the emergence of MR-CoNS disseminated within Staphylococcus cohnii, Staphylococcus epidermidis, Staphylococcus haemolyticus, M. sciuri, Staphylococcus warneri, Staphylococcus xylosus, M. lentus, Staphylococcus lugdunensis, Staphylococcus hominis, Staphylococcus saprophyticus, M. fleurettii, and Staphylococcus pasteuri [5,37,38,39,40,41,42]. This could explain the greater prevalence of these CoNS in animal-origin environments and increase the likelihood of MR-CoNS emergence.
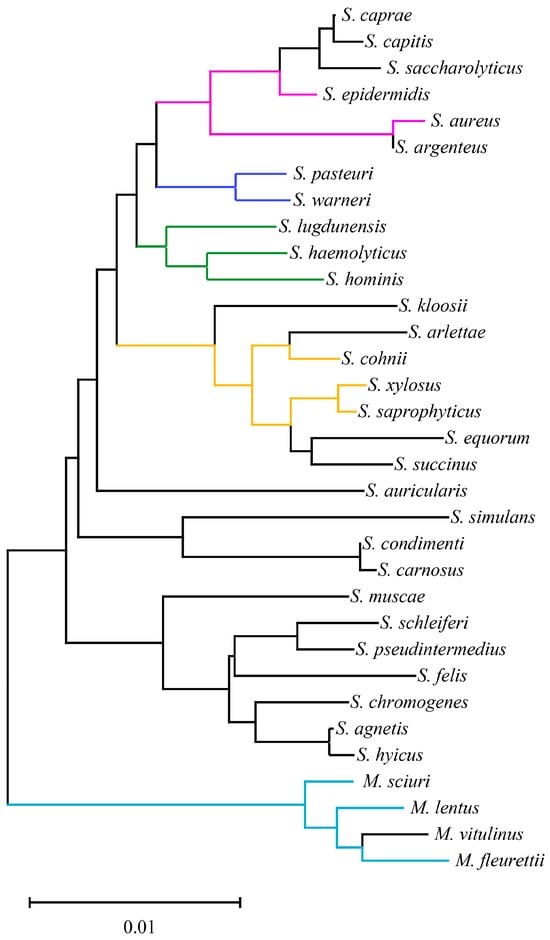
Figure 1.
Basic taxonomy of 33 staphylococci species based on 16S rRNA. Colored clades represent the dominant emergence of methicillin-resistant staphylococci in animals.
3.2. RSCU Profiles of mecA, ccrAB/C, and Core Genome
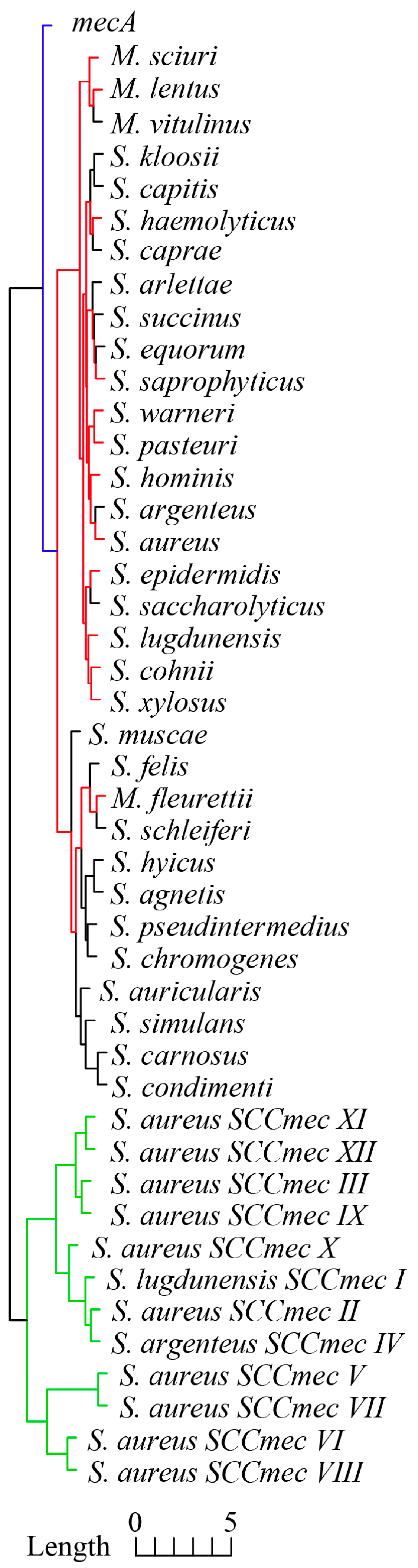
As shown in Figure 2, mecA and ccrAB/C genes within 12 SCCmec types and the core genome of 33 staphylococci species exhibited distinctive RSCU clustering. Generally speaking, the core genome of 33 staphylococci species had the most similar RSCU profiles compared with mecA and ccrAB/C, and the dominant species of the emerged MR-CoNS mentioned above exhibited more similar RSCU profiles (red branches) compared with other CoNS. This clustering result concurred with the basic taxonomy analysis; unfortunately, to the best of our knowledge, there is currently no theoretical statement or experimental evidence to explain this phenomenon. Importantly, mecA exhibited a more similar RSCU profile to the staphylococcal core genome than ccrAB/C; in other words, mecA exhibited more similar CUB to the core genome than ccrAB/C. The other two key CUB indexes, including the Codon Adaption Index (CAI) and GC3s [21], exhibited similar tendencies. In detail, the average CAI and GC3s values of ccrAB/C of 12 SCCmec types were 0.211 (95% CI: 0.205–0.217) and 0.265 (95% CI: 0.252–0.280), respectively, and the CAI and GC3s values of highly conserved mecA were 0.261 and 0.191, respectively. In contrast, the average CAI and GC3s values of the core genome were 0.300 (95% CI: 0.295–0.305) and 0.192 (95% CI: 0.181–0.202), respectively (the CAI and GC3s values are described in detail in the Supplementary Materials).
