Probiotic Supplementation Alleviates Corticosterone-Induced Fatty Liver Disease by Regulating Hepatic Lipogenesis and Increasing Gut Microbiota Diversity in Broilers
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval and Consent to Participate
2.2. Animals and Treatment
2.3. Histopathology
2.4. Plasma Biochemical Indicators Measurement and TG Concentration in Liver
2.5. Reverse Transcription and Real-Time PCR
2.6. Western Blotting Analysis
2.7. Extraction of Genome DNA and 16S rRNA Sequencing
2.8. Statistical Analysis
3. Results
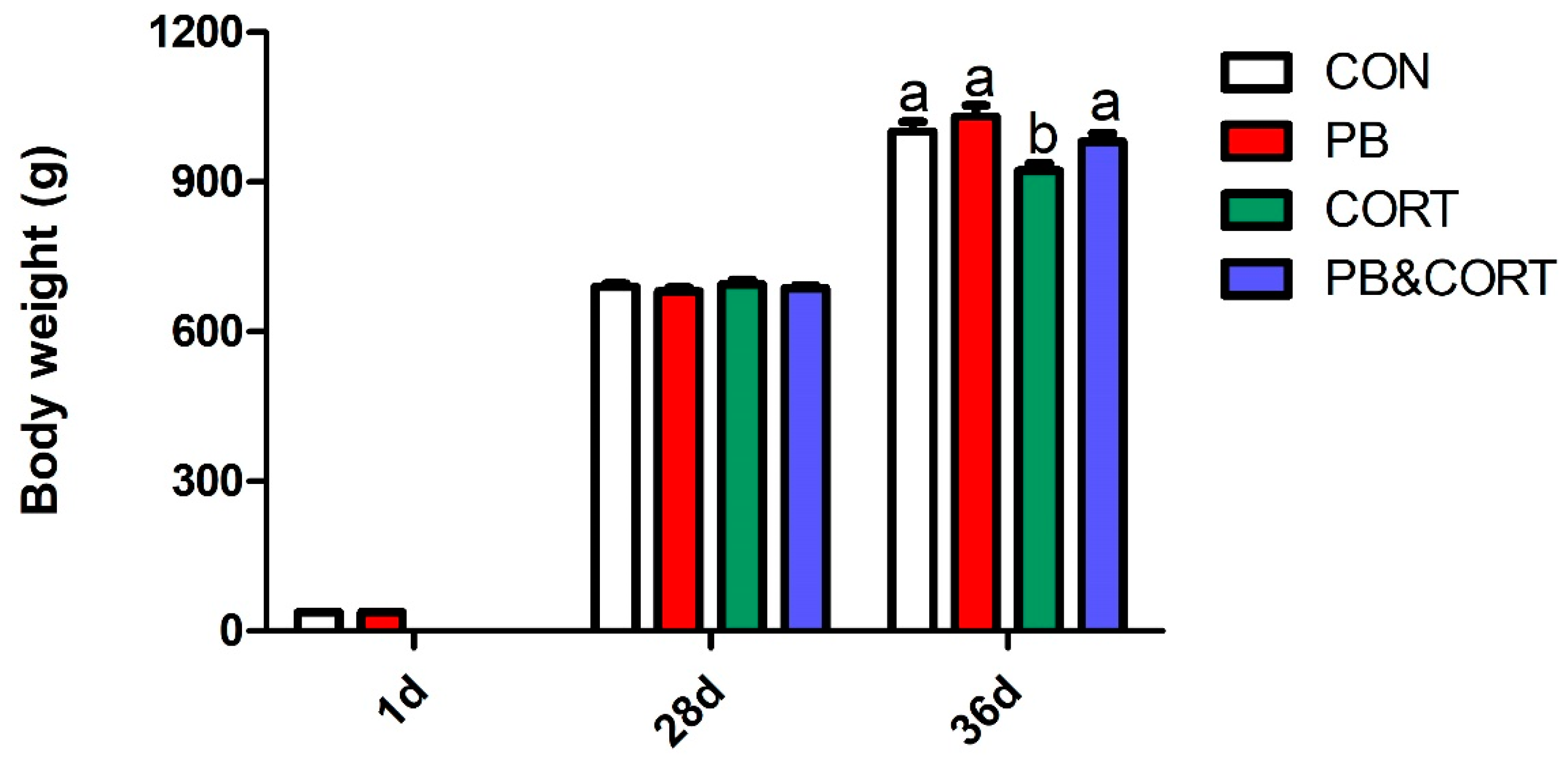
3.1. Body Weight and the Relative Weight of Immune Organs
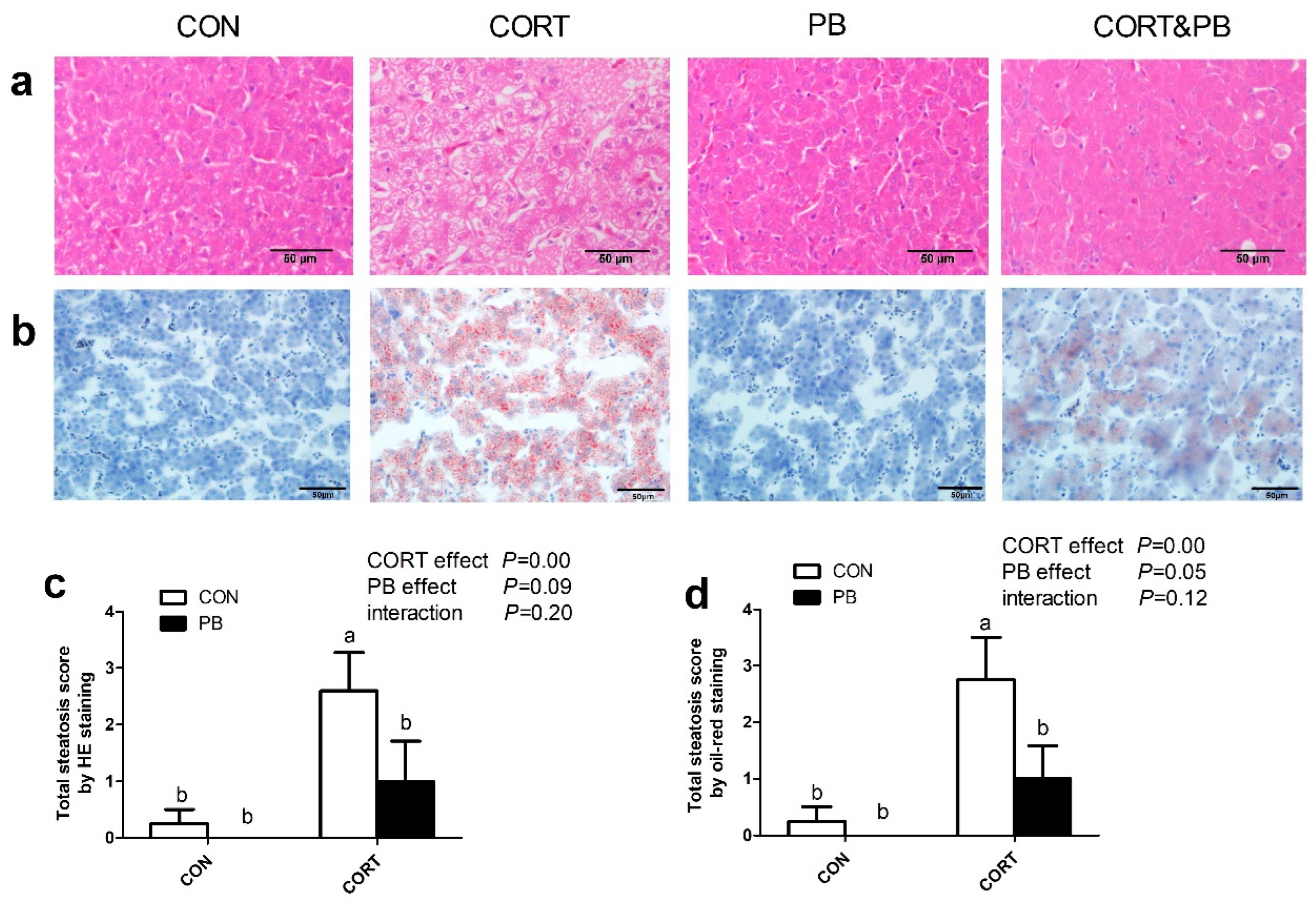
3.2. Hepatic Histological Analysis
3.3. TG Concentrations in Plasma and the Liver
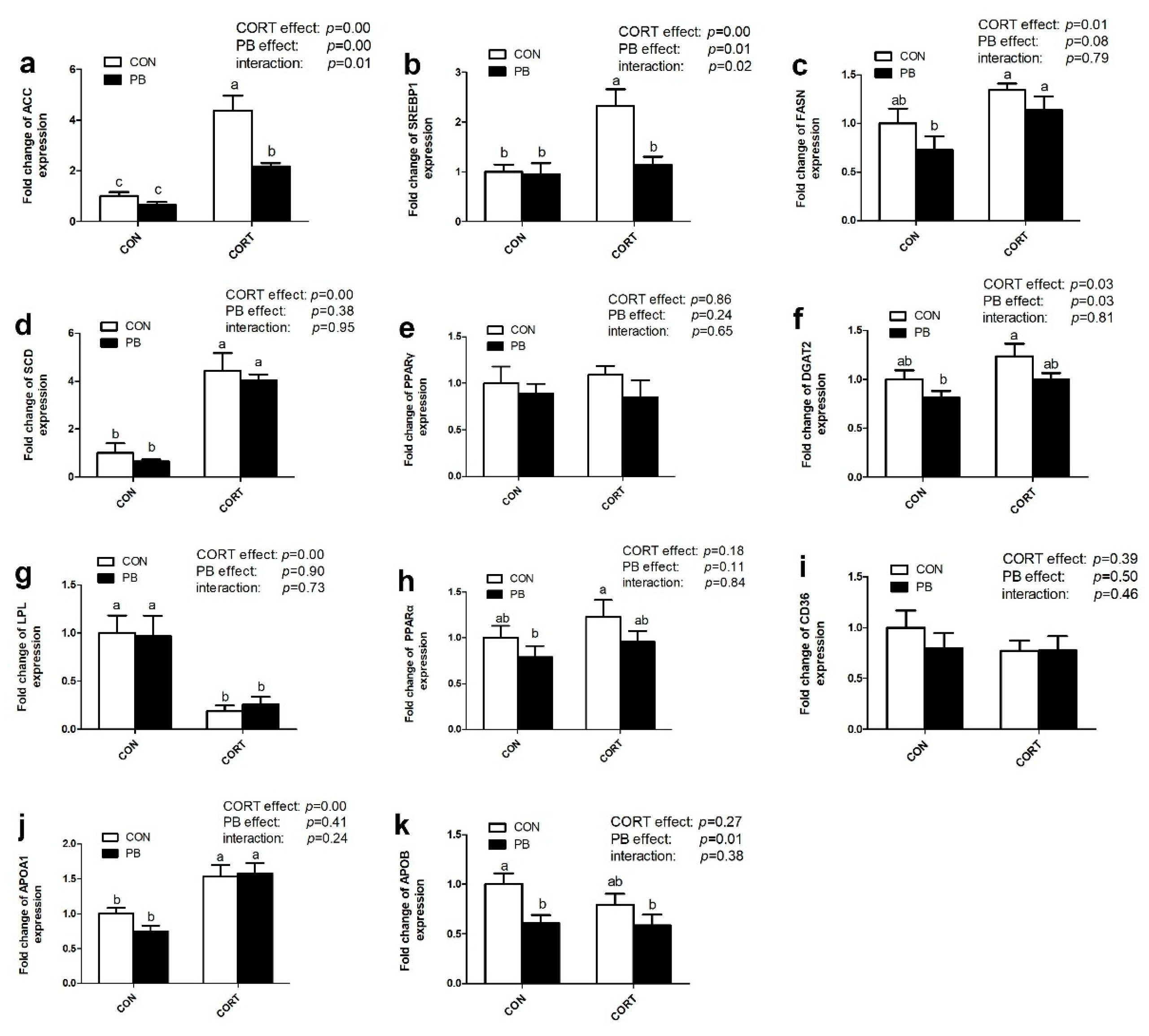
3.4. Expression of Genes Related to TG Metabolism in the Liver
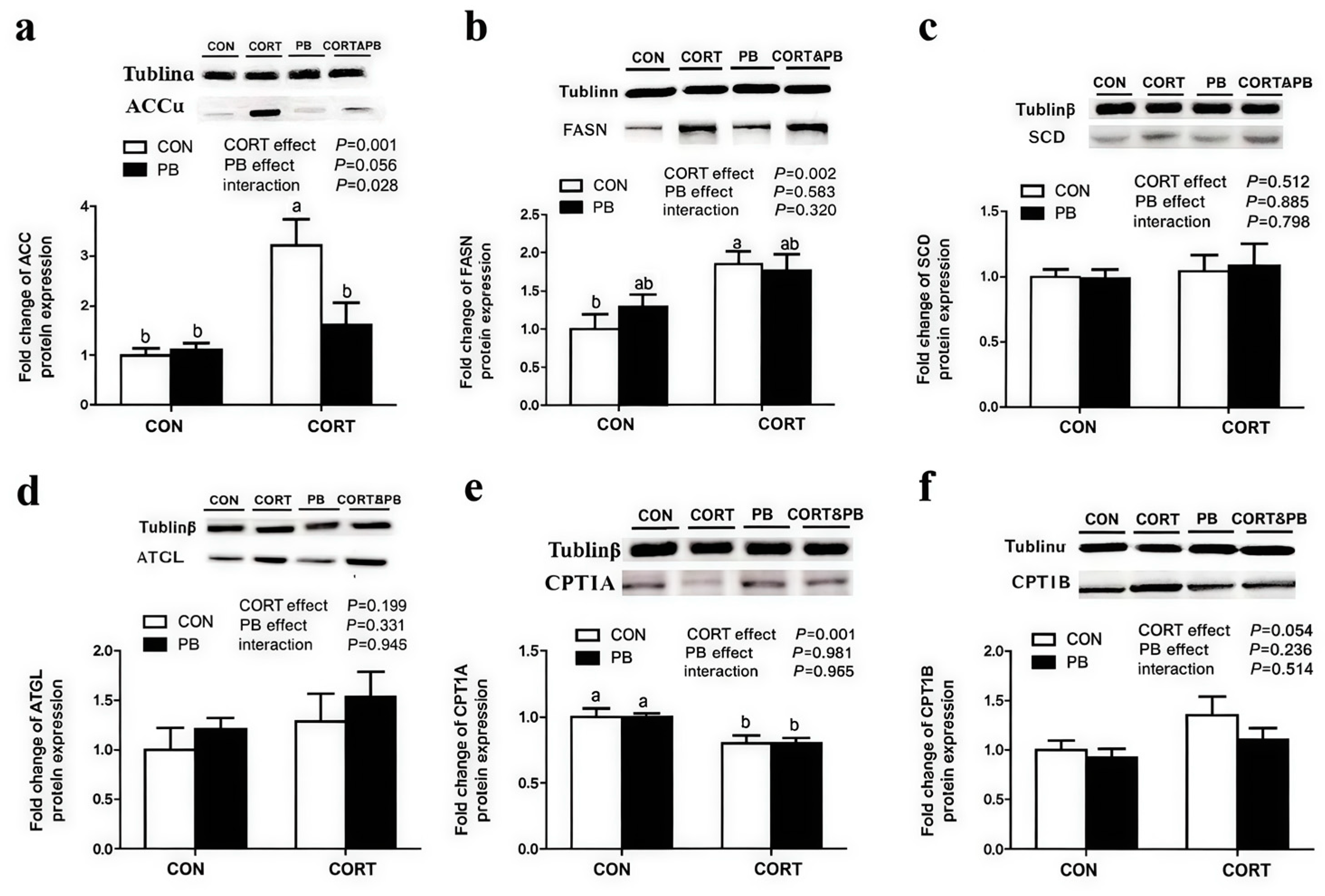
3.5. Expression of Protein Related to Lipid Metabolism in the Liver
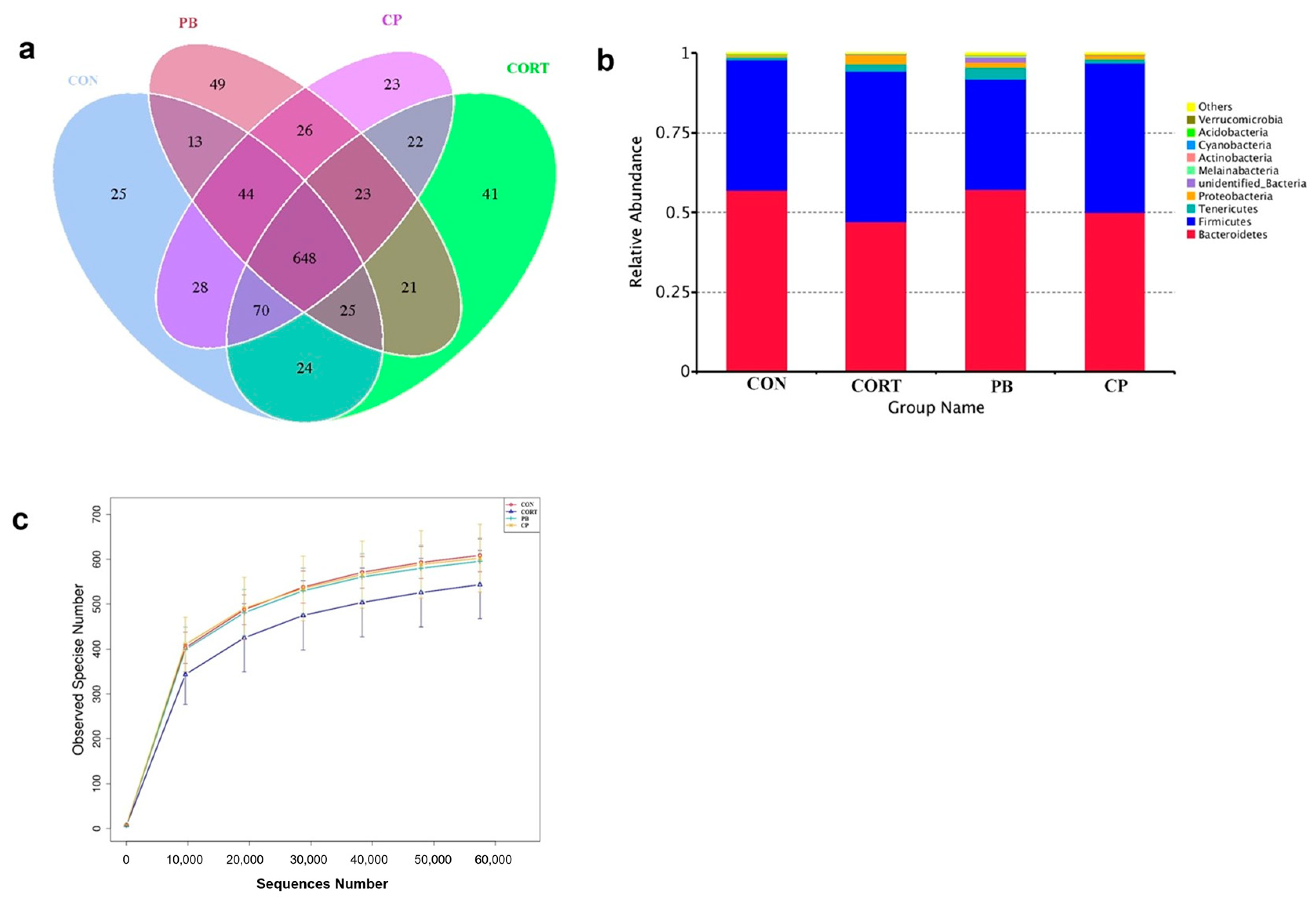
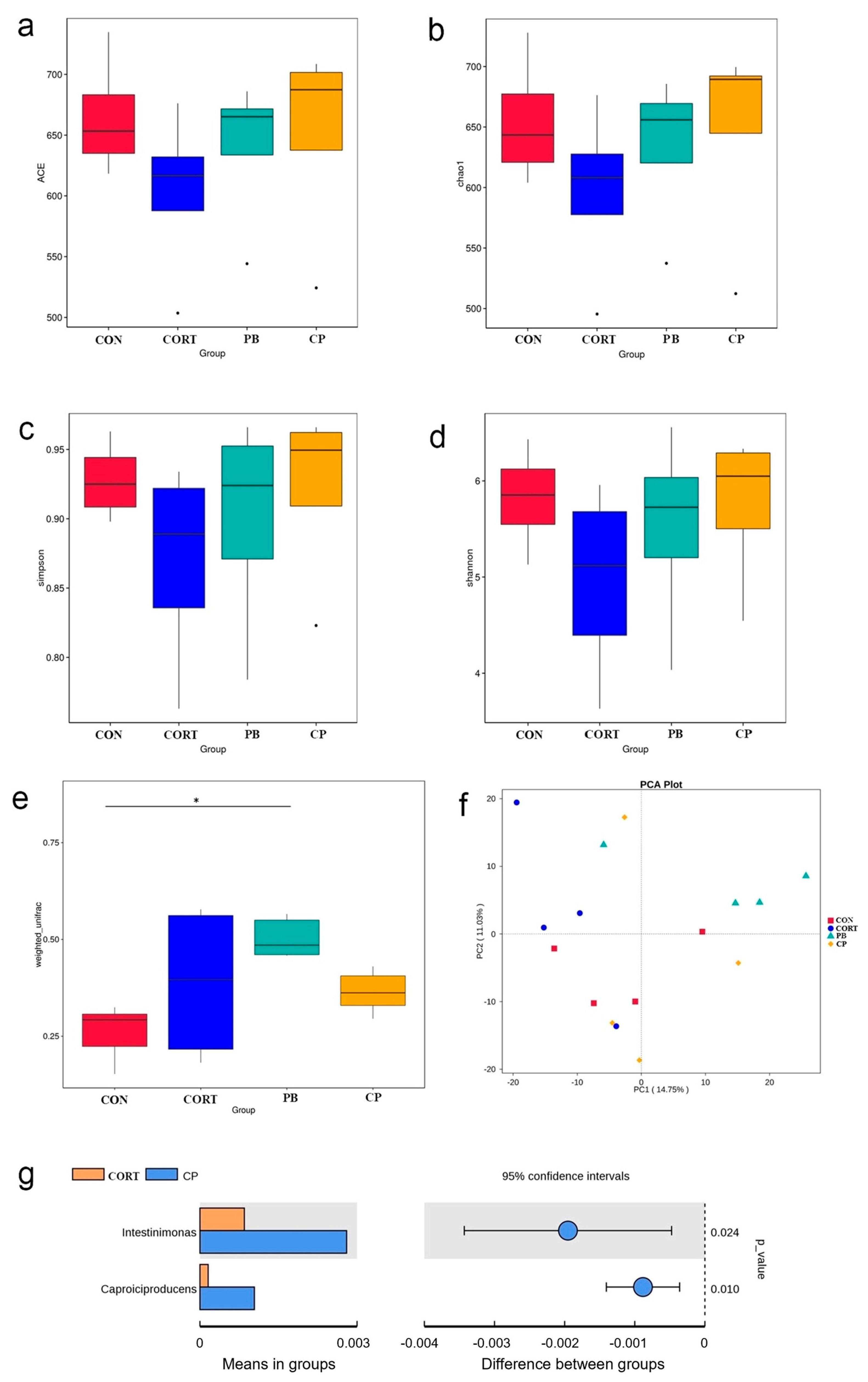
3.6. The Analysis of Cecum Microbiota Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Cohen, J.C.; Horton, J.D.; Hobbs, H.H. Human Fatty Liver Disease: Old Questions and New Insights. Science 2011, 332, 1519–1523. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P. Nonalcoholic Fatty Liver Disease. N. Engl. J. Med. 2002, 346, 1221–1231. [Google Scholar] [CrossRef]
- Choi, S.S.; Diehl, A.M. Hepatic triglyceride synthesis and nonalcoholic fatty liver disease. Curr. Opin. Lipidol. 2008, 19, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Paglialunga, S.; Dehn, C.A. Clinical assessment of hepatic de novo lipogenesis in non-alcoholic fatty liver disease. Lipids Health Dis. 2016, 15, 159. [Google Scholar] [CrossRef] [PubMed]
