Abstract
Alcohol use disorder (AUD) affects millions of people worldwide and can lead to deleterious physical and social consequences. Recent research has highlighted not only the effect of alcohol on the gut microbiome, but also the role of the gut microbiome and the gut–brain axis in the development and maintenance of alcohol use disorder. This review provides an overview of the reciprocal relationship between alcohol consumption and the gut microbiome, including the effects of alcohol on gut microbial composition, changes in gut microbial metabolites in response to alcohol consumption, and how gut microbial metabolites may modulate alcohol use behavior. We also discuss the gut-mediated mechanisms of neuroinflammation that contribute to and result from AUD, including disruption of the intestinal barrier, toll-like receptor signaling, and the activation of glial cells and immune cells. Finally, we review the current evidence on gut microbial-directed therapies for AUD and discuss the implications of this research for our understanding of the pathophysiology of AUD and future research directions.
1. Introduction
Alcohol use disorder (AUD) is a major global health concern characterized by persistent and excessive alcohol consumption, often marked by a loss of control, including the development of tolerance and intense cravings [1,2,3]. AUD is often comorbid with other physical and mental health issues [4] and the consequences of AUD include significant impairment in daily functioning, strained relationships, and reduced productivity. The etiology of AUD is multifactorial, involving genetic, prenatal, and environmental factors, as well as comorbid mental health conditions and stress. Emerging research implicates the gut microbiome as an additional key risk factor for AUD.
The gut–brain axis describes the complex, bidirectional communication network between the central nervous system and the gastrointestinal tract with its trillions of microbial inhabitants. Substances that are consumed orally, including food and alcohol, have a profound impact on the composition and function of the gut microbiota, which in turn can modulate brain activity and behavior, potentially perpetuating dietary habits [5,6]. For example, in response to food consumption, the gut microbiota can modulate the secretion of appetite-related hormones, such as glucagon-like peptide-1, peptide YY, and cholecystokinin, which, in turn, act on the central nervous system to increase satiety and decrease food intake [7,8,9,10]. This reciprocal relationship is also true in chronic heavy alcohol consumption and AUD.
Chronic alcohol consumption in individuals with and without alcoholic liver disease can disrupt the balance of gut microbiota, promoting the growth of aerobic and anaerobic bacteria and altering the composition of mucosa-associated microbiota. This disruption leads to changes in microbial metabolites, such as short-chain fatty acids, indoles, and tryptophan, which in turn influence the production and activity of neurotransmitters. These changes play a critical role in regulating mood, cognition, and addiction, and may contribute to both cognitive impairments and addictive behaviors [11,12,13]. These disruptions can trigger neuroinflammation through multiple mechanisms, including increased gut permeability, microglial activation, and astrocyte dysfunction [14,15]. Ultimately, these changes may contribute to the sustained pattern of alcohol consumption.
In this review, we will examine each stage of this process, from the impact of alcohol consumption on the gut microbiome to the downstream effects on brain function and behavior (Figure 1). We will conclude with a discussion of the therapeutic implications of the gut–brain axis and potential future directions for research on alcohol use disorder.
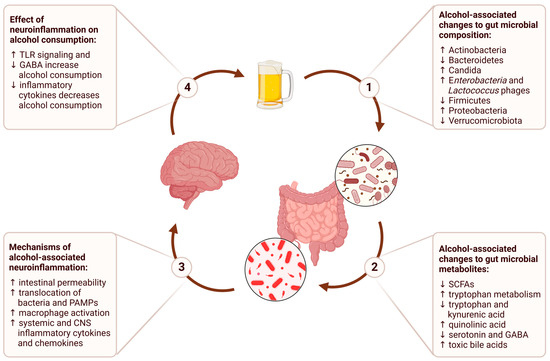
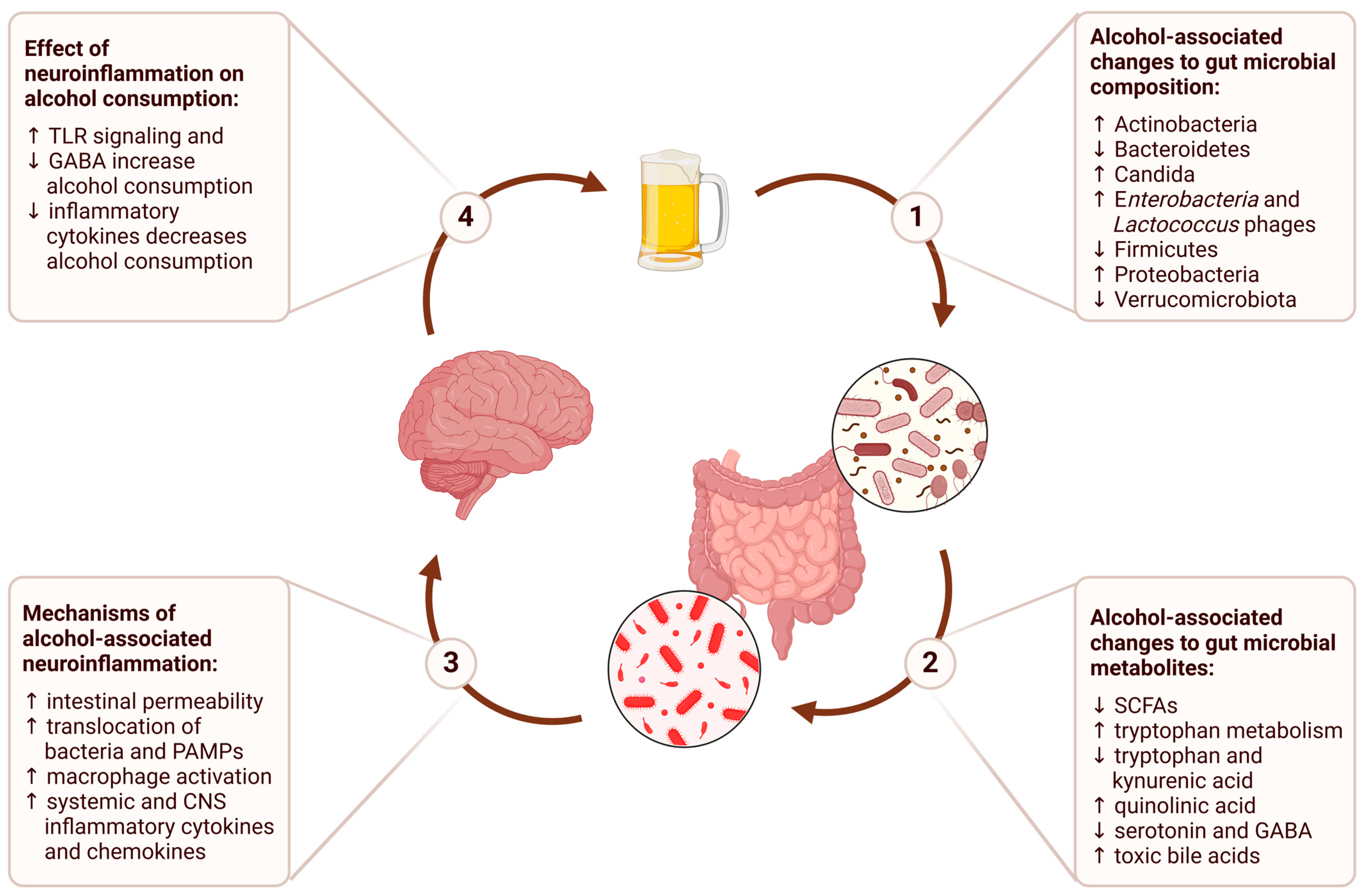
Figure 1.
The gut–brain axis in alcohol use disorder describes a bidirectional communication network where alcohol consumption alters gut microbial composition, leading to changes in gut microbial metabolites that contribute to alcohol-associated neuroinflammation, and ultimately perpetuate a cycle of persistent alcohol consumption.
2. Gut Microbial Composition in Alcohol Use Disorder
The gastrointestinal tract is home to a vast array of microorganisms, including bacteria, archaea, fungi, and viruses, collectively forming the gut microbiota [16,17]. These microbial communities are crucial for synthesizing essential vitamins and amino acids and facilitating the breakdown of macromolecules, while also playing key roles in energy production, drug and toxin metabolism, and the maintenance of the intestinal barrier [18,19,20,21]. Although there is a significant role of diet, lifestyle, and genetic predispositions on the gut microbiome of an individual, both acute and chronic alcohol consumption, even in the absence of liver disease, can profoundly disrupt composition and diversity of the bacterial [22,23], fungal [24], and viral microbiomes [25,26].
Early investigations documented that the levels of Gram-negative anaerobic bacteria in jejunal samples from individuals with alcohol use disorders are markedly higher than in healthy controls [27]. Subsequent studies have confirmed that chronic ethanol consumption leads to decreased abundance of the Bacteroidetes and Firmicutes phyla, alongside increases in Gram-negative Proteobacteria and Gram-positive Actinobacteria [28]. Chronic alcohol consumption is also associated with alterations in the gut fungal microbiome, evidenced by the elevated relative abundances of Candida, Debaryomyces, Pichia, Kluyveromyces, and Issatchenkia in the gut microbiota of individuals with AUD compared to healthy controls [24]. Furthermore, following a period of abstinence, the gut microbiota of individuals with AUD exhibited reduced abundances of Candida and Malassezia, suggesting a reversible impact of alcohol consumption on the gut fungal community. Lastly, analysis of the fecal virome in individuals with AUD revealed a positive correlation between the abundance of Enterobacteria and Lactococcus phages and the severity of liver disease [26].
Alcohol-induced gut dysbiosis leads to intestinal barrier dysfunction, which allows for increased translocation of microbial components to the liver and can exacerbate the progression of liver disease [29,30]. In the presence of concurrent liver disease, continued alcohol consumption is associated with a reduction in beneficial commensal bacteria [31] and an increase in microbiota linked to endotoxin production, such as cytolysin-producing Enterococcus faecalis [32] and candidalysin-producing Candida albicans [33]. Both cytolysin and candidalysin can cause direct hepatocyte injury and death, and also trigger immune activation that exacerbates tissue damage. Alcohol consumption can significantly alter the composition of the gut microbiome, with implications for host health and disease susceptibility.