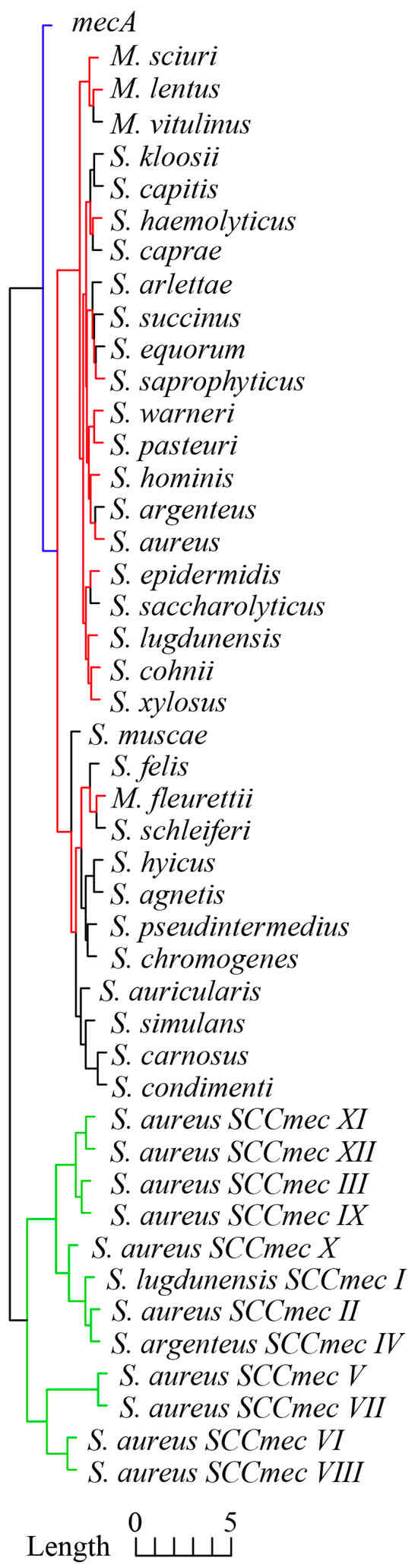
Figure 2.
Cluster dendrogram of RSCU profiles of mecA, 31 tandem housekeeping genes of 33 staphylococci species, and ccrAB/C of 12 SCCmec types. Colored clades represent the similarity clustering of RSCU profiles.
The results presented above indicate that ccrAB/C exhibited more explicit exogenous characteristics than mecA and support the previous assertion that exogenous ccrAB/C was later introduced into mecA-positive staphylococci, leading to the formation of SCCmec [4]. More importantly, exogenous mecA and ccrAB/C exhibited deviated CUB compared to the core genome, and ccrAB/C provided clearer discrimination than mecA among 12 SCCmec types [26], which can be employed to explore the horizontal transmission route of SCCmec based on sequence identity.
To our knowledge, there are few reports on CUB of SCCmec in the staphylococcal genome; however, a previous analysis of horizontal transfer-acquired genes of Pseudomonas aeruginosa proposed that the CUB deviation of acquired genes exhibited relatively stable long-term retention, and the synonymous mutations led to insignificant changes in the CUB of acquired genes [22]. This explained the high sequence conservation of mecA in these 176 strains across different countries and decades of evolution and provided solid theoretical support for further phylogenetic analysis in which ccrAB/C was representative of SCCmec, without implementing the sequence mutation of ccrAB/C to adapt the CUB of different receiving species.
