Animal Brucellosis in Egypt: Review on Evolution, Epidemiological Situation, Prevalent Brucella Strains, Genetic Diversity, and Assessment of Implemented National Control Measures
Abstract
:1. Introduction
2. Seroprevalence of Brucellosis in Egypt
2.1. Brucellosis Frequency and Control Between 2000 and 2010
2.1.1. Governmental Estimates
2.1.2. Research Estimates
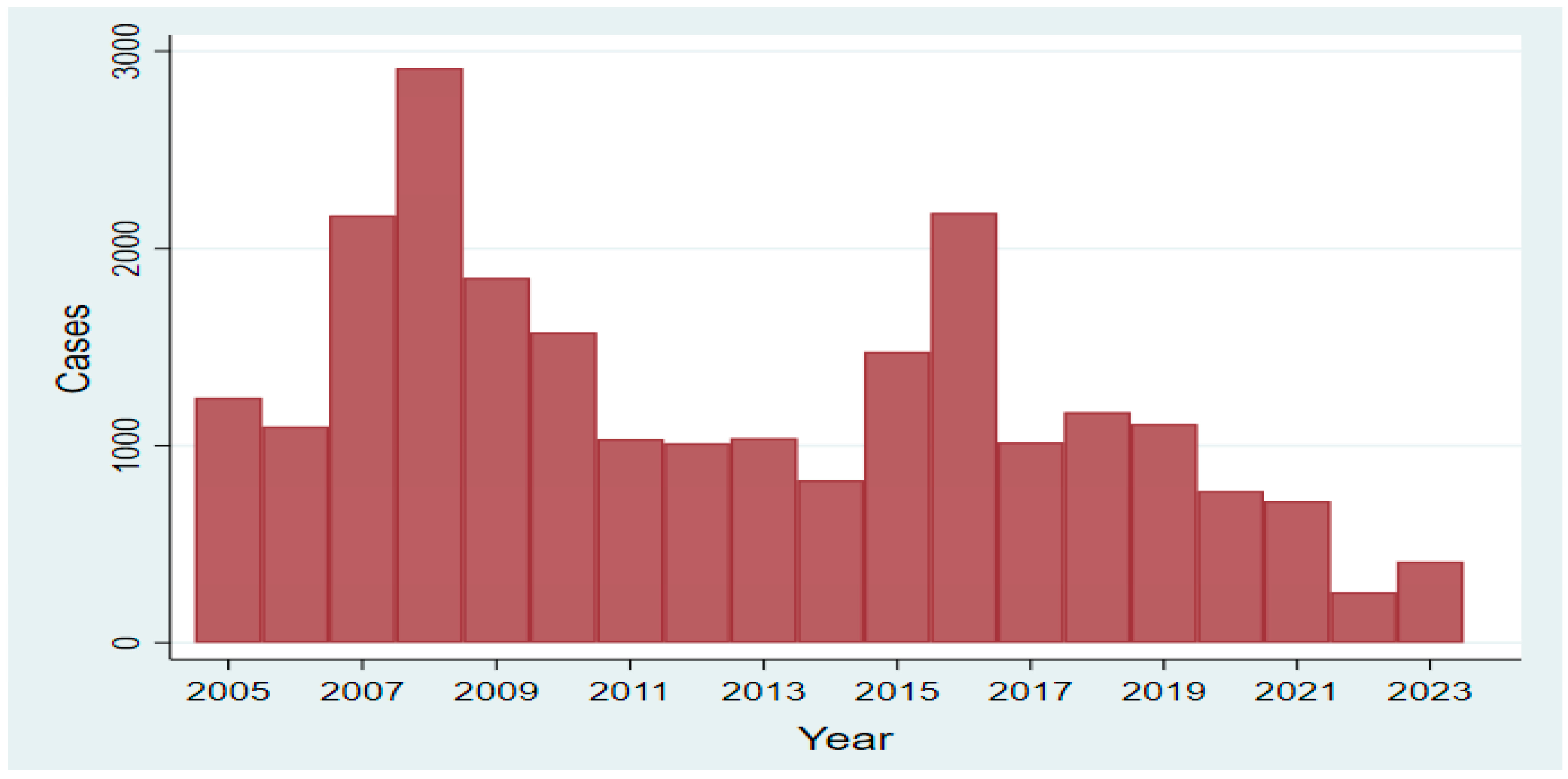
2.2. Brucellosis Frequency and Control Between 2011 and 2023
2.2.1. Governmental Estimates
2.2.2. Research Estimates
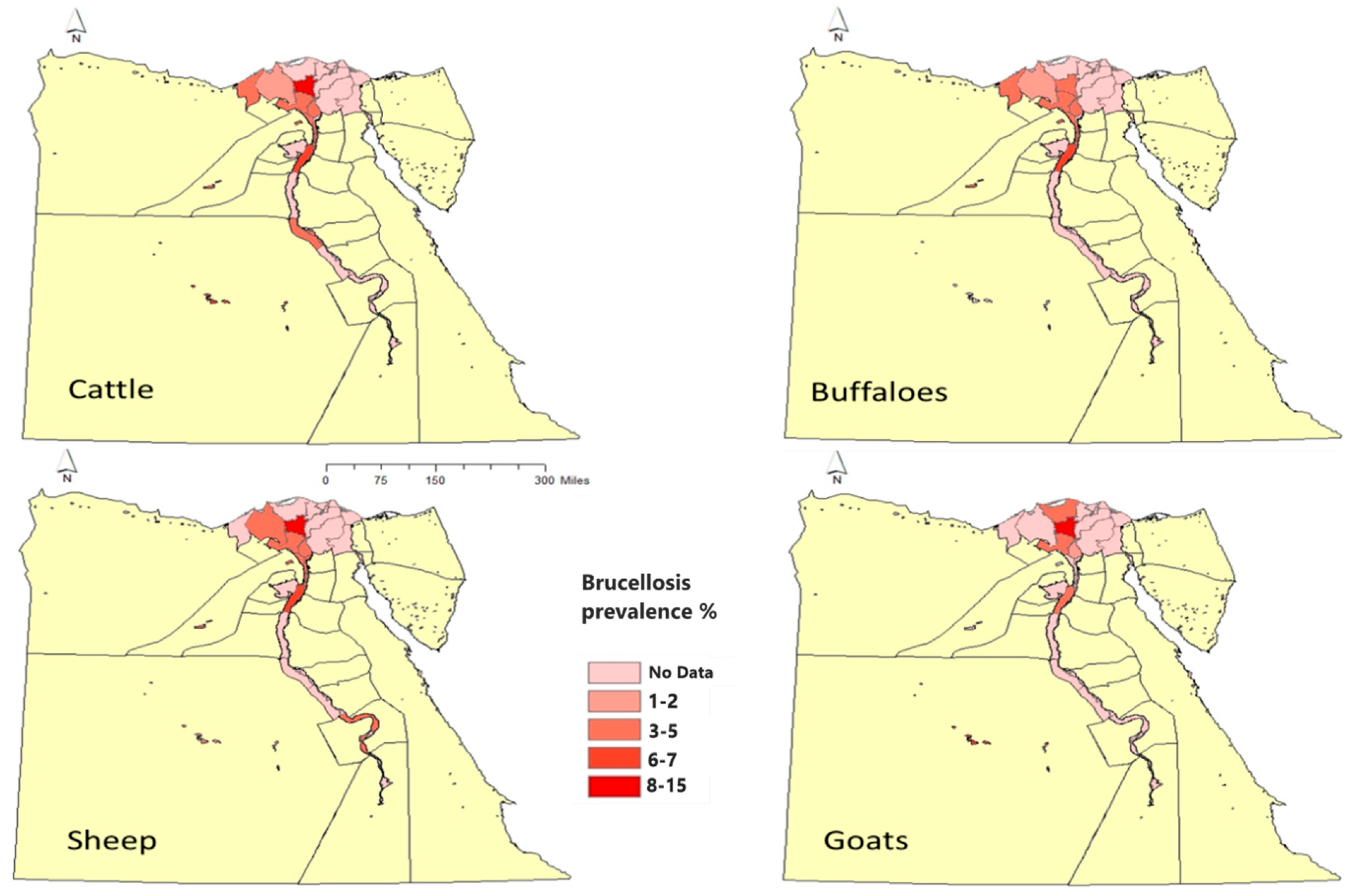
Cattle and Buffaloes
Sheep and Goats
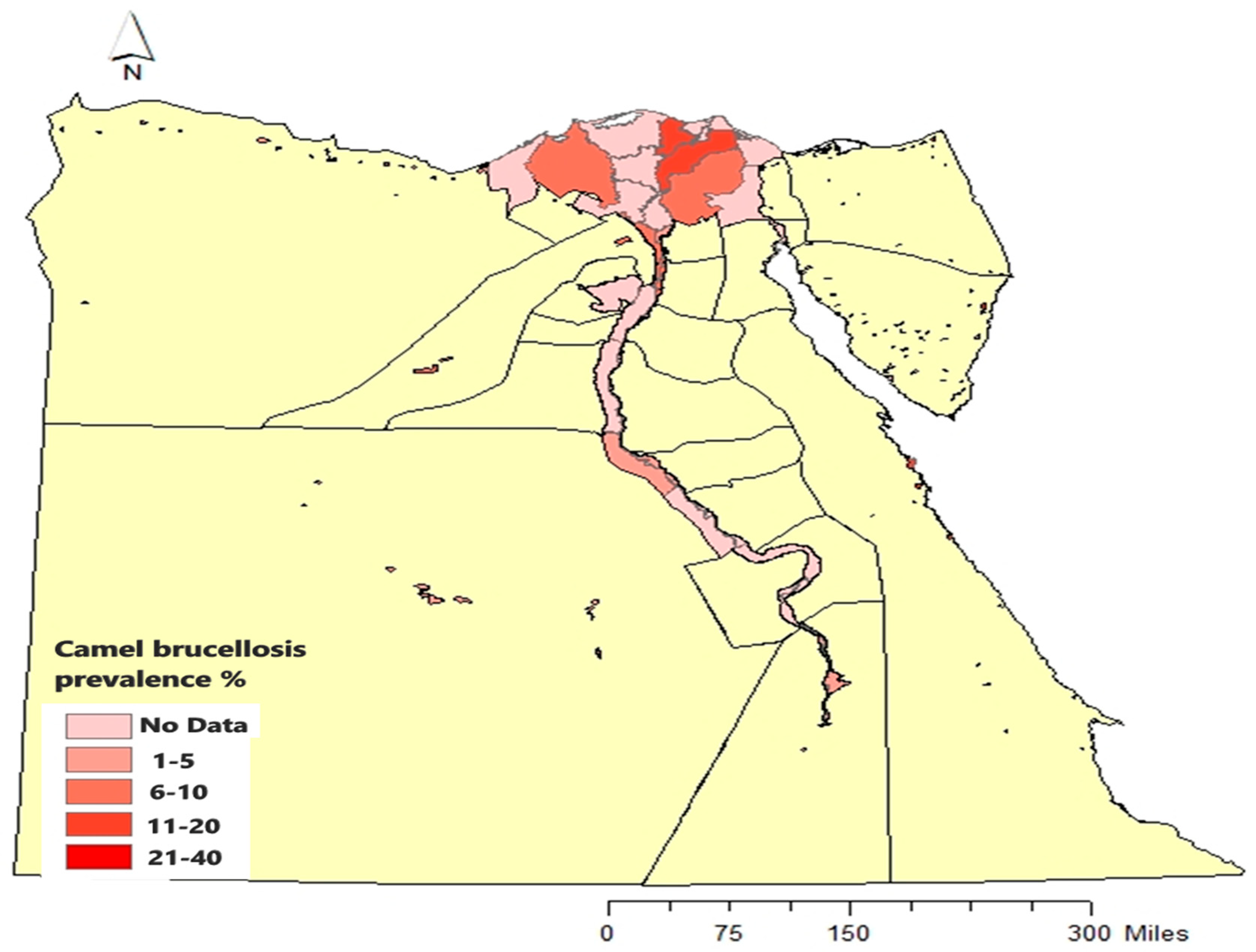
Camels
3. Causes of National Control Program Unsuccessfulness to Control Brucellosis in Egypt
3.1. Inappropriateness of the Planned Control Measures for Brucellosis Control in Egypt
3.2. Risk Practices by the Farmers (Shepherds) and Also Veterinarians
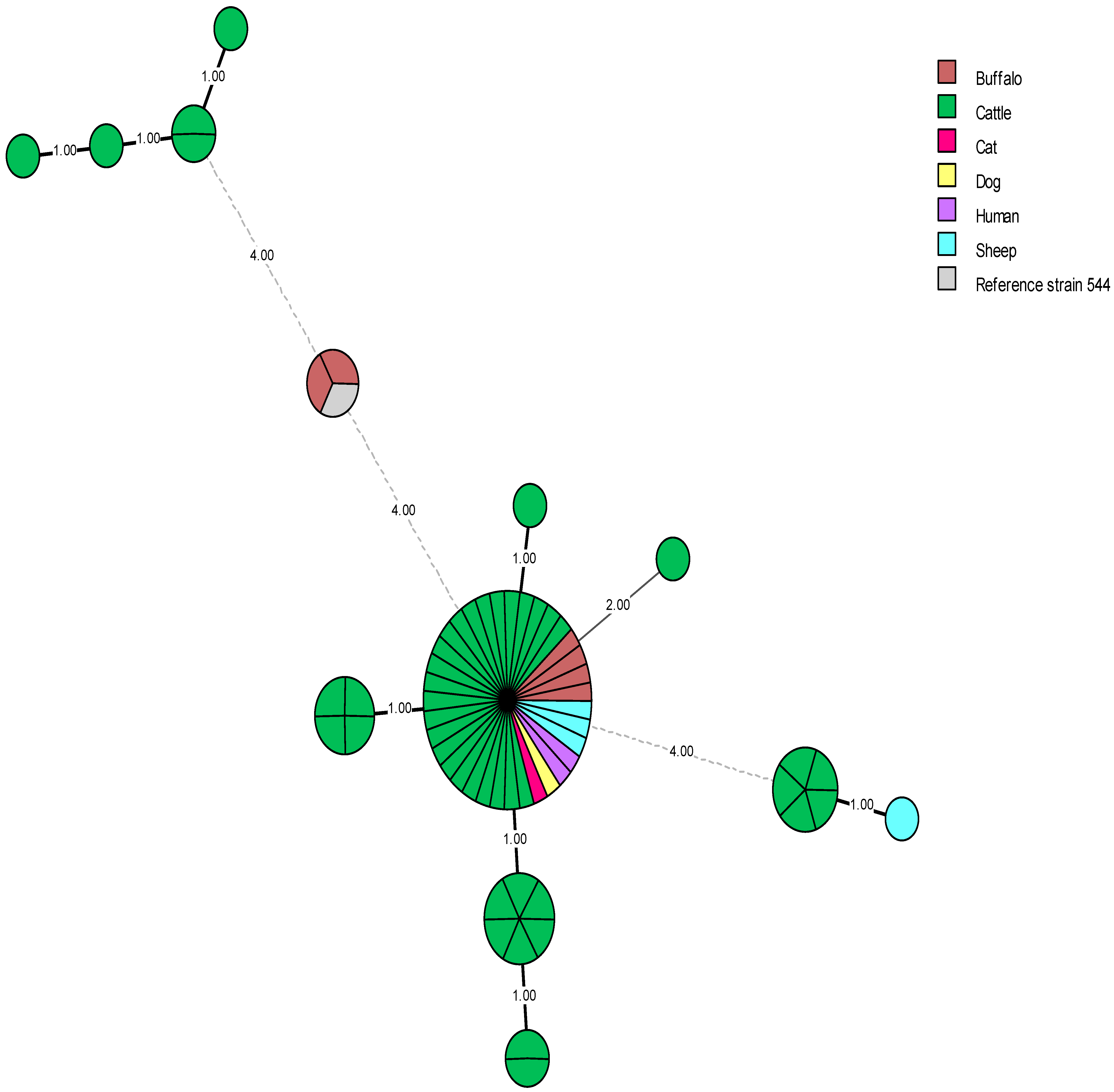
3.3. The Genetic Diversity and Strain Relatedness of B. melitensis in Egypt Under Various Husbandry Systems in Different Animal Species
3.4. Biological Causes
4. Prevalent Brucella Strains in Egypt
Bacteriological Identification and Biotyping
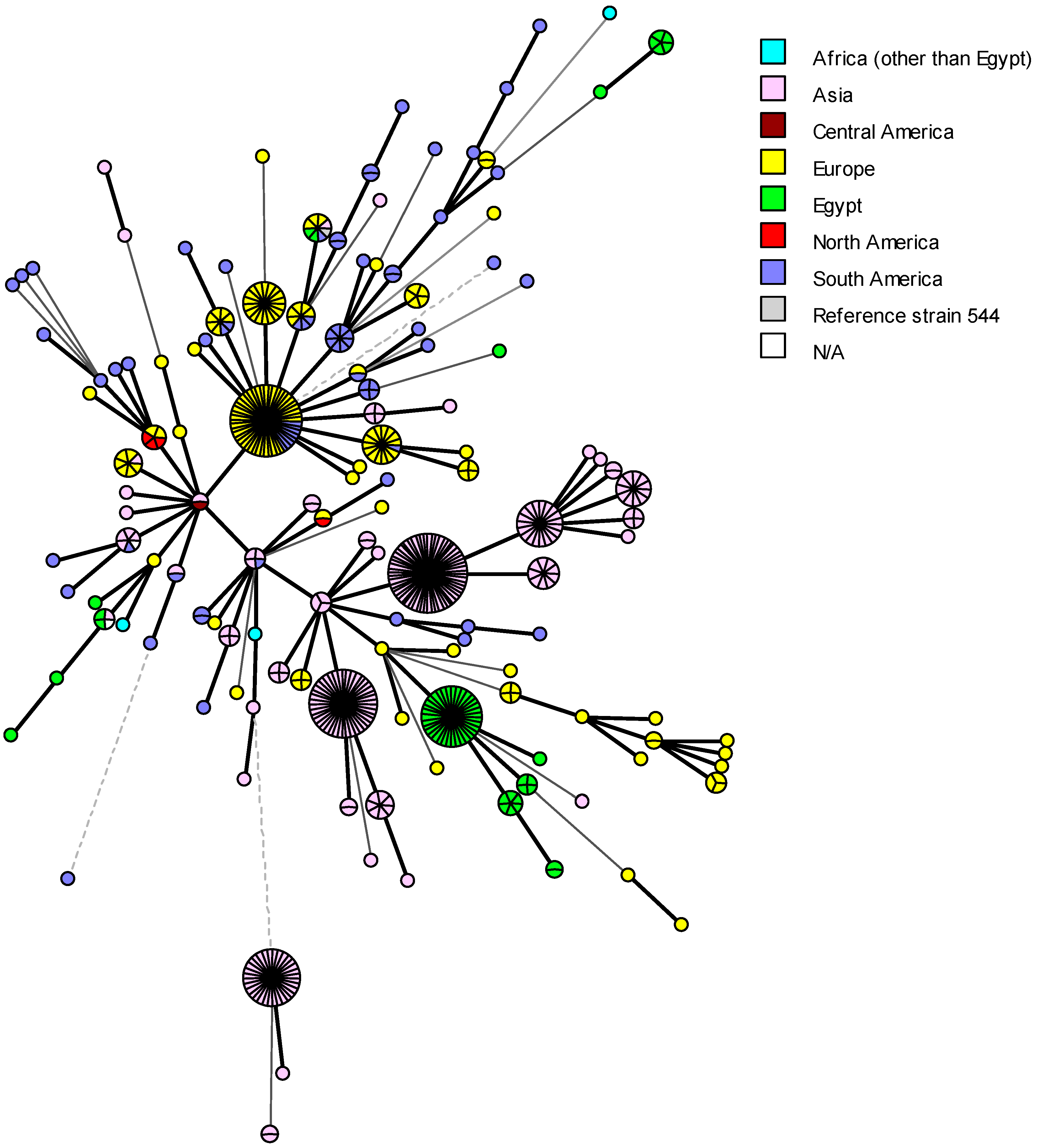
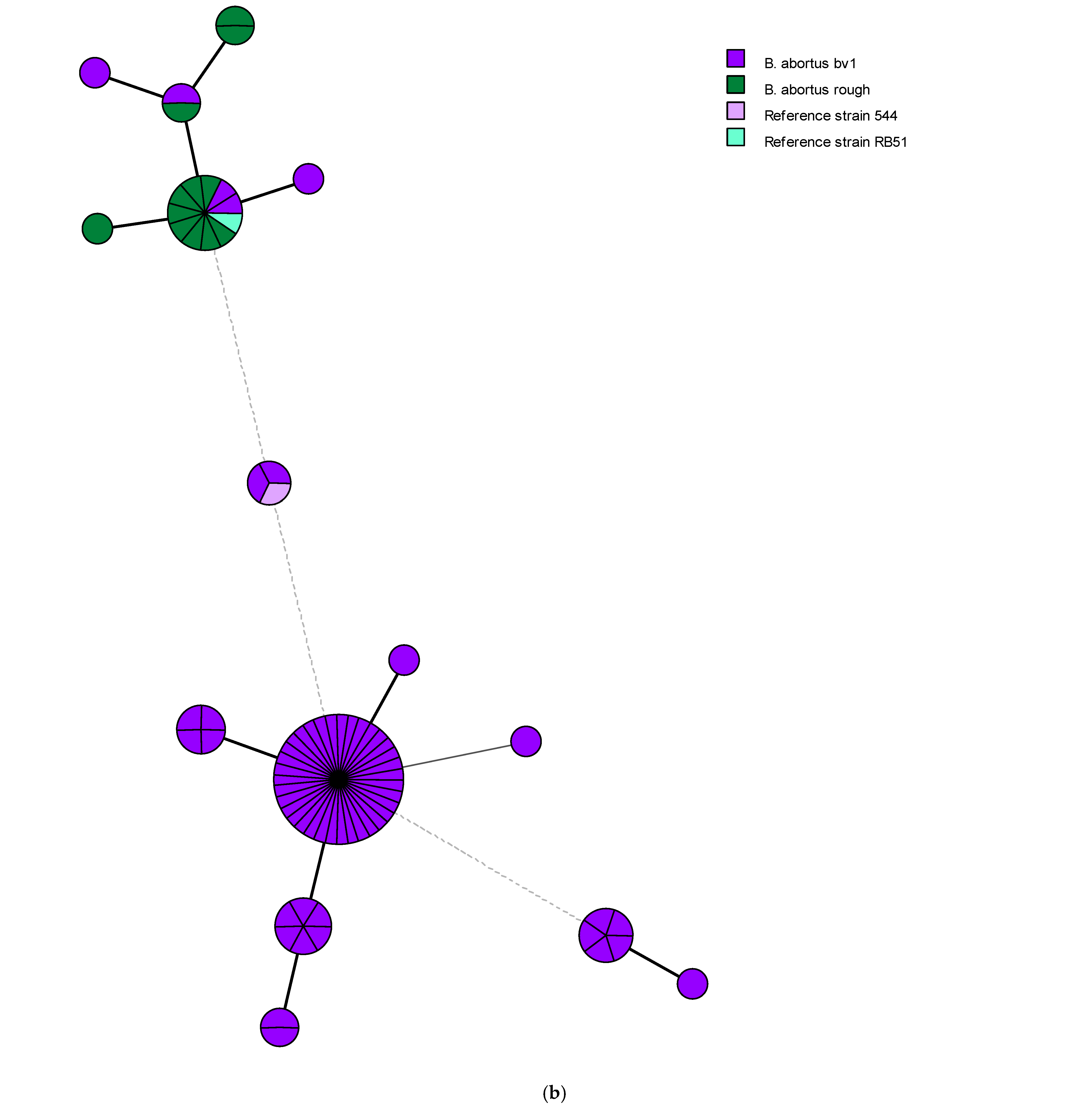
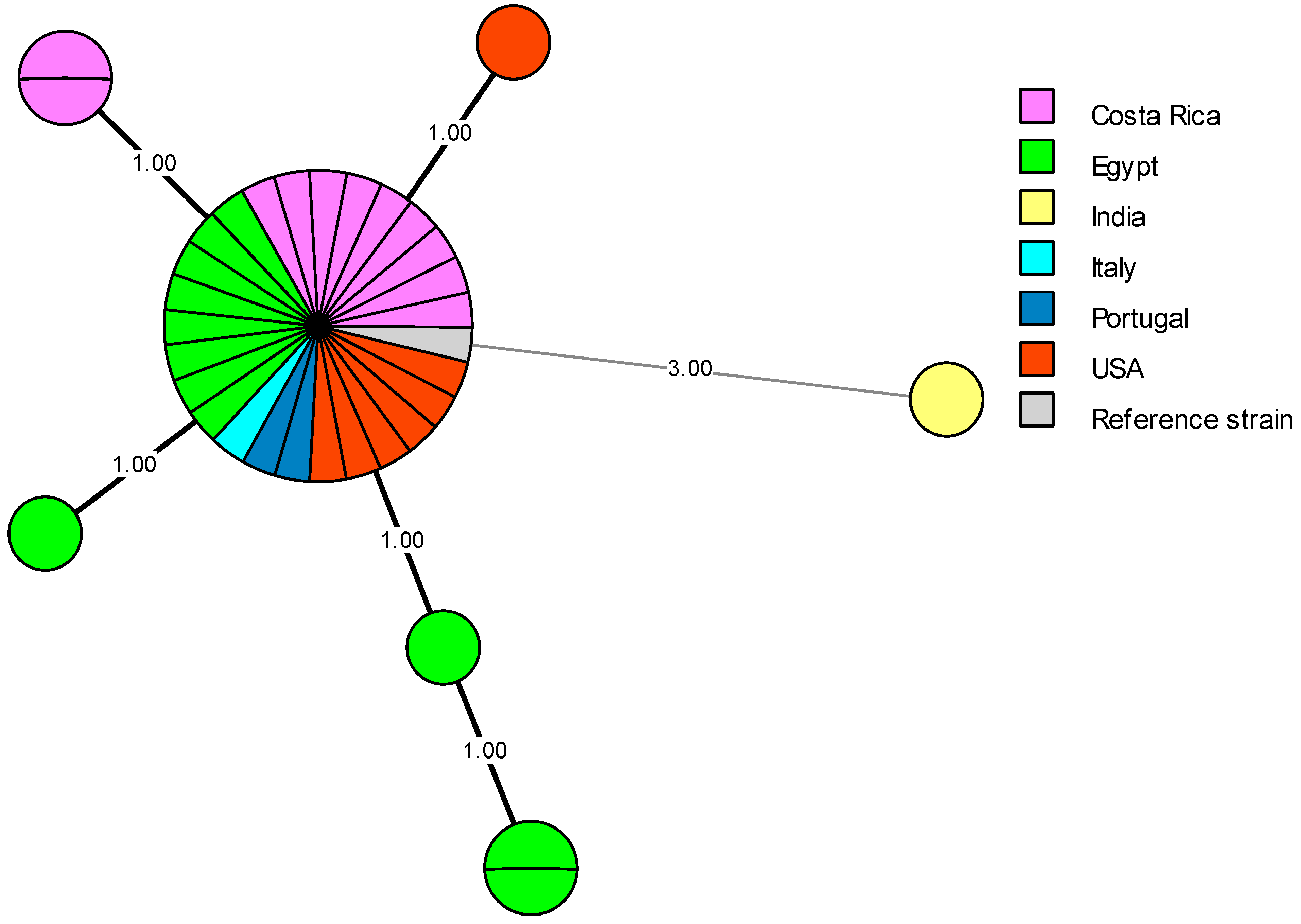
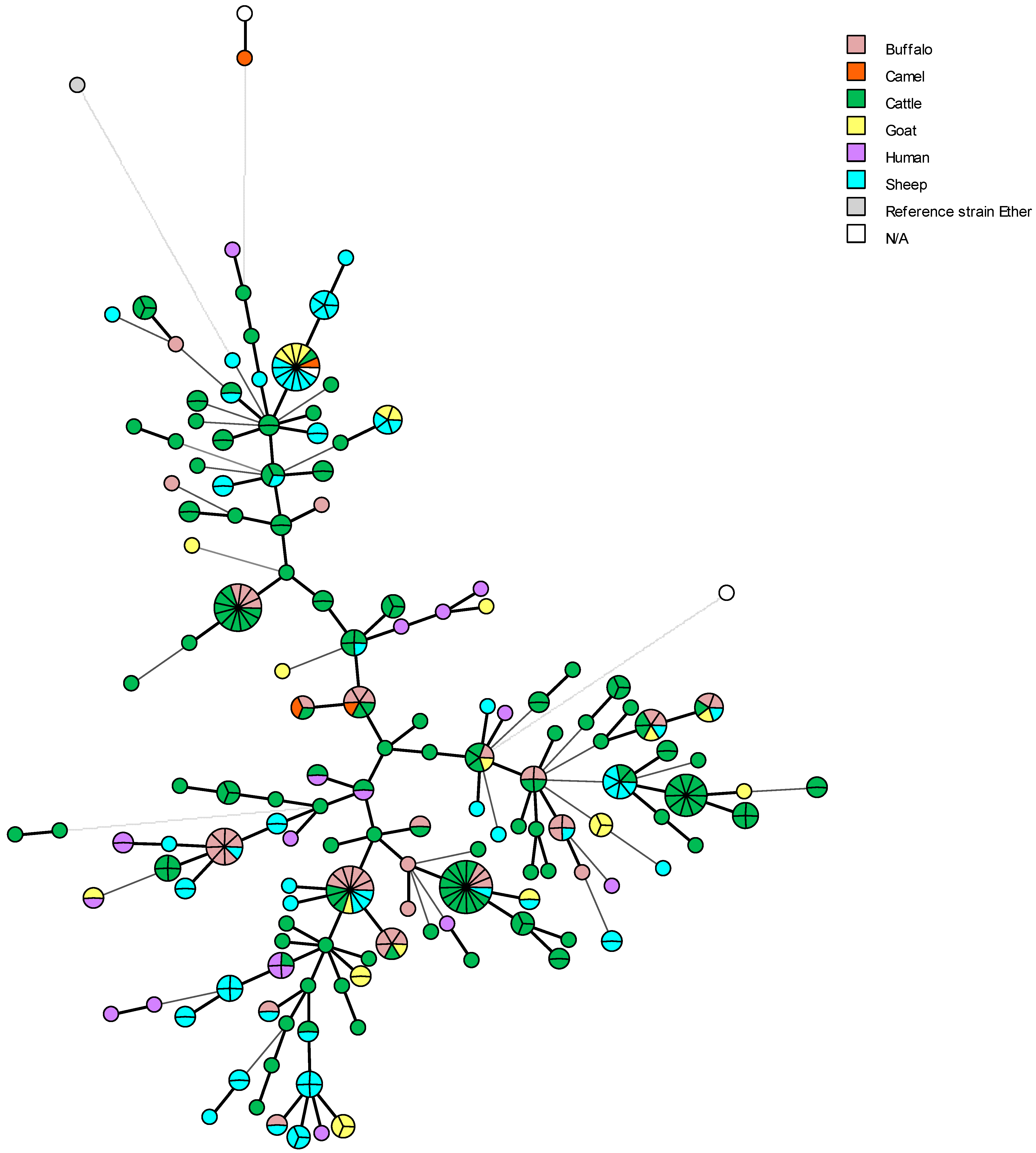
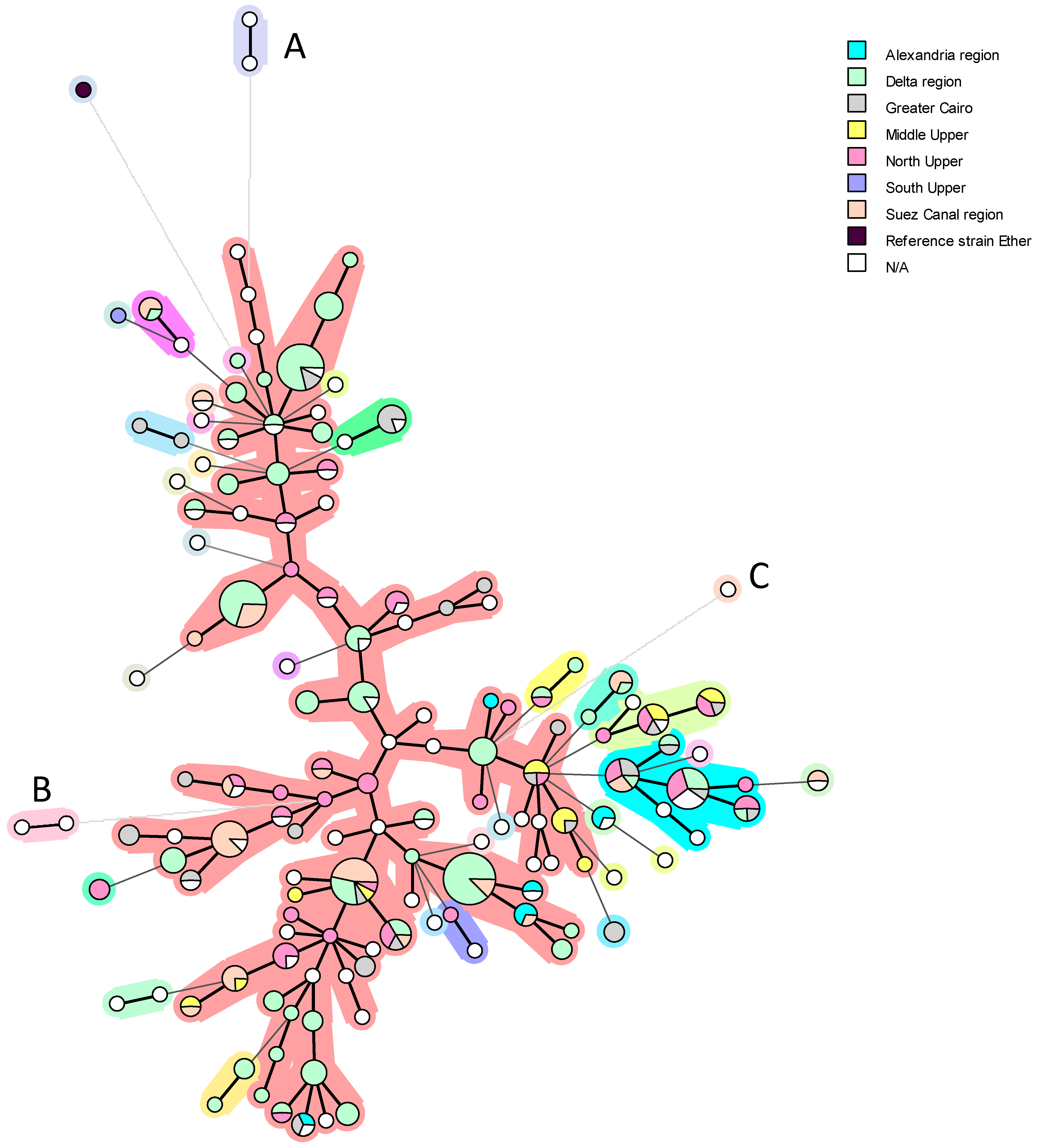
5. Genetic Diversity and Epidemiological Traceability of Local Egyptian Brucella Strains Using MLVA-16 and WGS
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WOAH. Brucellosis (infection with Brucella abortus, B. melitensis and B. suis). In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; World Health Organization for Animal Health: Paris, France, 2022; Chapter 3.1.4; pp. 1–48. [Google Scholar]
- Constable, P.D.; Hinchcliff, K.W.; Done, S.H.; Grünberg, W. Infectious diseases primarily affecting the reproductive system. In Veterinary Medicine: A Text Book of the Diseases of Cattle, Horses, Sheep, Pigs, and Goats, 11th ed.; Constable, P.D., Hinchcliff, K.W., Done, S.H., Grünberg, W., Eds.; Elsevier: St. Louis, MO, USA, 2017; pp. 1761–1784. [Google Scholar]
- Hull, N.C.; Schumaker, B.A. Comparisons of brucellosis between human and veterinary medicine. Infect. Ecol. Epidemiol. 2018, 8, 1500846. [Google Scholar] [CrossRef] [PubMed]
- Laine, C.G.; Johnson, V.E.; Scott, H.M.; Arenas-Gamboa, A.M. Global Estimate of Human Brucellosis Incidence. Emerg. Infect. Dis. 2023, 29, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Whatmore, A.M.; Davison, N.; Cloeckaert, A.; Al Dahouk, S.; Zygmunt, M.S.; Brew, S.D.; Perrett, L.L.; Koylass, M.S.; Vergnaud, G.; Quance, C.; et al. Brucella papionis sp. nov., isolated from baboons (Papio spp.). Int. J. Syst. Evol. Microbiol. 2014, 64 Pt 12, 4120–4128. [Google Scholar] [CrossRef]
- Scholz, H.C.; Revilla-Fernández, S.; Dahouk, S.A.; Hammerl, J.A.; Zygmunt, M.S.; Cloeckaert, A.; Koylass, M.; Whatmore, A.M.; Blom, J.; Vergnaud, G.; et al. Brucella vulpis sp. nov., isolated from mandibular lymph nodes of red foxes (Vulpes vulpes). Int. J. Syst. Evol. Microbiol. 2016, 66, 2090–2098. [Google Scholar] [CrossRef]
- Sayour, A.E.; Elbauomy, E.; Abdel-Hamid, N.H.; Mahrous, A.; Carychao, D.; Cooley, M.B.; Elhadidy, M. MLVA fingerprinting of Brucella melitensis circulating among livestock and cases of sporadic human illness in Egypt. Transbound. Emerg. Dis. 2020, 67, 2435–2445. [Google Scholar] [CrossRef] [PubMed]