3. Gut Microbial Metabolites in Alcohol Use Disorder
Alcohol consumption not only affects gut microbial composition, but also microbial functions and metabolites. The gut microbiome produces a vast array of metabolites, including short-chain fatty acids, amino acid derivatives, neurotransmitters, bile acids, and other bioactive compounds, which can exert effects locally in the gastrointestinal tract or systemically through absorption in the bloodstream. For example, short-chain fatty acids produced by the gut microbiome can modulate the immune system, regulate inflammation, and even influence brain function and behavior. Other metabolites, such as tryptophan-derived compounds, can affect the production of neurotransmitters and hormones essential for regulating mood, appetite, sleep, and other physiological processes. Alcohol-induced modifications in gut microbial metabolites can result in hepatic toxicity, increased intestinal permeability, and augmented inflammatory responses, which all play a multifaceted role in shaping brain function. Here, we will highlight some important changes in gut microbial metabolites and their downstream effects secondary to alcohol consumption.
3.1. Short-Chain Fatty Acids (SCFAs)
SCFAs, primarily acetate, propionate, and butyrate in the human gut, are key microbial metabolites produced by gut microbiota through the fermentation of dietary fiber. SCFAs are critical for enterocyte health and maintenance of the intestinal barrier. In both humans with alcohol use disorder (AUD) and animal models of AUD, scientists have found significantly reduced levels of fecal SCFAs in individuals with AUD, as well as a decrease in SCFA-producing bacteria alongside an increase in pro-inflammatory microbes [34,35]. SCFAs can regulate systemic inflammation directly through the production of various inflammatory mediators, including cytokines, chemokines, and eicosanoids, through their action on G protein-coupled receptors (GPCRs) such as FFAR2, FFAR3, GPR109, and Olfr78 [36,37]. SCFAs also indirectly regulate systemic inflammation through maintenance of the intestinal barrier—a reduction in fecal SCFAs leads to increased intestinal permeability, which can heighten the risk of liver injury and systemic inflammation [29]. Increased gut permeability and systemic inflammation, in turn, are positively correlated with symptoms such as depression and alcohol craving, which may contribute to the development and maintenance of alcohol dependence [38].
In addition to affecting gut permeability, SCFAs may also impact alcohol consumption in AUD via several different mechanisms. For example, acetate metabolized from ethanol can be converted to acetyl-CoA and deposited into the histones of neurons [39]. In the hippocampus, alcohol-induced histone acetylation has been found to upregulate transcriptional programs important for alcohol-related associative learning [39]. Acetate may also have an effect on cerebral blood flow, which could modulate neuroinflammation [40,41,42]. Although the effects of acetate on the epigenetic modulation of neuronal genes and its involvement in driving neuroinflammation have been extensively studied in mouse models, one of the limitations of these findings is that they still require validation in human studies.
SCFAs can also travel through the blood–brain barrier (BBB) to directly affect neuronal health. The endothelial cells of the BBB contain monocarboxylate transporters (MCTs) that enhance BBB permeability to SCFAs. Butyrate has been shown to benefit mice exposed to chronic stress by effectively counteracting depressive behaviors associated with prolonged stress exposure [43]. Furthermore, administration of butyrate resulted in elevated serotonin levels in the hippocampus and an increase in the expression of brain-derived neurotrophic factor (BDNF) [44]. Both depression symptoms and serotonin levels are tightly interconnected with alcohol consumption in people with AUD.
SCFAs can affect behaviors related to stress and substance use, including addiction. In rats, sodium butyrate has been found to mitigate alcohol-induced liver damage and inflammation while also exhibiting antidepressant-like effects [45]. One specific SCFA, valeric acid, has been shown to decrease binge drinking and anxiety-like behaviors. Mice that were supplemented with valeric acid exhibited a 40% reduction in alcohol consumption and had blood alcohol levels that were 53% lower [46]. Additionally, a recent study found that decreased levels of fecal isovalerate, an isomer of valeric acid, were associated with depression in humans [47]. Although the precise mechanisms through which SCFAs influence alcohol consumption and addiction remain unclear, they may involve the modulation of gene expression and epigenetic alterations in the brain.
3.2. Tryptophan Metabolites
Tryptophan, an essential amino acid, is not produced by animal cells and is primarily obtained through dietary intake. The gut microbiota play a crucial role in tryptophan metabolism, converting it into bioactive compounds such as aryl hydrocarbon receptor (AhR) ligands and serotonin when the indole ring remains intact [48]. However, when the indole ring is oxidatively cleaved, tryptophan is redirected into the kynurenine pathway, reducing its availability for neurotransmitter and indole synthesis [49].
In mammals, the majority of tryptophan is catabolized through the kynurenine pathway by indoleamine 2,3-dioxygenase 1 (IDO1) into kynurenine and downstream products such as kynurenic acid and quinolinic acid [50]. Gut microbiota are a critical stimulator of IDO1 activity—animals without gut microbiota demonstrate reduced tryptophan metabolism through the kynurenine pathway, an effect that is reversed upon recolonization [51]. Kynurenic acid is known for its neuroprotective and anticonvulsant effects. It acts as a competitive antagonist of N-methyl-D-aspartate (NMDA) receptors and activates G protein-coupled receptor 35, modulating cyclic AMP production and inhibiting N-type Ca2⁺ channels in sympathetic neurons and astrocytes to suppress inflammatory pathways [52,53]. In contrast, quinolinic acid is an NMDA receptor agonist that promotes inflammation and oxidative stress through the generation of reactive oxygen species, depletion of endogenous antioxidants, and promotion of lipid peroxidation [54,55].
In the context of AUD, alcohol consumption and chronic inflammation also stimulate the production of IDO1, leading to increased tryptophan metabolism via the kynurenine pathway [56,57]. This results in depletion of tryptophan and neuroprotective kynurenic acid, and the accumulation of neurotoxic quinolinic acid. One study observed significant changes in kynurenine metabolites following a 3-week detoxification program, including elevations in kynurenine and reductions in 3-hydroxykynurenine and xanthurenic acid, alongside increased quinolinic acid levels [58,59]. Furthermore, research in rats has shown that the metabolites 3-hydroxykynurenine, kynurenic acid, and 3-hydroxyanthranilic acid inhibit alcohol dehydrogenase, leading to the accumulation of toxic metabolite acetaldehyde and increased aversion to alcohol [60]. These findings suggest that the gut microbiota’s regulation of tryptophan metabolism via the kynurenine pathway in the context of alcohol consumption may contribute to alcohol-seeking behaviors through multiple mechanisms, including brain inflammation and acetaldehyde accumulation.
3.3. Neurotransmitters
The production and modulation of neurotransmitters such as serotonin, glutamate, and γ-aminobutyric acid (GABA) by gut microbiota is also affected by alcohol consumption. Most of the body’s serotonin is produced by enterochromaffin cells in the gut, and the gut microbiota play a crucial role in regulating serotonin production [61]. Studies utilizing germ-free mice have demonstrated that the absence of gut microbiota results in significantly reduced serotonin production in the colon and decreased circulating serotonin levels [62]. The gut microbiota is thought to modulate serotonin production directly through the induction of tryptophan hydroxylase 1 (TpH1) expression, a rate-limiting enzyme in the serotonin biosynthetic pathway [63]. Additionally, the production of secondary bile acids by gut microbiota may also contribute to the stimulation of serotonin biosynthesis. As discussed above, induction of tryptophan catabolism via the kynurenine pathway by alcohol consumption decreases tryptophan availability for serotonin synthesis, and this is exacerbated by alcohol-induced gut dysbiosis. For example, decreased levels of serotonin and serotonin metabolites were found in the hippocampus and serum of conventional mice compared to germ-free mice [64]. This dysregulation triggers neuroinflammation via serotonin binding to serotonin (5-HT) receptors on microglia [65].
Gut microbes can also regulate levels of GABA by consuming it via the GABA shunt, a metabolic pathway that converts GABA to succinate, as a carbon and nitrogen source, or by producing it as a way to modulate intracellular pH [66]. Germ-free animals exhibit significantly reduced luminal and serum, but not cerebral, GABA levels [67]. Chronic alcohol consumption leads to gut dysbiosis, which has been shown to impair the conversion of glutamate to GABA by beneficial bacteria like Lactobacillus and Bifidobacterium, subsequently reducing GABA levels and increasing neuronal excitability [68]. Additionally, the systemic inflammation caused by alcohol-induced increases in gut permeability and translocation of lipopolysaccharide products from the outer membrane of Gram-negative bacteria (discussed in more detail later in this review) attenuates GABA synthesis and receptor activity in the spinal dorsal horn [69]. A clinical study by Kirsten et al. found that brain GABA levels are inversely correlated with the severity of alcohol-associated liver disease, highlighting the importance of GABA in maintaining neural balance [70]. These findings suggest that alterations in the gut microbiota by alcohol consumption lead to dysregulation of neurotransmitters and may exacerbate the neuropsychiatric consequences of alcohol abuse.
3.4. Bile Acids
Primary bile acids are synthesized by hepatocytes and released via bile ducts into the gastrointestinal tract where they are metabolized by gut microbiota into secondary bile acids. The process of bile acid metabolism begins with bile acid deconjugation, or hydrolysis of the amino acid moiety, via gut bacterial bile salt hydrolases, which are highly conserved across all major gut microbial phyla [71]. Dysbiosis caused by chronic alcohol consumption disrupts bile acid homeostasis and leads to a systemic increase in bile acid levels [72]. Specifically, alcohol consumption is associated with elevated levels of toxic bile acids, including chenodeoxycholic acid, deoxycholic acid, and lithocholic acid, in enterohepatic circulation, which contribute to the pathogenesis of alcohol-associated liver injury. A recent study suggested that bile acids can not only affect the development of liver disease, but modulation of intrahepatic bile flow, including through the use of bile acid transport inhibitors, could also affect drinking behavior through the alteration of serum acetaldehyde levels [73].
4. Gut-Mediated Mechanisms of Neuroinflammation in Alcohol Use Disorder
Modulation of gut microbial composition and metabolites by chronic alcohol consumption can lead to neuroinflammation through a variety of different mechanisms (Figure 2). First, alcohol-induced gut dysbiosis promotes systemic inflammation and, indirectly, neuroinflammation, by increasing intestinal permeability and allowing for the direct translocation of bacteria and inflammatory products, as well as pathogen-associated molecular patterns that trigger inflammatory signaling cascades. Second, alcohol consumption more directly causes neuroinflammation via damage to the blood–brain barrier and activation of microglia and astrocytes. In this subsection, we will review how alcohol consumption can induce neuroinflammatory responses that elicit adverse effects on neuronal health and cognitive behavior.