3.3. Phylogenetic Relationship of ccrAB/C
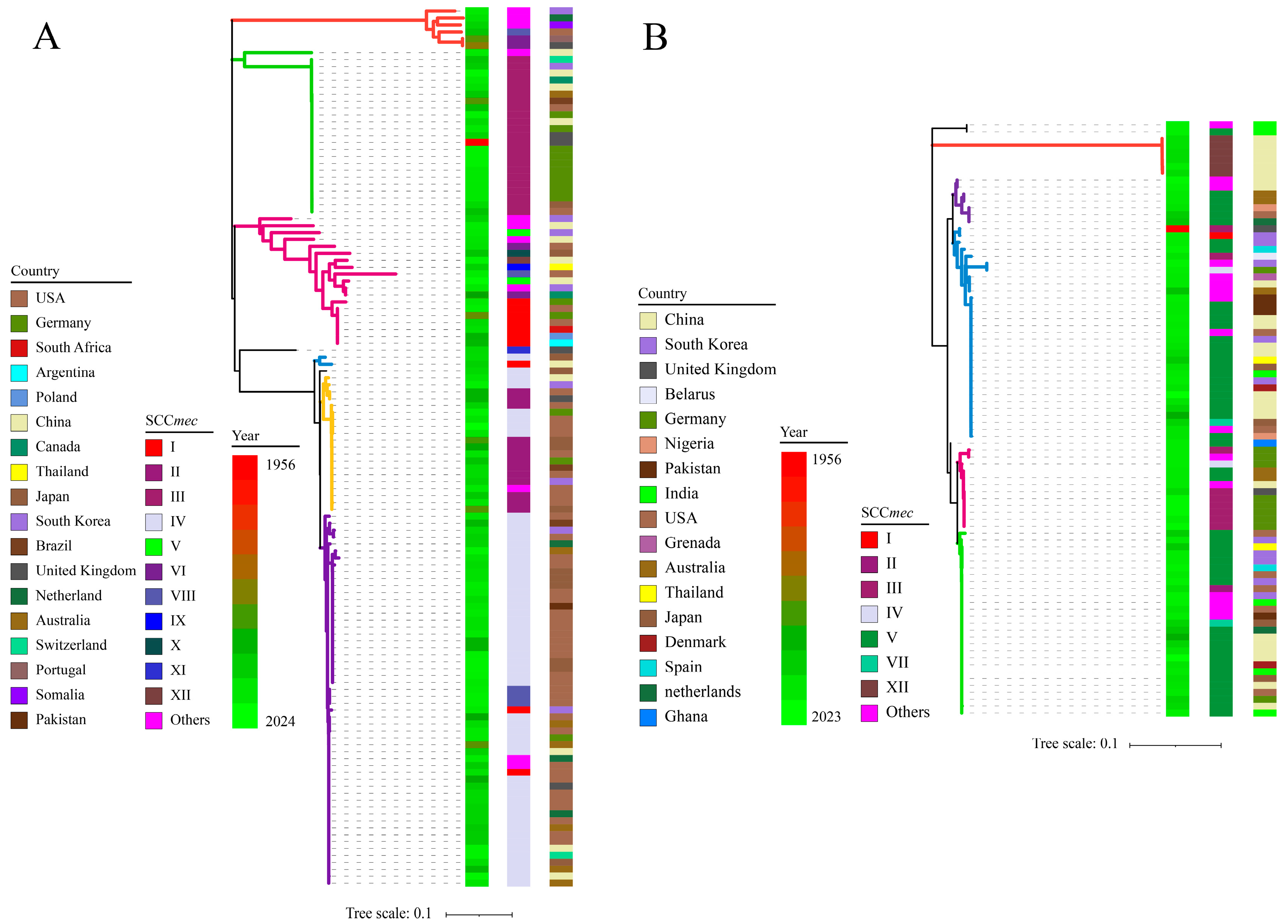
A total of 127 ccrAB and 86 ccrC of 176 mecA-positive staphylococcal strains (including 127 ccrAB strains and 63 ccrC strains (23 strains harboring 2 ccrC)) were sought to construct a phylogenetic tree, and were used in combination with information about SCCmec types, collection year, and locus. As shown in Figure 3, in general, there were no differentiable clades between S. aureus and CoNS; instead, the only correlation was observed between the sequence identities of ccrAB/C. S. aureus. NCTC9944 was the oldest strain (collected in 1956) that harbored ccrAB and ccrC simultaneously; however, other strains have been collected since the 1980s, and the majority of strains were obtained and sequenced in the 2000s. Clearly, ccrAB/C did not show a change in clade concentration as the number of collection years increased, which supported our CUB hypothesis for ccrAB/C, which states that exogenous genes can maintain stable sequence conservation in the long-term (this also explained our initial failed attempts to perform Bayesian analysis to explore the evolution of ccrAB/C).
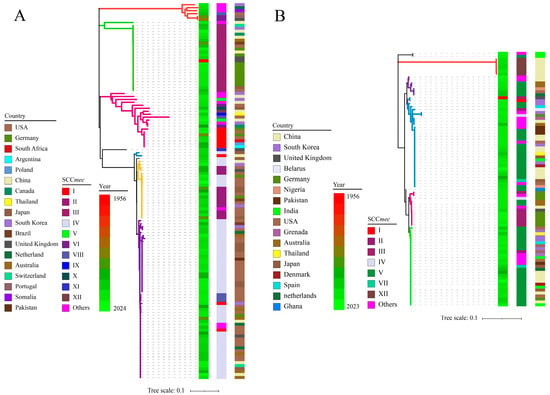
Figure 3.
Phylogenic relationship of 213 ccrAB/C corresponding to yearly, geographical, and SCCmec-type characteristics. (A,B) represent ccrAB and ccrC, respectively. The color strips in turn represent collection years, SCCmec types, and geographical locations from left to right.
