Benefits of Immobilized Bacteria in Bioremediation of Sites Contaminated with Toxic Organic Compounds
Abstract
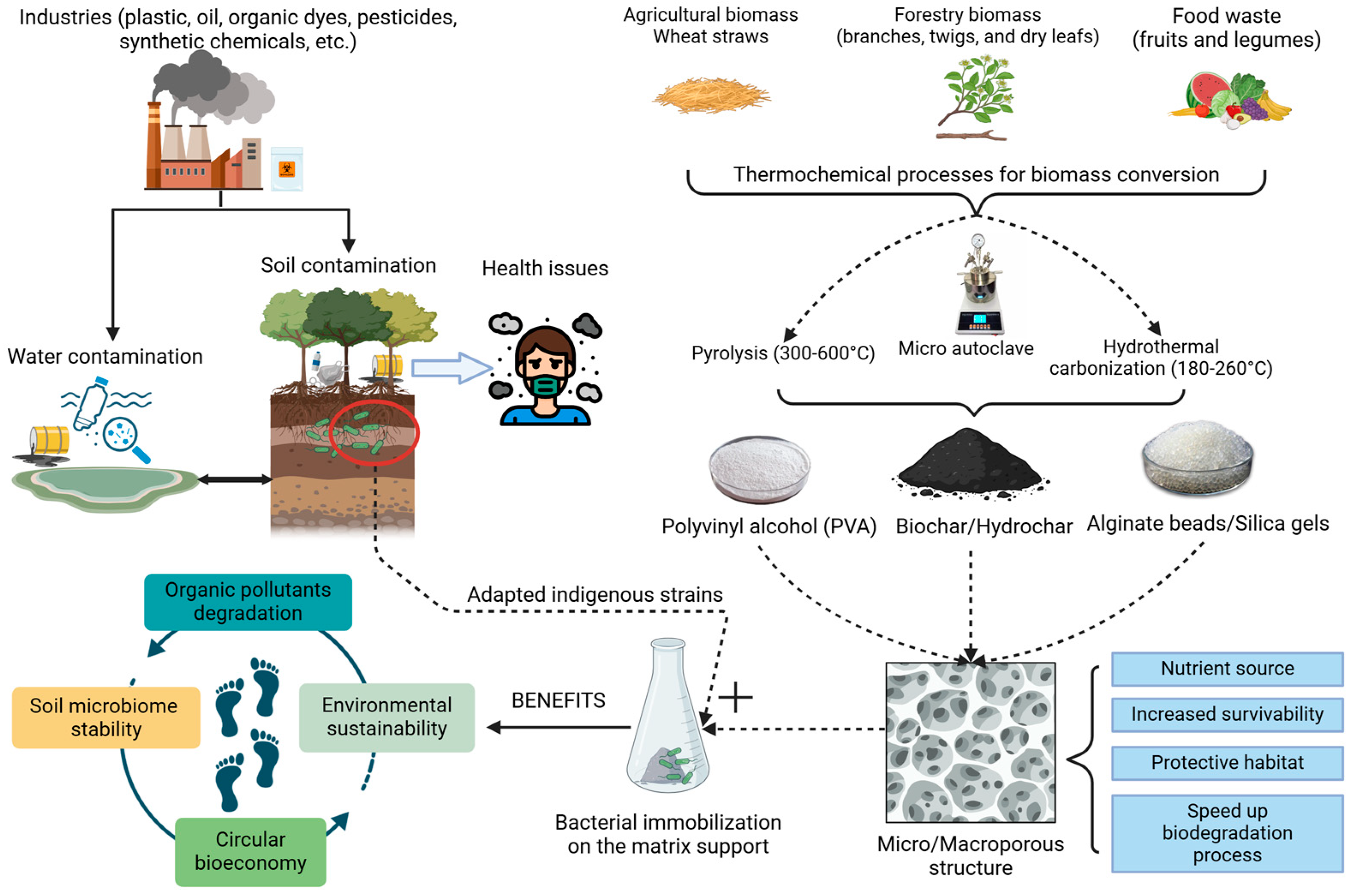
:1. Introduction
2. Types of Pollutants That Can Affect the Environment and Human Health
| Class | Examples | Sources | Effects | References |
|---|---|---|---|---|
| Industrial solvents | Acetone, tetrachloroethylene, toluene, trichloroethylene, dichloro-methane, tetrachloroethylene | Dry cleaning, metal degreasing, paint thinners, adhesives, dye manufacturing, paints and glues | Damage human liver, kidney, neural and immune systems; increase the level of volatile organic compounds indoors or outdoors | [55,56,57] |
| Polycyclic aromatic hydrocarbons (PAHs) | Benzo [a] pyrene, naphthalene, anthracene, chrysene, biphenyl, fluorene, tetracene | Incomplete combustion, power generation, agricultural waste, rubber manufacturing | Risk of lung cancer; increases cardiovascular disease, hypertension, and myocardial infarction; soil and water contamination | [38,58,59,60] |
| Polychlorinated biphenyls (PCBs) | Aroclor 1254, Aroclor 1260, Ascarel, Phenoclor, Clophen | Leaks or releases from electrical transformers, fuel combustion, chemical wastewater disposal sites, agriculture | Effect the immune system and reproductive system, neurobehavioral deficits, dementia, reduce aquatic life | [39,61,62] |
| Dioxins and furans | 2,3,7,8-tetrachlorodibenzo-p-dioxin, dibenzofurans, polychlorinated dibenzofurans | Incineration (waste), combustion, industrial materials and processes, volcanic eruptions | Influence marine and terrestrial organisms, effect tissues and cells, cancerogenic, effect reproductive system | [40,63,64] |
| Polyfluoroalkyl substances (PFASs) | Perfluorooctanoic acid, Perfluorooctanesulfonic acid | Oil exploitation activities, food packaging, chemical industry, cosmetics, ski wax, apparel | Alter the immune response, thyroid, breast cancer, liver damage, obesity | [65,66] |
| Volatile organic compounds (VOCs) | Benzene, toluene, xylene, formaldehyde | Paints, pesticide aerosol sprays, disinfectants, copiers, printers, markers, tobacco | Increase the risk of breast cancer, respiratory illnesses, leukemia, neural tube defects | [41,67,68] |
| Pesticides and herbicides | Dichlorodiphenyltrichloroethane, Chlordane, Aldrin, Glyphosate, Atrazine | Agriculture, industrial wastewater | Bioaccumulation in mammals; they can effect plant transpiration rate and plant growth | [69,70,71] |
| Pharmaceuticals and personal care products | Antibiotics, antidepressants, hormonal drugs, sunscreen agents | Pharmaceutical waste (e.g., expired and unused pills, body care and cleansing, cleansing pads, etc.), veterinary medicines, wastewater treatment plants | Increase antimicrobial resistance, contaminates soils and water bodies (eutrophication) | [72,73] |
| Endocrine-disrupting chemicals (EDCs) | Bisphenol A, phthalates, phenol, xenobiotics | Pharmaceuticals, estrogens and androgens, industrial chemicals, long-chain polymers, pesticides, plasticizers, organometals | Ovarian disorder, endocrine disruptor, interference with testosterone, sperm motility, testicular cancer, effect aquatic life | [74,75,76] |
| Fertilizers | Sewage sludge, green waste compost and mixed digestate | Agriculture, wastewater treatment plants, industrial plants | Alter the rhizosphere micro-ecological environment, cell inhibition, organ tumors, infections | [77,78,79] |
3. Bacterial Strains Used in Bioremediation Processes
4. Principles and Benefits of Immobilization
4.1. Physical Immobilization Methods
4.2. Chemical Immobilization Methods
5. Carriers Used for Bacterial Immobilization
| Carrier | Bacterial Genus | Experimental Conditions | Targeted Pollutant and Initial Concentration | Degradation Efficacy | References | |
|---|---|---|---|---|---|---|
| Non-Immobilized | Immobilized | |||||
| Biochar | Bacillus | Simulated sewage | Chlortetracycline (73.75 mg/L) | 66% | 83% | [168] |
| Polyvinyl alcohol–sodium alginate–kaolin | Bacillus | Synthetic medium | Trinitrotoluene (120 mg/L) | 72% | 99% | [169] |
| Modified peanut shell powder | Mycobacterium | Simulated polluted water | Pyrene (50 mg/L) | 45% | 70% | [170] |
| Rice straw biochar | Mycobacterium | Real contaminated soil | Phenanthrene (50 mg/L) | 43% | 69% | [171] |
| Nanocellulose fibers | Arthrobacter | Spiked medium | Diuron (10 mg/L) | 86% | 99% | [167] |
| Expanded polystyrene | Arthrobacter | Synthetic medium | Pentane (50 mL/500 mL) | 70% | 90% | [172] |
| Sunflower seeds husk | Rhodococcus | Real soil | Crude oil (25 g/kg) | 28% | 66% | [173] |
| Magnetic nanoparticles | Rhodococcus | Spiked medium | Chlorophenol (0.50 mM) | 50% | 80% | [166] |
| Sodium alginate + polyvinyl alcohol | Flavobacterium | Real soil | Ammonia nitrogen (50 mg/L) | 25% | >80% | [174] |
| Polyurethane | Flavobacterium | Spiked medium | Pentachloro-phenol 300 (mg/L) | 20% | 90% | [175] |
| Polyvinyl alcohol | Acinetobacter | Spiked medium | Phenol (1100 mg/L) | 50% | >99% | [176] |
| Membrane Bioreactor | Pseudomonas | Real wastewater | Phenanthrene (20 mg/L) | 77% | 96% | [177] |
| Alginate, agar, and polyacrylamide | Pseudomonas | Spiked medium | Ethylbenzene (1 mL/L) | 2% | 60% | [178] |
| Modified biochar | Pseudomonas | Real wastewater | Triclocarban (10 mg/L) | 32% | 80% | [179] |
| Bamboo charcoal and wood charcoal | Bacterial consortium | Real wastewater | Nonylphenol (50 mg/L) | 20% | 70% | [180] |
| Coco-peat and rice hull powder | Bacterial consortium | In vitro sea water | DDT (pesticide) (0.75 mL/150 mL) | 25% | 86% | [181] |
6. Conclusions, Challenges, and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Geetha, D.; Nagarajan, E.R. Chapter 3—Impact and Issues of Organic Pollutants. In Management of Contaminants of Emerging Concern (CEC) in Environment; Elsevier: Oxford, UK, 2021; pp. 93–126. [Google Scholar] [CrossRef]
- Mishra, A.; Kumari, M.; Kumar, R.S.; Iqbal, K.; Thakur, S.I. Persistent organic pollutants in the environment: Risk assessment, hazards, and mitigation strategies. J. Bioresour. Bioprod. 2022, 19, 101143. [Google Scholar] [CrossRef]
- Pariatamby, A.; Kee, Y.L. Persistent Organic Pollutants Management and Remediation. Procedia Environ. Sci. 2016, 31, 842–848. [Google Scholar] [CrossRef]