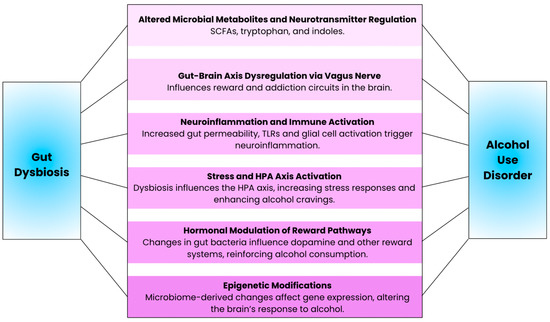
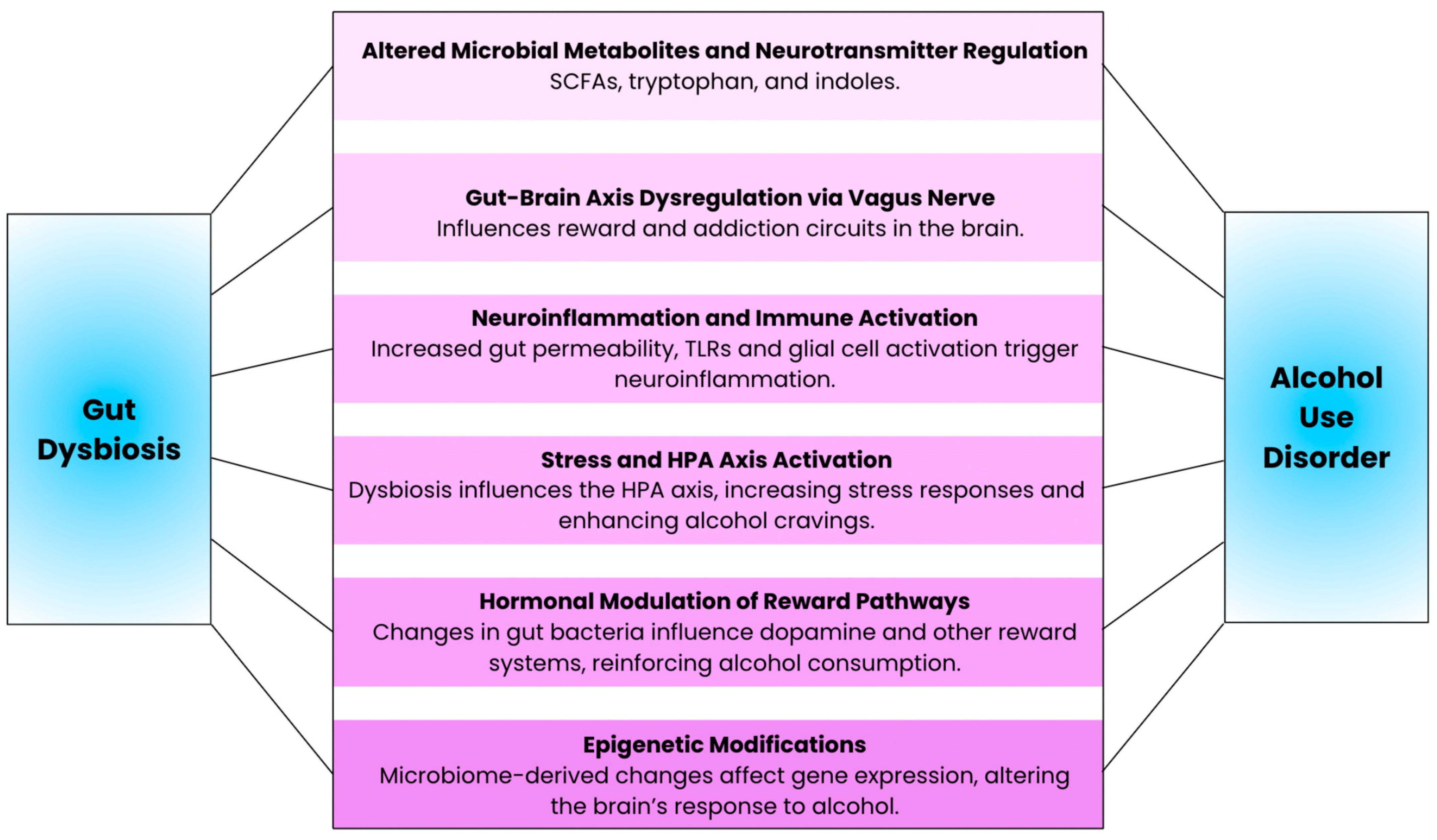
Figure 2.
Illustration of the key mechanisms underlying the connection between gut dysbiosis and alcohol use disorder.
4.1. Disruption of the Intestinal Barrier
Several studies show that individuals with alcohol dependence exhibit increased gut permeability, which correlates with elevated levels of depression, anxiety, and cravings for alcohol [74,75]. Studies using animal models of alcohol-associated liver disease have demonstrated that modulating the gut microbiome with dietary fibers or probiotics such as Lactobacillus GG can reduce endotoxemia, inflammation, and liver damage by reducing gut permeability [76]. The intestinal barrier consists of tight junctions and adherens junctions between intestinal epithelial cells. Proteins such as claudin and occludin help maintain this barrier, allowing for the selective passage of nutrients while preventing the entry of harmful bacteria into the bloodstream. Alcohol consumption disrupts the intestinal barrier by altering the expression of tight junction-associated proteins such as ZO-1, claudin-1, claudin-5, and claudin-7 through the activation of PKCα [77,78]. Alcohol consumption also induces the production of reactive oxygen species and nitric oxide [79]. Additionally, alcohol upregulates CYP2E1 expression in gut epithelial cells and activates the nuclear factor kappa B (NF-κB) pathway, leading to cytoskeletal disruption and increased intestinal permeability [80]. As a result, harmful bacterial endotoxins, including lipopolysaccharides and peptidoglycans, are allowed to enter the systemic circulation, triggering systemic inflammatory responses [38,81].
Key cytokines, including tumor necrosis factor (TNF), interferon-gamma (IFN-γ), and interleukin-1β (IL-1β), act as pivotal mediators in this process. Chronic and acute ethanol exposure have been shown to increase TNF-α production, which can lead to glutamatergic excitotoxicity [82]. Additionally, IFN-γ enhances the effects of TNF-α by increasing the expression of TNF receptors on epithelial cells, amplifying intestinal permeability [83]. The upregulation of adhesion molecules such as ICAM-1, driven by IFN-γ, facilitates neutrophil migration into subepithelial spaces, exacerbating tissue damage and inflammation [84].
Alcohol also affects intestinal immune cells, which play an essential role in gut permeability. For example, alcohol induces M2b polarization of macrophages, which can contribute to inflammation and tissue damage [85]. Recent research highlights the importance of intestinal macrophages in regulating inflammation during dysbiosis and maintaining epithelial barrier function. These immune cells not only promote the survival of FOXP3+ regulatory T cells, which are essential for maintaining immune tolerance, but they also contribute to the preservation of epithelial barrier function [86].
In a study of patients with AUD, a subset of individuals exhibited increased gut permeability, and this was associated with altered gut microbiota composition and activity as well as higher scores of depression, anxiety, and alcohol craving after abstinence [74]. These results suggest the importance of gut barrier function in the development and maintenance of alcohol dependence.
4.2. Toll-like Receptors
Toll-like receptors (TLRs) play a crucial role in the innate immune response, serving as key sensors for microbial detection and maintaining intestinal homeostasis. Among the various TLRs, TLR2, TLR3, TLR4, and TLR7 have been shown to be significantly affected by alcohol consumption [87]. Following ethanol intake, these receptors exhibit increased expression in the prefrontal cortex, a region vital for cognitive function, highlighting the impact of alcohol on neuroimmune signaling [88,89].
Concomitantly, these TLRs are also involved in alcohol addiction and AUD. Alcohol-induced activation of TLR3 has been shown to trigger a pro-inflammatory response in the hippocampus, characterized by elevated levels of cytokines such as IL-1β, IL-6, and TNF-α, which contribute to the development of neuroinflammation. Conversely, activation of TLR3 enhances voluntary alcohol consumption, suggesting a feedback mechanism that may contribute to continued alcohol consumption [90].
Acute activation of TLR7 has been shown to reduce ethanol intake and preference, likely due to an acute sickness response, whereas chronic activation of TLR7 via treatment with an agonist leads to tolerance and increased ethanol consumption [91]. Postmortem analysis of human brains from individuals with AUD has revealed elevated TLR7 expression and increased microglial activation, supporting the connection between alcohol, TLR signaling, and neuroinflammation [92].
Many studies have shown that inhibition of TLR4 decreases binge drinking behaviors [93,94]. For example, mice treated with lipopolysaccharide, a bacterial endotoxin, demonstrated persistent increases in alcohol consumption, whereas mice lacking CD14, a key component of TLR4 signaling, showed no increases in alcohol intake after treatment with lipopolysaccharide [95]. Additionally, rodents lacking TLR2 and TLR4 show protection against ethanol-induced neuroinflammatory responses and cognitive impairments, underscoring the importance of these receptors in mediating alcohol’s detrimental effects on the central nervous system [96,97].
4.3. Glial Cells and Neuroimmune Function
Glial cells, which include astrocytes and microglia, are non-neuronal cells that play a crucial role in maintaining the health and function of the nervous system by providing structural and metabolic support to neurons, regulating neurotransmitter activity, removing waste products, maintaining the blood–brain barrier, and contributing to the neuroimmune response. Many studies have highlighted their role in the gut–brain axis in the pathophysiology of AUD.
Astrocytes play a crucial role in regulating various aspects of AUD, including arousal, cortical sensory processing, and the experience of reward [98]. Studies in both mice and humans have shown that the ethanol-dependent brain exhibits significant dysregulation of genes associated with astrocyte function [99,100,101]. One such gene, glial fibrillary acidic protein (GFAP), an intermediate filament-III protein involved in astroglial cell activation following CNS injury and neurodegeneration, exhibits elevated expression in response to both acute and chronic ethanol exposure [102]. The expression of other genes, such as toll-like receptors and receptors for inflammatory cytokines, are also upregulated in astrocytes in response to alcohol. TNF-α and IL-1β disrupt astrocytic glutamate uptake, resulting in an accumulation of extracellular glutamate and the induction of neurotoxicity. The development and function of astrocytes are influenced by gut microbiota. The administration of lactic acid bacteria during the early developmental stages of rats significantly influences astrocyte maturation [103]. Moreover, changes in fecal microbiota and metabolites can affect astrocyte phenotypes and the development of age-related cognitive decline in mice [104].