- John, K.; Marino, J.S.; Sanchez, E.R.; Hinds, T.D. The glucocorticoid receptor: Cause of or cure for obesity? Am. J. Physiol. Endocrinol. Metab. 2016, 310, E249–E257. [Google Scholar] [CrossRef] [PubMed]
- Pasieka, A.M.; Rafacho, A. Impact of Glucocorticoid Excess on Glucose Tolerance: Clinical and Preclinical Evidence. Metabolites 2016, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Buettner, C. Mechanisms of Glucocorticoid-Induced Insulin Resistance Focus on Adipose Tissue Function and Lipid Metabolism. Endocrinol. Metab. Clin. N. Am. 2014, 43, 75–102. [Google Scholar]
- Mei, W.Q.; Hao, Y.R.; Xie, H.L.; Ni, Y.D.; Zhao, R.Q. Hepatic Inflammatory Response to Exogenous LPS Challenge is Exacerbated in Broilers with Fatty Liver Disease. Animals 2020, 10, 514. [Google Scholar] [CrossRef]
- Hu, Y.; Sun, Q.W.; Liu, J.; Jia, Y.M.; Cai, D.M.; Idriss, A.A.; Omer, N.A.; Zhao, R.Q. In ovo injection of betaine alleviates corticosterone-induced fatty liver in chickens through epigenetic modifications. Sci. Rep. 2017, 7, 40251. [Google Scholar] [CrossRef]
- Hu, Y.; Sun, Q.W.; Hu, Y.; Hou, Z.; Zong, Y.B.; Omer, N.A.; Abobaker, H.; Zhao, R.Q. Corticosterone-induced Lipogenesis activation and Lipophagy inhibition in chicken liver are alleviated by maternal Betaine supplementation. J. Nutr. 2018, 148, 316–325. [Google Scholar] [CrossRef]
- Usami, M.; Miyoshi, M.; Yamashita, H. Gut microbiota and host metabolism in liver cirrhosis. World J. Gastroenterol. 2015, 21, 11597–11608. [Google Scholar] [CrossRef]
- Iacono, A.; Raso, G.M.; Canani, R.B.; Calignano, A.; Meli, R. Probiotics as an emerging therapeutic strategy to treat NAFLD: Focus on molecular and biochemical mechanisms. J. Nutr. Biochem. 2011, 22, 699–711. [Google Scholar] [CrossRef]