- Elmonir, W.; Abdel-Hamid, N.H.; Hamdy, M.E.R.; Beleta, E.I.M.; El-Diasty, M.; Melzer, F.; Wareth, G.; Neubauer, H. Isolation and molecular confirmation of Brucella suis biovar 2 from slaughtered pigs: An unanticipated biovar from domestic pigs in Egypt. BMC Vet. Res. 2022, 18, 224. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, M.E.R.; Abdel-Haleem, M.H.; Dawod, R.E.; Ismail, R.I.; Hazem, S.S.; Fahmy, H.A.; Abdel-Hamid, N.H. First seroprevalence and molecular identification report of Brucella canis among dogs in Greater Cairo region and Damietta Governorate of Egypt. Vet. World 2023, 16, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, N.H.; El-bauomy, E.M.; Ghobashy, H.M.; Shehata, A.A. Genetic variation of Brucella isolates at strain level in Egypt. Vet. Med. Sci. 2020, 6, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Pappas, G. The changing Brucella ecology: Novel reservoirs, new threats. Int. J. Antimicrob. Agents 2010, 36, S8–S11. [Google Scholar] [CrossRef]
- Hegazy, Y.M.; Abdel-Hamid, N.H.; Eldehiey, M.; Oreiby, A.F.; Algabbary, M.H.; Hamdy, M.E.R.; Beleta, E.I.; Martínez, I.; Shahein, M.A.; García, N.; et al. Trans-species transmission of Brucellae among ruminants hampering brucellosis control efforts in Egypt. J. Appl. Microbiol. 2021, 132, 90–100. [Google Scholar] [CrossRef]
- Wareth, G.; Melzer, F.; Tomaso, H.; Roesler, U.; Neubauer, H. Detection of Brucella abortus DNA in aborted goats and sheep in Egypt by real-time PCR. BMC Res. Notes 2015, 8, 212. [Google Scholar] [CrossRef] [PubMed]
- Wareth, G.; El-Diasty, M.; Abdel-Hamid, N.H.; Holzer, K.; Hamdy, M.E.R.; Moustafa, S.; Shahein, M.A.; Melzer, F.; Beyer, W.; Pletz, M.W.; et al. Molecular characterization and antimicrobial susceptibility testing of clinical and non-clinical Brucella melitensis and Brucella abortus isolates from Egypt. One Health 2021, 13, 100255. [Google Scholar] [CrossRef] [PubMed]
- Godfroid, J. Brucella spp. at the Wildlife-Livestock Interface: An Evolutionary Trajectory through a Livestock-to-Wildlife “Host Jump”? Vet. Sci. 2018, 5, 81. [Google Scholar] [CrossRef] [PubMed]
- Refai, M. Incidence and control of brucellosis in the Near East region. Vet. Microbiol. 2002, 90, 81–110. [Google Scholar] [CrossRef] [PubMed]
- Refai, M. Brucellosis in animals and man in Egypt. Egypt. J. Vet. Sci. 2003, 37, 1–31. [Google Scholar]
- Yang, X.; Skyberg, J.A.; Cao, L.; Clapp, B.; Thornburg, T.; Pascual, D.W. Progress in Brucella vaccine development. Front. Biol. 2012, 8, 60–77. [Google Scholar] [CrossRef] [PubMed]
- Ali, A. Self-Declaration by Egypt of Compartment Free from Infection with Brucella with Vaccination. 2021. Available online: https://www.woah.org/app/uploads/2022/03/2022-03-egypt-compartment-free-infection-brucella-with-vaccination-selfd.pdf (accessed on 18 November 2023).
- Wareth, G.; Melzer, F.; Böttcher, D.; El-Diasty, M.; El-Beskawy, M.; Rasheed, N.; Schmoock, G.; Roesler, U.; Sprague, L.D.; Neubauer, H. Molecular typing of isolates obtained from aborted foetuses in Brucella -free Holstein dairy cattle herd after immunisation with Brucella abortus RB51 vaccine in Egypt. Acta Trop. 2016, 164, 267–271. [Google Scholar] [CrossRef]
- Abdel-Hamid, N.H.; Elbauomy, E.; Ghobashy, H.; Sayour, A.; Ismail, R.; Hazem, S.; Abdel-Haleem, M.H. Role of sheep and goat mobile flocks in the transmission of brucellosis to the household ruminants and the disease prevalence in these flocks. Anim. Health Res. J. 2017, 5, 95–105. [Google Scholar]
- Abdel-Hamid, N.; Al-Saftawy, M.; Moawad, A.; Zaki, H.; Abdelhalem, M. Brucellosis Seroprevalence, REP-PCR-Based Genotyping, and Virulence-Associated Genes Distribution Among Brucella melitensis Strains Isolated from Ruminants in Kafr Elsheikh Governorate of Egypt. Egypt. J. Vet. Sci. 2024, 55, 979–990. [Google Scholar] [CrossRef]
- Eltholth, M.; El-Wahab, E.A.; Salem, M.; Abdel-Hamid, N.; El-Diasty, M.; Eldehiey, M.; Badr, Y.; Elsobky, Y.; Ahmed, E.; Zaffan, M.; et al. Prevalence of brucellosis in ruminants and the risk of human exposure in rural delta of Egypt. Egypt. J. Vet. Sci. 2024, 55, 1257–1269. [Google Scholar] [CrossRef]
- Hegazy, Y.; Elmonir, W.; Abdel-Hamid, N.H.; Elbauomy, E.M. Seroprevalence and “Knowledge, Attitudes and Practices” (KAPs) survey of endemic ovine brucellosis in Egypt. Acta Vet. Scand. 2015, 58, 1. [Google Scholar] [CrossRef] [PubMed]