The host microbiota also play a crucial role in maintaining microglial homeostasis. For example, germ-free mice exhibit widespread abnormalities in their microglia, including altered cell subtypes and an immature phenotype, which impairs innate immune responses [105,106]. Moreover, temporary removal of the host microbiota significantly alters microglial properties, and a limited diversity of microbiota leads to dysfunctional microglia [107]. The gut microbiome also influences the reciprocal transformation of microglial subpopulations in the prefrontal cortex and hippocampus [108]. Notably, this interaction is bidirectional, with microglia expressing pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6, as well as trophic factors that mediate the interactions between the host microbiome and the brain [109].
In the setting of alcohol exposure, microglia, the resident immune cells of the CNS, play a pivotal role in neuroimmune activation and neuroinflammation [110]. Microglial activation, as evidenced by increased expression of ionized calcium-binding adaptor molecule 1 (IBA1), is a hallmark of both acute and chronic ethanol exposure in murine models, as well as in postmortem brain tissue from individuals with AUD [111,112]. This activation is mediated by a complex interplay of immune signaling pathways, including the activation of NF-κB, NADPH oxidase, and the production of reactive oxygen species [112]. Microglia are also activated by cytokine and TLR signaling, triggered by the recognition of pathogen-associated molecular patterns (PAMPs), and this is further exacerbated by the increased gut permeability and translocation of systemic inflammatory mediators caused by alcohol exposure.
Neuroinflammation is associated with mood and behavioral changes, such as the development of depression [113]. Postmortem brain tissue analyses have shown that individuals with depression exhibit increased microglial activation in cortical regions that are commonly implicated in depression [114]. Further, the administration of the IL-1β receptor antagonist IL-1Ra could mitigate anhedonic stress-induced behavior in mice [115]. While the exact neuronal mechanisms underlying this phenomenon are not yet fully understood, evidence suggests that neuroinflammation may plausibly contribute to symptoms of alcohol withdrawal and alcohol craving [116].
5. Gut Microbial Therapies for AUD
These recent advances in the understanding the gut–brain axis have led to several human clinical trials investigating the therapeutic use of gut microbiota-targeting interventions for the treatment of AUD and other mental health conditions that are often associated with increased alcohol consumption, with varying benefits (Table 1 and Table 2, ordered chronologically).
In the realm of prebiotics, one published trial using the prebiotic inulin in patients with severe AUD did not demonstrate any effect on alcohol craving [117]. Another study investigating the use of short chain fatty acid prebiotics in people living with HIV with and without AUD on intestinal barrier function, systemic inflammation, and brain pathology is currently recruiting (https://clinicaltrials.gov/study/NCT06139224, accessed on 16 November 2024). Colonic delivery of SCFAs also appeared to improve responses to stressors in healthy individuals [118,119]. Supplementation with probiotics also appeared to reduced symptoms of stress, anxiety, and psychological distress in stressed adults and improve depressive symptoms in patients with major depression disorder [120,121,122].
Therapies that directly modify gut microbiota include one study which used the probiotic Lactobacillus rhamnosus GG in patients with AUD and moderate alcohol-associated hepatitis, which showed improvements in alcohol craving [123], and another study using the probiotic VSL#3 in patients with AUD and alcohol-associated liver disease (https://clinicaltrials.gov/study/NCT05007470, accessed on 16 November 2024), which is still ongoing. Two studies using fecal microbiota transplantation (FMT) in patients with alcohol use disorder and cirrhosis also demonstrated improvements in alcohol craving [124,125]. Indirect modification of the gut microbiota using the antibiotic minocycline did not demonstrate any effect on alcohol craving in patients with AUD in one study [126], and another study examining the effects of minocycline on neuroinflammation, alcohol cue reactivity, neurocognitive performance, and alcohol use has been completed but not yet published (https://clinicaltrials.gov/study/NCT04210713, accessed on 16 November 2024). Larger and more comprehensive studies are warranted to determine the optimal delivery methods, treatment duration, and specific gut microbial targets for the effective treatment of AUD and associated mental health disorders.
Another important area of research is the development of biomarkers that can be used to (1) identify patients with AUD who are more likely to have a positive response to specific microbiome-based therapies, (2) monitoring changes in the composition and function of the gut microbiome in response to therapy, and (3) predict those who are more likely to have severe or life-threatening outcomes, such as severe alcohol withdrawal or alcohol-associated liver disease. Additionally, better understanding the impact of existing approved medications for AUD (naltrexone, acamprosate, and disulfiram in the United States, and additionally nalmefene in Europe) on the gut microbiome could provide valuable insights into the variability of treatment responses in patients with AUD.

Table 1.
Published Human Clinical Trials Targeting the Gut Microbiome for the Treatment of AUD.
Table 1.
Published Human Clinical Trials Targeting the Gut Microbiome for the Treatment of AUD.
| Reference | Study Design | Outcomes | Results | Limitations |
|---|---|---|---|---|
| Petrakis, et al., 2019 [126] | Randomized, double-blind, placebo-controlled study of 49 heavy drinkers (≥7/14 standard alcoholic drinks per week for females/males) who received placebo (n = 20), 100 mg (n = 12), or 200 mg (n = 17) of minocycline daily for 10 days |
|
|
|
| Bajaj, et al., 2021 [124] | Randomized, double-blind clinical trial of 20 patients with AUD and alcohol-associated cirrhosis who received one placebo (n = 10) or FMT (n = 10) enema from from a donor enriched in Lachnospiraceae and Ruminococcaceae |
|
|
|
| Amadieu, et al., 2022 [117] | Randomized, double-blind, placebo-controlled study of 43 patients with severe AUD (DSM-5 ≥ 6 criteria) who received daily inulin (uptitration to 16 g per day, n = 22) or placebo (maltodextrin, n = 21) daily for 17 days |
|
|
|
| Philips, et al., 2022 [125] | Retrospective analysis of 61 patients with severe alcohol-associated hepatitis who underwent FMT (n = 35) or standard of care (n = 26) |
|
|
|
| Vatsalya, et al., 2023 [123] | Randomized, double-blind, placebo-controlled study of 46 patients with AUD and moderate alcohol-associated hepatitis (MELD between 12 and 20) who received daily oral Lactobacillus rhamnosus GG (n = 24) or placebo (n = 22) for 6 months |
|
|
|
ALT, alanine aminotransferase; AST, aspartate aminotransferase; DSM, Diagnostic and Statistical Manual of Mental Disorders; FMT, fecal microbiota transplantation; MELD, Model for End-Stage Liver Disease.

Table 2.
Published Human Clinical Trials Targeting the Gut Microbiome for the Treatment of Mental Health Conditions Associated with AUD.
Table 2.
Published Human Clinical Trials Targeting the Gut Microbiome for the Treatment of Mental Health Conditions Associated with AUD.
| Reference | Study Design | Results |
|---|---|---|
| Chong, 2019 [120] | Randomized, double-blind, placebo-controlled study of 111 stressed adults (based on moderate stress levels using the PSS-10 questionnaire) who received either Lactobacillus plantarum DR7 (109 CFU/day, n = 56) or placebo (n = 55) daily for 12 weeks |
|
| Rudzki, 2019 [121] | Randomized, double-blind, placebo-controlled study of 60 patients with major depression disorder who received either SSRIs with the probiotic Lactobacillus Plantarum 299v (n = 30) or SSRIs with placebo (n = 30) for 8 weeks |
|
| Dalile, 2020 [118] | Randomized, triple-blind, placebo-controlled study of 65 healthy males who received colonic SCFA mixture containing 10 g (n = 22) or 20 g (n = 21) of arabinoxylan oligosaccharides or placebo daily (n = 22) for one week |
|
| Tian, 2022 [122] | Randomized, double-blind, placebo-controlled study of 45 patients with major depression disorder who received Bifidobacterium breve CCFM1025 (freeze-dried, 1010 CFU of viable bacteria, n = 20) or placebo (maltodextrin, n = 25) daily for four weeks |
|
| Dalile, 2024 [119] | Randomized, triple-blind, placebo-controlled study of 71 healthy males who received colon-delivery capsules of 5.28 g of butyrate (n = 35) or placebo (n = 36) daily for one week |
|
CFU, colony forming unit; SCFA, short chain fatty acid; SSRI, selective serotonin reuptake inhibitor.
6. Limitations to Existing Research on the Gut–Brain Axis
Despite advances in the understanding of the gut–brain axis in AUD, there are several limitations to the gut microbiome studies carried out in both preclinical models and human studies. Rodent models are hindered by their inability to recapitulate some human-specific social, environmental, and subjective factors, and more human-specific symptoms, such as alcohol craving [127]. Additionally, there are many important differences between rodent and human gastrointestinal tract anatomy and gut microbial composition that may affect the translatability of results in rodent models. For example, the murine stomach features a non-glandular forestomach, absent in humans, which stores food and harbors a high abundance of Lactobacillus species due to its relatively higher pH (pH 3–4) compared to the human stomach (pH 1) [128]. This, along with other anatomical differences, contributes to distinct gut microbial compositions between humans and mice. For instance, healthy humans harbor a higher abundance of the beneficial Faecalibacterium species, which are often reduced in individuals with excessive alcohol consumption, while Faecalibacterium are very rarely found in laboratory mice [129]. Notably, despite these differences, human and murine gut microbiota do share about 90% similarity in phyla, predominantly comprising Bacteroidetes and Firmicutes [130]. Nevertheless, when applying results from rodent models to human disease, these anatomic and gut microbial differences should be considered. Gnotobiotic mice offer a means to approximate the human microbiome in a murine model, but they do not perfectly replicate the conditions of the human gut [131]. Germ-free mice are raised in sterile environments and are immunocompromised, even after colonization with human microbiota [132]. Furthermore, only a subset of the human microbiota inoculum (approximately 50%) is retained in the recipient mouse microbiota, with certain genera such as Faecalibacterium and Bifidobacterium being significantly reduced or lost, while others, like Bacteroides, exhibit a marked increase in relative abundance following inoculation [133]. Notwithstanding these limitations, the majority of metabolomic features present in donor samples are successfully recapitulated in colonized gnotobiotic mice, suggesting that the functional properties of the gut microbiota can be effectively replicated in this model [134]. And indeed, germ-free mice colonized with microbiota from humans with cirrhosis and AUD who showed improved clinical outcomes following FMT exhibited reduced alcohol consumption and preference, suggesting that AUD-related microbiome alterations are transmissible to a gnotobiotic mouse model and can be utilized to investigate the therapeutic effects of microbiome modulation [135].