- Yang, Q. Oral Administration of Compound Probiotics Ameliorates HFD-Induced Gut Microbe Dysbiosis and Chronic Metabolic Inflammation via the G Protein-Coupled Receptor 43 in Non-alcoholic Fatty Liver Disease Rats. Probiotics Antimicrob. Proteins 2019, 11, 175–185. [Google Scholar]
- Xin, J.G.; Zeng, D.; Wang, H.S.; Ni, X.Q.; Yi, D.; Pan, K.C.; Jing, B. Preventing non-alcoholic fatty liver disease through Lactobacillus johnsonii BS15 by attenuating inflammation and mitochondrial injury and improving gut environment in obese mice. Appl. Microbiol. Biot. 2014, 98, 6817–6829. [Google Scholar] [CrossRef]
- Cortez-Pinto, H.; Borralho, P.; Machado, J.; Lopes, M.T.; Gato, I.V.; Santos, A.M.; Guerreiro, A.S. Microbiota Modulation With Synbiotic Decreases Liver Fibrosis in a High Fat Choline Deficient Diet Mice Model of Non-Alcoholic Steatohepatitis (NASH). GE Port. J. Gastroenterol. 2016, 23, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liao, M.; Zhou, N.; Bao, L.; Ma, K.; Zheng, Z.; Wang, Y.; Liu, C.; Wang, W.; Wang, J.; et al. Parabacteroides distasonis Alleviates Obesity and Metabolic Dysfunctions via Production of Succinate and Secondary Bile Acids. Cell Rep. 2019, 26, 222–235.e5. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Dong, H.; Zhang, Z.; Liu, J.; Hu, Y.; Ni, Y.; Grossmann, R.; Zhao, R. Activation of epithelial proliferation induced by Eimeria acervulina infection in the duodenum may be associated with cholesterol metabolism. Oncotarget 2016, 7, 27627–27640. [Google Scholar] [CrossRef][Green Version]