- Wareth, G.; Hikal, A.; Refai, M.; Melzer, F.; Roesler, U.; Neubauer, H. Animal brucellosis in Egypt. J. Infect. Dev. Ctries. 2014, 8, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, Y.M.; Ridler, A.L.; Guitian, F.J. Assessment and simulation of the implementation of brucellosis control programme in an endemic area of the Middle East. Epidemiol. Infect. 2009, 137, 1436–1448. [Google Scholar] [CrossRef] [PubMed]
- Samaha, H.; Al-Rowaily, M.; Khoudair, R.M.; Ashour, H.M. Multicenter study of Brucellosis in Egypt. Emerg. Infect. Dis. 2008, 14, 1916–1918. [Google Scholar] [CrossRef]
- Menshawy, A.M.S.; Perez-Sancho, M.; Garcia-Seco, T.; Hosein, H.I.; García, N.; Martinez, I.; Sayour, A.E.; Goyache, J.; Azzam, R.A.A.; Dominguez, L.; et al. Assessment of Genetic Diversity of Zoonotic Brucella spp. Recovered from Livestock in Egypt Using Multiple Locus VNTR Analysis. BioMed Res. Int. 2014, 2014, 353876. [Google Scholar] [CrossRef]
- Elmonir, W.; Hegazy, Y.; Abdel-Hamid, N.; Elbauomy, E. Brucellosis at the Human-Animal Interface in Kafrelsheikh Governorate, Egypt. Alex. J. Vet. Sci. 2016, 50, 1–7. [Google Scholar] [CrossRef]
- Abdel-Hamid, N.H.; Ghobashy, H.M.; Beleta, E.I.; Elbauomy, E.M.; Ismail, R.I.; Nagati, S.F.; Hassan, S.K.; Elmonir, W. Risk factors and Molecular genotyping of Brucella melitensis strains recovered from humans and their owned cattle in Upper Egypt. One Health 2021, 13, 100281. [Google Scholar] [CrossRef]
- Jennings, G.J.; Hajjeh, R.A.; Girgis, F.Y.; Fadeel, M.A.; Maksoud, M.A.; Wasfy, M.O.; Sayed, N.E.; Srikantiah, P.; Luby, S.P.; Earhart, K.; et al. Brucellosis as a cause of acute febrile illness in Egypt. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 707–713. [Google Scholar] [CrossRef]
- Abdelbaset, A.E.; Abushahba, M.F.N.; Hamed, M.I.; Rawy, M.S. Sero-diagnosis of brucellosis in sheep and humans in Assiut and El-Minya governorates, Egypt. Int. J. Vet. Sci. Med. 2018, 6 (Suppl. S1), S63–S67. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, Y.M.; Moawad, A.; Osman, S.; Ridler, A.; Guitian, J. Ruminant Brucellosis in the Kafr el Sheikh Governorate of the Nile Delta, Egypt: Prevalence of a neglected zoonosis. PLoS Neglected Trop. Dis. 2011, 5, e944. [Google Scholar] [CrossRef] [PubMed]
- Holt, H.R.; Eltholth, M.M.; Hegazy, Y.M.; El-Tras, W.F.; Tayel, A.A.; Guitian, J. Brucella spp. infection in large ruminants in an endemic area of Egypt: Cross-sectional study investigating seroprevalence, risk factors and livestock owner’s knowledge, attitudes and practices (KAPs). BMC Public Health 2011, 11, 341. [Google Scholar] [CrossRef]
- El-Wahab, E.W.A.; Hegazy, Y.M.; El-Tras, W.F.; Mikheal, A.; Kabapy, A.F.; Abdelfatah, M.; Bruce, M.; Eltholth, M.M. A multifaceted risk model of brucellosis at the human–animal interface in Egypt. Transbound. Emerg. Dis. 2019, 66, 2383–2401. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.U.; Shell, W.S.; Melzer, F.; Sayour, A.E.; Ramadan, E.S.; Elschner, M.C.; Moawad, A.A.; Roesler, U.; Neubauer, H.; El-Adawy, H. Identification, Genotyping and Antimicrobial Susceptibility Testing of Brucella spp. Isolated from Livestock in Egypt. Microorganisms 2019, 7, 603. [Google Scholar] [CrossRef]
- El-Tras, W.F.; Tayel, A.A.; Eltholth, M.M.; Guitian, J. Brucella infection in fresh water fish: Evidence for natural infection of Nile catfish, Clarias gariepinus, with Brucella melitensis. Vet. Microbiol. 2009, 141, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Wareth, G.; Melzer, F.; El-Diasty, M.; Schmoock, G.; Elbauomy, E.; Abdel-Hamid, N.; Sayour, A.; Neubauer, H. Isolation of Brucella abortus from a Dog and a Cat Confirms their Biological Role in Re-emergence and Dissemination of Bovine Brucellosis on Dairy Farms. Transbound. Emerg. Dis. 2016, 64, e27–e30. [Google Scholar] [CrossRef] [PubMed]