Human studies of the gut–brain axis in AUD are also subject to limitations. Most studies rely on 16S rRNA gene sequencing to compare gut microbial composition between patients with AUD and healthy controls. While this approach provides a snapshot of microbial diversity, it lacks functional insights [136,137]. To gain a deeper understanding of the gut–brain axis, future studies should employ a more comprehensive range of techniques, including metagenomic, meta-transcriptomic, and metabolomic analyses, to assess both gut microbial composition and function. Another limitation of current research is its reliance on stool samples, which neglects the distinct bacterial populations residing in the small intestine and mucosal communities [138]. Furthermore, patients with AUD often exhibit altered dietary habits and are at increased risk of malnutrition [139], which may be a confounding variable in gut microbiome analyses. For example, a study of 50 patients with AUD found that they had lower intake of essential nutrients and dietary fibers and consumed more ultra-processed foods compared to healthy subjects, which was associated with higher anxiety, lower sociability, and intestinal discomfort [140]. Chronic alcohol consumption can also injure the small intestinal mucosa and damage the brush border membrane of enterocytes where nutrients are absorbed, leading to disruption of macronutrient and micronutrient absorption [141]. Therefore, it is essential to accurately assess dietary and nutritional status in patients with AUD and consider it as a potential confounding factor in future studies.
To enhance the validity and reliability of future findings, several key concerns should be addressed. Variability in results can stem from factors such as sample size, sample timing and methodology, DNA extraction, sequencing techniques, and data analysis [142]. Moreover, studies should strive to account for confounding variables, including age, gender, antibiotic or probiotic use, diet, and supplements, to the best of their ability. Careful selection of control groups is also crucial to avoid introducing bias. By addressing these limitations, future studies can provide more accurate and reliable insights into the gut–brain axis in AUD.
7. Conclusions
The gut–brain axis plays a critical role in the pathophysiology of AUD, with alcohol-induced gut dysbiosis profoundly affecting microbial composition, metabolite production, and neuroimmune pathways. These disruptions contribute to neuroinflammation and addiction cycles, underscoring the gut as a promising therapeutic target. Emerging interventions, including probiotics, prebiotics, and fecal microbiota transplantation, have shown potential in reducing alcohol cravings and improving neuropsychiatric symptoms. While promising, these therapies require larger clinical trials to validate their efficacy and determine optimal application strategies. Identifying microbial biomarkers may pave the way for personalized treatments, enabling tailored interventions and better patient outcomes. However, significant knowledge gaps persist, particularly in the mechanistic understanding of gut–brain interactions in AUD. Addressing these gaps through targeted research could clarify the bidirectional impacts of alcohol on gut and brain health. Such advancements will be vital to improving therapeutic strategies and transforming our approach to this complex disorder.
Funding
This work was supported by grant K99 AA031328 from the NIH/NIAAA and grant #CTORA23-208366 from the American Association for the Study of Liver Diseases Foundation (to C.L.H).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Grant, B.F.; Goldstein, R.B.; Saha, T.D.; Chou, S.P.; Jung, J.; Zhang, H.; Pickering, R.P.; Ruan, W.J.; Smith, S.M.; Huang, B.; et al. Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry 2015, 72, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef]
- Cargiulo, T. Understanding the health impact of alcohol dependence. Am. J. Health Pharm. 2007, 64 (Suppl. S3), S5–S11. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Ross, F.C.; Patangia, D.; Grimaud, G.; Lavelle, A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. The interplay between diet and the gut microbiome: Implications for health and disease. Nat. Rev. Microbiol. 2024, 22, 671–686. [Google Scholar] [CrossRef]
- Han, H.; Yi, B.; Zhong, R.; Wang, M.; Zhang, S.; Ma, J.; Yin, Y.; Yin, J.; Chen, L.; Zhang, H. From gut microbiota to host appetite: Gut microbiota-derived metabolites as key regulators. Microbiome 2021, 9, 162. [Google Scholar] [CrossRef]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef]
- Farzi, A.; Ip, C.K.; Reed, F.; Enriquez, R.; Zenz, G.; Durdevic, M.; Zhang, L.; Holzer, P.; Herzog, H. Lack of peptide YY signaling in mice disturbs gut microbiome composition in response to high-fat diet. FASEB J. 2021, 35, e21435. [Google Scholar] [CrossRef]
- Dockray, G.J. Cholecystokinin and gut–brain signalling. Regul. Pept. 2009, 155, 6–10. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Gut microbiota-derived metabolites mediate the neuroprotective effect of melatonin in cognitive impairment induced by sleep deprivation. Microbiome 2023, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Du, H.; Li, Z.; Xiong, J.; Liu, Y.; Li, Y.; Zhang, W.; Liang, F.; He, J.; Liu, X.; et al. Decoding the contributions of gut microbiota and cerebral metabolism in acute liver injury mice with and without cognitive dysfunction. CNS Neurosci. Ther. 2022, 29 (Suppl. S1), 31–42. [Google Scholar] [CrossRef]
- Qu, S.; Yu, Z.; Zhou, Y.; Wang, S.; Jia, M.; Chen, T.; Zhang, X. Gut microbiota modulates neurotransmitter and gut-brain signaling. Microbiol. Res. 2024, 287, 127858. [Google Scholar] [CrossRef] [PubMed]
- Engen, P.A.; Green, S.J.; Voigt, R.M.; Forsyth, C.B.; Keshavarzian, A. The Gastrointestinal Microbiome: Alcohol Effects on the Composition of Intestinal Microbiota. Alcohol. Res. 2015, 37, 223–236. [Google Scholar] [PubMed]
- Shukla, S.; Saxena, A.; Shukla, S.K.; Nazir, A. Modulation of Neurotransmitter Pathways and Associated Metabolites by Systemic Silencing of Gut Genes in C. elegans. Diagnostics 2023, 13, 2322. [Google Scholar] [CrossRef]
- Brussow, H. The human microbiome project at ten years—Some critical comments and reflections on “our third genome”, the human virome. Microbiome Res. Rep. 2023, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef]
- Agus, A.; Clément, K.; Sokol, H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut 2021, 70, 1174–1182. [Google Scholar] [CrossRef]
- Weersma, R.K.; Zhernakova, A.; Fu, J. Interaction between drugs and the gut microbiome. Gut 2020, 69, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Ragsdale, S.W. Not a “they” but a “we”: The microbiome helps promote our well-being. J. Biol. Chem. 2022, 298, 101511. [Google Scholar] [CrossRef] [PubMed]
- Ames, N.J.; Barb, J.J.; Schuebel, K.; Mudra, S.; Meeks, B.K.; Tuason, R.T.S.; Brooks, A.T.; Kazmi, N.; Yang, S.; Ratteree, K.; et al. Longitudinal gut microbiome changes in alcohol use disorder are influenced by abstinence and drinking quantity. Gut Microbes 2020, 11, 1608–1631. [Google Scholar] [CrossRef] [PubMed]
- Piacentino, D.; Vizioli, C.; Barb, J.J.; Grant-Beurmann, S.; Bouhlal, S.; Battista, J.T.; Jennings, O.; Lee, M.R.; Schwandt, M.L.; Walter, P.; et al. Gut microbial diversity and functional characterization in people with alcohol use disorder: A case-control study. PLoS ONE 2024, 19, e0302195. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, P.; Lang, S.; Zeng, S.; Duan, Y.; Zhang, X.; Wang, Y.; Bondareva, M.; Kruglov, A.; Fouts, D.E.; Stärkel, P.; et al. Dynamic Changes of the Fungal Microbiome in Alcohol Use Disorder. Front. Physiol. 2021, 12, 699253. [Google Scholar] [CrossRef]
- Hsu, C.L.; Lang, S.; Demir, M.; Fouts, D.E.; Stärkel, P.; Schnabl, B. Any alcohol use in NAFLD patients is associated with significant changes to the intestinal virome. Hepatology 2023, 77, 2073–2083. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Zhang, X.; Jiang, L.; Lang, S.; Hartmann, P.; Pride, D.; Fouts, D.E.; Stärkel, P.; Schnabl, B. Intestinal virome in patients with alcohol use disorder and after abstinence. Hepatol. Commun. 2022, 6, 2058–2069. [Google Scholar] [CrossRef] [PubMed]
- Bode, J.C.; Bode, C.; Heidelbach, R.; Dürr, H.K.; Martini, G.A. Jejunal microflora in patients with chronic alcohol abuse. Hepatogastroenterology 1984, 31, 30–34. [Google Scholar]
- Bull-Otterson, L.; Feng, W.; Kirpich, I.; Wang, Y.; Qin, X.; Liu, Y.; Gobejishvili, L.; Joshi-Barve, S.; Ayvaz, T.; Petrosino, J.; et al. Metagenomic Analyses of Alcohol Induced Pathogenic Alterations in the Intestinal Microbiome and the Effect of Lactobacillus rhamnosus GG Treatment. PLoS ONE 2013, 8, e53028. [Google Scholar] [CrossRef]
- Jew, M.H.; Hsu, C.L. Alcohol, the gut microbiome, and liver disease. J. Gastroenterol. Hepatol. 2023, 38, 1205–1210. [Google Scholar] [CrossRef]