- Yu, S.; Tan, Z.; Lai, Y.; Li, Q.; Liu, J. Nanoparticulate pollutants in the environment: Analytical methods, formation, and transformation. Eco-Environ. Health. 2023, 2, 61–67. [Google Scholar] [CrossRef]
- World Health Organization; United Nations Children’s Fund (UNICEF); United Nations Development Programme; United Nations Environment Programme. Compendium of WHO and Other UN Guidance in Health and Environment—2024 Update; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Wang, Z.; Walker, G.W.; Muir, D.C.; Nagatani-Yoshida, K. Toward a global understanding of chemical pollution: A first comprehensive analysis of national and regional chemical inventories. Environ. Sci. Technol. 2020, 54, 2575–2584. [Google Scholar] [CrossRef]
- Ali, S.; Sharma, B.; Deoli, K.; Saini, D.; Bisht, M. An Overview on Bioremediation Strategies for Waste Water Treatment and Environmental Sustainability. Appl. Ecol. Environ. Sci. 2023, 11, 64–70. [Google Scholar]
- Saeed, U.M.; Hussain, N.; Sumrin, A.; Shahbaz, A.; Noor, S.; Bilal, M.; Aleya, L.; Iqbal, H.M.N. Microbial bioremediation strategies with wastewater treatment potentialities—A review. Sci. Total Environ. 2022, 808, 151754. [Google Scholar] [CrossRef]
- Norfazilah, W.I.W.; Umairah, M.S. Various Methods for Removal, Treatment, and Detection of Emerging Water Contaminants. In Emerging Contaminants; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Y.; Kamyab, H.; Rajasimman, M.; Rajamohan, N.; Ngo, H.G.; Xia, C. Physico-chemical and biological remediation techniques for the elimination of endocrine-disrupting hazardous chemicals. Environ. Res. 2023, 232, 116363. [Google Scholar] [CrossRef] [PubMed]
- Lawniczak, L.; Woźniak-Karczewska, M.; Loibner, A.P.; Heipieper, H.J.; Chrzanowski, L. Microbial degradation of hydrocarbons—Basic principles for bioremediation: A review. Molecules 2020, 25, 856. [Google Scholar] [CrossRef]
- Bala, S.; Garg, D.; Thirumalesh, B.V.; Sharma, M.; Sridhar, K.; Inbaraj, B.S.; Tripathi, M. Recent Strategies for Bioremediation of Emerging Pollutants: A Review for a Green and Sustainable Environment. Toxics 2022, 10, 484. [Google Scholar] [CrossRef] [PubMed]
- Atashgahi, S.; Sánchez-Andrea, I.; Heipieper, H.J.; van der Meer, J.R.; Stams, A.J.M.; Smidt, H. Prospects for harnessing biocide resistance for bioremediation and detoxification. Science 2018, 360, 743–746. [Google Scholar] [CrossRef]
- Shen, L.; Yu, W.; Li, L.; Zhang, T.; Abshir, I.Y.; Luo, P.; Liu, Z. Microorganism, Carriers, and Immobilization Methods of the Microbial Self-Healing Cement-Based Composites: A Review. Materials 2021, 14, 5116. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, I. Biofilm exopolysaccharides: A strong and sticky framework. Microbiology 2001, 147, 3–9. [Google Scholar] [CrossRef]
- Beveridge, T.J.; Makin, S.A.; Kadurugamuwa, J.L.; Li, Z. Interactions between biofilms and the environment. FEMS Microbiol. Rev. 1997, 20, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Schertzer, J.W.; Whiteley, M. A bilayer-couple model of bacterial outer membrane vesicle biogenesis. mBio 2012, 3, e00297-11. [Google Scholar] [CrossRef] [PubMed]
- Eberlein, C.; Baumgarten, T.; Starke, S.; Heipieper, H.J. Immediate response mechanisms of Gram-negative solvent tolerant bacteria to cope with environmental stress: Cis-trans isomerization of unsaturated fatty acids and outer membrane vesicle secretion. Appl. Microbiol. Biotechnol. 2018, 102, 2583–2593. [Google Scholar] [CrossRef] [PubMed]
- Schooling, S.R.; Beveridge, T.J. Membrane vesicles: An overlooked component of the matrices of biofilms. J. Bacteriol. 2006, 188, 5945–5957. [Google Scholar] [CrossRef]
- Ceri, H.; Olson, M.E.; Stremick, C.; Read, R.R.; Morck, D.; Buret, A. The Calgary Biofilm Device: New technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 1999, 37, 1771–1776. [Google Scholar] [CrossRef]
- Carvalho, G.G.P.; Pires, A.J.V.; Garcia, R.; Veloso, C.M.; Silva, R.R.; Mendes, F.B.L.; Pinheiro, A.A.; Souza, D.R. In situ degradability of dry matter, crude protein and fibrous fraction of concentrate and agroindustrial by-products. Ciência Anim. Bras. 2009, 10, 689–697. [Google Scholar]
- Heipieper, H.J.; Keweloh, H.; Rehm, H.J. Influence of phenols on growth and membrane permeability of free and immobilized Escherichia coli. Appl. Environ. Microbiol. 1991, 57, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.I.; Lee, Y.H.; Kim, K.H.; Oh, Y.K.; Moon, Y.H.; Kwak, W.S. Effects of supplementing microbially-fermented spent mushroom substrates on growth performance and carcass characteristics of Hanwoo steers (a field study). Asian-Aust. J. Anim. Sci. 2012, 25, 1575–1581. [Google Scholar] [CrossRef]
- Weitere, M.; Scherwass, A.; Sieben, K.T.; Arndt, H. Planktonic food web structure and potential carbon flow in the lower river Rhine with a focus on the role of protozoans. River Res. Appl. 2005, 21, 535–549. [Google Scholar] [CrossRef]
- Baumgarten, T.; Sperling, S.; Seifert, J.; Bergen, M.; Steiniger, F.; Wick, L.Y.; Heipieper, H.J. Membrane vesicle formation as a multiple-stress response mechanism enhances Pseudomonas putida DOT-T1E cell surface hydrophobicity and biofilm formation. Appl. Environ. Microbiol. 2012, 78, 6217–6224. [Google Scholar] [CrossRef] [PubMed]
- Keweloh, H.; Heipieper, H.J.; Rehm, H.J. Protection of Bacteria Against Toxicity of Phenol by Immobilization in Calcium Alginate. Appl. Microbiol. Biotechnol. 1989, 31, 383–389. [Google Scholar] [CrossRef]
- Heipieper, H.J.; Loffeld, B.; Keweloh, H.; Bont, J.A.M. The cis/trans isomerisation of unsaturated fatty acids in Pseudomonas putida S12: An indicator for environmental stress due to organic compounds. Chemosphere 1995, 30, 1041–1051. [Google Scholar] [CrossRef]
- Balciunas, E.M.; Kappelmeyer, U.; Harms, H.; Heipieper, H.J. Increasing ibuprofen degradation in constructed wetlands by bioaugmentation with gravel containing biofilms of an ibuprofen-degrading Sphingobium yanoikuyae. Eng. Life Sci. 2020, 20, 160–167. [Google Scholar] [CrossRef]
- Berillo, D.; Al-Jwaid, A.; Caplin, J. Polymeric Materials Used for Immobilisation of Bacteria for the Bioremediation of Contaminants in Water. Polymers 2021, 13, 1073. [Google Scholar] [CrossRef]
- Shetty, S.S.; Deepthi, D.; Harshitha, S.; Sonkusare, S.; Naik, P.B.; Kumari, N.S.; Madhyastha, H. Environmental pollutants and their effects on human health. Heliyon 2023, 9, 19496. [Google Scholar] [CrossRef]