- Wareth, G.; Abdel-Hamid, N.H.; Hamdy, M.E.R.; Elmonir, W.; Beleta, E.I.M.; El-Diasty, M.; Abdel-Glil, M.Y.; Melzer, F.; Neubauer, H. Whole-genome sequencing (WGS) analysis of Brucella suis biovar 2 isolated from domestic pigs in Egypt for epidemiological and genetic diversity tracing. Vet. Microbiol. 2022, 277, 109637. [Google Scholar] [CrossRef] [PubMed]
- Eltholth, M.M.; Hegazy, Y.M.; El-Tras, W.F.; Bruce, M.; Rushton, J. Temporal analysis and costs of Ruminant Brucellosis control programme in Egypt between 1999 and 2011. Transbound. Emerg. Dis. 2016, 64, 1191–1199. [Google Scholar] [CrossRef]
- Eltholth, M.M.; El-Wahab, A.; Hegazy, E.W.; El-Tras, Y.M. Assessing Impacts and Costs of Brucellosis Control Programme in an Endemic Area of the Nile Delta, Egypt. Worlds Vet. J. 2015, 5, 74–81. [Google Scholar] [CrossRef]
- Sherbini, A.E.; Kabbash, I.; Schelling, E.; Shennawy, S.E.; Shalapy, N.; Elnaby, G.H.; Helmy, A.A.; Eisa, A. Seroprevalences and local variation of human and livestock brucellosis in two villages in Gharbia Governorate, Egypt. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 923–928. [Google Scholar] [CrossRef]
- Kaoud, H.; Zaki, M.; El-Dahshan, A.; Nasr, S. Epidemiology of brucellosis among farm animals. Nat. Sci. 2010, 8, 190–197. [Google Scholar]
- Sayed, A.; Ismail, A.A.; Omran, E. Epidemiological Study on Brucellosis in Animals and Man in New Valley Governorate. Assiut Vet. Med. J. 2010, 56, 1–13. [Google Scholar] [CrossRef]
- Hegazy, Y.M.; Molina-Flores, B.; Shafik, H.; Ridler, A.L.; Guitian, F.J. Ruminant brucellosis in Upper Egypt (2005–2008). Prev. Vet. Med. 2011, 101, 173–181. [Google Scholar] [CrossRef]
- El-Raz, K.A.A.; Desouky, H.M.; Ahmed, W.M. Investigations on Brucellosis in Egyptian Baladi Does with Emphasis on Evaluation of Diagnostic Techniques. Pak. J. Biol. Sci. 2007, 10, 342–348. [Google Scholar] [CrossRef] [PubMed]
- El-Taweel, A.H. Country Reports; Brucellosis Information Workshop: Ramallah, Palestine, 1999. [Google Scholar]
- Abdel-Moghney, A.F. A preliminary study on brucellosis on camels on Behira province. Assiut Univ. Bull. Environ. Res. 2004, 7, 39–43. [Google Scholar] [CrossRef]
- Nell, A.J.; van Vugt, F. BOCI-Egypt: Brucellosis and Tuberculosis Control 21–25 March 2011; Centre for Development Innovation: Wageningen, The Netherlands, 2011; 20p. [Google Scholar]
- Abdallah, O.; Kilany, O.; Saied, S.E. Clinicopathological studies in Brucellosis diseased cows and humans. Suez Canal Vet. Med. J. 2014, 19, 277–288. [Google Scholar] [CrossRef]
- Selim, A.; Gaber, A.; Moustafa, A. Diagnosis of Brucellosis in Ruminant in kafr el sheikh Governorate, Egypt. Int. J. Adv. Res. 2015, 3, 345–350. [Google Scholar]
- El-Diasty, M.M.; Ahmed, H.A.; Sayour, A.E.; Hofy, F.I.E.; Tahoun, A.B.M.B.; Shafik, S.M. Seroprevalence of Brucella spp. in Cattle, Molecular Characterization in Milk, and the Analysis of Associated Risk Factors with Seroprevalence in Humans, Egypt. Vector Borne Zoonotic Dis. 2016, 16, 758–764. [Google Scholar] [CrossRef]
- Khalafallah, S.; Zaki, H.; Seada, A. Epidemiological studies on brucellosisin dairy farms in Nile delta, Egypt. Benha Vet. Med. J. 2020, 39, 75–78. [Google Scholar] [CrossRef]
- Salem, L.; Khalifa, N.; Moustafa, S. Sero-diagnosis of brucellosis in Gharbiya governorate, Egypt. Benha Vet. Med. J. 2016, 31, 10–16. [Google Scholar] [CrossRef]
- Hashem, M.; El-Mandrawy, S.; El-Diasty, M.; Zidan, A. Hematological, biochemical and immunological studies on brucellosis in cows and ewes in Dakahlia and Damietta governorates, Egypt. Zagazig Vet. J. 2020, 48, 23–35. [Google Scholar] [CrossRef]
- Fereig, R.M.; Mazeed, A.M.; Alharbi, A.S.; Abdelraheem, M.Z.; Omar, M.A.; Almuzaini, A.M.; El-Diasty, M.; Elsharkawy, H.I.; Sobhy, K.; Frey, C.F.; et al. Seroprevalence of Antibodies to Brucella spp. and Neospora caninum in Cattle from Delta Region of Egypt: Correlation of Seropositivity with Abortion History. Immuno 2024, 4, 374–384. [Google Scholar] [CrossRef]