- Hsu, C.L.; Wang, Y.; Duan, Y.; Chu, H.; Hartmann, P.; Llorente, C.; Zhou, R.; Schnabl, B. Differences in Bacterial Translocation and Liver Injury in Ethanol Versus Diet-Induced Liver Disease. Dig. Dis. Sci. 2023, 68, 3059–3069. [Google Scholar] [CrossRef]
- Hsu, C.L.; Schnabl, B. The gut–liver axis and gut microbiota in health and liver disease. Nat. Rev. Microbiol. 2023, 21, 719–733. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Llorente, C.; Lang, S.; Brandl, K.; Chu, H.; Jiang, L.; White, R.C.; Clarke, T.H.; Nguyen, K.; Torralba, M.; et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature 2019, 575, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Duan, Y.; Lang, S.; Jiang, L.; Wang, Y.; Llorente, C.; Liu, J.; Mogavero, S.; Bosques-Padilla, F.; Abraldes, J.G.; et al. The Candida albicans exotoxin candidalysin promotes alcohol-associated liver disease. J. Hepatol. 2020, 72, 391–400. [Google Scholar] [CrossRef]
- Couch, R.D.; Dailey, A.; Zaidi, F.; Navarro, K.; Forsyth, C.B.; Mutlu, E.; Engen, P.A.; Keshavarzian, A. Alcohol induced alterations to the human fecal VOC metabolome. PLoS ONE 2015, 10, e0119362. [Google Scholar] [CrossRef]
- Bjørkhaug, S.T.; Aanes, H.; Neupane, S.P.; Bramness, J.G.; Malvik, S.; Henriksen, C.; Skar, V.; Medhus, A.W.; Valeur, J. Characterization of gut microbiota composition and functions in patients with chronic alcohol overconsumption. Gut Microbes 2019, 10, 663–675. [Google Scholar] [CrossRef]
- Pluznick, J. A novel SCFA receptor, the microbiota, and blood pressure regulation. Gut Microbes 2014, 5, 202–207. [Google Scholar] [CrossRef]
- Mishra, S.P.; Karunakar, P.; Taraphder, S.; Yadav, H. Free Fatty Acid Receptors 2 and 3 as Microbial Metabolite Sensors to Shape Host Health: Pharmacophysiological View. Biomedicines 2020, 8, 154. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, S.; Cani, P.D.; Neyrinck, A.M.; Stärkel, P.; Jamar, F.; Mikolajczak, M.; Delzenne, N.M.; De Timary, P. Role of intestinal permeability and inflammation in the biological and behavioral control of alcohol-dependent subjects. Brain Behav. Immun. 2012, 26, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Mews, P.; Egervari, G.; Nativio, R.; Sidoli, S.; Donahue, G.; Lombroso, S.I.; Alexander, D.C.; Riesche, S.L.; Heller, E.A.; Nestler, E.J.; et al. Alcohol metabolism contributes to brain histone acetylation. Nature 2019, 574, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, J.; Neff, S.; Sutton, B.; Ellis, S.; Patten, L.; Brown, M.S.; Hoffman, P.L.; Tabakoff, B.; Burnham, E.L. Effects of acetate on cerebral blood flow, systemic inflammation, and behavior in alcohol use disorder. Alcohol Clin. Exp. Res. 2021, 45, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.A.; Speed, N.M.; Gross, M.D.; Lucey, M.R.; Bazakis, A.M.; Hariharan, M.; Beresford, T.P. Acute effects of alcohol administration on regional cerebral blood flow: The role of acetate. Alcohol Clin. Exp. Res. 1993, 17, 1119–1123. [Google Scholar] [CrossRef]
- Reisenauer, C.J.; Bhatt, D.P.; Mitteness, D.J.; Slanczka, E.R.; Gienger, H.M.; Watt, J.A.; Rosenberger, T.A. Acetate supplementation attenuates lipopolysaccharide-induced neuroinflammation. J. Neurochem. 2011, 117, 264–274. [Google Scholar] [CrossRef]
- van de Wouw, M.; Boehme, M.; Lyte, J.M.; Wiley, N.; Strain, C.; O’Sullivan, O.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Short-chain fatty acids: Microbial metabolites that alleviate stress-induced brain-gut axis alterations. J. Physiol. 2018, 596, 4923–4944. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, J.; Wang, F.; Hong, G.; Pang, M.; Xu, H.; Li, H.; Tian, F.; Fang, R.; Yao, Y.; Liu, J. Antidepressant-like effects of sodium butyrate and its possible mechanisms of action in mice exposed to chronic unpredictable mild stress. Neurosci. Lett. 2016, 618, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, J.; Liu, C.-P.; Guo, M.; Gao, C.-L.; Zhou, L.-P.; Long, Y.; Xu, Y. Butyrate ameliorates alcoholic fatty liver disease via reducing endotoxemia and inhibiting liver gasdermin D-mediated pyroptosis. Ann. Transl. Med. 2021, 9, 873. [Google Scholar] [CrossRef]
- Bokoliya, S.C.; Russell, J.; Dorsett, Y.; Panier, H.A.; Singh, V.; Daddi, L.; Yuan, H.; Dedon, L.R.; Liu, Z.; Zhou, Y.; et al. Short-chain fatty acid valerate reduces voluntary alcohol intake in male mice. Microbiome 2024, 12, 108. [Google Scholar] [CrossRef]
- Szczesniak, O.; Hestad, K.A.; Hanssen, J.F.; Rudi, K. Isovaleric acid in stool correlates with human depression. Nutr. Neurosci. 2015, 19, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef] [PubMed]
- Fiore, A.; Murray, P.J. Tryptophan and indole metabolism in immune regulation. Curr. Opin. Immunol. 2021, 70, 7–14. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 2017, 112 Pt B, 399–412. [Google Scholar] [CrossRef]
- Moroni, F.; Cozzi, A.; Sili, M.; Mannaioni, G. Kynurenic acid: A metabolite with multiple actions and multiple targets in brain and periphery. J. Neural Transm. 2012, 119, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W. Does kynurenic acid act on nicotinic receptors? An assessment of the evidence. J. Neurochem. 2020, 152, 627–649. [Google Scholar] [CrossRef]
- Stone, T.; Perkins, M. Quinolinic acid: A potent endogenous excitant at amino acid receptors in CNS. Eur. J. Pharmacol. 1981, 72, 411–412. [Google Scholar] [CrossRef]
- Schwarcz, R.; Bruno, J.P.; Muchowski, P.J.; Wu, H.-Q. Kynurenines in the mammalian brain: When physiology meets pathology. Nat. Rev. Neurosci. 2012, 13, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.; Mendonca, R.; Gazzard, L.; Pastor, R.; Goon, L.; Gustafson, A.; VanderPorten, E.; Hatzivassiliou, G.; Dement, K.; Cass, R.; et al. Aminoisoxazoles as Potent Inhibitors of Tryptophan 2,3-Dioxygenase 2 (TDO2). ACS Med. Chem. Lett. 2018, 9, 417–421. [Google Scholar] [CrossRef]
- Gao, J.; Deng, F.; Jia, W. Inhibition of Indoleamine 2,3-Dioxygenase Enhances the Therapeutic Efficacy of Immunogenic Chemotherapeutics in Breast Cancer. J. Breast Cancer 2019, 22, 196–209. [Google Scholar] [CrossRef]
- Leclercq, S.; Stärkel, P.; Delzenne, N.M.; de Timary, P. The gut microbiota: A new target in the management of alcohol dependence? Alcohol 2019, 74, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, S.; Schwarz, M.; Delzenne, N.M.; Stärkel, P.; de Timary, P. Alterations of kynurenine pathway in alcohol use disorder and abstinence: A link with gut microbiota, peripheral inflammation and psychological symptoms. Transl. Psychiatry 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Badawy, A.A.-B.; Bano, S.; Steptoe, A. Tryptophan in alcoholism treatment I: Kynurenine metabolites inhibit the rat liver mitochondrial low km aldehyde dehydrogenase activity, elevate blood acetaldehyde concentration and induce aversion to alcohol. Alcohol Alcohol. 2011, 46, 651–660. [Google Scholar] [CrossRef]
- Gershon, M.D. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr. Opin. Endocrinol. Diabetes Obes 2013, 20, 14–21. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Reigstad, C.S.; Salmonson, C.E.; Rainey, J.F., III; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015, 29, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 2013, 18, 666–673. [Google Scholar] [CrossRef]
- Glebov, K.; Löchner, M.; Jabs, R.; Lau, T.; Merkel, O.; Schloss, P.; Steinhäuser, C.; Walter, J. Serotonin stimulates secretion of exosomes from microglia cells. Glia 2015, 63, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Feehily, C.; Karatzas, K. Role of glutamate metabolism in bacterial responses towards acid and other stresses. J. Appl. Microbiol. 2013, 114, 11–24. [Google Scholar] [CrossRef]
- Matsumoto, M.; Kibe, R.; Ooga, T.; Aiba, Y.; Sawaki, E.; Koga, Y.; Benno, Y. Cerebral low-molecular metabolites influenced by intestinal microbiota: A Pilot Study. Front. Syst. Neurosci. 2013, 7, 9. [Google Scholar] [CrossRef]
- Davies, M. The role of GABAA receptors in mediating the effects of alcohol in the central nervous system. J. Psychiatry Neurosci. 2003, 28, 263–274. [Google Scholar]
- Zhang, P.; Yang, M.; Chen, C.; Liu, L.; Wei, X.; Zeng, S. Toll-Like Receptor 4 (TLR4)/Opioid Receptor Pathway Crosstalk and Impact on Opioid Analgesia, Immune Function, and Gastrointestinal Motility. Front. Immunol. 2020, 11, 1455. [Google Scholar] [CrossRef] [PubMed]
- Morley, K.C.; Lagopoulos, J.; Logge, W.; Baillie, A.; Adams, C.; Haber, P.S. Brain GABA levels are reduced in alcoholic liver disease: A proton magnetic resonance spectroscopy study. Addict. Biol. 2020, 25, e12702. [Google Scholar] [CrossRef] [PubMed]