- Panchal, S.K.; Poudyal, H.; Iyer, A.; Nazer, R.; Alam, M.A.; Diwan, V.; Kauter, K.; Sernia, C.; Campbell, F.; Ward, L.; et al. High-carbohydrate, high-fat diet-induced metabolic syndrome and cardiovascular remodeling in rats. J. Cardiovasc. Pharmacol. 2011, 57, 611–624. [Google Scholar] [CrossRef]
- Li, L.; Guo, W.L.; Zhang, W.; Xu, J.X.; Qian, M.; Bai, W.D.; Zhang, Y.Y.; Rao, P.F.; Ni, L.; Lv, X.C. Grifola frondosa polysaccharides ameliorate lipid metabolic disorders and gut microbiota dysbiosis in high-fat diet fed rats. Food Funct. 2019, 10, 2560–2572. [Google Scholar] [CrossRef]
- Lin, H.; Sui, S.; Jiao, H.; Buyse, J.; Decuypere, E. Impaired development of broiler chickens by stress mimicked by corticosterone exposure. Comp. Biochem. Physiol. Part. A Mol. Integr. Physiol. 2006, 143, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.Z.; Ho, Y.W.; Abdullah, N.; Jalaludin, S. Digestive and bacterial enzyme activities in broilers fed diets supplemented with Lactobacillus cultures. Poult. Sci. 2000, 79, 886–891. [Google Scholar] [CrossRef]
- Kalavathy, R.; Abdullah, N.; Jalaludin, S.; Ho, Y.W. Effects of Lactobacillus cultures on growth performance, abdominal fat deposition, serum lipids and weight of organs of broiler chickens. Br. Poult. Sci. 2003, 44, 139–144. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Wang, B.; Xu, H.; Mei, X.; Xu, X.; Zhang, X.; Ni, J.; Li, W. Bacillus amyloliquefaciens SC06 Protects Mice Against High-Fat Diet-Induced Obesity and Liver Injury via Regulating Host Metabolism and Gut Microbiota. Front. Microbiol. 2019, 10, 1161. [Google Scholar] [CrossRef]
- Zhao, N.; Yang, S.; Jia, Y.; Sun, B.; He, B.; Zhao, R. Maternal betaine supplementation attenuates glucocorticoid-induced hepatic lipid accumulation through epigenetic modification in adult offspring rats. J. Nutr. Biochem. 2018, 54, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Yalçin, S.; Güçer, Ş.; Yalçin, S.; Onbaşilar, İ.; Kale, G.; Coşkun, T. Effects of probiotic (Primalac 454) on nonalcoholic fatty liver disease in broilers. Rev. Med. Vet. 2011, 162, 371–376. [Google Scholar]