Based on the classification of SCCmec types, generally, close phylogenetic relationships were observed within the same SCCmec type. As shown in Figure 3, regular phylogenetic distribution should be achieved for 12 SCCmec types, according to the classification rule for ccrAB/C of SCCmec mentioned above; nevertheless, more distant clades appeared in several SCCmec types, and nearby clades appeared among other SCCmec types. For instance, SCCmec IV (ccrA2B2) displayed two major clades, i.e., yellow and purple, and had nearby clades with II (ccrA2B2) and VIII (ccrA4B4), respectively. Meanwhile, SCCmec V (ccrC1) and VII (ccrC1) exhibited divergent distribution due to significant sequence variation in ccrC1, with the exception of XII (ccrC2). In addition, SCCmec V and XII appeared in the ccrAB phylogenetic tree, and SCCmec I, II, III, and IV appeared in the ccrC phylogenetic tree. The presence of a certain number of untyped SCCmec genes (others) limited further typing analysis for SCCmec in this study. These unexpected divergences could contribute to the low-resolution discrimination or ambiguous outputs for SCCmec typing achieved using SCCmecFinder, resulting from sequence variation in ccrAB/C within the same SCCmec type and the presence of multiple forms of ccrAB/C within SCCmec. According to the preliminary sequence screening for ccrAB/C, dramatic sequence variation occurred within the same SCCmec type; conversely, high sequence identity was observed among different SCCmec types (the conserved sequences of ccrAB/C are described in detail in the Supplementary Materials). This also explained the more distant clades within same SCCmec types. Based on the high sequence conservation of mecA [27], the phylogenetic relationship of ccrAB/C provided more phylogenetic information to balance the SCCmec types and sequence identity of ccrAB/C.
Regarding the collection locus of these 176 strains, the distribution of these strains on a geographical scale was mainly dependent on the number of isolates obtained from native environments. As shown in Figure 3A, the majority of the ccrAB strains (110/127) were obtained from the USA (46), Germany (15), China (13), Japan (13), South Korea (10), Australia (7), and the United Kingdom (6), with the USA exhibiting centralized distribution of SCCmec II and IV. A previous study on the genomic evolution of 386 MRSA in America showed that the two dominant linkages were cluster 7 (all composed of SCCmec IV) and cluster 8 (containing 146/209 SCCmec II and 37/209 SCCmec IV) [19]. Meanwhile, a review emphasized the dominant presence of hospital-associated MRSA (HA-MRSA) SCCmec II in the USA/Canada [25]. Additionally, 10 S. epidermidis strains from Germany were grouped into type III, which was mainly attributed to our centralized selection of S. epidermidis strains from the same environments [43,44]. Notably, 5 SCCmec IV strains from Australia exhibited nearby clades, which could be attributed to the dominant community-associated MRSA (CA-MRSA) SCCmec IV disseminated in Australia/New Zealand [25]. In contrast, China, Japan, South Korea, and the United Kingdom exhibited dispersed distribution of either SCCmec types or phylogenetic clades, as summarized in terms of the varied presence of SCCmec types in Asia and Europe [25].