- Guzior, D.V.; Quinn, R.A. Review: Microbial transformations of human bile acids. Microbiome 2021, 9, 140. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Zhang, Q.; Ruan, Y.; Hu, M.; Liu, Z.; Gong, L. Chronic Alcohol Consumption Increased Bile Acid Levels in Enterohepatic Circulation and Reduced Efficacy of Irinotecan. Alcohol Alcohol. 2020, 55, 264–277. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Mackowiak, B.; Lin, Y.-H.; Maccioni, L.; Lehner, T.; Pan, H.; Guan, Y.; Godlewski, G.; Lu, H.; Chen, C.; et al. Coordinated action of a gut–liver pathway drives alcohol detoxification and consumption. Nat. Metab. 2024, 6, 1380–1396. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, S.; Matamoros, S.; Cani, P.D.; Neyrinck, A.M.; Jamar, F.; Stärkel, P.; Windey, K.; Tremaroli, V.; Bäckhed, F.; Verbeke, K.; et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc. Natl. Acad. Sci. USA 2014, 111, E4485–E4493. [Google Scholar] [CrossRef]
- Bishehsari, F.; Magno, E.; Swanson, G.; Desai, V.; Voigt, R.M.; Forsyth, C.B.; Keshavarzian, A. Alcohol and Gut-Derived Inflammation. Alcohol Res. Curr. Rev. 2017, 38, 163–171. [Google Scholar]
- Forsyth, C.B.; Farhadi, A.; Jakate, S.M.; Tang, Y.; Shaikh, M.; Keshavarzian, A. Lactobacillus GG treatment ameliorates alcohol-induced intestinal oxidative stress, gut leakiness, and liver injury in a rat model of alcoholic steatohepatitis. Alcohol 2009, 43, 163–172. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, J.; Chang, B.; Wang, B.; Zhang, D.; Wang, B. Effects of alcohol on intestinal epithelial barrier permeability and expression of tight junction-associated proteins. Mol. Med. Rep. 2014, 9, 2352–2356. [Google Scholar] [CrossRef]
- Simet, S.M.; Wyatt, T.A.; DeVasure, J.; Yanov, D.; Allen-Gipson, D.; Sisson, J.H. Alcohol increases the permeability of airway epithelial tight junctions in Beas-2B and NHBE cells. Alcohol Clin. Exp. Res. 2012, 36, 432–442. [Google Scholar] [CrossRef]
- Kuo, C.; Wu, L.; Chen, H.; Yu, J.; Wu, C. Direct effects of alcohol on gut-epithelial barrier: Unraveling the disruption of physical and chemical barrier of the gut-epithelial barrier that compromises the host–microbiota interface upon alcohol exposure. J. Gastroenterol. Hepatol. 2024, 39, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, C.B.; Voigt, R.M.; Shaikh, M.; Tang, Y.; Cederbaum, A.I.; Turek, F.W.; Keshavarzian, A. Role for intestinal CYP2E1 in alcohol-induced circadian gene-mediated intestinal hyperpermeability. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G185–G195. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, S.; De Saeger, C.; Delzenne, N.; de Timary, P.; Stärkel, P. Role of inflammatory pathways, blood mononuclear cells, and gut-derived bacterial products in alcohol dependence. Biol. Psychiatry 2014, 76, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Blaine, S.K.; Ridner, C.M.; Campbell, B.R.; Crone, L.; Claus, E.D.; Wilson, J.R.; West, S.N.; McClanahan, A.J.; Siddiq, A.S.; Layman, I.M.; et al. IL-6, but not TNF-α, response to alcohol cues and acute consumption associated with neural cue reactivity, craving, and future drinking in binge drinkers. Brain Behav. Immun.-Health 2023, 31, 100645. [Google Scholar] [CrossRef] [PubMed]
- Meyer, F.; Wendling, D.; Demougeot, C.; Prati, C.; Verhoeven, F. Cytokines and intestinal epithelial permeability: A systematic review. Autoimmun. Rev. 2023, 22, 103331. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Yuan, B.; Lu, X.; Zheng, D.; Zhang, K.; Duan, X. GVS-12 attenuates non-alcoholic steatohepatitis by suppressing inflammatory responses via PPARgamma/STAT3 signaling pathways. RSC Adv. 2019, 9, 9555–9564. [Google Scholar] [CrossRef]
- Zhang, Z.; Leng, Z.; Kang, L.; Yan, X.; Shi, J.; Ji, Y.; Xu, M. Alcohol inducing macrophage M2b polarization in colitis by modulating the TRPV1-MAPK/NF-kappaB pathways. Phytomedicine 2024, 130, 155580. [Google Scholar] [CrossRef]
- Jacobse, J.; Li, J.; Rings, E.H.H.M.; Samsom, J.N.; Goettel, J.A. Intestinal Regulatory T Cells as Specialized Tissue-Restricted Immune Cells in Intestinal Immune Homeostasis and Disease. Front. Immunol. 2021, 12, 716499. [Google Scholar] [CrossRef]
- Crews, F.T.; Sarkar, D.K.; Qin, L.; Zou, J.; Boyadjieva, N.; Vetreno, R.P. Neuroimmune Function and the Consequences of Alcohol Exposure. Alcohol Res. 2015, 37, 331–351. [Google Scholar] [PubMed]
- Alfonso-Loeches, S.; Pascual-Lucas, M.; Blanco, A.M.; Sanchez-Vera, I.; Guerri, C. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J. Neurosci. 2010, 30, 8285–8295. [Google Scholar] [CrossRef]
- Czerwińska-Błaszczyk, A.; Pawlak, E.; Pawłowski, T. The Significance of Toll-Like Receptors in the Neuroimmunologic Background of Alcohol Dependence. Front. Psychiatry 2021, 12, 797123. [Google Scholar] [CrossRef]
- Warden, A.S.; Azzam, M.; DaCosta, A.; Mason, S.; Blednov, Y.A.; Messing, R.O.; Mayfield, R.D.; Harris, R.A. Toll-like receptor 3 activation increases voluntary alcohol intake in C57BL/6J male mice. Brain Behav. Immun. 2018, 77, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Grantham, E.; Warden, A.; McCarthy, G.; DaCosta, A.; Mason, S.; Blednov, Y.; Mayfield, R.; Harris, R. Role of toll-like receptor 7 (TLR7) in voluntary alcohol consumption. Brain Behav. Immun. 2020, 89, 423–432. [Google Scholar] [CrossRef]
- Coleman, L.G., Jr.; Zou, J.; Crews, F.T. Microglial-derived miRNA let-7 and HMGB1 contribute to ethanol-induced neurotoxicity via TLR7. J. Neuroinflamm. 2017, 14, 1–15. [Google Scholar] [CrossRef]
- Foster, K.L.; McKay, P.F.; Seyoum, R.; Milbourne, D.; Yin, W.; Sarma, P.V.V.S.; June, H.L. GABA(A) and opioid receptors of the central nucleus of the amygdala selectively regulate ethanol-maintained behaviors. Neuropsychopharmacology 2004, 29, 269–284. [Google Scholar] [CrossRef]
- Balan, I.; Warnock, K.T.; Puche, A.; Gondre-Lewis, M.C.; June, H.; Aurelian, L. The GABA(A) Receptor alpha2 Subunit Activates a Neuronal TLR4 Signal in the Ventral Tegmental Area that Regulates Alcohol and Nicotine Abuse. Brain Sci. 2018, 8, 72. [Google Scholar] [CrossRef]
- Blednov, Y.; Benavidez, J.; Geil, C.; Perra, S.; Morikawa, H.; Harris, R. Activation of inflammatory signaling by lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice. Brain Behav. Immun. 2011, 25, S92–S105. [Google Scholar] [CrossRef]
- Montesinos, J.; Pascual, M.; Pla, A.; Maldonado, C.; Rodríguez-Arias, M.; Miñarro, J.; Guerri, C. TLR4 elimination prevents synaptic and myelin alterations and long-term cognitive dysfunctions in adolescent mice with intermittent ethanol treatment. Brain Behav. Immun. 2015, 45, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Pascual, M.; Montesinos, J.; Montagud-Romero, S.; Forteza, J.; Rodríguez-Arias, M.; Miñarro, J.; Guerri, C. TLR4 response mediates ethanol-induced neurodevelopment alterations in a model of fetal alcohol spectrum disorders. J. Neuroinflamm. 2017, 14, 145. [Google Scholar] [CrossRef] [PubMed]
- Riveros, M.E.; Leibold, N.K.; Retamal, M.A.; Ezquer, F. Role of histaminergic regulation of astrocytes in alcohol use disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2024, 133, 111009. [Google Scholar] [CrossRef] [PubMed]
- Ponomarev, I.; Wang, S.; Zhang, L.; Harris, R.A.; Mayfield, R.D. Gene coexpression networks in human brain identify epigenetic modifications in alcohol dependence. J. Neurosci. 2012, 32, 1884–1897. [Google Scholar] [CrossRef]
- Erickson, E.K.; Farris, S.P.; Blednov, Y.A.; Mayfield, R.D.; Harris, R.A. Astrocyte-specific transcriptome responses to chronic ethanol consumption. Pharmacogenomics J. 2018, 18, 578–589. [Google Scholar] [CrossRef]
- Erickson, E.K.; Grantham, E.K.; Warden, A.S.; Harris, R. Neuroimmune signaling in alcohol use disorder. Pharmacol. Biochem. Behav. 2019, 177, 34–60. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, K.K. Glial fibrillary acidic protein: From intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci. 2015, 38, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Ushakova, G.; Fed’Kiv, O.; Prykhod’Ko, O.; Pierzynowski, S.; Kruszewska, D. The effect of long-term lactobacilli (lactic acid bacteria) enteral treatment on the central nervous system of growing rats. J. Nutr. Biochem. 2009, 20, 677–684. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, Z.; Liu, X.; Jin, G.; Wang, Y.; He, L.; Li, M.; Pang, X.; Yan, B.; Jia, Z.; et al. Dietary emulsifier polysorbate 80 exposure accelerates age-related cognitive decline. Brain Behav. Immun. 2024, 119, 171–187. [Google Scholar] [CrossRef] [PubMed]
- Erny, D.; Dokalis, N.; Mezö, C.; Castoldi, A.; Mossad, O.; Staszewski, O.; Frosch, M.; Villa, M.; Fuchs, V.; Mayer, A.; et al. Microbiota-derived acetate enables the metabolic fitness of the brain innate immune system during health and disease. Cell Metab. 2021, 33, 2260–2276.e7. [Google Scholar] [CrossRef]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Prinz, M. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Warden, A.S.; Triplett, T.A.; Lyu, A.; Grantham, E.K.; Azzam, M.M.; DaCosta, A.; Mason, S.; Blednov, Y.A.; Ehrlich, L.I.; Mayfield, R.D.; et al. Microglia depletion and alcohol: Transcriptome and behavioral profiles. Addict. Biol. 2021, 26, e12889. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, J.; Zhang, H.; Li, Y.; Wen, L.; Tan, X.; Cheng, K.; Liu, Y.; Pu, J.; Liu, L.; et al. The gut microbiome modulates the transformation of microglial subtypes. Mol. Psychiatry 2023, 28, 1611–1621. [Google Scholar] [CrossRef] [PubMed]