- Leveille, G.A. In vitro hepatic lipogenesis in the hen and chick. Comp. Biochem. Physiol. 1969, 28, 431–435. [Google Scholar] [CrossRef]
- Kemper, J.K.; Choi, S.-E.; Kim, D.H. Sirtuin 1 deacetylase: A key regulator of hepatic lipid metabolism. Vitam. Horm. 2013, 91, 385–404. [Google Scholar] [PubMed]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ni, X.; Qing, X.; Zeng, D.; Luo, M.; Liu, L.; Li, G.; Pan, K.; Jing, B. Live Probiotic Lactobacillus johnsonii BS15 Promotes Growth Performance and Lowers Fat Deposition by Improving Lipid Metabolism, Intestinal Development, and Gut Microflora in Broilers. Front. Microbiol. 2017, 8, 1073. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.; Carvalho, D.; Freitas, P. Gut Microbiota: Association with NAFLD and Metabolic Disturbances. Biomed. Res. Int. 2015, 2015, 979515. [Google Scholar] [CrossRef] [PubMed]
- Hamid, H.; Zhang, J.Y.; Li, W.X.; Liu, C.; Li, M.L.; Zhao, L.H.; Ji, C.; Ma, Q.G. Interactions between the cecal microbiota and non-alcoholic steatohepatitis using laying hens as the model. Poult. Sci. 2019, 98, 2509–2521. [Google Scholar] [CrossRef]
- Ritze, Y.; Bárdos, G.; Claus, A.; Ehrmann, V.; Bergheim, I.; Schwiertz, A. and Bischoff, S.C. Lactobacillus rhamnosus GG Protects against Non-Alcoholic Fatty Liver Disease in Mice. PLoS ONE 2014, 9, e80169. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1131. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Huang, M.H.; Yang, J.C.; Nien, C.K.; Yang, C.C.; Yeh, Y.H.; Yueh, S.K. Prevalence and Risk Factors of Nonalcoholic Fatty Liver Disease in an Adult Population of Taiwan: Metabolic Significance of Nonalcoholic Fatty Liver Disease in Nonobese Adults. J. Clin. Gastroenterol. 2006, 40, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.L.; Zheng, T.Y.; Chen, H.T.; Li, Y.Q.; Huang, H.L.; Chen, W.J.; Du, Y.L.; He, J.; Li, Y.Y.; Cao, J.; et al. Microbial Intervention as a Novel Target in Treatment of Non-Alcoholic Fatty Liver Disease Progression. Cell. Physiol. Biochem. 2018, 51, 2123–2135. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [PubMed]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Schnabl, B.; Brenner, D.A. Interactions Between the Intestinal Microbiome and Liver Diseases. Gastroenterology 2014, 146, 1513–1524. [Google Scholar] [CrossRef] [PubMed]
- Jiao, N.; Baker, S.S.; Nugent, C.A.; Tsompana, M.; Cai, L.T.; Wang, Y.; Buck, M.J.; Genco, R.J.; Baker, R.D.; Zhu, R.X.; et al. Gut microbiome may contribute to insulin resistance and systemic inflammation in obese rodents: A meta-analysis. Physiol. Genom. 2018, 50, 244–254. [Google Scholar] [CrossRef] [PubMed]
- de Moreno de LeBlanc, A.D.; LeBlanc, J.G. Effect of probiotic administration on the intestinal microbiota, current knowledge and potential applications. World J. Gastroenterol. 2014, 20, 16518–16528. [Google Scholar] [CrossRef]

| Ingredient % | Content | Composition | Content |
|---|---|---|---|
| Corn | 57.61 | ME (MJ/kg) | 12.56 |
| Soybean meal | 31.00 | CP (g/kg) | 211.00 |
| Corn protein powder | 3.29 | Ca (g/kg) | 10.00 |
| Soybean oil | 3.11 | P (g/kg) | 4.60 |
| Limestone | 1.20 | Lys (g/kg) | 12.00 |
| Dicalcium P | 2.00 | Met (g/kg) | 5.00 |
| L- Lysine | 0.34 | Met + Cys (g/kg) | 8.50 |
| DL-Met | 0.15 | ||