- Lyu, S.; Shen, X.; Bi, Y. The Dually Negative Effect of Industrial Polluting Enterprises on China’s Air Pollution: A Provincial Panel Data Analysis Based on Environmental Regulation Theory. Int. J. Environ. Res. Public Health 2020, 17, 7814. [Google Scholar] [CrossRef] [PubMed]
- Speight, J.G. Sources and Types of Inorganic Pollutants. In Environmental Inorganic Chemistry for Engineers; Butterworth-Heinemann: Oxford, UK, 2017; pp. 231–282. [Google Scholar] [CrossRef]
- Mehrotra, T.; Dev, S.; Banerjee, A.; Chatterjee, A.; Singh, R.; Aggarwal, S. Use of immobilized bacteria for environmental bioremediation: A review. J. Environ. Chem. Eng. 2021, 9, 105920. [Google Scholar] [CrossRef]
- Wilson, F.M. Agriculture and Industry as Potential Origins for Chemical Contamination in the Environment. A Review of the Potential Sources of Organic Contamination. Curr. Org. Chem. 2013, 17, 2972–2975. [Google Scholar] [CrossRef]
- Kumar, A.R.; Singh, I.; Ambekar, K. Chapter 1—Occurrence, Distribution, and Fate of Emerging Persistent Organic Pollutants In Management of Contaminants of Emerging Concern (CEC) in Environment; Elsevier: Oxford, UK, 2021; pp. 1–69. [Google Scholar] [CrossRef]
- Singh, A.K.; Kumar, A.; Chandra, R. Environmental pollutants of paper industry wastewater and their toxic effects on human health and ecosystem. Bioresour. Technol. 2022, 20, 101250. [Google Scholar] [CrossRef]
- Girolkar, S.; Thawale, P.; Juwarkar, A. Chapter 12—Bacteria-assisted phytoremediation of heavy metals and organic pollutants: Challenges and future prospects. In Bioremediation for Environmental Sustainability; Elsevier: Amsterdam, The Netherlands, 2021; pp. 247–267. [Google Scholar] [CrossRef]
- Patel, A.B.; Shaikh, S.; Jain, K.R.; Desai, C.; Madamwar, D. Polycyclic Aromatic Hydrocarbons: Sources, Toxicity, and Remediation Approaches. Front. Microbiol. 2020, 11, 562813. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.V.A.; Moniruzzaman, M.; Aminabhavi, M.T. Polychlorinated biphenyls (PCBs) in the environment: Recent updates on sampling, pretreatment, cleanup technologies and their analysis. Chem. Eng. J. 2019, 358, 1186–1207. [Google Scholar] [CrossRef]
- Kirkok, S.K.; Kibet, J.K.; Kinyanjui, T.K.; Okanga, F.I. A review of persistent organic pollutants: Dioxins, furans, and their associated nitrogenated analogues. SN Appl. Sci. 2020, 2, 1729. [Google Scholar] [CrossRef]
- Chaleshtari, Z.A.; Foudazi, R. A Review on Per- and Polyfluoroalkyl Substances (PFAS) Remediation: Separation Mechanisms and Molecular Interactions. ACS EST Water 2022, 2, 2258–2272. [Google Scholar] [CrossRef]
- David, E.; Niculescu, V.C. Volatile Organic Compounds (VOCs) as Environmental Pollutants: Occurrence and Mitigation Using Nanomaterials. Int. J. Environ. Res. Public Health 2021, 18, 13147. [Google Scholar] [CrossRef]
- Rajmohan, K.S.; Chandrasekaran, R.; Varjani, S. A Review on Occurrence of Pesticides in Environment and Current Technologies for Their Remediation and Management. Indian J. Microbiol. 2020, 60, 125–138. [Google Scholar] [CrossRef]
- Wilms, W.; Woźniak-Karczewska, M.; Corvini, P.F.X.; Chrzanowski, Ł. Nootropic drugs: Methylphenidate, modafinil and piracetam–Population use trends, occurrence in the environment, ecotoxicity and removal methods—A review. Chemosphere 2019, 233, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Wydro, U.; Wołejko, E.; Luarasi, L.; Puto, K.; Tarasevičienė, Ž.; Jabłońska-Trypuć, A. A Review on Pharmaceuticals and Personal Care Products Residues in the Aquatic Environment and Possibilities for Their Remediation. Sustainability 2024, 16, 169. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef] [PubMed]
- Semerád, J.; Hatasová, N.; Grasserová, A.; Černá, T.; Filipová, A.; Hanč, A.; Innemanová, P.; Pivokonský, M.; Cajthaml, T. Screening for 32 per- and polyfluoroalkyl substances (PFAS) including GenX in sludges from 43 WWTPs located in the Czech Republic—Evaluation of potential accumulation in vegetables after application of biosolids. Chemosphere 2020, 261, 128018. [Google Scholar] [CrossRef]
- Guo, W.; Pan, B.; Sakkiah, S.; Yavas, G.; Ge, W.; Zou, W.; Tong, W.; Hong, H. Persistent Organic Pollutants in Food: Contamination Sources, Health Effects and Detection Methods. Int. J. Environ. Res. Public Health 2019, 16, 4361. [Google Scholar] [CrossRef]
- Khare, A.; Jadhao, P.; Paliya, S.; Kumari, K. Toxicity and Structural Activity Relationship of Persistent Organic Pollutants. Front. Enzym. Inhib. 2020, 30, 174–203. [Google Scholar] [CrossRef]
- Gessesse, K.; Tekle, T.; Ebrahim, M.A.; Kamaraj, M.; Fassil, A. Factors Influencing the Bacterial Bioremediation of Hydrocarbon Contaminants in the Soil: Mechanisms and Impacts. J. Chem. 2021, 2021, 9823362. [Google Scholar] [CrossRef]
- Laane, C.; Boeren, S.; Vos, K.; Veeger, C. Rules for optimization of biocatalysis in organic solvents. Biotechnol. Bioeng. 1987, 30, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Leahy, J.G.; Colwell, R.R. Microbial degradation of hydrocarbons in the environment. Microbiol. Rev. 1990, 54, 305–315. [Google Scholar] [CrossRef]
- Parus, A.; Ciesielski, T.; Woźniak-Karczewska, M.; Ślachciński, M.; Owsianiak, M.; Ławniczak, L.; Loibner, P.A.; Heipieper, H.J.; Chrzanowski, L. Basic principles for biosurfactant-assisted (bio)remediation of soils contaminated by heavy metals and petroleum hydrocarbons—A critical evaluation of the performance of rhamnolipids. J. Hazard. Mater. 2023, 443, 130171. [Google Scholar] [CrossRef]
- Kuppan, K.; Padman, M.; Mahadeva, M.; Srinivasan, S.; Devarajan, R. A comprehensive review of sustainable bioremediation techniques: Eco friendly solutions for waste and pollution management. Waste Manag. Bull. 2024, 2, 154–171. [Google Scholar] [CrossRef]
- Hogstedt, C.; Axelson, O. Long-term health effects of industrial solvents—A critical review of the epidemiological research. Med. Lav. 1986, 77, 11–22. [Google Scholar] [PubMed]
- Tobiszewski, M.; Namieśnik, J.; Pena-Pereira, F. Environmental risk-based ranking of solvents using the combination of a multimedia model and multi-criteria decision analysis. Green. Chem. 2017, 19, 1034–1042. [Google Scholar] [CrossRef]