- Hickman, S.E.; El Khoury, J. TREM2 and the neuroimmunology of Alzheimer’s disease. Biochem. Pharmacol. 2014, 88, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Melbourne, J.K.; Thompson, K.R.; Peng, H.; Nixon, K. Its complicated: The relationship between alcohol and microglia in the search for novel pharmacotherapeutic targets for alcohol use disorders. Prog. Mol. Biol. Transl. Sci. 2019, 167, 179–221. [Google Scholar] [PubMed]
- He, J.; Crews, F.T. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp. Neurol. 2008, 210, 349–358. [Google Scholar] [CrossRef]
- Qin, L.; Crews, F.T. NADPH oxidase and reactive oxygen species contribute to alcohol-induced microglial activation and neurodegeneration. J. Neuroinflamm. 2012, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Berk, M.; Williams, L.J.; Jacka, F.N.; O’Neil, A.; Pasco, J.A.; Moylan, S.; Maes, M. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013, 11, 200. [Google Scholar] [CrossRef]
- Rajkowska, G.; Stockmeier, C.A. Astrocyte pathology in major depressive disorder: Insights from human postmortem brain tissue. Curr. Drug Targets 2013, 14, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Goshen, I.; Kreisel, T.; Ben-Menachem-Zidon, O.; Licht, T.; Weidenfeld, J.; Ben-Hur, T.; Yirmiya, R. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol. Psychiatry 2008, 13, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Gorky, J.; Schwaber, J. The role of the gut–brain axis in alcohol use disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2015, 65, 234–241. [Google Scholar] [CrossRef]
- Amadieu, C.; Coste, V.; Neyrinck, A.M.; Thijssen, V.; Leyrolle, Q.; Bindels, L.B.; Piessevaux, H.; Stärkel, P.; de Timary, P.; Delzenne, N.M.; et al. Restoring an adequate dietary fiber intake by inulin supplementation: A pilot study showing an impact on gut microbiota and sociability in alcohol use disorder patients. Gut Microbes 2022, 14, 2007042. [Google Scholar] [CrossRef] [PubMed]
- Dalile, B.; Vervliet, B.; Bergonzelli, G.; Verbeke, K.; Van Oudenhove, L. Colon-delivered short-chain fatty acids attenuate the cortisol response to psychosocial stress in healthy men: A randomized, placebo-controlled trial. Neuropsychopharmacology 2020, 45, 2257–2266. [Google Scholar] [CrossRef] [PubMed]
- Dalile, B.; Fuchs, A.; La Torre, D.; Vervliet, B.; Van Oudenhove, L.; Verbeke, K. Colonic butyrate administration modulates fear memory but not the acute stress response in men: A randomized, triple-blind, placebo-controlled trial. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2024, 131, 110939. [Google Scholar] [CrossRef] [PubMed]
- Chong, H.X.; Yusoff, N.A.A.; Hor, Y.Y.; Lew, L.C.; Jaafar, M.H.; Choi, S.-B.; Yusoff, M.S.B.; Wahid, N.; Bin Abdullah, M.F.I.L.; Zakaria, N.; et al. Lactobacillus plantarum DR7 alleviates stress and anxiety in adults: A randomised, double-blind, placebo-controlled study. Benef. Microbes 2019, 10, 355–373. [Google Scholar] [CrossRef]
- Rudzki, L.; Ostrowska, L.; Pawlak, D.; Małus, A.; Pawlak, K.; Waszkiewicz, N.; Szulc, A. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology 2019, 100, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Chen, Y.; Zhu, H.; Wang, L.; Qian, X.; Zou, R.; Zhao, J.; Zhang, H.; Qian, L.; Wang, Q.; et al. Bifidobacterium breve CCFM1025 attenuates major depression disorder via regulating gut microbiome and tryptophan metabolism: A randomized clinical trial. Brain Behav. Immun. 2022, 100, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Vatsalya, V.; Feng, W.; Kong, M.; Hu, H.; Szabo, G.; McCullough, A.; Dasarathy, S.; Nagy, L.E.; Radaeva, S.; Barton, B.; et al. The Beneficial Effects of Lactobacillus GG Therapy on Liver and Drinking Assessments in Patients with Moderate Alcohol-Associated Hepatitis. Am. J. Gastroenterol. 2023, 118, 1457–1460. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Gavis, E.A.; Fagan, A.; Wade, J.B.; Thacker, L.R.; Fuchs, M.; Patel, S.; Davis, B.; Meador, J.; Puri, P.; et al. A Randomized Clinical Trial of Fecal Microbiota Transplant for Alcohol Use Disorder. Hepatology 2021, 73, 1688–1700. [Google Scholar] [CrossRef] [PubMed]
- Philips, C.A.; Ahamed, R.; Rajesh, S.; Abduljaleel, J.K.; Augustine, P. Long-term Outcomes of Stool Transplant in Alcohol-associated Hepatitis-Analysis of Clinical Outcomes, Relapse, Gut Microbiota and Comparisons with Standard Care. J. Clin. Exp. Hepatol. 2022, 12, 1124–1132. [Google Scholar] [CrossRef]
- Petrakis, I.L.; Ralevski, E.; Gueorguieva, R.; Sloan, M.E.; Devine, L.; Yoon, G.; Arias, A.J.; Sofuoglu, M. Targeting neuroinflammation with minocycline in heavy drinkers. Psychopharmacology 2019, 236, 3013–3021. [Google Scholar] [CrossRef]
- Barkley-Levenson, A.M.; Crabbe, J.C. Bridging Animal and Human Models: Translating From (and to) Animal Genetics. Alcohol Res. 2012, 34, 325–335. [Google Scholar]
- Hugenholtz, F.; de Vos, W.M. Mouse models for human intestinal microbiota research: A critical evaluation. Cell Mol. Life Sci. 2018, 75, 149–160. [Google Scholar] [CrossRef]
- Krych, L.; Hansen, C.H.F.; Hansen, A.K.; Berg, F.W.J.V.D.; Nielsen, D.S. Quantitatively different, yet qualitatively alike: A meta-analysis of the mouse core gut microbiome with a view towards the human gut microbiome. PLoS ONE 2013, 8, e62578. [Google Scholar] [CrossRef] [PubMed]
- Rawls, J.F.; Mahowald, M.A.; Ley, R.E.; Gordon, J.I. Reciprocal gut microbiota transplants from zebrafish and mice to germ-free recipients reveal host habitat selection. Cell 2006, 127, 423–433. [Google Scholar] [CrossRef]
- Wahl, A.; Yao, W.; Liao, B.; Chateau, M.; Richardson, C.; Ling, L.; Franks, A.; Senthil, K.; Doyon, G.; Li, F.; et al. A germ-free humanized mouse model shows the contribution of resident microbiota to human-specific pathogen infection. Nat. Biotechnol. 2024, 42, 905–915. [Google Scholar] [CrossRef]
- Chung, H.; Pamp, S.J.; Hill, J.A.; Surana, N.K.; Edelman, S.M.; Troy, E.B.; Reading, N.C.; Villablanca, E.J.; Wang, S.; Mora, J.R.; et al. Gut immune maturation depends on colonization with a host-specific microbiota. Cell 2012, 149, 1578–1593. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, R.; Toft, M.F.; Metzdorff, S.B.; Hansen, C.H.F.; Licht, T.R.; Bahl, M.I.; Hansen, A.K. Human microbiota-transplanted C57BL/6 mice and offspring display reduced establishment of key bacteria and reduced immune stimulation compared to mouse microbiota-transplantation. Sci. Rep. 2020, 10, 7805. [Google Scholar] [CrossRef] [PubMed]
- Marcobal, A.; Kashyap, P.C.; Nelson, T.A.; Aronov, P.A.; Donia, M.S.; Spormann, A.; Fischbach, M.A.; Sonnenburg, J.L. A metabolomic view of how the human gut microbiota impacts the host metabolome using humanized and gnotobiotic mice. ISME J. 2013, 7, 1933–1943. [Google Scholar] [CrossRef] [PubMed]
- Wolstenholme, J.T.; Saunders, J.M.; Smith, M.; Kang, J.D.; Hylemon, P.B.; González-Maeso, J.; Fagan, A.; Zhao, D.; Sikaroodi, M.; Herzog, J.; et al. Reduced alcohol preference and intake after fecal transplant in patients with alcohol use disorder is transmissible to germ-free mice. Nat. Commun. 2022, 13, 6198. [Google Scholar] [CrossRef]
- Rizal, N.S.M.; Neoh, H.-M.; Ramli, R.; Periyasamy, P.R.A.K.; Hanafiah, A.; Samat, M.N.A.; Tan, T.L.; Wong, K.K.; Nathan, S.; Chieng, S.; et al. Advantages and Limitations of 16S rRNA Next-Generation Sequencing for Pathogen Identification in the Diagnostic Microbiology Laboratory: Perspectives from a Middle-Income Country. Diagnostics 2020, 10, 816. [Google Scholar]
- Ng, Q.X.; Lim, Y.L.; Yaow, C.Y.L.; Ng, W.K.; Thumboo, J.; Liew, T.M. Effect of Probiotic Supplementation on Gut Microbiota in Patients with Major Depressive Disorders: A Systematic Review. Nutrients 2023, 15, 1351. [Google Scholar] [CrossRef] [PubMed]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the Human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef]
- White, B.; Sirohi, S. A Complex Interplay between Nutrition and Alcohol use Disorder: Implications for Breaking the Vicious Cycle. Curr. Pharm. Des. 2024, 30, 1822–1837. [Google Scholar] [CrossRef]
- Amadieu, C.; Leclercq, S.; Coste, V.; Thijssen, V.; Neyrinck, A.M.; Bindels, L.B.; Cani, P.D.; Piessevaux, H.; Stärkel, P.; de Timary, P.; et al. Dietary fiber deficiency as a component of malnutrition associated with psychological alterations in alcohol use disorder. Clin. Nutr. 2021, 40, 2673–2682. [Google Scholar] [CrossRef] [PubMed]
- Butts, M.; Sundaram, V.L.; Murughiyan, U.; Borthakur, A.; Singh, S. The Influence of Alcohol Consumption on Intestinal Nutrient Absorption: A Comprehensive Review. Nutrients 2023, 15, 1571. [Google Scholar] [CrossRef]
- Allaband, C.; Lingaraju, A.; Ramos, S.F.; Kumar, T.; Javaheri, H.; Tiu, M.D.; Machado, A.C.D.; Richter, R.A.; Elijah, E.; Haddad, G.G.; et al. Time of sample collection is critical for the replicability of microbiome analyses. Nat. Metab. 2024, 6, 1282–1293. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).