| NaCl | 0.30 | ||
| Premix 1 | 1.00 |
| Genes | Genbank Accession | Primer Sequences (5′ to 3′) | Fragment Size |
|---|---|---|---|
| ACC | NM_205505.1 | F: TGTGGGCTTTAGGAGATAA | 136 |
| R: GGAACATTCAGGATACGC | |||
| APOA1 | NM_205525.4 | F: ATGCCATCGCCCAGTTCG | 165 |
| R: GCAGAGCCTCGGTGTCCTT | |||
| APOB | NM_001044633.1 | F: CAGAGGTAGAGGCAGGAC | 82 |
| R: TCATCGGAGAAGTTAGGA | |||
| CD36 | NM_001030731.1 | F: GCATCATTTCCTCCATTT | 110 |
| R: ATTCCCTTCACGGTCTTA | |||
| DGAT2 | XM_419374.6 | F: GCTCTTCTCCTCGAACACG | 184 |
| R: CAACCCGAACCTGCCTTT | |||
| FASN | NM_205155.3 | F: GGGAATGTCACACCTTGCTC | 164 |
| R: GGAAATGGGTATTGTCGCTC | |||
| LPL | NM_205282.1 | F: CGGTGACAGGAATGTATGA | 140 |
| R: CTTCGTGTAAGCAGCAGA | |||
| PPAR-α | AF163809.1 | F: TTGTCGCTGCCATCATTT | 147 |
| R: GAAGTTTCGGGAAGAGGA | |||
| PPAR-γ | NM_001001460.1 | F: CAGTGCAGGAGATTACAG | 87 |
| R: CATATTTCAGGAGGGTTA | |||
| SCD | NM_204890.1 | F: GTTTCCACAACTACCACCAT | 173 |
| R: ATCTCCAGTCCGCATTTT | |||
| SREBP1 | NM_204126.2 | F: GGCAGAGGAAGACAAAGGC | 123 |
| R: AGCAGCAGTGACTCCGAGC | |||
| β-actin | L08165.1 | F: CCCTGTATGCCTCTGGTC | 194 |
| R: CTCGGCTGTGGTGGTGAA |
| Parameters | CON | CORT | PB | CORT&PB | p-Value | ||
|---|---|---|---|---|---|---|---|
| CT | PB | Interaction | |||||
| Liver weight (g) | 26.37 ± 0.65 b | 32.71 ± 1.83 a | 23.71 ± 1.11 b | 32.16 ± 1.78 a | 0.00 | 0.27 | 0.47 |
| Liver index (%) | 2.72 ± 0.05 b | 3.54 ± 0.17 a | 2.37 ± 0.07 b | 3.28 ± 0.16 a | 0.00 | 0.02 | 0.74 |
| Thymus weight(g) | 3.58 ± 0.24 b | 0.91 ± 0.07 a | 3.92 ± 0.52 b | 1.30 ± 0.12 a | 0.00 | 0.25 | 0.93 |
| Thymus index (%) | 0.36 ± 0.03 b | 0.11 ± 0.01 a | 0.39 ± 0.04 b | 0.13 ± 0.01 a | 0.00 | 0.32 | 0.95 |