As for ccrC distribution (Figure 3B), due to the sequence variation of ccrC1 and high sequence identity of ccrC2, ccrC1 exhibited diverged clades, with the exception of ccrC2, which was distributed into a single clade (red). Interestingly, the 20 additional ccrC of 23 strains were mainly distributed in another major clade (green). Regarding the collection countries, most of the ccrC strains (44/63) were obtained from China (17), Germany (9), South Korea (6), India (6), and the USA (6). However, due to the presence of ccrAB and ccrC, eight strains from Germany exhibited unexpected corresponding relationships with SCCmec types. Disregarding the ambiguous SCCmec types observed via SCCmecFinder, 13 strains from China had centralized distribution and close clades in SCCmec V (7) and XII (6), which were mainly attributed to the high sequence identity of ccrC [45,46]. Comparatively, ccrC strains from other countries commonly exhibited sequence variation of ccrC and the presence of multiple forms of ccr, leading to the more distant phylogenetic clades and dispersed distribution of SCCmec types. The original phylogenetic trees of ccrAB and ccrC are listed in the Supplementary Materials.
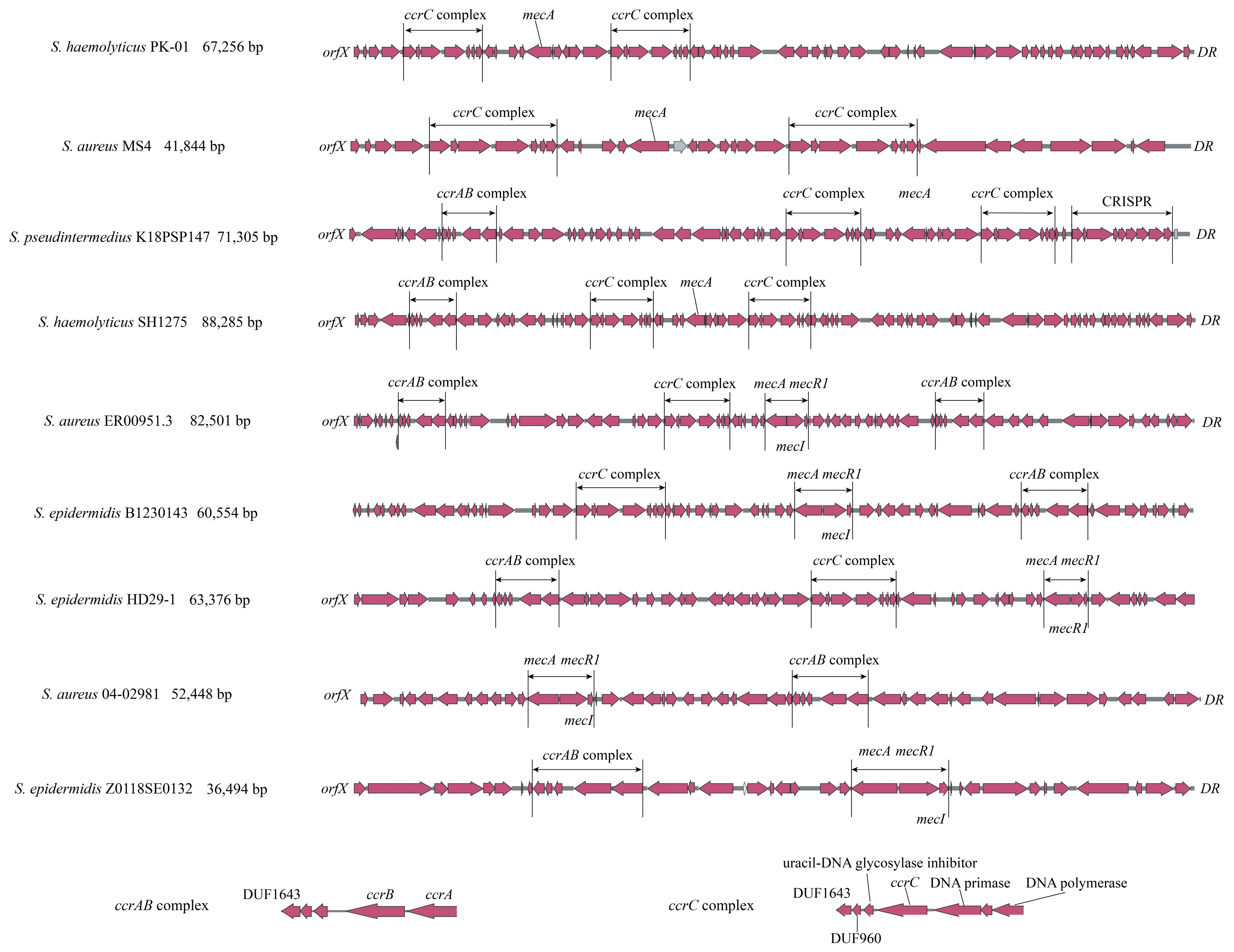
As mentioned above, there are multiple forms of ccrAB/C (e.g., ccrAB-ccrC, ccrC-ccrC, ccrAB-ccrAB-ccrC, ccrAB-ccrC-ccrC) close to mecA in 35 strains with complete or incomplete SCCmec structures. This could explain the low identity or ambiguous outputs observed using SCCmecFinder, resulting in the presence of unexpected SCCmec types in phylogenetic trees. As shown in Figure 4, nine representative strains were selected to demonstrate these phenomena. S. haemolyticus PK-01 and S. aureus MS4 exhibit ccrC-ccrC and a complete SCCmec structure; S. pseudintermedius K18PSP147 and S. haemolyticus SH1275 exhibit ccrAB-ccrC-ccrC and a complete SCCmec structure, whereas all four strains exhibit a loss of mecR1 and mecI in the mecA complex. S. aureus ER00951.3 exhibits ccrAB-ccrC-ccrAB and complete SCCmec structure with a complete mecA complex. S. epidermidis B1230143 and S. epidermidis HD29-1 exhibit ccrAB-ccrC and an incomplete SCCmec structure with a complete mecA complex. S. aureus 04-02981 and S. epidermidis Z0118SE0132 were introduced as regular SCCmec types that exhibit ccrAB and ccrC with a complete mecA complex, respectively. The ccrAB and ccrC complexes in these nine strains have the same ORF structures. Regarding the distant geographical locus and collection years of these nine strains, it is suggested that there may have been a fixed transmission model for ccrAB and ccrC complexes compared with the mecA complex. The excision and reintegration of ccrAB/C might be more complicated than previously believed (i.e., these processes may involve targeting upstream and downstream DRs of SCCmec).
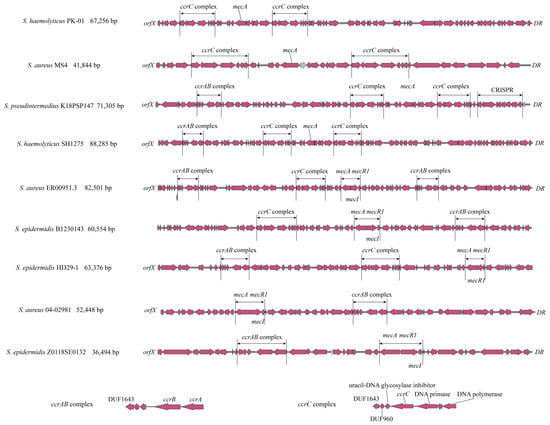
Figure 4.
Duplicate appearance of ccrAB/C within complete SCCmec and incomplete SCCmec structures in staphylococci. S. haemolyticus PK-01, S. aureus MS4, S. pseudintermedius K18PSP147, S. haemolyticus SH1275, and S. aureus ER00951.3 have duplicate ccrAB/C complexes, while S. epidermidis B1230143 and S. epidermidis HD29-1 have incomplete SCCmec structures compared with regular SCCmec structures of S. aureus 04-02981 and S. epidermidis Z0118SE0132.