- Baker, E.L.; Smith, T.J.; Landrigan, P.J. The neurotoxicity of industrial solvents: A review of the literature. Am. J. Ind. Med. 1985, 8, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Eberlein, C.; Johannes, J.; Mouttaki, H.; Sadeghi, M.; Golding, B.T.; Boll, M.; Meckenstock, R.U. Ring reductases involved in naphthalene degradation. Environ. Microbiol. 2013, 15, 1832–1841. [Google Scholar] [CrossRef] [PubMed]
- Mallah, M.A.; Changxing, L.; Mallah, M.A.; Noreen, S.; Liu, Y.; Saeed, M.; Xi, H.; Ahmed, B.; Feng, F.; Mirjat, A.A.; et al. Polycyclic aromatic hydrocarbon and its effects on human health: An overeview. Chemosphere 2022, 296, 133948. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.K. Sources, Distribution and Toxicity of Polyaromatic Hydrocarbons (PAHs) in Particulate Matter. In Air Pollution; Sciyo: Rijeka, Croatia, 2010. [Google Scholar] [CrossRef]
- Montano, L.; Pironti, C.; Pinto, G.; Ricciardi, M.; Buono, A.; Brogna, C.; Venier, M.; Piscopo, M.; Amoresano, A.; Motta, O. Polychlorinated Biphenyls (PCBs) in the Environment: Occupational and Exposure Events, Effects on Human Health and Fertility. Toxics 2022, 10, 365. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, D.O. Polychlorinated biphenyls (PCBs): Routes of exposure and effects on human health. Rev. Environ. Health 2006, 21, 1–23. [Google Scholar] [CrossRef]
- Loganathan, B.G.; Masunaga, S. Chapter 18—PCBs, Dioxins, and Furans: Human Exposure and Health Effects. In Handbook of Toxicology of Chemical Warfare Agents; Elsevier: London, UK, 2009; pp. 245–253. [Google Scholar] [CrossRef]
- Kanan, S.; Samara, F. Dioxins and furans: A review from chemical and environmental perspectives. Trends Environ. Anal. Chem. 2018, 17, 1–13. [Google Scholar] [CrossRef]
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.N.C.; Smith, J.S.; Roberts, S.M. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ. Toxicol. Chem. 2021, 40, 606–630. [Google Scholar] [CrossRef] [PubMed]
- Schiavone, C.; Portesi, C. PFAS: A Review of the State of the Art, from Legislation to Analytical Approaches and Toxicological Aspects for Assessing Contamination in Food and Environment and Related Risks. Appl. Sci. 2023, 13, 6696. [Google Scholar] [CrossRef]
- Li, A.J.; Pal, V.K.; Kannan, K. A review of environmental occurrence, toxicity, biotransformation and biomonitoring of volatile organic compounds. Environ. Chem. Ecotoxicol. 2021, 3, 91–116. [Google Scholar] [CrossRef]
- Baskaran, D.; Dhamodharan, D.; Behera, U.S.; Byun, H.S. A comprehensive review and perspective research in technology integration for the treatment of gaseous volatile organic compounds. Environ. Res. 2024, 251, 118472. [Google Scholar] [CrossRef] [PubMed]
- Santacruz, G.; Bandala, E.R.; Torres, L.G. Chlorinated pesticides (2,4-D and DDT) biodegradation at high concentrations using immobilized Pseudomonas fluorescens. J. Environ. Sci. Health B 2005, 40, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Yañez-Ocampo, G.; Sanchez-Salinas, E.; Jimenez-Tobon, G.A.; Penninckx, M.; Ortiz-Hernández, M.L. Removal of two organophosphate pesticides by a bacterial consortium immobilized in alginate or tezontle. J. Hazard. Mater. 2009, 168, 1554–1561. [Google Scholar] [CrossRef]
- Kalyabina, V.P.; Esimbekova, E.N.; Kopylova, K.V.; Kratasyuk, V.A. Pesticides: Formulants, distribution pathways and effects on human health—A review. Toxicol. Rep. 2021, 6, 1179–1192. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ok, Y.S.; Kim, K.H.; Kwon, E.E.; Tsang, Y.F. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review. Sci. Total Environ. 2017, 596–597, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.; Nnorom, M.A.; Tsang, Y.F.; Knapp, C.W. Pharmaceuticals and personal care products’ (PPCPs) impact on enriched nitrifying cultures. Environ. Sci. Pollut. Res. Int. 2021, 28, 60968–60980. [Google Scholar] [CrossRef]
- Metcalfe, C.D.; Bayen, S.; Desrosiers, M.; Muñoz, G.; Sauvé, S.; Yargeau, V. An introduction to the sources, fate, occurrence and effects of endocrine disrupting chemicals released into the environment. Environ. Res. 2022, 207, 112658. [Google Scholar] [CrossRef]
- Thacharodi, A.; Hassan, S.; Hegde, T.A.; Thacharodi, D.D.; Brindhadevi, K.; Pugazhendhi, A. Water a major source of endocrine-disrupting chemicals: An overview on the occurrence, implications on human health and bioremediation strategies. Environ. Res. 2023, 231, 116097. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Han, D.; Currell, M.; Song, X.; Zhang, Y. Review of Endocrine Disrupting Compounds (EDCs) in China’s water environments: Implications for environmental fate, transport and health risks. Water Res. 2023, 245, 120645. [Google Scholar] [CrossRef]
- Thomas, D.; Bloem, E. Visible intruders: Tracing (micro-) plastic in organic fertilizers. Sci. Total Environ. 2024, 947, 174311. [Google Scholar] [CrossRef] [PubMed]
- Krein, D.D.C.; Rosseto, M.; Cemin, F.; Massuda, L.A.; Dettmer, A. Recent trends and technologies for reduced environmental impacts of fertilizers: A review. Int. J. Environ. Sci. Technol. 2023, 20, 12903–12918. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Jiang, L.; Chen, X.; Zhao, Y.; Shi, W.; Xing, Z. From organic fertilizer to the soils: What happens to the microplastics? A critical review. Sci. Total Environ. 2024, 919, 170217. [Google Scholar] [CrossRef] [PubMed]
- Stubbendieck, M.R.; Vargas-Bautista, C. Straight, Bacterial Communities: Interactions to Scale. Front. Microbiol. 2016, 7, 1234. [Google Scholar] [CrossRef] [PubMed]
- Hibbing, M.E.; Fuqua, C.; Parsek, M.R.; Peterson, S.B. Bacterial competition: Surviving and thriving in the microbial jungle. Nat. Rev. Microbiol. 2010, 8, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Ayilara, M.S.; Babalola, O.O. Bioremediation of environmental wastes: The role of microorganisms. Front. Agron. 2023, 5, 1183691. [Google Scholar] [CrossRef]
- Dell’ Anno, F.; Rastelli, E.; Sansone, C.; Brunet, C.; Ianora, A.; Dell’ Anno, A. Bacteria, Fungi and Microalgae for the Bioremediation of Marine Sediments Contaminated by Petroleum Hydrocarbons in the Omics Era. Microorganisms 2021, 9, 1695. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, W.L.; Letti, J.A.L.; Penha, O.R.; Soccol, T.V.; Rodrigues, C.; Soccol, R.C. Bacillus genus industrial applications and innovation: First steps towards a circular bioeconomy. Biotechnol. Adv. 2024, 70, 108300. [Google Scholar] [CrossRef] [PubMed]