- Hady, A.M.E.; Ahmed, M.S. Seroprevalence and molecular epidemiology of brucellosis in cattle in Egypt. Adv. Dairy Res. 2016, 4, 153. [Google Scholar] [CrossRef]
- Ramadan, E.; Nassar, N.; Ibrahim, I.; Zayed, A. Epidemiological and Zoonotic Surveillance of Brucellosis in Beni-Suef Governorate. Alex. J. Vet. Sci. 2019, 61, 22. [Google Scholar] [CrossRef]
- Khalafallah, S.; Zaki, H.; Seada, A. Some epidemiological studies on brucellosis in dairy farms in Gharbia governorate, Egypt. Benha Vet. Med. J. 2020, 39, 15–19. [Google Scholar] [CrossRef]
- Koriem, A.M.; Al-Habaty, S.H.; Makar, N.H.; Abd El-Kader, H.A. Seroprevalence of Brucellosis in Slaughtered Animals at Assiut Governorate. Assiut Vet. Med. J. 2013, 59, 72–77. [Google Scholar] [CrossRef]
- Rabah, I.; Nossair, M.; Abdou, E.; Elkamshishi, M.; Khalifa, E. Serological and molecular epidemiological study on brucellosis in Camels and human in Matrouh Province. Damanhour J. Vet. Sci. 2020, 4, 1–6. [Google Scholar] [CrossRef]
- Wassif, I.M.; Mohamed, R.H. Studies on ruminant brucellosis in El Salam canal area, Egypt. Benha J. Appl. Sci. 2017, 2, 151–155. [Google Scholar] [CrossRef]
- Shalaby, N.; Gafer, J.; Badran, B. Serological and Molecular Differentiation Between Brucella and Yersinia enterocolitica O:9 Infections in Cattle. Assiut Vet. Med. J. 2013, 59, 1–8. [Google Scholar] [CrossRef]
- Gwida, M.; Ashker, M.E.; Awad, A.; Khan, I.; Gohary, A.E. Seroprevalence of brucellosis and coxiellosis in a linked study population in Egypt. Asian J. Res. Anim. Vet. Sci. 2020, 3, 360–367. [Google Scholar] [CrossRef]
- Abd-El Halim, M.H.; Mohamed, A.A.E.; Shalaby, N.A. Prevalence of brucellosis in buffaloes and its control measures. J. Vet. Med. Res. 2017, 24, 84–93. [Google Scholar] [CrossRef]
- Diab, M.S.; Elnaker, Y.F.; Ibrahim, N.A.; Sedeek, E.K.; Zidan, S.A. Seroprevalence and Associated Risk Factors of Brucellosis in Sheep and Human in Four Regions in Matrouh Governorate, Egypt. World Vet. J. 2018, 8, 65–72. [Google Scholar]
- Nayel, M.; Ibrahim, R.; Zaghawa, A. Seroprevalence and Associated Risk Factors of Brucellosis among Sheep, Goats and Camels in North Western Coastal Area of Egypt. J. Curr. Vet. Res. 2020, 2, 25–34. [Google Scholar] [CrossRef]
- Nagati, S.F.; Hassan, S.K. Diagnosis of Brucella Infection in Sheep and Goat and Evaluation of the associated Practices in Animal Contacts. Am. J. Infect. Dis. 2016, 4, 95–101. [Google Scholar] [CrossRef]
- Ghanem, Y.M.A. A Seroprevalence Study of Brucellosis in Goats at Kafrelsheikh Governorate, EGYPT. Kafrelsheikh Vet. Med. J. 2011, 9, 229–247. [Google Scholar] [CrossRef]
- Al-Habaty, S.H.; Abuo-Gazia, K.A.; Ammar, M.A.M. Prevalence Study on Brucellosis in Some Ruminants Slaughtered out of Abattoirs in Assiut Governorate. Assiut Vet. Med. J. 2015, 61, 65–72. [Google Scholar]
- Ibrahim, H.H.; Rouby, S.; Menshawy, A.; Ghazy, N. Seroprevalence of Camel Brucellosis and Molecular Characterization of Brucella melitensis Recovered from Dromedary Camels in Egypt. Res. J. Vet. Pract. 2016, 4, 17–24. [Google Scholar] [CrossRef]
- El-Sayed, A.; El-Diasty, M.; Elbeskawy, M.; Zakaria, M.; Younis, E. Prevalence of Camel Brucellosis at Al-Shalateen Area. Mansoura Vet. Med. J. 2017, 18, 33–43. [Google Scholar] [CrossRef]
- Elsohaby, I.; Kostoulas, P.; Elsayed, A.M.; Ahmed, H.A.; El-Diasty, M.M.; Wareth, G.; Ghanem, F.M.; Arango-Sabogal, J.C. Bayesian Evaluation of Three Serological Tests for Diagnosis of Brucella infections in Dromedary Camels Using Latent Class Models. Prev. Vet. Med. 2022, 208, 105771. [Google Scholar] [CrossRef] [PubMed]
- Fereig, R.M.; Mazeed, A.M.; Tawab, A.A.A.E.; El-Diasty, M.; Elsayed, A.; Shaapan, R.M.; Abdelbaset, A.E.; Frey, C.F.; Alawfi, B.S.; Altwaim, S.A.; et al. Exposure to Brucella Species, Coxiella burnetii, and Trichinella Species in Recently Imported Camels from Sudan to Egypt: Possible Threats to Animal and Human Health. Pathogens 2024, 13, 179. [Google Scholar] [CrossRef] [PubMed]