4. Conclusions
In this work, we made a novel attempt to explore the phylogenetic relationship of SCCmec in terms of ccrAB/C and demonstrated the sequence conservation and exogenous characteristics of ccrAB/C across SCCmec types, geographical limits, and decades of evolution. Currently, SCCmec typing is commonly based on the composition of a single mecA complex and a single ccrAB/C complex, and the SCCmec region is commonly located from upstream attR to downstream attL. Due to the presence of multiple forms of ccrAB/C near mecA loci and incomplete SCCmec structures observed in this study, the variation in ccrAB/C could be utilized as an additional or alternative classification marker for SCCmec, especially for the ambiguous definition of SCCmec types and omission of incomplete SCCmec structures. The fixed composition of the ccrAB/C complex in multi-ccrAB/C SCCmec suggests there might be an undiscovered transmission mechanism for ccrAB/C. More importantly, regarding the large proportion (60/176) of incomplete SCCmec structures observed in this study, we have ascertained that there might be other potential recognition sites for ccrAB/C except attR/L. However, these observations are in need of further experimental validation to identify the variation in ccrAB/C within same the SCCmec type, the same recombinase function of multiple forms of ccrAB/C within SCCmec, and the possible new recognition sites of ccrAB/C.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/microorganisms13010153/s1: The 16S rRNA and 33 housekeeping genes sequences of 33 staphylococci species, RSCU values of mecA, ccrAB/C of 12 SCCmec types and 31 tandem housekeeping genes of 33 staphylococci species, and SCCmec types, conserved domain sequences of 213 ccrAB/C, collection year and locus of 176 mecA-positive staphylococcal strains are provided in Supplementary Data S1. xlsx. The original phylogenetic trees of ccrAB and ccrC are provided in Supplementary Data S2. docx. The SCCmec sequences of 176 staphylococcal strains are provided in Supplementary Data S3. docx. The annotation information of 35 multiple ccrAB/C staphylococcal strains is provided in Supplementary Data S4. docx.
Author Contributions
Conceptualization, H.W.; methodology, H.W.; software, H.W.; validation, H.W.; data curation, H.W.; writing—original draft preparation, H.W.; writing—review and editing, H.W.; supervision, J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 32102025; the College (Institute) Talent Scientific Research Project, grant number 2023RCKY233.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article and in Supplementary Materials.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- Hiramatsu, K.; Cui, L.; Kuroda, M.; Ito, T. The emergence and evolution of methicillin-resistant Staphylococcus aureus. Trends Microbiol. 2001, 9, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, A.M.; Ericson Sollid, J.U. SCCmec in staphylococci: Genes on the move. Pathog. Dis. 2006, 46, 8–20. [Google Scholar]
- Tsubakishita, S.; Kuwahara-Arai, K.; Sasaki, T.; Hiramatsu, K. Origin and molecular evolution of the determinant of methicillin resistance in staphylococci. Antimicrob. Agents Chemother. 2010, 54, 4352–4359. [Google Scholar] [CrossRef]
- Rolo, J.; Worning, P.; Nielsen, J.B.; Bowden, R.; Bouchami, O.; Damborg, P.; Guardabassi, L.; Perreten, V.; Tomasz, A.; Westh, H. Evolutionary origin of the staphylococcal cassette chromosome mec (SCCmec). Antimicrob. Agents Chemother. 2017, 61, e02302–e02316. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Heilmann, C.; Peters, G. Coagulase-negative staphylococci. Clin. Microbiol. Rev. 2014, 27, 870–926. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Coagulase-negative staphylococci as reservoirs of genes facilitating MRSA infection. Bioessays 2013, 35, 4–11. [Google Scholar] [CrossRef]
- Madhaiyan, M.; Wirth, J.S.; Saravanan, V.S. Phylogenomic analyses of the Staphylococcaceae family suggest the reclassification of five species within the genus Staphylococcus as heterotypic synonyms, the promotion of five subspecies to novel species, the taxonomic reassignment of five Staphylococcus species to Mammaliicoccus gen. nov., and the formal assignment of Nosocomiicoccus to the family Staphylococcaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 5926–5936. [Google Scholar] [CrossRef]
- Couto, I.; de Lencastre, H.; Severina, E.; Kloos, W.; Webster, J.A.; Hubner, R.J.; Sanches, I.S.; Tomasz, A. Ubiquitous presence of a mecA homologue in natural isolates of Staphylococcus sciuri. Microb. Drug Resist. 1996, 2, 377–391. [Google Scholar] [CrossRef]
- Wu, S.; Piscitelli, C.; de Lencastre, H.; Tomasz, A. Tracking the evolutionary origin of the methicillin resistance gene: Cloning and sequencing of a homologue of mecA from a methicillin susceptible strain of Staphylococcus sciuri. Microb. Drug Resist. 1996, 2, 435–441. [Google Scholar] [CrossRef]