- Polpass, A.J.; Maharshi, A.; Jha, B. Actinobacteria in natural products research: Progress and prospects. Microbiol. Res. 2021, 26, 126708. [Google Scholar]
- Pan, X.; Raaijmakers, M.J.; Carrión, J.V. Importance of Bacteroidetes in host–microbe interactions and ecosystem functioning. Trends Microbiol. 2023, 31, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Wang, P.; Li, Y.; Ling, J.; Ruan, Y.; Yu, J.; Zhang, L. Bioremediation of environmental organic pollutants by Pseudomonas aeruginosa: Mechanisms, methods and challenges. Environ. Res. 2023, 239, 117211. [Google Scholar] [CrossRef] [PubMed]
- Mokrani, S.; Houali, K.; Yadav, K.K.; Arabi, A.I.A.; Eltayeb, B.L.; AwjanAlreshidi, M.; Benguerba, Y.; Cabral-Pinto, M.M.S.; Nabti, E.H. Bioremediation techniques for soil organic pollution: Mechanisms, microorganisms, and technologies—A comprehensive review. Ecol. Eng. 2024, 207, 107338. [Google Scholar] [CrossRef]
- Mateescu, C.; Lungulescu, E.-M.; Nicula, N.-O. Effectiveness of Biological Approaches for Removing Persistent Organic Pollutants from Wastewater: A Mini-Review. Microorganisms 2024, 12, 1632. [Google Scholar] [CrossRef]
- Ferreira, L.; Rosales, E.; Danko, S.A.; Sanromán, A.M.; Pazos, M.M. Bacillus thuringiensis a promising bacterium for degrading emerging pollutants. Process Saf. Environ. Prot. 2016, 101, 19–26. [Google Scholar] [CrossRef]
- Wongbunmak, A.; Khiawjan, S.; Suphantharika, M.; Pongtharangkul, T. BTEX biodegradation by Bacillus amyloliquefaciens subsp. plantarum W1 and its proposed BTEX biodegradation pathways. Sci. Rep. 2020, 10, 17408. [Google Scholar] [CrossRef] [PubMed]
- Kostka, J.E.; Prakash, O.; Overholt, W.A.; Green, S.J.; Freyer, G.; Canion, A.; Delgardio, J.; Norton, N.; Hazen, T.C.; Huettel, M. Hydrocarbon-Degrading Bacteria and the Bacterial Community Response in Gulf of Mexico Beach Sands Impacted by the Deepwater Horizon Oil Spill. Appl. Environ. Microbiol. 2011, 77, 22. [Google Scholar] [CrossRef]
- Youssef, N.; Simpson, D.R.; Duncan, K.E.; McInerney, M.J.; Folmsbee, M.; Fincher, T.; Knapp, R.M. In Situ Biosurfactant Production by Bacillus Strains Injected into a Limestone Petroleum Reservoir. Appl. Environ. Microbiol. 2007, 73, 4. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Jeong, R.B.; Glick, R.B. Potential use of Bacillus spp. as an effective biostimulant against abiotic stresses I crops—A review. Curr. Res. Biotechnol. 2023, 5, 100128. [Google Scholar] [CrossRef]
- Kim, S.J.; Kweon, O.; Cerniglia, C. Degradation of Polycyclic Aromatic Hydrocarbons by Mycobacterium Strains. In Handbook of Hydrocarbon and Lipid Microbiology; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1865–1879. [Google Scholar] [CrossRef]
- Dudhagara, D.R.; Bharti, P.D. Mycobacterium as Polycyclic Aromatic Hydrocarbons (PAHs) Degrader. In Mycobacterium—Research and Development; InTech: Rijeka, Croatia, 2018. [Google Scholar] [CrossRef]
- Qutob, M.; Rafatullah, M.; Muhammad, S.A.; Alosaimi, A.M.; Alorfi, H.S.; Hussein, M.A. A Review of Pyrene Bioremediation Using Mycobacterium Strains in a Different Matrix. Fermentation 2022, 8, 260. [Google Scholar] [CrossRef]
- Guo, X.; Xie, C.; Wang, L.; Li, Q.; Wang, Y. Biodegradation of persistent environmental pollutants by Arthrobacter sp. Environ. Sci. Pollut. Res. 2019, 26, 8429–8443. [Google Scholar] [CrossRef]
- Verma, J.P.; Jaiswal, D.K. Book review: Advances in biodegradation and bioremediation of industrial waste. Front. Microbiol. 2016, 6, 1555. [Google Scholar] [CrossRef]
- Megharaj, M.; Ramakrishnan, B.; Venkateswarlu, K.; Sethunathan, N.; Naidu, R. Bioremediation approaches for organic pollutants: A critical perspective. Environ. Int. 2011, 37, 1362–1375. [Google Scholar] [CrossRef] [PubMed]
- Sagarkar, S.; Bhardwaj, P.; Storck, V.; Devers-Lamrani, M.; Martin-Laurent, F.; Kapley, A. s-triazine degrading bacterial isolate Arthrobacter sp. AK-YN10, a candidate for bioaugmentation of atrazine contaminated soil. Appl. Microbiol. Biotechnol. 2016, 100, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Krivoruchko, A.; Kuyukina, M.; Ivshina, I. Advanced Rhodococcus Biocatalysts for Environmental Biotechnologies. Catalysts 2019, 9, 236. [Google Scholar] [CrossRef]
- Ivshina, I.; Bazhutin, G.; Tyumina, E. Rhodococcus strains as a good biotool for neutralizing pharmaceutical pollutants and obtaining therapeutically valuable products: Through the past into the future. Front. Microbiol. 2022, 13, 967127. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.C.C.R.; Costa, S.S.; Fernandes, P.; Couto, I.; Viveiros, M. Membrane transport systems and the biodegradation potential and pathogenicity of genus Rhodococcus. Front. Physiol. 2014, 5, 133. [Google Scholar] [CrossRef]
- Kuyukina, M.S.; Ivshina, I.B. Application of Rhodococcus in bioremediation of contaminated environments. In Biology of Rhodococcus; Springer Nature: Cham, Switzerland, 2010; pp. 231–262. [Google Scholar] [CrossRef]
- Bissett, A.; Bowman, P.J.; Burke, M.C. Flavobacterial response to organic pollution. Aquat. Microb. Ecol. 2008, 51, 31–43. [Google Scholar] [CrossRef]
- Guerrero Ramírez, J.R.; Ibarra Muñoz, L.A.; Balagurusamy, N.; Frías Ramírez, J.E.; Alfaro Hernández, L.; Carrillo Campos, J. Microbiology and Biochemistry of Pesticides Biodegradation. Int. J. Mol. Sci. 2023, 24, 15969. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Singh, D.; Kumari, A.; Sharma, G.; Rajput, S.; Arora, S.; Kaur, R. Pesticide Residues Degradation Strategies in Soil and Water: A Review. Int. J. Environ. Sci. Technol. 2023, 20, 3537–3560. [Google Scholar] [CrossRef]
- Mekonnen, B.A.; Aragaw, T.A.; Genet, M.B. Bioremediation of petroleum hydrocarbon contaminated soil: A review on principles, degradation mechanisms, and advancements. Front. Environ. Sci. 2024, 12, 1354422. [Google Scholar] [CrossRef]
- Chaudhary, D.K.; Kim, D.U.; Kim, D.; Kim, J. Flavobacterium petrolei sp. nov., a novel psychrophilic, diesel-degrading bacterium isolated from oil-contaminated Arctic soil. Sci. Rep. 2019, 9, 4134. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Park, W. Acinetobacter species as model microorganisms in environmental microbiology: Current state and perspectives. Appl. Microbiol. Biotechnol. 2015, 99, 2533–2548. [Google Scholar] [CrossRef] [PubMed]