- Eisa, M.I.; Monazie, A.M.; Khoudair, R.M.; El-Shymaa, A.A. Serological Diagnosis of Camel Brucellosis at Sharkia Governorate, Egypt. Zagazig Vet. J. 2014, 42, 117–123. [Google Scholar] [CrossRef]
- Abd El Tawab, A.A.; Agag, M.A.; Khoudair, R.M.; Anwer, B.R. Some studies on Brucella among camels with reference to isolated strains. Benha Vet. Med. J. 2018, 35, 12–21. [Google Scholar] [CrossRef]
- El-Khadrawy, H.; Abo-Elmaaty, A.; Zaabal, M.; Zaki, H.; Ahmed, W.; Hanafi, E. Updates of Brucellosis in Egyptian Cattle and Camels with Emphasis on Some Associated Biochemical Values and Genetic Markers. Middle East. J. Sci. Res. 2021, 29, 12–20. [Google Scholar] [CrossRef]
- Hazem, S.; Ismail, R.; Elsharkawy, H.; Kholi, M.A.; Younis, E.; El-Madawy, S.; Dep, B. Overview on Brucellosis in Camels. Egypt. J. Anim. Health 2023, 3, 185–199. [Google Scholar] [CrossRef]
- Abdelgawad, H.A.; Sultan, S.; Abdel-Aal, A.M.M.; Abd Al-Azeem, M.W. Serological and Molecular Detection of Brucella Species in Camel. Benha Vet. Med. J. 2017, 33, 314–319. [Google Scholar]
- Khan, A.U.; Sayour, A.E.; Melzer, F.; El-Soally, S.A.G.E.; Elschner, M.C.; Shell, W.S.; Moawad, A.A.; Mohamed, S.A.; Hendam, A.; Roesler, U.; et al. Seroprevalence and Molecular Identification of Brucella spp. in Camels in Egypt. Microorganisms 2020, 8, 1035. [Google Scholar] [CrossRef]
- Gwida, M.M.; El-Gohary, A.H.; Melzer, F.; Tomaso, H.; Rösler, U.; Wernery, U.; Wernery, R.; Elschner, M.C.; Khan, I.; Eickhoff, M.; et al. Comparison of diagnostic tests for the detection of Brucella spp. in camel sera. BMC Res. Notes 2011, 4, 525. [Google Scholar] [CrossRef] [PubMed]
- Elbauomy, E.; Abdel-Haleem, M.; Abdel-Hamid, N. Epidemiological situation of brucellosis in five related ovine farms with suggestion of appropriate programmes for disease control. Anim. Health Res. J. 2014, 2, 129–142. [Google Scholar]
- Eldeihy, M.; Hegazy, Y.; Oreiby, A.; Al-Gaabary, M. Knowledge, Attitude and Practices (KAPS) of Veterinarians and Farmers Regarding Brucellosis with Assessment of the Current National Control Program in Egypt. Glob. Vet. 2017, 18, 197–202. [Google Scholar]
- StataCorp. Stata Statistical Software, Release 18; StataCorp LLC: College Station, TX, USA, 2023. [Google Scholar]
- Holzer, K.; El-Diasty, M.; Wareth, G.; Abdel-Hamid, N.H.; Hamdy, M.E.R.; Moustafa, S.A.; Linde, J.; Bartusch, F.; Sayour, A.E.; Elbauomy, E.M.; et al. Tracking the Distribution of Brucella abortus in Egypt Based on Core Genome SNP Analysis and In Silico MLVA-16. Microorganisms 2021, 9, 1942. [Google Scholar] [CrossRef]
- Aidaros, H. Global perspectives—The Middle East: Egypt. Rev. Sci. Tech. 2005, 24, 589–596. [Google Scholar]
- Holzer, K.; Wareth, G.; El-Diasty, M.; Abdel-Hamid, N.H.; Hamdy, M.E.R.; Moustafa, S.A.; Linde, J.; Bartusch, F.; Abdel-Glil, M.Y.; Sayour, A.E.; et al. Tracking the distribution, genetic diversity and lineage of Brucella melitensis recovered from humans and animals in Egypt based on core-genome SNP analysis and in silico MLVA-16. Transbound. Emerg. Dis. 2022, 69, 3952–3963. [Google Scholar] [CrossRef] [PubMed]
- Corbel, M.J. Brucellosis in Humans and Animals; World Health Organization: Geneva, Switzerland, 2006; pp. 1–102. [Google Scholar]
- Etman, R.; Barsoum, S.; Ibrahim, I.; El-Ashmawy, W.; Abou-Gazia, K. Evaluation of efficacy of some serological tests used for diagnosis of brucellosis in cattle in Egypt using latent class analysis. Sokoto J. Vet. Sci. 2014, 12, 1–7. [Google Scholar] [CrossRef]
- Afifi, M.; Abdul-Raouf, U.M.; Hussein, H.A.M. Isolation and Biotyping of Brucella melitensis from Upper Egypt. J. Am. Sci. 2011, 7, 653–659. [Google Scholar]
- El-Diasty, M.; Salah, K.; El-Hofy, F.I.; Tawab, A.A.A.E.; Soliman, E.A. Investigation of an outbreak of brucellosis in a mixed dairy farm and evaluation of a test and slaughter strategy to release the herd out of the quarantine. Ger. J. Vet. Res. 2022, 2, 1–9. [Google Scholar] [CrossRef]