- Rolo, J.; Worning, P.; Boye Nielsen, J.; Sobral, R.; Bowden, R.; Bouchami, O.; Damborg, P.; Guardabassi, L.; Perreten, V.; Westh, H.; et al. Evidence for the evolutionary steps leading to mecA-mediated beta-lactam resistance in staphylococci. PLoS Genet. 2017, 13, e1006674. [Google Scholar] [CrossRef]
- Katayama, Y.; Ito, T.; Hiramatsu, K. A New Class of Genetic Element, Staphylococcus Cassette Chromosome mec, Encodes Methicillin Resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 2000, 44, 1549–1555. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Ma, X.X.; Takeuchi, F.; Okuma, K.; Yuzawa, H.; Hiramatsu, K. Novel type V staphylococcal cassette chromosome mec driven by a novel cassette chromosome recombinase, ccrC. Antimicrob Agents Chemother. 2004, 48, 2637–2651. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, A.M.; Kjeldsen, G.; Sollid, J.U.E. Local Variants of Staphylococcal Cassette Chromosome mec in Sporadic Methicillin-Resistant Staphylococcus aureus and Methicillin-Resistant Coagulase-Negative Staphylococci: Evidence of Horizontal Gene Transfer? Antimicrob. Agents Chemother. 2003, 48, 285–296. [Google Scholar] [CrossRef]
- Tsubakishita, S.; Kuwahara-Arai, K.; Baba, T.; Hiramatsu, K. Staphylococcal cassette chromosome mec-like element in Macrococcus caseolyticus. Antimicrob. Agents Chemother. 2010, 54, 1469–1475. [Google Scholar] [CrossRef]
- Miragaia, M. Factors Contributing to the Evolution of mecA-Mediated beta-lactam Resistance in Staphylococci: Update and New Insights From Whole Genome Sequencing (WGS). Front. Microbiol. 2018, 9, 2723. [Google Scholar] [CrossRef]
- Nubel, U.; Dordel, J.; Kurt, K.; Strommenger, B.; Westh, H.; Shukla, S.K.; Zemlickova, H.; Leblois, R.; Wirth, T.; Jombart, T.; et al. A timescale for evolution, population expansion, and spatial spread of an emerging clone of methicillin-resistant Staphylococcus aureus. PLoS Pathog. 2010, 6, e1000855. [Google Scholar] [CrossRef]
- McAdam, P.R.; Templeton, K.E.; Edwards, G.F.; Holden, M.T.; Feil, E.J.; Aanensen, D.M.; Bargawi, H.J.; Spratt, B.G.; Bentley, S.D.; Parkhill, J.; et al. Molecular tracing of the emergence, adaptation, and transmission of hospital-associated methicillin-resistant Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 2012, 109, 9107–9112. [Google Scholar] [CrossRef]
- Strauß, L.; Stegger, M.; Akpaka, P.E.; Alabi, A.; Breurec, S.; Coombs, G. Origin, evolution, and global transmission of community-acquired Staphylococcus aureus ST8. Proc. Natl. Acad. Sci. USA 2017, 114, E10596–E10604. [Google Scholar] [CrossRef]
- Smith, J.T.; Eckhardt, E.M.; Hansel, N.B.; Eliato, T.R.; Martin, I.W.; Andam, C.P. Genome Evolution of Invasive Methicillin-Resistant Staphylococcus aureus in the Americas. Microbiol. Spectr. 2022, 10, e0020122. [Google Scholar] [CrossRef]
- Bianco, C.M.; Moustafa, A.M.; O’Brien, K.; Martin, M.A.; Read, T.D.; Kreiswirth, B.N.; Planet, P.J. Pre-epidemic evolution of the MRSA USA300 clade and a molecular key for classification. Front. Cell. Infect. Microbiol. 2023, 13, 1081070. [Google Scholar] [CrossRef]
- Parvathy, S.T.; Udayasuriyan, V.; Bhadana, V. Codon usage bias. Mol. Biol. Rep. 2021, 49, 539–565. [Google Scholar] [CrossRef]
- Callens, M.; Scornavacca, C.; Bedhomme, S.J.M.G. Evolutionary responses to codon usage of horizontally transferred genes in Pseudomonas aeruginosa: Gene retention, amelioration and compensatory evolution. Microb. Genom. 2021, 7, 000587. [Google Scholar] [CrossRef] [PubMed]
- Haaber, J.; Penadés, J.R.; Ingmer, H. Transfer of Antibiotic Resistance in Staphylococcus aureus. Trends Microbiol. 2017, 25, 893–905. [Google Scholar] [CrossRef] [PubMed]
- Juhas, M. Horizontal gene transfer in human pathogens. Crit. Rev. Microbiol. 2015, 41, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Lakhundi, S.; Zhang, K. Methicillin-Resistant Staphylococcus aureus: Molecular Characterization, Evolution, and Epidemiology. Clin. Microbiol. Rev. 2018, 31, e00020-18. [Google Scholar] [CrossRef]
- Wolska-Gębarzewska, M.; Międzobrodzki, J.; Kosecka-Strojek, M. Current types of staphylococcal cassette chromosome mec (SCCmec) in clinically relevant coagulase-negative staphylococcal (CoNS) species. Crit. Rev. Microbiol. 2023, 50, 1020–1036. [Google Scholar] [CrossRef]
- Zhan, X.Y.; Zhu, Q.Y. Evolution of methicillin-resistant Staphylococcus aureus: Evidence of positive selection in a penicillin-binding protein (PBP) 2a coding gene mecA. Infect. Genet. Evol. 2018, 59, 16–22. [Google Scholar] [CrossRef]
- MacFadyen, A.C.; Paterson, G.K. Methicillin resistance in Staphylococcus pseudintermedius encoded within novel staphylococcal cassette chromosome mec (SCCmec) variants. J. Antimicrob. Chemother. 2024, 79, 1303–1308. [Google Scholar] [CrossRef]