- Czarny, J.; Staninska-Pięta, J.; Piotrowska-Cyplik, A.; Juzwa, W.; Wolniewicz, A.; Marecik, R.; Ławniczak, L.; Chrzanowski, L. Acinetobacter sp. as the key player in diesel oil degrading community exposed to PAHs and heavy metals. J. Hazard. Mater. 2020, 383, 121168. [Google Scholar] [CrossRef] [PubMed]
- Berardinis, V.; Durot, M.; Weissenbach, J.; Salanoubat, M. Acinetobacter baylyi ADP1 as a model for metabolic system biology. Curr. Opin. Microbiol. 2009, 12, 568–576. [Google Scholar] [CrossRef]
- Seitz, P.; Blokesch, M. Cues and regulatory pathways involved in natural competence and transformation in pathogenic and environmental Gram-negative bacteria. FEMS Microbiol. Rev. 2012, 37, 336–363. [Google Scholar] [CrossRef]
- Wasi, S.; Tabrez, S.; Ahmad, M. Use of Pseudomonas spp. for the bioremediation of environmental pollutants: A review. Environ. Monit. Assess. 2012, 185, 8147–8155. [Google Scholar] [CrossRef] [PubMed]
- Nogales, J.; García, J.L.; Díaz, E. Degradation of Aromatic Compounds in Pseudomonas: A Systems Biology View. In Aerobic Utilization of Hydrocarbons, Oils and Lipids; Rojo, F., Ed.; Handbook of Hydrocarbon and Lipid Microbiology Series; Springer: Cham, Switzerland, 2017; pp. 1–49. [Google Scholar] [CrossRef]
- Mandelbaum, R.T.; Allan, D.L.; Wackett, L.P. Isolation and Characterization of a Pseudomonas sp. That Mineralizes the s-Triazine Herbicide Atrazine. Appl. Environ. Microbiol. 1995, 61, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, J.; Liu, Y.; Li, Z.; Nan, J. Optimization of entrapping conditions of nitrifying bacteria and selection of entrapping agent. Procedia Environ. Sci. 2011, 8, 166–172. [Google Scholar] [CrossRef]
- Armanu, G.E.; Volf, I. Natural carriers for bacterial immobilization used in bioremediation. Bul. Inst. Polit. Iasi 2022, 68, 109–122. [Google Scholar] [CrossRef]
- Bayat, Z.; Hassanshahian, M.; Cappello, S. Immobilization of microbes for bioremediation of crude oil polluted environments: A mini review. Open Microbiol. J. 2015, 9, 48–54. [Google Scholar] [PubMed]
- Bouabidi, Z.B.; El-Naas, M.H.; Zhang, Z. Immobilization of microbial cells for the biotreatment of wastewater: A review. Environ. Chem. Lett. 2019, 17, 241–257. [Google Scholar] [CrossRef]
- Berillo, D.; Malika, T.; Baimakhanova, B.B.; Sadanov, A.K.; Berezin, V.E.; Trenozhnikova, L.P.; Baimakhanova, G.B.; Amangeldi, A.A.; Kerimzhanova, B. An Overview of Microorganisms Immobilized in a Gel Structure for the Production of Precursors, Antibiotics, and Valuable Products. Gels 2024, 10, 646. [Google Scholar] [CrossRef]
- Singh, R.; Paul, D.; Jain, R.K. Biofilms: Implications in bioremediation. Trends Microbiol. 2006, 14, 389–397. [Google Scholar] [CrossRef]
- Vemmer, M.; Patel, V.A. Review of encapsulation methods suitable for microbial biological control agents. Biol. Control 2013, 67, 380–389. [Google Scholar] [CrossRef]
- Ogundolie, A.F.; Babalola, O.O.; Adetunji, O.C.; Aruwa, E.C.; Manjia, M.J.; Muftaudeen, K.T. A review on bioremediation by microbial immobilization-an effective alternative for wastewater treatment. AIMS Environ. Sci. 2024, 11, 918–939. [Google Scholar] [CrossRef]
- Purbasha, S.; Rao, K.V.B. Immobilization as a powerful bioremediation tool for abatement of dye pollution: A review. Environ. Rev. 2021, 29, 277–299. [Google Scholar] [CrossRef]
- Kierek-Pearson, K.; Karatan, E. Biofilm development in bacteria. Adv. Appl. Microbiol. 2005, 57, 79–111. [Google Scholar] [CrossRef] [PubMed]
- Schommer, A.V.; Nazari, T.M.; Melara, F.; Braun, A.C.J.; Rempel, A.; dos Santos, L.F.; Ferrari, V.; Colla, M.L.; Dettmer, A.; Piccin, S.J. Techniques and mechanisms of bacteria immobilization on biochar for further environmental and agricultural applications. Microbiol. Res. 2024, 278, 127534. [Google Scholar] [CrossRef]
- Yoetz-Kopelman, T.; Dror, Y.; Shacham-Diamand, Y.; Freeman, A. “Cells-on-Beads”: A novel immobilization approach for the construction of whole-cell amperometric biosensors. Sens. Actuators B Chem. 2016, 232, 758–764. [Google Scholar] [CrossRef]
- Zhu, Y.; Ren, X.; Liu, Y.; Wei, Y.; Qing, L.; Liao, X. Covalent immobilization of porcine pancreatic lipase on carboxyl-activated magnetic nanoparticles: Characterization and application for enzymatic inhibition assays. Mat. Sci. Eng. C 2014, 38, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Dzionek, A.; Wojcieszyńska, D.; Guzi, U. Natural carriers in bioremediation: A review. Electron. J. Biotechnol. 2016, 19, 5. [Google Scholar] [CrossRef]
- Trevan, M.D.; Zdarta, J.; Krajewska, B. Enzyme immobilization by adsorption: A review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef]
- Armanu, G.E.; Secula, M.S.; Cimpoesu, C.; Heipieper, H.J.; Volf, I. A biobased nano/micro-structured material for microorganisms’ immobilization. In Proceedings of the 24th International Multidisciplinary Scientific GeoConference SGEM, Albena, Bulgaria, 29 June–8 July 2024; p. 24. [Google Scholar]
- Cassidy, M.B.; Lee, H.; Trevors, J.T. Immobilized microbial cells: A review. J. Ind. Microbiol. Biotechnol. 1996, 16, 79–101. [Google Scholar] [CrossRef]
- Raj, D.S.; Kumar, G.; Vickram, A.S.; Rani, S.R.; Dong, C.D.; Rohini, K.; Anbarasu, K.; Thanigaivel, S.; Ponnusamy, V.K. Efficiency of various biofilm carriers and microbial interactions with substrate in moving bed-biofilm reactor for environmental wastewater treatment. Bioresour. Technol. 2022, 359, 127421. [Google Scholar] [CrossRef]
- Ławniczak, Ł.; Kaczorek, E.; Olszanowski, A. The influence of cell immobilization by biofilm forming on the biodegradation capabilities of bacterial consortia. World J. Microbiol. Biotechnol. 2011, 27, 1183–1188. [Google Scholar] [CrossRef]
- Mandeep, D.; Shukla, P. Microbial Nanotechnology for Bioremediation of Industrial Wastewater. Front. Microbiol. 2020, 11, 590631. [Google Scholar] [CrossRef]
- Hou, L.; Hu, K.; Huang, F.; Pan, Z.; Jia, X.; Liu, W.; Yao, X.; Yang, Z.; Tang, P.; Li, J. Advances in immobilized microbial technology and its application to wastewater treatment: A review. Bioresour. Technol. 2024, 413, 131518. [Google Scholar] [CrossRef]
- Sharma, I. Bioremediation Techniques for Polluted Environment: Concept, Advantages, Limitations, and Prospects. In Trace Metals in the Environment; IntechOpen: London, UK, 2020; p. 12. [Google Scholar] [CrossRef]