| Spleen weight (g) | 1.84 ± 0.09 ac | 1.28 ± 0.09 b | 2.07 ± 0.20 a | 1.55 ± 0.17 bc | 0.00 | 0.11 | 0.88 |
| Spleen index (%) | 0.18 ± 0.01 ac | 0.14 ± 0.01 b | 0.21 ± 0.02 a | 0.16 ± 0.02 bc | 0.00 | 0.14 | 0.94 |
| Bursa of fabricius weight(g) | 3.08 ± 0.19 a | 1.22 ± 0.15 b | 3.37 ± 0.37 a | 1.30 ± 0.11 b | 0.00 | 0.41 | 0.66 |
| Bursa of fabricius index (%) | 0.31 ± 0.02 a | 0.12 ± 0.01 b | 0.34 ± 0.03 a | 0.13 ± 0.01 b | 0.00 | 0.32 | 0.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Mei, W.; Chen, Q.; Chen, X.; Ni, Y.; Lei, M.; Liu, J. Probiotic Supplementation Alleviates Corticosterone-Induced Fatty Liver Disease by Regulating Hepatic Lipogenesis and Increasing Gut Microbiota Diversity in Broilers. Microorganisms 2025, 13, 200. https://doi.org/10.3390/microorganisms13010200
Feng Y, Mei W, Chen Q, Chen X, Ni Y, Lei M, Liu J. Probiotic Supplementation Alleviates Corticosterone-Induced Fatty Liver Disease by Regulating Hepatic Lipogenesis and Increasing Gut Microbiota Diversity in Broilers. Microorganisms. 2025; 13(1):200. https://doi.org/10.3390/microorganisms13010200
Chicago/Turabian StyleFeng, Yuyan, Wenqing Mei, Qu Chen, Xiaojing Chen, Yingdong Ni, Mingming Lei, and Jie Liu. 2025. "Probiotic Supplementation Alleviates Corticosterone-Induced Fatty Liver Disease by Regulating Hepatic Lipogenesis and Increasing Gut Microbiota Diversity in Broilers" Microorganisms 13, no. 1: 200. https://doi.org/10.3390/microorganisms13010200
APA StyleFeng, Y., Mei, W., Chen, Q., Chen, X., Ni, Y., Lei, M., & Liu, J. (2025). Probiotic Supplementation Alleviates Corticosterone-Induced Fatty Liver Disease by Regulating Hepatic Lipogenesis and Increasing Gut Microbiota Diversity in Broilers. Microorganisms, 13(1), 200. https://doi.org/10.3390/microorganisms13010200