- International Working Group on the Classification of Staphylococcal Cassette Chromosome Elements. Classification of staphylococcal cassette chromosome mec (SCCmec): Guidelines for reporting novel SCCmec elements. Antimicrob. Agents Chemother. 2009, 53, 4961–4967. [Google Scholar] [CrossRef]
- Uehara, Y. Current Status of Staphylococcal Cassette Chromosome mec (SCCmec). Antibiotics 2022, 11, 86. [Google Scholar] [CrossRef]
- Bitrus, A.A.; Zunita, Z.; Khairani-Bejo, S.; Othman, S.; Ahmad Nadzir, N.A. Staphylococcal cassette chromosome mec (SCCmec) and characterization of the attachment site (attB) of methicillin resistant Staphylococcus aureus (MRSA) and methicillin susceptible Staphylococcus aureus (MSSA) isolates. Microb. Pathog. 2018, 123, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Maree, M.; Thi Nguyen, L.T.; Ohniwa, R.L.; Higashide, M.; Msadek, T.; Morikawa, K. Natural transformation allows transfer of SCCmec-mediated methicillin resistance in Staphylococcus aureus biofilms. Nat. Commun. 2022, 13, 2477. [Google Scholar] [CrossRef] [PubMed]
- Kaya, H.; Hasman, H.; Larsen, J.; Stegger, M.; Johannesen, T.B.; Allesoe, R.L.; Lemvigh, C.K.; Aarestrup, F.M.; Lund, O.; Larsen, A.R. SCCmecFinder, a Web-Based Tool for Typing of Staphylococcal Cassette Chromosome mec in Staphylococcus aureus Using Whole-Genome Sequence Data. mSphere 2018, 3, e00612-17. [Google Scholar] [CrossRef] [PubMed]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid Annotations using Subsystems Technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef]
- Overbeek, R.; Olson, R.; Pusch, G.D.; Olsen, G.J.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Parrello, B.; Shukla, M.; et al. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res. 2014, 42, D206–D214. [Google Scholar] [CrossRef]
- Brettin, T.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Olsen, G.J.; Olson, R.; Overbeek, R.; Parrello, B.; Pusch, G.D.; et al. RASTtk: A modular and extensible implementation of the RAST algorithm for building custom annotation pipelines and annotating batches of genomes. Sci. Rep. 2015, 5, 8365. [Google Scholar] [CrossRef]
- Kawano, J.; Shimizu, A.; Saitoh, Y.; Yagi, M.; Saito, T.; Okamoto, R. Isolation of methicillin-resistant coagulase-negative staphylococci from chickens. J. Clin. Microbiol. 1996, 34, 2072–2077. [Google Scholar] [CrossRef]
- Bhargava, K.; Zhang, Y. Multidrug-resistant coagulase-negative Staphylococci in food animals. J. Appl. Microbiol. 2012, 113, 1027–1036. [Google Scholar] [CrossRef]
- Bonvegna, M.; Grego, E.; Sona, B.; Stella, M.C.; Nebbia, P.; Mannelli, A.; Tomassone, L. Occurrence of Methicillin-Resistant Coagulase-Negative Staphylococci (MRCoNS) and Methicillin-Resistant Staphylococcus aureus (MRSA) from Pigs and Farm Environment in Northwestern Italy. Antibiotics 2021, 10, 676. [Google Scholar] [CrossRef]
- Zhang, Y.; Agidi, S.; LeJeune, J.T. Diversity of staphylococcal cassette chromosome in coagulase-negative staphylococci from animal sources. J. Appl. Microbiol. 2009, 107, 1375–1383. [Google Scholar] [CrossRef]
- Founou, L.L.; Founou, R.C.; Essack, S.Y.; Djoko, C.F. Mannitol-fermenting methicillin-resistant staphylococci (MRS) in pig abattoirs in Cameroon and South Africa: A serious food safety threat. Int. J. Food Microbiol. 2018, 285, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Huber, H.; Ziegler, D.; Pflüger, V.; Vogel, G.; Zweifel, C.; Stephan, R. Prevalence and characteristics of methicillin-resistant coagulase-negative staphylococci from livestock, chicken carcasses, bulk tank milk, minced meat, and contact persons. BMC Vet. Res. 2011, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Papan, C.; Schröder, M.; Hoffmann, M.; Knoll, H.; Last, K.; Albrecht, F.; Geisel, J.; Fink, T.; Gärtner, B.C.; Mellmann, A.; et al. Combined antibiotic stewardship and infection control measures to contain the spread of linezolid-resistant Staphylococcus epidermidis in an intensive care unit. Antimicrob. Resist. Infect. Control 2021, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Peschel, A.; Both, A.; Huang, J.; Qi, M.; Lausmann, C.; Weißelberg, S.; Büttner, H.; Lezius, S.; Failla, A.V.; Christner, M.; et al. Distinct clonal lineages and within-host diversification shape invasive Staphylococcus epidermidis populations. PLoS Pathog. 2021, 17, e1009304. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, G.; Yang, W.; Chen, F.; Qi, Y.; Lou, Z. Investigation into the prevalence of enterotoxin genes and genetic background of Staphylococcus aureus isolates from retain foods in Hangzhou, China. BMC Microbiol. 2023, 23, 294. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, J.; Yu, S.; Wu, Q.; Guo, W.; Huang, J.; Cai, S. Prevalence of Staphylococcus aureus and Methicillin-Resistant Staphylococcus aureus in Retail Ready-to-Eat Foods in China. Front. Microbiol. 2016, 7, 816. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).