- Lago, A.; Rocha, V.; Barros, O.; Silva, B.; Tavares, T. Bacterial biofilm attachment to sustainable carriers as a clean-up strategy for wastewater treatment: A review. J. Water Process Eng. 2024, 63, 105368. [Google Scholar] [CrossRef]
- Al-Amshawee, S.; Yunus, M.B.Y.M. Geometry of biofilm carriers: A systematic review deciding the best shape and pore size. Groundw. Sustain. Dev. 2021, 12, 100520. [Google Scholar] [CrossRef]
- Winnicki, T.; Szetela, R.; Wisniewski, J. Immobilized microorganisms in wastewater treatment. Stud. Environ. Sci. 1982, 19, 341–352. [Google Scholar] [CrossRef]
- Gryta, A.; Skic, K.; Adamczuk, A.; Skic, A.; Marciniak, M.; Józefaciuk, G.; Boguta, P. The Importance of the Targeted Design of Biochar Physicochemical Properties in Microbial Inoculation for Improved Agricultural Productivity—A Review. Agriculture 2024, 14, 37. [Google Scholar] [CrossRef]
- Armanu, E.-G.; Secula, M.S.; Tofanica, B.-M.; Volf, I. The Impact of Biomass Composition Variability on the Char Features and Yields Resulted through Thermochemical Processes. Polymers 2024, 16, 2334. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, K.M.; Sar, T.; Gowd, C.S.; Rajendran, K.; Kumar, V.; Sarsaiya, S.; Li, Y.; Sindhu, R.; Binod, P.; Zhang, Z.; et al. A comprehensive review on thermochemical, and biochemical conversion methods of lignocellulosic biomass into valuable end product. Fuel 2023, 342, 127790. [Google Scholar] [CrossRef]
- White, R.; Budarin, V.; Luque, R.; Clark, J.; Macquarrie, D. Tuneable porous carbonaceous materials from renewable resources. Chem. Soc. Rev. 2009, 38, 3401–3418. [Google Scholar] [CrossRef] [PubMed]
- Harpreet, S.K.; Animesh, D. A comparative review of biochar and hydrochar in terms of production, physic chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, Z.; Zhang, G.; Qin, L.; Fang, J. Application progress of microbial immobilization technology based on biomass materials. BioResources 2021, 16, 8509–8524. [Google Scholar] [CrossRef]
- Zhu, H.; An, Q.; Nasir, A.S.M.; Babin, A.; Saucedo, S.L.; Vallenas, A.; Li, L.; Baldwin, S.A.; Lau, A.; Bi, X. Emerging applications of biochar: A review on techno-environmental-economic aspects. Bioresour. Technol. 2023, 388, 129745. [Google Scholar] [CrossRef]
- Gong, Y.Z.; Niu, Q.Y.; Liu, G.Y.; Dong, J.; Xia, M.M. Development of multifarious carrier materials and impact conditions of immobilised microbial technology for environmental remediation: A review. Environ. Pollut. 2022, 314, 120232. [Google Scholar] [CrossRef] [PubMed]
- Chioti, A.G.; Tsioni, V.; Patsatzis, S.; Filidou, E.; Banti, D.; Samaras, P.; Economou, E.A.; Kostopoulou, E.; Sfetsas, T. Characterization of Biofilm Microbiome Formation Developed on Novel 3D-Printed Zeolite Biocarriers during Aerobic and Anaerobic Digestion Processes. Fermentation 2022, 8, 746. [Google Scholar] [CrossRef]
- Bhaskar, S.; Hossain, K.M.A.; Lachemi, M.; Wolfaardt, G.; Kroukamp, O. Effect of self-healing on strength and durability of zeolite-immobilized bacterial cementitious mortar composites. Cem. Concr. Compos. 2017, 82, 23–33. [Google Scholar] [CrossRef]
- Asghar, N.; Hussain, A.; Nguyen, D.A.; Ali, S.; Hussain, I.; Junejo, A.; Ali, A. Advancement in nanomaterials for environmental pollutants remediation: A systematic review on bibliometrics analysis, material types, synthesis pathways, and related mechanisms. J. Nanobiotechnol. 2024, 22, 26. [Google Scholar] [CrossRef]
- Yunoki, A.; Tsuchiya, E.; Fukui, Y.; Fujii, A.; Maruyama, T. Preparation of inorganic/organic polymer hybrid microcapsules with high encapsulation efficiency by an electrospray technique. ACS Appl. Mater. Interfaces 2014, 6, 11973–11979. [Google Scholar] [CrossRef] [PubMed]
- Kourkoutas, Y.; Bekatorou, A.; Banat, I.M.; Marchant, R.; Koutinas, A.A. Immobilization technologies and support materials suitable in alcohol beverages production: A review. Food Microbiol. 2004, 21, 377–397. [Google Scholar] [CrossRef]
- Leilei, Z.; Mingxin, H.; Suiyi, Z. Biodegradation of p-nitrophenol by immobilized Rhodococcus sp. strain Y-1. Chem. Biochem. Eng. Q. 2012, 26, 137–144. [Google Scholar]
- Dan, S.U.; Pei-jun, L.I.; Stagnitti, F.; Xiong, X.-Z. Biodegradation of benzo[a]pyrene in soil by Mucor sp. SF06 and Bacillus sp. SB02 co-immobilized on vermiculite. J. Environ. Sci. 2006, 18, 1204–1209. [Google Scholar] [CrossRef]
- Gentili, A.R.; Cubitto, M.A.; Ferrero, M.; Rodriguéz, M.S. Bioremediation of crude oil polluted seawater by a hydrocarbon-degrading bacterial strain immobilized on chitin and chitosan flakes. Int. Biodeterior. Biodegrad. 2006, 57, 222–228. [Google Scholar] [CrossRef]
- Paliwal, R.; Uniyal, S.; Rai, J.P.N. Evaluating the potential of immobilized bacterial consortium for black liquor biodegradation. Environ. Sci. Pollut. Res. 2015, 22, 6842–6853. [Google Scholar] [CrossRef] [PubMed]
- Pongkua, W.; Dolphen, R.; Thiravetyan, P. Bioremediation of gaseous methyl tert-butyl ether by combination of sulfuric acid modified bagasse activated carbon-bone biochar beads and Acinetobacter indicus screened from petroleum contaminated soil. Chemosphere 2020, 239, 124724. [Google Scholar] [CrossRef]
- Liu, D.; Yang, X.; Zhang, L.; Tang, Y.; He, H.; Liang, M.; Tu, Z.; Zhu, H. Immobilization of Biomass Materials for Removal of Refractory Organic Pollutants from Wastewater. Int. J. Environ. Res. Public Health 2022, 19, 13830. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Nie, M.; Diwu, Z.; Wang, L.; Yan, H.; Bai, X. Immobilization of Rhodococcus qingshengii strain FF on the surface of polyethylene and its adsorption and biodegradation of mimic produced water. J. Hazard. Mater. 2021, 403, 124075. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, Y.; Singh, B.G.; Mathur, A.; Srivastava, S.; Gupta, S.; Gupta, N. Biodegradation of carbazole by Pseudomonas sp. GBS.5 immobilized in polyvinyl alcohol beads. J. Biochem. Tech. 2015, 6, 1003–1007. [Google Scholar]
- Del Prado-Audelo, M.L.; García Kerdan, I.; Escutia-Guadarrama, L.; Reyna-González, J.M.; Magaña, J.J.; Leyva-Gómez, G. Nanoremediation: Nanomaterials and Nanotechnologies for Environmental Cleanup. Front. Environ. Sci. 2021, 9, 793765. [Google Scholar] [CrossRef]
- Chaudhary, P.; Ahamad, L.; Chaudhary, A.; Kumar, G.; Chen, W.J.; Chen, S. Nanoparticle-mediated bioremediation as a powerful weapon in the removal of environmental pollutants. J. Environ. Chem. Eng. 2023, 11, 109591. [Google Scholar] [CrossRef]