- Blasco, J.M. Control and eradication strategies for Brucella melitensis infection in sheep and goats. Prilozi 2010, 31, 145–165. [Google Scholar]
- Verger, J.M.; Garin-Bastuji, B.; Grayon, M.; Mahé, A.M. Bovine brucellosis caused by Brucella melitensis in France. Ann. Rech. Vet. 1989, 20, 93–102. [Google Scholar]
- Al-Sherida, Y.; El-Gohary, A.H.; Mohamed, A.; El-Diasty, M.; Wareth, G.; Neubauer, H.; Abdelkhalek, A. Sheep Brucellosis in Kuwait: A Large-Scale Serosurvey, identification of Brucella species and zoonotic significance. Vet. Sci. 2020, 7, 132. [Google Scholar] [CrossRef] [PubMed]
- Hosein, H.; Hamdy, M.; Zaitoun, A.; Menshawy, A.; Rouby, S.; Madkour, B.; Mazeed, A.; Abdel-Raouf, A. Brucella Prevalent Strains Circulating in Egypt during 2020–2021: Bacteriological and Molecular Study. J. Vet. Med. Res. 2021, 28, 12–20. [Google Scholar] [CrossRef]
- De Massis, F.; Ali, R.M.; Serrani, S.; Toro, M.; Sferrella, A.; D’Aurelio, N.; Janowicz, A.; Zilli, K.; Romualdi, T.; Felicioni, E.; et al. Genetic Diversity of Brucella melitensis Isolated from Domestic Ruminants in Iraq. Microorganisms 2024, 12, 475. [Google Scholar] [CrossRef]
- El-Diasty, M.; Wareth, G.; Melzer, F.; Mustafa, S.; Sprague, L.; Neubauer, H. Isolation of Brucella abortus and Brucella melitensis from Seronegative Cows is a Serious Impediment in Brucellosis Control. Vet. Sci. 2018, 5, 28. [Google Scholar] [CrossRef]
- El-Diasty, M.; El-Said, R.; Abdelkhalek, A. Seroprevalence and molecular diagnosis of sheep brucellosis in Dakahlia governorate, Egypt. Ger. J. Vet. Res. 2021, 1, 34–39. [Google Scholar] [CrossRef]
- Wareth, G.; El-Diasty, M.; Melzer, F.; Schmoock, G.; Moustafa, S.A.; El-Beskawy, M.; Khater, D.F.; Hamdy, M.E.R.; Zaki, H.M.; Ferreira, A.C.; et al. MLVA-16 Genotyping of Brucella abortus and Brucella melitensis Isolates from Different Animal Species in Egypt: Geographical Relatedness and the Mediterranean Lineage. Pathogens 2020, 9, 498. [Google Scholar] [CrossRef] [PubMed]
- Salah, K.; El-Diasty, M.; El-Hofy, F.I.; Wareth, G.; Abd El Tawab, A.A. Case Study: B. abortus Outbreak in Egyptian Dairy Farm with a Special Reference to Control Programs. J. Adv. Vet. Res. 2022, 12, 462–465. [Google Scholar]
- Abdel-Hamid, N.H.; Shell, W.S.; Khafagi, M.H.M. Comprehensive Serological, Bacteriological, and Molecular Perspective of Brucellosis in Cattle and Buffaloes in Some Governorates. Int. J. Recent Sci. Res. 2017, 8, 20226–20234. [Google Scholar]
- Hegab, D.H.; El-Mashad, E.A.; Mustafa, S.A.; Zaki, H.M.; Amin, A.A. Pathological and molecular studies on Brucellosis in cattle and buffaloes. Benha Vet. Med. J. 2022, 43, 6–11. [Google Scholar]
- Elhaig, M.M.; Wahdan, A. Seroprevalence, associated risk factors, and molecular detection of bovine brucellosis in rural areas of Egypt. Comp. Immunol. Microbiol. Infect. Dis. 2023, 95, 101971. [Google Scholar] [CrossRef]
- WHO. The Control of Neglected Zoonotic Diseases—A Route to Poverty Alleviation. 2005. Available online: https://iris.who.int/bitstream/handle/10665/43485/9789241594301_eng.pdf?sequence=1 (accessed on 9 January 2025).
- Salem, A.A.; Hosein, H.I. Brucella Strains Prevalent in Egypt. Assiut Vet. Med. J. 1990, 22, 160–163. [Google Scholar]
- Ibrahim, S.I. Studies on swine brucellosis in Egypt. J. Egypt. Vet. Med. Assoc. 1996, 56, 1–12. [Google Scholar]
- Khan, A.U.; Melzer, F.; El-Soally, S.A.G.E.; Elschner, M.C.; Mohamed, S.A.; Ahmed, M.A.S.; Roesler, U.; Neubauer, H.; El-Adawy, H. Serological and Molecular Identification of Brucella spp. in Pigs from Cairo and Giza Governorates, Egypt. Pathogens 2019, 8, 248. [Google Scholar] [CrossRef]