- Hou, J.; Liu, F.; Wu, N.; Ju, J.; Yu, B. Efficient biodegradation of chlorophenols in aqueous phase by magnetically immobilized aniline-degrading Rhodococcus rhodochrous strain. J. Nanobiotechnol. 2016, 14, 5. [Google Scholar] [CrossRef]
- Liu, J.; Morales-Narváez, E.; Vicent, T.; Merkoçi, A.; Zhong, H.G. Microorganism-decorated nanocellulose for efficient diuron removal. Chem. Eng. J. 2018, 354, 1083–1091. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Wang, S.; Leng, S. Effective removal of chlortetracycline and treatment of simulated sewage by Bacillus cereus LZ01 immobilized on erding medicine residues biochar. Biomass Convers. Biorefin. 2022, 14, 2281–2291. [Google Scholar] [CrossRef]
- Lin, H.; Chen, Z.; Megharaj, M.; Naidu, R. Biodegradation of TNT using Bacillus mycoides immobilized in PVA–sodium alginate–kaolin. Appl. Clay Sci. 2013, 83–84, 336–342. [Google Scholar] [CrossRef]
- Deng, F.; Liao, C.; Yang, C.; Guo, C.; Dangm, Z. Enhanced biodegradation of pyrene by immobilized bacteria on modified biomass materials. Int. Biodeterior. Biodegrad. 2016, 110, 46–52. [Google Scholar] [CrossRef]
- Xiong, B.; Zhang, Y.; Hou, Y.; Arp, H.P.H.; Reid, B.J.; Cai, C. Enhanced biodegradation of PAHs in historically contaminated soil by M. gilvum inoculated biochar. Chemosphere 2017, 40, 2. [Google Scholar] [CrossRef] [PubMed]
- Ionata, E.; de Blasio, P.; La Cara, F. Microbiological degradation of pentane by immobilized cells of Arthrobacter sp. Biodegradation 2005, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cubitto, M.A.; Gentili, A.R. Bioremediation of Crude Oil–Contaminated Soil By Immobilized Bacteria on an Agroindustrial Waste—Sunflower Seed Husks. Bioremediat. J. 2015, 19, 277–286. [Google Scholar] [CrossRef]
- Sun, P.; Wei, J.; Gao, Y.; Zhu, Z.; Huang, X. Biochar/Clay Composite Particle Immobilized Compound Bacteria: Preparation, Collaborative Degradation Performance and Environmental Tolerance. Water 2023, 15, 2959. [Google Scholar] [CrossRef]
- O’Reilly, K.T.; Crawford, R.L. Degradation of pentachlorophenol by polyurethane-immobilized Flavobacterium cells. Appl. Environ. Microbiol. 1989, 55, 2113–2118. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, Y.; Han, B.; Zhao, H.B.; Bi, J.N.; Cai, B.L. Biodegradation of phenol by free and immobilized Acinetobacter sp. strain PD12. J. Environ. Sci. 2007, 19, 222–225. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, H.; Wu, M. Pseudomonas sp. LZ-Q continuously degrades phenanthrene under hypersaline and hyperalkaline condition in a membrane bioreactor system. Biophys. Rep. 2015, 1, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Parameswarappa, S.; Karigar, C.; Nagenahalli, M. Degradation of ethylbenzene by free and immobilized Pseudomonas fluorescens-CS2. Biodegradation 2008, 19, 137–144. [Google Scholar] [CrossRef]
- Jenjaiwit, S.; Supanchaiyamat, N.; Hunt, J.A.; Ngernyen, Y.; Ratpukdi, T.; Siripattanakul-Ratpukdi, S. Removal of triclocarban from treated wastewater using cell-immobilized biochar as a sustainable water treatment technology. J. Clean. Prod. 2021, 320, 128919. [Google Scholar] [CrossRef]
- Lou, L.; Huangm, Q.; Lou, Y.; Lu, J.; Hu, B.; Lin, Q. Adsorption and degradation in the removal of nonylphenol from water by cells immobilized on biochar. Chemosphere 2019, 228, 676–684. [Google Scholar] [CrossRef]
- Nunal, S.N.; Santander-de Leon, S.M.S.; Bacolod, E.; Koyama, J.; Uno, S.; Hidaka, M.; Yoshikawa, T.; Maeda, H. Bioremediation of Heavily Oil-Polluted Seawater by a Bacterial Consortium Immobilized in Cocopeat and Rice Hull Powder. Biocontrol Sci. 2014, 19, 11–22. [Google Scholar] [CrossRef] [PubMed]


| Type of Immobilization | Costs | Benefits | Potential Problems | References |
|---|---|---|---|---|
| Entrapment | Low costs due to the manufacturing process and materials | Prevents leaking of cells into the environment; diffusion of pollutants and various metabolic products is facilitated by the porous structure of the matrix; protective barrier against pollutants | Leaking effects when the pores are larger than the immobilized cells; requires high costs of maintenance; limits the exchange of nutrients with the exterior environment | [120,122] |
| Encapsulation | Moderate costs, depends on the materials and processes used | Prevents biocatalyst leakage; has a long-term effectiveness; can be tailored for specific pollutants | High risks of leaching in case of improper encapsulation of polymeric gels or other materials | [123,124] |
| Covalent binding | High costs due to the materials and the applied processes | Durable solution; strong covalent bonds that prevent the leaking of molecules into the environment | Can often be toxic and effect cell viability or enzyme activity | [125,126] |
| Adsorption | Low costs due to cost of materials and processes applied | Absorbents can be regenerated and reused; does not require chemical additives | High risk of leakage from the matrix due to weak binding forces and unstable interactions | [127,128] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armanu, E.G.; Bertoldi, S.; Chrzanowski, Ł.; Volf, I.; Heipieper, H.J.; Eberlein, C. Benefits of Immobilized Bacteria in Bioremediation of Sites Contaminated with Toxic Organic Compounds. Microorganisms 2025, 13, 155. https://doi.org/10.3390/microorganisms13010155
Armanu EG, Bertoldi S, Chrzanowski Ł, Volf I, Heipieper HJ, Eberlein C. Benefits of Immobilized Bacteria in Bioremediation of Sites Contaminated with Toxic Organic Compounds. Microorganisms. 2025; 13(1):155. https://doi.org/10.3390/microorganisms13010155
Chicago/Turabian StyleArmanu, Emanuel Gheorghita, Simone Bertoldi, Łukasz Chrzanowski, Irina Volf, Hermann J. Heipieper, and Christian Eberlein. 2025. "Benefits of Immobilized Bacteria in Bioremediation of Sites Contaminated with Toxic Organic Compounds" Microorganisms 13, no. 1: 155. https://doi.org/10.3390/microorganisms13010155
APA StyleArmanu, E. G., Bertoldi, S., Chrzanowski, Ł., Volf, I., Heipieper, H. J., & Eberlein, C. (2025). Benefits of Immobilized Bacteria in Bioremediation of Sites Contaminated with Toxic Organic Compounds. Microorganisms, 13(1), 155. https://doi.org/10.3390/microorganisms13010155