- Gwida, M.; El-Gohary, A.; Melzer, F.; Khan, I.; Rösler, U.; Neubauer, H. Brucellosis in camels. Res. Vet. Sci. 2011, 92, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Shokry, K.; Hashad, M.; Abdelhalem, M.H.; Ahmed, Z.; Sayour, A.E.E.; Selim, S.; Abdel-Hamid, N.H. ERIC PCR-Based Genotyping and Antimicrobial Susceptibility of Brucella melitensis Isolates Recovered from Slaughtered Camels in Egypt. Egypt. J. Vet. Sci. 2024, 1–11. [Google Scholar] [CrossRef]
- Le Flèche, P.L.; Jacques, I.; Grayon, M.; Dahouk, S.A.; Bouchon, P.; Denoeud, F.; Nöckler, K.; Neubauer, H.; Guilloteau, L.A.; Vergnaud, G. Evaluation and selection of tandem repeat loci for a Brucella MLVA typing assay. BMC Microbiol. 2006, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Maquart, M.; Flèche, P.L.; Foster, G.; Tryland, M.; Ramisse, F.; Djønne, B.; Dahouk, S.A.; Jacques, I.; Neubauer, H.; Walravens, K.; et al. MLVA-16 typing of 295 marine mammal Brucella isolates from different animal and geographic origins identifies 7 major groups within Brucella ceti and Brucella pinnipedialis. BMC Microbiol. 2009, 9, 145. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Esquivel, M.; Chaves-Olarte, E.; Moreno, E.; Guzmán-Verri, C. Brucella Genomics: Macro and Micro Evolution. Int. J. Mol. Sci. 2020, 21, 7749. [Google Scholar] [CrossRef] [PubMed]
- Her, M.; Kang, S.I.; Kim, J.W.; Kim, J.Y.; Hwang, I.Y.; Jung, S.C.; Park, S.H.; Park, M.Y.; Yoo, H. A genetic comparison of Brucella abortus isolates from animals and humans by using an MLVA assay. J. Microbiol. Biotechnol. 2010, 20, 1750–1755. [Google Scholar]
- USDA Animal and Plant Health Inspection Service. USDA Updates National Bovine Brucellosis Surveillance Plan; USDA Animal and Plant Health Inspection Service: Riverdale, MD, USA, 2022. Available online: https://content.govdelivery.com/accounts/USDAAPHIS/bulletins/337453d (accessed on 8 December 2023).
- Lounes, N.; Cherfa, M.-A.; Carrou, G.L.; Bouyoucef, A.; Jay, M.; Garin-Bastuji, B.; Mick, V. Human Brucellosis in Maghreb: Existence of a Lineage Related to Socio-Historical Connections with Europe. PLoS ONE 2014, 9, e115319. [Google Scholar] [CrossRef]
- Godfroid, J. Brucellosis in livestock and wildlife: Zoonotic diseases without pandemic potential in need of innovative one health approaches. Arch. Public Health 2017, 75, 34. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menshawy, A.M.S.; Vicente, A.F.; Hegazy, Y.M.; Djokic, V.; Hamdy, M.E.R.; Freddi, L.; Elbauomy, E.M.; Sayour, A.E.; Ponsart, C.; Abdel-Hamid, N.H. Animal Brucellosis in Egypt: Review on Evolution, Epidemiological Situation, Prevalent Brucella Strains, Genetic Diversity, and Assessment of Implemented National Control Measures. Microorganisms 2025, 13, 170. https://doi.org/10.3390/microorganisms13010170
Menshawy AMS, Vicente AF, Hegazy YM, Djokic V, Hamdy MER, Freddi L, Elbauomy EM, Sayour AE, Ponsart C, Abdel-Hamid NH. Animal Brucellosis in Egypt: Review on Evolution, Epidemiological Situation, Prevalent Brucella Strains, Genetic Diversity, and Assessment of Implemented National Control Measures. Microorganisms. 2025; 13(1):170. https://doi.org/10.3390/microorganisms13010170
Chicago/Turabian StyleMenshawy, Ahmed M. S., Acacia Ferreira Vicente, Yamen M. Hegazy, Vitomir Djokic, Mahmoud E. R. Hamdy, Luca Freddi, Essam M. Elbauomy, Ashraf E. Sayour, Claire Ponsart, and Nour H. Abdel-Hamid. 2025. "Animal Brucellosis in Egypt: Review on Evolution, Epidemiological Situation, Prevalent Brucella Strains, Genetic Diversity, and Assessment of Implemented National Control Measures" Microorganisms 13, no. 1: 170. https://doi.org/10.3390/microorganisms13010170
APA StyleMenshawy, A. M. S., Vicente, A. F., Hegazy, Y. M., Djokic, V., Hamdy, M. E. R., Freddi, L., Elbauomy, E. M., Sayour, A. E., Ponsart, C., & Abdel-Hamid, N. H. (2025). Animal Brucellosis in Egypt: Review on Evolution, Epidemiological Situation, Prevalent Brucella Strains, Genetic Diversity, and Assessment of Implemented National Control Measures. Microorganisms, 13(1), 170. https://doi.org/10.3390/microorganisms13010170




